Introduction
The nipple array is a nano-scale structure found on the surface of some metazoans, and is usually comprised of protuberances of about 100 nm or less in height. This structure was originally described as nipples on the compound eyes of a night moth (Bernhard, Reference Bernhard1967) and known to form a gradient of refractivity, resulting in a reduction of light reflectance (moth-eye effect) (Bernhard, Reference Bernhard1967; Wilson & Hutley, Reference Wilson and Hutley1982). The presence of the nipple array has been reported in marine invertebrates across various taxa, such as tunicates (Hirose et al., Reference Hirose, Saito, Hashimoto and Watanabe1990, Reference Hirose, Nishikawa, Saito and Watanabe1992, Reference Hirose, Lambert, Kusakabe and Nishikawa1997, Reference Hirose, Kimura, Itoh and Nishikawa1999), echinoderms (Holland, Reference Holland, Bereiter-Hahn, Matoltsy and Richards1984), annelids (Hausen, Reference Hausen2005), parasitic copepods (Hirose & Uyeno, Reference Hirose and Uyeno2014, Reference Hirose and Uyeno2016; Uyeno & Hirose, Reference Uyeno and Hirose2018) and entoprocts (Iseto & Hirose, Reference Iseto and Hirose2010). Because of the histological differences in integumentary tissues among the taxa, the nipple arrays of phylogenetically distant taxa would have convergently evolved in each lineage. Moreover, the comprehensive survey in ascidians showed that the nipple array usually occurs in many stolidobranchs and the species of Polyclinidae, Clavelinidae, Agneziidae with some exceptions that have a very thick cuticular layer (Hirose et al., Reference Hirose, Lambert, Kusakabe and Nishikawa1997). It is absent in other ascidian families such as Cionidae and Ascidiidae, but Ueki et al. (Reference Ueki, Koike, Fukuba and Yamaguchi2018) found the protrusions in juveniles (but not adults) of Ascidia sydneiensis samea (Ascidiidae). In salps, the protrusions were found in the species occurring in the shallow layers of the water column throughout the day but not in the species performing diel vertical migration and distributing in deeper and darker layers in the daytime (Hirose et al., Reference Hirose, Sakai, Shibata, Nishii, Mayama, Miyauchi and Nishikawa2015). These findings indicate that considerable function(s) of the nipple array exist in an aquatic environment.
Reduction in reflectance should be an important function to camouflage the body from predators. According to our simulation in some tunicates (salps and ascidians), the moth-eye effect can be expected on a nipple array under water, but the effect is much smaller than that in an aerial environment because of the small difference in refractive indices between seawater and the body surface (Hirose et al., Reference Hirose, Sakai, Shibata, Nishii, Mayama, Miyauchi and Nishikawa2015; Kakiuchida et al., Reference Kakiuchida, Sakai, Nishikawa and Hirose2017; Sakai et al., Reference Sakai, Kakiuchida, Nishikawa and Hirose2018). Moreover, anti-reflection properties would rarely be needed for meso- and endoparasites whose bodies are always covered with host tissues (Hirose & Uyeno, Reference Hirose and Uyeno2014, Reference Hirose and Uyeno2016; Uyeno & Hirose, Reference Uyeno and Hirose2018), and thus, the nipple array is expected to have additional functions, such as bubble repellency (Hirose et al., Reference Hirose, Mayama and Miyauchi2013) and suppression of the activities of phagocytic haemocytes (Ballarin et al., Reference Ballarin, Franchi, Gasparini, Caicci, Miyauchi and Hirose2015). In this study, we hypothesize that the nipple array may prevent the settlement of epibionts that are often a nuisance and can potentially cause serious problems for the host. We tested substrate selection during ascidian larval settlement using MOSMITE™, which is a biomimetic, anti-reflective film that mimics the moth-eye structure (https://www.m-chemical.co.jp/en/products/departments/mcc/hp-films-pl/product/1201267_7568.html).
Materials and methods
MOSMITE™ (E075M2N) and flat film were generously provided by Mitsubishi Chemical (Tokyo, Japan). The base material of both films was polyethylene terephthalate coated with acrylic resin. On one side of the film was the moth-eye structure, an array of nipples about 100 nm in height, but there were no structures on the flat film (Figure 1). The surface image of MOSMITE™ was closely similar in size and density to the nipple array on stolidobranch ascidians (Figure 1C). Because the surface wettability affects the larval settlement rate in ascidians (Cloney, Reference Cloney1990; Gerhart et al., Reference Gerhart, Rittschof, Hooper, Eisenman, Meyer, Baier and Young1992; Rittschof et al., Reference Rittschof, Forward, Cannon, Welch, McClary, Holm, Clare, Conova, McKelvey, Bryan and Van Dover1998), we measured the wettability of MOSMITE™ and the flat film. Wettability was determined on the contact angles of water in the air and those of air and mineral oil in artificial seawater (Marine Art SF-1; Osakayakken, Osaka, Japan) with a contact angle meter, LSE-ME2 (NiCK, Kawaguchi, Japan). Regarding the measurements in seawater, we suspended the films, test side down, in the artificial seawater and measured the contact angles of the air bubbles or oil droplets. We measured the contact angles 10 times at different points on the films in each of the measurements.

