INTRODUCTION
Our understanding of the neuropsychological aspects of MS was quite different 30 years ago than it is today. Most medical textbooks in the 1980s viewed “intellectual” changes to be a rare occurrence in MS patients. The most common estimate was 5%, with the understanding that such changes only occurred in patients with extensive physical disability and with a long disease duration (>30 years). These opinions were fairly universally accepted throughout the 20th century, despite the astute clinical observations in 1877 by the French neurologist, Jean Charcot, that most patients with MS had a “marked enfeeblement of the memory” and “conceptions that are formed slowly” (Charcot, Reference Charcot1877).
Two major scientific events occurred during the decade between 1985 and 1995 that changed how the disease is viewed from a neuropsychological perspective. The first was the publication of large-scale, controlled studies using batteries of standardized neuropsychological measures that demonstrated the prevalence of cognitive dysfunction in MS to be in the 40–65% range (for a review, see Rao, Reference Rao1995). Furthermore, these studies showed that cognitive dysfunction is weakly correlated with physical disability (e.g., ambulation) and disease duration (Rao, Leo, Bernardin, & Unverzagt, Reference Rao, Leo, Bernardin and Unverzagt1991); is most commonly observed on measures of information processing speed (Rao, St Aubin-Faubert, & Leo, Reference Rao, St Aubin-Faubert and Leo1989) and episodic memory (Rao, Leo, et al., Reference Rao, Leo, Haughton, St Aubin-Faubert and Bernardin1989); and can have a meaningful and specific impact on activities of daily living (Rao, Leo, Ellington, et al., Reference Rao, Leo, Ellington, Nauertz, Bernardin and Unverzagt1991).
The second event was the introduction of magnetic resonance imaging (MRI). Because of its unique sensitivity to changes in water content in tissue, MS-related white matter (WM) lesions could be readily observed as WM hyperintensities (WMH) on T2-weighted scans. Moderate correlations between severity of cerebral WMH load and degree of cognitive dysfunction were observed (Rao, Leo, Haughton, St Aubin-Faubert, & Bernardin, Reference Rao, Leo, Haughton, St Aubin-Faubert and Bernardin1989), clearly indicating that cognitive dysfunction could be related to underlying MS-related brain pathology rather than non-specific disease-related factors, such as fatigue, depression, or anxiety.
Since these early studies, the neuropsychology of MS has continued to evolve. This review is not designed to be an exhaustive appraisal of this large body of scientific literature. Rather, the intent is to highlight major innovations and scientific discoveries in the areas of neuropathology, neuroimaging, diagnosis, and treatment that pertain to our understanding of the neuropsychological aspects of MS. Finally, we highlight trends for potential future innovations over the next decade.
NEUROPATHOLOGY
Traditionally, MS has been thought to be a disease of WM. This view was supported by early pathological studies that used standard histochemical myelin stains, such as luxol fast-blue (LFB). However, LFB works well in WM where there is an abundance of myelin, but is considerably less sensitive to detecting scarce amounts of myelin as contained in gray matter (GM). Only recently have investigators begun to appreciate that GM demyelination is a common pathological feature of MS. This newly found appreciation was made possible with the development of more advanced immunohistochemistry techniques based on myelin basic protein and proteolipid protein. Studies using these techniques have shown that around 15% of the neocortex is demyelinated at autopsy, with some outliers up to 70% of the total neocortical volume (Haider et al., Reference Haider, Simeonidou, Steinberger, Hametner, Grigoriadis, Deretzi and Frischer2014; Kutzelnigg et al., Reference Kutzelnigg, Lucchinetti, Stadelmann, Bruck, Rauschka, Bergmann and Lassmann2005).