Fig. 1. Scanning electron micrographs of the moth-eye structures on MOSMITE™ (A), the flat surface on the flat film (B), and the nipple arrays on the tunic cuticle of the colonial ascidian Botryllus delicatus (Stolidobranchia: Botryllidae) (C). Scale bars = 1 µm.
The solitary ascidian Phallusia philippinensis Millar, 1975 is commonly found in the tropical Indo-Pacific (Vandepas et al., Reference Vandepas, Oliveira, Lee, Hirose, Rocha and Swalla2015). This ascidiid species does not have nipple array, while its body is usually clean of epibionts (Hirose, Reference Hirose1999). The ascidians were collected at Ginowan Port Marina (Okinawa, Japan) and mature gametes were obtained from gonoducts by dissection. Eggs were inseminated with sperm from another individual in the artificial seawater. Following artificial fertilization, the embryos were incubated at 25–26°C for 23–24 h until the larvae hatched. One MOSMITE™ and one flat film (18 × 36 mm for each) were floated on top of the artificial seawater (pH 8.2), with the test side down, in a plastic dish (60 mm diameter) of which the inner surface was coated with 1.5% agar in order to avoid the attachment of the ascidian larvae on the dish wall (e.g. Matsunobu & Sasakura, Reference Matsunobu and Sasakura2015). Then, 500–2000 larvae were added to the dish. After incubation for 48 h at 25–26°C in continuous darkness, we counted the settled larva on the MOSMITE™ and the flat film. Because the edges of the films may have unexpected effects on larval settlement, we did not count the settled larvae on the 0.5 mm margin of the films. The area for counting was 17 × 35 mm for each film. Eight sets of testing were carried out and the numbers of settled larva were compared between the MOSMITE™ and the flat film by the paired t-test, following the Shapiro–Wilk test for normality using R (R Core Team, 2018).
Some of the larvae which had settled on the films were photographed under a light microscope 5, 12 and 24 h after hatching. To increase the depth of field, we combined 15–20 micrographs for each image using the post-processing image software Helicon Focus Pro 6.72 (Helicon Soft, Ltd, Kharkov, Ukraine).
Some settled larvae after 5 h from hatching were fixed with 2.5% glutaraldehyde in 0.45 M sucrose–0.1 M cacodylate buffer (pH 7.5) at 4°C for 2 h. They were rinsed with 0.45 M sucrose and 0.1 M cacodylate buffer (pH 7.5) and post-fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.5) at 4°C for 1.5 h. The specimens were dehydrated through an ethanol series and embedded in an epoxy resin (Epon 812, TAAB Laboratories). Thin sections were stained with uranyl acetate and lead citrate, and examined in a transmission electron microscope (JEM-1011; JEOL, Tokyo, Japan) at 80 kV.
Results
The wettability of the films, represented by the contact angle, is summarized in Table 1. While both films are hydrophilic in the air (small contact angle of water) and seawater (large contact angle of air) and have high oil-repellency in the seawater (large contact angle of oil) (Figure 2), the MOSMITE™ is more hydrophilic and oil repellent than the flat film.
Table 1. Contact angles (°) of water, air and mineral oil on MOSMITE™ and flat film


Fig. 2. Water droplets, air bubbles and oil droplets on the MOSMITE™ and on the flat film.
In the substrate selection test, larvae settled on both films (Figure 3A, B), but the number of settled larvae on the flat film was consistently higher than on the MOSMITE™ in the eight sets of testing (Figure 3C, Table 2). The numbers were significantly different between the film types (paired t-test, P < 0.01). In the whole-mount specimens, we could not find significant morphological differences between settled larvae on the MOSMITE™ and the flat film within 24 h (Figure 4).

Fig. 3. Larval settlement in the substrate selection test. (A) Settled larvae on the MOSMITE™. The open circle indicates one larva settled on the film. (B) Settled larvae on the flat film. (C) Comparison of the settled larvae on the 17 × 35 mm areas of the MOSMITE™ and flat film. Dotted line indicates equal numbers on both films. Scale bars = 1 mm.