Furthermore, deep GM nuclei can be heavily affected, with an average of approximately 30% demyelination. GM demyelination is more common in the cerebellum (Gilmore, DeLuca, et al., Reference Gilmore, DeLuca, Bo, Owens, Lowe, Esiri and Evangelou2009; Kutzelnigg et al., Reference Kutzelnigg, Faber-Rod, Bauer, Lucchinetti, Sorensen, Laursen and Lassmann2007), hippocampus (Geurts et al., Reference Geurts, Bo, Roosendaal, Hazes, Daniels, Barkhof and van der Valk2007), and spinal cord GM (Gilmore, DeLuca, et al., Reference Gilmore, DeLuca, Bo, Owens, Lowe, Esiri and Evangelou2009; Gilmore, Geurts, et al., Reference Gilmore, Geurts, Evangelou, Bot, van Schijndel, Pouwels and Bo2009), but essentially no areas within the central nervous system are spared. Cerebellar cortex can be almost entirely demyelinated in chronic MS cases (Kutzelnigg et al., Reference Kutzelnigg, Faber-Rod, Bauer, Lucchinetti, Sorensen, Laursen and Lassmann2007). Although the thalamus is the most frequently affected subcortical GM structure, lesions have been detected within the caudate, putamen, pallidum, claustrum, amygdala, hypothalamus, and substantia nigra (Gilmore, Donaldson, et al., Reference Gilmore, Donaldson, Bo, Owens, Lowe and Evangelou2009; Huitinga et al., Reference Huitinga, De Groot, Van der Valk, Kamphorst, Tilders and Swaab2001; Vercellino et al., Reference Vercellino, Masera, Lorenzatti, Condello, Merola, Mattioda and Cavalla2009).
Whereas deep GM damage develops early in the disease, neocortical demyelination predominates in later stages (Haider et al., Reference Haider, Simeonidou, Steinberger, Hametner, Grigoriadis, Deretzi and Frischer2014). Cortical lesions are generally characterized by an intact blood–brain barrier and a lack of inflammatory cell infiltration (Bo, Vedeler, Nyland, Trapp, & Mork, Reference Bo, Vedeler, Nyland, Trapp and Mork2003; Peterson, Bo, Mork, Chang, & Trapp, Reference Peterson, Bo, Mork, Chang and Trapp2001; van Horssen, Brink, de Vries, van der Valk, & Bo, Reference van Horssen, Brink, de Vries, van der Valk and Bo2007; Wegner, Esiri, Chance, Palace, & Matthews, Reference Wegner, Esiri, Chance, Palace and Matthews2006). Neuronal loss is generally limited to cortical lesions in contrast to the widespread changes observed in neurodegenerative diseases like Alzheimer’s disease. Nevertheless, a neuronal density reduction of 18–23% has been reported (Vercellino et al., Reference Vercellino, Plano, Votta, Mutani, Giordana and Cavalla2005).
Furthermore, loss of glia cells (~36%) and synapses (~47%) have also been found (Wegner et al., Reference Wegner, Esiri, Chance, Palace and Matthews2006). On occasion, signs of cortical inflammation have been observed in MS. One post-mortem study found rims of activated microglia at the border of cortical lesions (Kooi, Strijbis, van der Valk, & Geurts, Reference Kooi, Strijbis, van der Valk and Geurts2012), and two others found meningeal B-cell follicle-like structures, associated with cortical demyelination (Howell et al., Reference Howell, Reeves, Nicholas, Carassiti, Radotra, Gentleman and Reynolds2011; Magliozzi et al., Reference Magliozzi, Howell, Vora, Serafini, Nicholas, Puopolo and Aloisi2007).
Neuropsychological investigators have come to realize that the presence and severity of cognitive impairment in MS may not be fully explained by WM pathology alone. Our understanding of the role of GM pathology in the development of cognitive dysfunction, however, is dependent on our ability to visualize these lesions in vivo using novel neuroimaging technologies.