Table 2. Number of settled larvae on MOSMITE™ and flat film

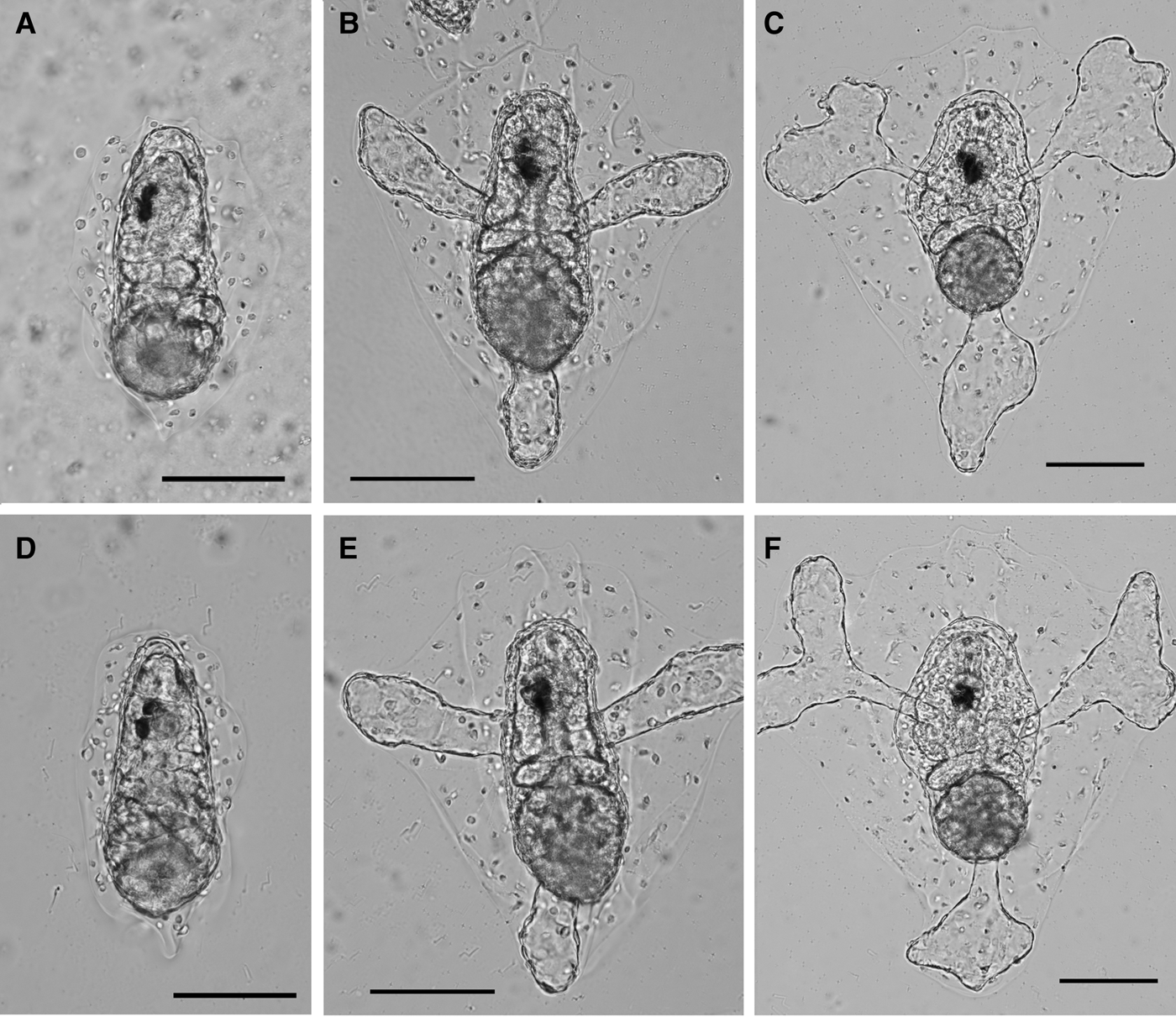
Fig. 4. Settled larvae on the MOSMITE™ (A–C) and flat film (D–F) after 5 h (A, D), 12 h (B, E) and 24 h (C, F) of hatching. Scale bars = 100 µm.
During settlement, the larvae firstly adhere to the substrate with adhesive papillae, and then the integumentary matrix (tunic) of the body directly adheres to the substrate surface. On the MOSMITE™, the adhesive material secreted from the papillae penetrates into the nipple array layer and fills the space among the nipples, while the tunic cuticle of the metamorphosing larvae only adheres to the nipple tips but does not penetrate into the space among the nipples (Figure 5). The metamorphosing larvae do not have the nipple arrays on the tunic cuticle.