NEUROIMAGING
Initial attempts to image GM lesions using conventional T2-weighted and three-dimensional (3D) fluid-attenuated inversion recovery (FLAIR) imaging were largely unsuccessful, with detection of only 5% of the GM lesions observed at autopsy (Geurts, Bo, et al., Reference Geurts, Bo, Pouwels, Castelijns, Polman and Barkhof2005). Newly developed MRI sequences, such as 3D-double inversion recovery (see Figure 1A) (Calabrese & Gallo, Reference Calabrese and Gallo2009; Geurts, Pouwels, et al., Reference Geurts, Pouwels, Uitdehaag, Polman, Barkhof and Castelijns2005; Geurts et al., Reference Geurts, Roosendaal, Calabrese, Ciccarelli, Agosta, Chard and Group2011; Nelson et al., Reference Nelson, Poonawalla, Hou, Huang, Wolinsky and Narayana2007), phase-sensitive inversion recovery (Nelson et al., Reference Nelson, Poonawalla, Hou, Huang, Wolinsky and Narayana2007; Sethi et al., Reference Sethi, Yousry, Muhlert, Ron, Golay, Wheeler-Kingshott and Chard2012), 3D MPRAGE (Nelson, Poonawalla, Datta, Wolinsky, & Narayana, Reference Nelson, Poonawalla, Datta, Wolinsky and Narayana2014; Nelson, Poonawalla, Hou, Wolinsky, & Narayana, Reference Nelson, Poonawalla, Hou, Wolinsky and Narayana2008), and T2* imaging (Nielsen et al., Reference Nielsen, Kinkel, Tinelli, Benner, Cohen-Adad and Mainero2012) have been more successful, but still detected only the tip of the pathological iceberg (Seewann et al., Reference Seewann, Vrenken, Kooi, van der Valk, Knol, Polman and Geurts2011). Using higher MR field strengths (7T) and quantitative MRI methods, investigators have shown that subpially located cortical GM lesions, which form the bulk of the GM demyelination in progressive MS, are especially hard to find at lower field strengths (see Figure 1B) (Kilsdonk et al., Reference Kilsdonk, Jonkman, Klaver, van Veluw, Zwanenburg, Kuijer and Geurts2016; Mainero et al., Reference Mainero, Louapre, Govindarajan, Gianni, Nielsen, Cohen-Adad and Kinkel2015).
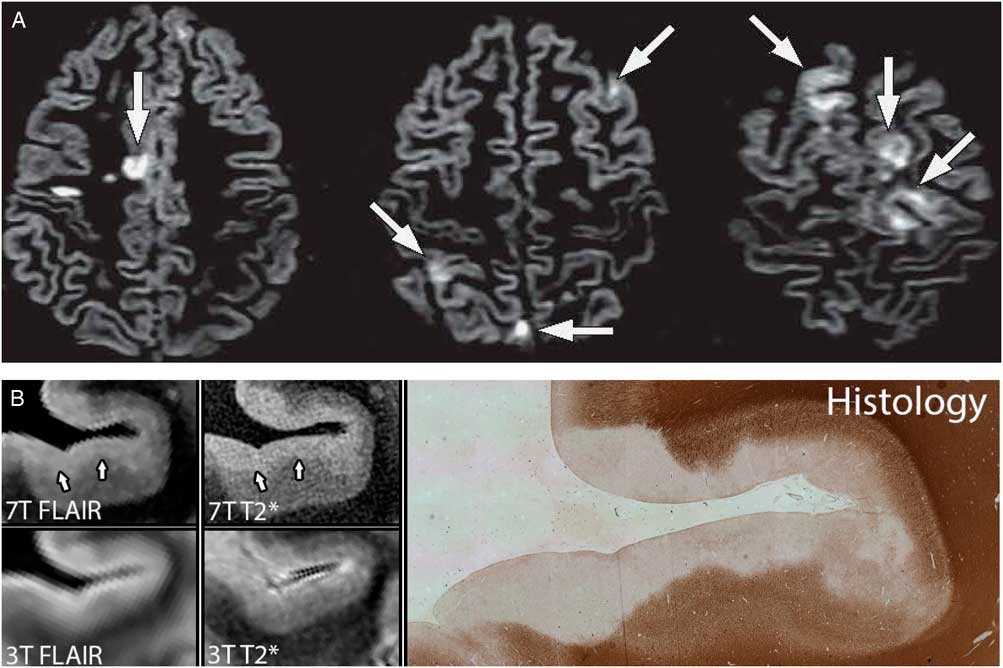
Fig. 1 (A) Intracortical lesions and mixed GM and WM lesions are shown (arrows) using double inversion recovery MR images obtained at 3 Tesla (T) field strength; reprinted with permission (Geurts, Calabrese, Fisher, & Rudick, Reference Geurts, Calabrese, Fisher and Rudick2012). (B) Post-mortem GM lesions detected at 7T FLAIR and 7T T2* images and on histology, but not detected on 3T images; reprinted with permission (Kilsdonk et al., Reference Kilsdonk, Jonkman, Klaver, van Veluw, Zwanenburg, Kuijer and Geurts2016).