Fig. 5. Transmission electron micrographs of larval settlement on the MOSMITE™. (A) An adhesive papilla adheres onto the MOSMITE™ (mo). (B) Adhesive material (am) secreted from the papilla penetrates among the nipples. (C) The attachment of the tunic (tu) of metamorphosing larva on the nipple array. Two-way arrows, nipple array (moth-eye structure) on the MOSMITE™; facing arrows, cuticular layer of the tunic. Scale bars: A = 2 µm; B, C = 0.1 µm.
Discussion
Epibionts are often nuisances for the host organisms, unless there are any mutualists, as they cause resistance against motility, inhibit the translocation of molecules via the body surface, and are potential competitors for food resources. The hosts are occasionally killed by the epibionts that cover the host's mouth and/or cloaca. Anti-fouling should be an important function for many organisms, and various types of bioinspired surfaces have been investigated to control biofouling (Scardino & de Nys, Reference Scardino and de Nys2011). The present study demonstrates that a larger number of ascidian larvae settled on the flat film than the MOSMITE™. This indicates that the nipple array on the surface of some marine invertebrates may protect their body surface from the settlement of epibionts. The nipple array cannot serve as absolute protection against settlers because the ascidian larvae settled on both films. The nipple array could accomplish the prevention of settlers in combination with other countermeasures, such as chemical defences (e.g. Stoecker, Reference Stoecker1980; Slattery et al., Reference Slattery, McClintock and Heine1995; Teo & Ryland, Reference Teo and Ryland1995; Qian et al., Reference Qian, Xu and Fusetani2009). In this case, the host organism may be able to deter the settlers with a smaller amount of repellent molecules on the nipple array than that on the flat surface.
The test films used here are made of a hydrophilic material, which is suitable for this test since the body surface of many marine organisms does not repel water. The MOSMITE™ is more hydrophilic and oil-repellent than the flat film, probably because the water among the nipples makes another hydrophilic surface. In seawater, the bubbles and oil droplets apparently adhere to the nipple tips but do not penetrate into the space among the nipples, i.e. Cassie–Baxter state (Hirose et al., Reference Hirose, Mayama and Miyauchi2013). The single choice substrate test showed that the settlement rate of ascidian larvae is negatively correlated with the water-wettability of the substrate surface (Gerhart et al., Reference Gerhart, Rittschof, Hooper, Eisenman, Meyer, Baier and Young1992; Rittschof et al., Reference Rittschof, Forward, Cannon, Welch, McClary, Holm, Clare, Conova, McKelvey, Bryan and Van Dover1998). Hence, the higher wettability on the MOSMITE™ should be one of the reasons for the fewer settlers on the MOSMITE™ than the flat sheet.
At the beginning of larval settlement in ascidians, material is secreted from the adhesion papillae and adheres to the substratum. On the MOSMITE™, adhesive material fills the space among the nipples, increasing the effective area for adhesion compared with the flat surface. Accordingly, the larvae can adhere to the nipple array more tightly than the flat film, although this may be inconsistent with the larval preference of substrates. Thereafter, the juvenile ascidian directly adheres to the substratum with the tunic that is an integumentary matrix of ascidians. The ascidians on the MOSMITE™ have a smaller effective area for adhesion than the flat surface, because the tunic adheres only to the nipple tips. Therefore, the ascidians would adhere to the nipple array less tightly than the flat surface, and this may be a possible reason why the larvae prefer the flat surface to the nipple array.
The nano-scale nipple array has been found on various metazoans. While this structure is expected to be multifunctional, the major function may be different depending upon the species. The present study demonstrated the anti-fouling property of the nipple array, which is an important function especially for sessile organisms. In aquatic environments, adsorbed organic matter and microorganisms may easily cover up the nano-structures and alter the surface properties (Mihm et al., Reference Mihm, Banta and Loeb1981), but the body surface of many organisms is often free from epibionts and debris. Biological activities, such as chemical defences, would help to maintain the clean surface and functional properties. It would also be possible that the nano-structure may affect the biofilm formation on the body surface and this should be evaluated in future studies.
Author ORCID
Euichi Hirose, 0000-0001-7002-4524
Acknowledgements
We thank Yoshihiro Uozu and Yuusuke Nakai (Mitsubishi Chemical) for providing the MOSMITE™ and the flat film.
Financial support
This research was partly supported by Okinawa Research Core for Highly Innovative Discipline Science Project from University of the Ryukyus to EH.