Despite these technical limitations, several imaging studies have demonstrated that GM pathology is clinically relevant to the study of cognitive deficits in MS. Calabrese and colleagues found that, although patients may have similar T2-weighted WMH loads, cognitively impaired patients had more cortical lesions than patients who were cognitively intact (Calabrese et al., Reference Calabrese, Agosta, Rinaldi, Mattisi, Grossi, Favaretto and Filippi2009). Other studies have shown strong correlations between cortical lesions and worse performance on measures of information processing speed, episodic memory, and learning capacity (Mike et al., Reference Mike, Glanz, Hildenbrand, Meier, Bolden, Liguori and Guttmann2011; Nelson et al., Reference Nelson, Datta, Garcia, Rozario, Perez, Cutter and Wolinsky2011; Roosendaal et al., Reference Roosendaal, Moraal, Pouwels, Vrenken, Castelijns, Barkhof and Geurts2009, Reference Roosendaal, Moraal, Vrenken, Castelijns, Pouwels, Barkhof and Geurts2008).
Because of the difficulty in visualizing GM lesions with current imaging technologies (Geurts et al., Reference Geurts, Roosendaal, Calabrese, Ciccarelli, Agosta, Chard and Group2011), MS researchers have focused on comparing GM and WM atrophy, measurements of which can be reliably reproduced across imaging centers despite disease heterogeneity. In a 4-year longitudinal study, Fisher et al. (Fisher, Lee, Nakamura, & Rudick, Reference Fisher, Lee, Nakamura and Rudick2008) reported that WM atrophy increased three-fold, but this rate of change was similar across disease stages (relapsing-remitting, secondary progressive, and primary progressive MS, and clinically isolated syndrome). In contrast, GM atrophy increased in proportion to disease stage, that is, three-fold increase in early stage (clinically isolated syndrome converting to relapsing remitting MS) versus a 14-fold increase in late stage (secondary progressive MS).
MRI-based atrophy measures have been assumed to reflect a neurodegenerative process. This assumption was recently confirmed using combined post-mortem whole-brain in situ MRI imaging and histopathology in brain donors with chronic MS (Popescu et al., Reference Popescu, Klaver, Voorn, Galis-de Graaf, Knol, Twisk and Geurts2015). Neuronal and axonal pathology were identified as the predominant substrates of MRI-measured cortical volume.
Furthermore, GM atrophy has been shown to be regionally specific, with early volume loss of the thalamus and basal ganglia, as well as within the limbic system (Audoin et al., Reference Audoin, Zaaraoui, Reuter, Rico, Malikova, Confort-Gouny and Ranjeva2010; Bergsland et al., Reference Bergsland, Horakova, Dwyer, Dolezal, Seidl, Vaneckova and Zivadinov2012; Schoonheim et al., Reference Schoonheim, Popescu, Rueda Lopes, Wiebenga, Vrenken, Douw and Barkhof2012; Zivadinov et al., Reference Zivadinov, Havrdova, Bergsland, Tyblova, Hagemeier, Seidl and Horakova2013), and to be of relevance for understanding cognitive impairment (Damjanovic et al., Reference Damjanovic, Valsasina, Rocca, Stromillo, Gallo, Enzinger and Filippi2017; Preziosa et al., Reference Preziosa, Rocca, Pagani, Stromillo, Enzinger, Gallo and Group2016; Tillema et al., Reference Tillema, Hulst, Rocca, Vrenken, Steenwijk and Damjanovic2016).
Regional GM atrophy appears to be topographically associated with lesion pathology in the adjacent WM, but only in relapse-onset MS. For primary progressive MS, no such correlation was found (Riccitelli et al., Reference Riccitelli, Rocca, Pagani, Rodegher, Rossi, Falini and Filippi2011). This, however, may be largely a technical issue, as a diffusion tensor approach did show a spatial association between GM atrophy and adjoining diffuse WM damage (Bodini et al., Reference Bodini, Khaleeli, Cercignani, Miller, Thompson and Ciccarelli2009). The latter finding was later confirmed longitudinally (Bodini et al., Reference Bodini, Veronese, Garcia-Lorenzo, Battaglini, Poirion, Chardain and Stankoff2016), and it was suggested that WM damage causes GM atrophy more so than the other way around. While this may certainly be true, second-order effects may start playing a role in later disease stages.
This means that WM lesions or more subtle WM damage may initially lead to local GM damage, but later GM damage will also be affected by connected, damaged cortical or deep GM, leading to an increasing GM atrophy rate over time. This would entail that WM damage will have less of an effect on the total GM damage over time, which was indeed shown (Steenwijk et al., Reference Steenwijk, Geurts, Daams, Tijms, Wink, Balk and Pouwels2016). In other words, GM damage (demyelination and atrophy) seems to grow into an independent pathological process, with potential implications for the rate of progression of cognitive dysfunction.
Numerous studies have demonstrated correlations between cognitive testing and brain volume measures (Batista et al., Reference Batista, Zivadinov, Hoogs, Bergsland, Heininen-Brown, Dwyer and Benedict2012; Benedict, Ramasamy, Munschauer, Weinstock-Guttman, & Zivadinov, Reference Benedict, Ramasamy, Munschauer, Weinstock-Guttman and Zivadinov2009; Calabrese et al., Reference Calabrese, Agosta, Rinaldi, Mattisi, Grossi, Favaretto and Filippi2009; Rao et al., Reference Rao, Glatt, Hammeke, McQuillen, Khatri, Rhodes and Pollard1985). For example, Benedict and colleagues (Benedict et al., Reference Benedict, Ramasamy, Munschauer, Weinstock-Guttman and Zivadinov2009; Houtchens et al., Reference Houtchens, Benedict, Killiany, Sharma, Jaisani, Singh and Bakshi2007) showed that mesial temporal lobe (hippocampus and amygdala) and deep GM (thalamus and caudate) volumes correlated with recall and recognition memory, respectively. Amato and colleagues showed that overall normalized cortical volume was related to neuropsychological tests of verbal memory and fluency, as well as attention/concentration (Amato et al., Reference Amato, Bartolozzi, Zipoli, Portaccio, Mortilla, Guidi and De Stefano2004).
In addition to structural imaging studies, functional MRI (fMRI) is gaining increasing attention in the MS field. Combining functional and structural imaging data are indispensable to understanding the brain mechanisms responsible for cognitive decline. Researchers have begun to look at the effects of MS related tissue changes on cognition, integrating information from multimodal imaging and demonstrating widespread functional network abnormalities to be present in MS (Rocca et al., Reference Rocca, Pravata, Valsasina, Radaelli, Colombo, Vacchi and Filippi2015).
As an example, thalamic volume together with thalamic activation were identified as the best predictors of impaired information processing speed and executive function in RR-MS patients (Koini et al., Reference Koini, Filippi, Rocca, Yousry, Ciccarelli and Tedeschi2016). In another fMRI study involving recognition memory, WMH load was shown to correlate positively with retrieval activation, but not with encoding activation, suggesting that the retrieval stage is more affected by disease burden than the encoding stage (Bobholz et al., Reference Bobholz, Rao, Lobeck, Elsinger, Gleason, Kanz and Maas2006).
However, subsequent work showed that the association between brain pathology and retrieval difficulties was mediated by the strength or integrity of initial learning (DeLuca, Leavitt, Chiaravalloti, & Wylie, Reference DeLuca, Leavitt, Chiaravalloti and Wylie2013). In a recent fMRI study (Nelson et al., Reference Nelson, Akhtar, Zuniga, Perez, Hasan, Wilken and Steinberg2017), reduced activation of prefrontal cortex was observed in cognitively impaired MS patients on a working memory task compared to MS patients who were cognitively intact.
NEUROPSYCHOLOGICAL ASSESSMENT
Beatty and colleagues demonstrated that frequently used tests for senile dementia, including the Folstein Mini-Mental State Exam (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975), were insensitive to MS cognitive disorders (Beatty & Goodkin, Reference Beatty and Goodkin1990). Considerable effort has focused on identifying the most psychometrically sound and valid tests for assessing cognition in MS, with the primary emphasis on the two domains, processing speed and learning/memory, that are most commonly impaired based on large-scale, controlled neuropsychological studies. In 2001 (Benedict et al., Reference Benedict, Fischer, Archibald, Arnett, Beatty, Bobholz and Munschauer2002), a consensus conference (including RHBB, SR, and JD) identified what was dubbed the Minimal Assessment of Cognitive Function in MS (MACFIMS), a battery that is typically administered in roughly 90 min. The MACFIMS was subsequently validated in numerous studies (e.g., Benedict et al., Reference Benedict, Cookfair, Gavett, Gunther, Munschauer, Garg and Weinstock-Guttman2006).
The MACFIMS panel recognized several confounds to the interpretation of neuropsychological data. Major depression is related to WM and GM abnormalities in the frontotemporal region (Feinstein et al., Reference Feinstein, O’Connor, Akbar, Moradzadeh, Scott and Lobaugh2010, Reference Feinstein, Roy, Lobaugh, Feinstein, O’Connor and Black2004), and likely accounts for some impairment on cognitive testing (Arnett et al., Reference Arnett, Rao, Bernardin, Grafman, Yetkin and Lobeck1994). The influence of dysphoria among MS patients not in the throes of a major depression episode is less certain. Fatigue, another common subjective complaint is usually not correlated with performance based neurocognitive test results (Morrow, Weinstock-Guttman, Munschauer, Hojnacki, & Benedict, Reference Morrow, Weinstock-Guttman, Munschauer, Hojnacki and Benedict2009).
One cognitive test, in particular, the Symbol Digit Modalities Test (SDMT) (Smith, Reference Smith1982) has repeatedly been found to be exceptionally reliable and sensitive, and correlated most robustly with other outcomes, such as brain MRI metrics and employment. As noted above, deep GM atrophy is a particularly relevant MRI abnormality in MS. Third ventricle widening and thalamus volume loss are robustly correlated with SDMT performance defcits, even when analyses account for the effects of depression and education (Benedict, Carone, & Bakshi, Reference Benedict, Carone and Bakshi2004; Houtchens et al., Reference Houtchens, Benedict, Killiany, Sharma, Jaisani, Singh and Bakshi2007; Minagar et al., Reference Minagar, Barnett, Benedict, Pelletier, Pirko, Sahraian and Zivadinov2013). As a result, the SDMT has figured prominently in brief, widely used international monitoring tools (Benedict et al., Reference Benedict, Amato, Boringa, Brochet, Foley, Fredrikson and Langdon2012) and composites for neurological performance in clinical trials (Benedict et al., Reference Benedict, DeLuca, Phillips, LaRocca, Hudson and Rudick2017). While the Brief International Cognitive Assessment for MS (BICAMS) was not designated a GM battery per se, deficits on the battery are strongly correlated with deep GM atrophy (Batista et al., Reference Batista, Zivadinov, Hoogs, Bergsland, Heininen-Brown, Dwyer and Benedict2012). A self-administered, computerized version of the test has recently been adapted to the iPad platform (Rao et al., Reference Rao, Losinski, Mourany, Schindler, Mamone, Reece and Alberts2017).
Surprisingly, well-controlled longitudinal studies of MS cognitive function are few, and have yielded inconsistent results. Considering the plethora of studies on brain MRI abnormalities in MS, involving demyelinating lesions and GM atrophy, one may wonder why more patients do not suffer from cognitive impairment and evidence clear decline within a few years. Part of the answer may reside in the cognitive reserve theory which was first investigated in MS by Sumowski and colleagues (Sumowski, Chiaravalloti, Wylie, & Deluca, Reference Sumowski, Chiaravalloti, Wylie and Deluca2009).
In a cross-sectional study, the investigators showed that the correlation between brain atrophy and cognitive function is moderated by cognitive reserve (Sumowski et al., Reference Sumowski, Chiaravalloti, Wylie and Deluca2009). The results were soon replicated in a longitudinal study showing decline on SDMT over 5 years, only in patients with low versus high cognitive reserve (Benedict, Morrow, Weinstock Guttman, Cookfair, & Schretlen, Reference Benedict, Morrow, Weinstock Guttman, Cookfair and Schretlen2010). Since then, other aspects of reserve including estimated brain growth capacity, leisure activities, and personality traits such as Neuroticism and Conscientiousness have also been shown to influence the trajectory of cognitive decline in this disease (Roy et al., Reference Roy, Schwartz, Duberstein, Dwyer, Zivadinov, Bergsland and Benedict2016; Sandroff, Schwartz, & DeLuca, Reference Sandroff, Schwartz and DeLuca2016).
Traditionally, MS is not thought of as a chronic, progressive neurodegenerative disease, but a disease characterized by episodes of acute exacerbation followed by remission. Until recently, MS relapses have been defined by transient neurologic signs, not mental status changes. Serendipitously, Morrow et al. discovered that during a year-long observational study, including surveillance of mental status by monthly SDMT assessments, relapses were in fact accompanied by declines in SDMT (Morrow, Jurgensen, Forrestal, Munchauer, & Benedict, Reference Morrow, Jurgensen, Forrestal, Munchauer and Benedict2011). Subsequently, in studies including more carefully defined MS samples and non-relapsing controls, neuropsychological assessments demonstrate that cognitive relapses do occur (Benedict, Rodgers, Emmert, Kininger, & Weinstock-Guttman, Reference Benedict, Rodgers, Emmert, Kininger and Weinstock-Guttman2014; Pardini et al., Reference Pardini, Uccelli, Grafman, Yaldizli, Mancardi and Roccatagliata2014), and that the patient recovery falls short of baseline levels of function. In this way, neuropsychology is providing an important tool for redefining MS disease activity.
Some investigators have tried to better characterize the nature of the cognitive disruption in MS. In a particularly creative study of episodic memory, DeLuca and colleagues (DeLuca, Barbieri-Berger, & Johnson, Reference DeLuca, Barbieri-Berger and Johnson1994) found that the primary MS deficit was in the acquisition or learning aspect of memory performance. When MS patients and controls were tested to a criterion level of performance, patients required more learning trials, but then recalled and recognized the learned information normally. In other words, the episodic memory deficit was ameliorated by overcoming a deficit in acquisition, subsequently found to be associated with slowed processing speed and executive dysfunction. As noted previously, while an fMRI study (Bobholz et al., Reference Bobholz, Rao, Lobeck, Elsinger, Gleason, Kanz and Maas2006) noted that lesion burden correlated more with activation during retrieval than encoding, subsequent work found that the strength of initial learning mediated the relationship between brain atrophy and retrieval (DeLuca et al., Reference DeLuca, Leavitt, Chiaravalloti and Wylie2013). Future studies are needed to resolve these differences.
COGNITIVE DYSFUNCTION IN PEDIATRIC MS
Traditionally, MS has been thought to be a disease of adult onset. Until recently, neuropsychological studies focused exclusively on adults with MS. In recent years, epidemiological studies, with improved diagnostic criteria, have called attention to pediatric onset MS, defined as symptoms beginning before 18 years of age. While relatively rare (only 2–5% of the MS population), the majority of pediatric MS patients (80–90%) are adolescents who have already reached puberty, but children as young as 20–25 months can present with their initial episode of MS (L.B.K.’s clinical practice; Sivaraman & Moodley, Reference Sivaraman and Moodley2016).
Cognitive deficits have been identified in approximately one third of individuals, with the most prominent deficits in motor and cognitive processing speed as well as in attention, verbal and visual memory, expressive and receptive language, and visuo-motor integration (Amato et al., Reference Amato, Goretti, Ghezzi, Hakiki, Niccolai and Lori2014; Charvet et al., Reference Charvet, O’Donnell, Belman, Chitnis, Ness and Parrish2014; Pardini et al., Reference Pardini, Uccelli, Grafman, Yaldizli, Mancardi and Roccatagliata2014; Till et al., Reference Till, Racine, Araujo, Narayanan, Collins, Aubert-Broche and Banwell2013). These studies suggest that language acquisition may be more vulnerable to disruption in children than in adults with MS.
A recent multimodal imaging study (Rocca et al., Reference Rocca, Absinta, Amato, Moiola, Ghezzi, Veggiotti and Filippi2014) identified several brain MRI differences among pediatric MS participants classified as cognitively preserved versus cognitively impaired. The two groups differed most on verbal and visual memory performance as measured by the Brief Repeatable Neuropsychological Battery (Rao, Reference Rao1991). On multivariate analysis, cognitive impairment was linked to diffusivity abnormalities in the cingulum and corpus callosum, and reduced resting state functional connectivity of the precuneus. Increases in resting state functional connectivity of the anterior cingulate cortex were noted among cognitively preserved pediatric MS participants relative to healthy controls (Rocca et al., Reference Rocca, Absinta, Amato, Moiola, Ghezzi, Veggiotti and Filippi2014).
Among pediatric-onset MS patients who underwent fMRI while completing the SDMT (Akbar et al., Reference Akbar, Banwell, Sled, Binns, Doesburg, Rypma and Till2016), those with MS and normal performance showed increased activation in the frontal lobes relative to healthy controls, with faster response times associated with increased activation. The increased activation could reflect adaptive changes used to overcome MS associated brain injury.
Results from longitudinal studies of cognitive functioning in pediatric MS have been variable. In a United States cohort tested twice over a mean of 1.6 years, approximately one-third were impaired relative to published normative data both at baseline and at follow-up, but little change in test scores occurred over time (Charvet et al., Reference Charvet, O’Donnell, Belman, Chitnis, Ness and Parrish2014). In contrast, among an Italian cohort of pediatric MS patients serially assessed at baseline and at 2 and 5 years, more than half declined at the 5-year follow-up (Amato et al., Reference Amato, Goretti, Ghezzi, Hakiki, Niccolai and Lori2014).
In the only study in which the same set of both healthy controls and pediatric MS patients were tested twice over approximately 12 months (Till et al., Reference Till, Racine, Araujo, Narayanan, Collins, Aubert-Broche and Banwell2013), healthy controls compared to those with MS were much more likely to improve on repeated testing (69% vs. 18%), with 25% of pediatric MS demonstrating a decline in performance over time. Neither clinical features nor neuroimaging findings predicted who declined. However, those whose parents had 16 years or more of education were more likely to show improvement. Taken together, it is reasonable to conclude that many children and adolescents with MS show cognitive deficits at any point in time and that they are less likely than healthy controls to show age appropriate gains on serial cognitive testing.
Cognitive difficulties take a toll. Interviews of the parents with pediatric MS (Amato et al., Reference Amato, Goretti, Ghezzi, Hakiki, Niccolai and Lori2014) uncovered that a third (all of whom had cognitive impairment) were having serious academic problems requiring grade retention or tutorial support (Amato et al., Reference Amato, Goretti, Ghezzi, Hakiki, Niccolai and Lori2014). This study also showed that 75% of cognitively impaired children experience negative effects on family or social relationships. Other studies (Charvet, Cersosimo, Schwarz, Belman, & Krupp, Reference Charvet, Cersosimo, Schwarz, Belman and Krupp2016) indicated that pediatric MS patients with cognitive dysfunction are more likely to report behavioral and emotional problems than those without cognitive impairment. Securing support services and special school accommodations can therefore be very helpful.
COGNITIVE REHABILITATION
Given the frequency and severity of cognitive impairments in MS and the impact on everyday life, the next question is its rehabilitation. The latest Cochrane review (das Nair, Martin, & Lincoln, Reference das Nair, Martin and Lincoln2016) indicates that there is significant support for memory rehabilitation for immediate and long-term follow-up, which significantly improves quality of life. For instance, a randomized control trial by Chiaravalloti and colleagues (Chiaravalloti, Moore, Nikelshpur, & DeLuca, Reference Chiaravalloti, Moore, Nikelshpur and DeLuca2013) provided class I evidence that a behavioral intervention (i.e., modified Story Memory Technique, mSMT) designed to improve the acquisition of new learning, significantly improved learning and memory as well as reported everyday life functioning. Several other authors have shown similar improvements from behavioral interventions in persons with MS focusing on attention, working memory, and executive functions (for a review, see Sandry, Akbar, Zuppichini, & DeLuca, Reference Sandry, Akbar, Zuppichini and DeLuca2016).
The success of the mSMT is based on the concept of compensation or memory strategy training. Specifically, persons with MS are trained to use specific techniques (i.e., context and imagery) to improve or strengthen the learning of new information, thus improving retrieval. Several other memory strategy training techniques have been shown to be effective in improving the acquisition and retrieval of newly learned information, including spaced learning, self-generated learning, and retrieval practice (or the Testing effect) (Sandry et al., Reference Sandry, Akbar, Zuppichini and DeLuca2016).
Other studies have focused on restoring or strengthening cognitive constructs such as memory, attention and executive functions, or even preventing cognitive decline by holding it stable. Such restorative cognitive training programs have primarily used computer interventions, including home-based programs (Stuifbergen et al., Reference Stuifbergen, Becker, Perez, Morison, Kullberg and Todd2012). The majority of these studies have shown positive effects in improving cognition (Sandry et al., Reference Sandry, Akbar, Zuppichini and DeLuca2016). However, such computerized programs that have been designed to serve as rehabilitation intervention tools must be distinguished from commercially available programs that advertise cognitive improvements. These products have generally not been studied in persons with MS and, thus, should not be viewed as rehabilitation or treatment (for a review, see Simons et al., Reference Simons, Boot, Charness, Gathercole, Chabris, Hambrick and Stine-Morrow2016).
Numerous studies have shown that cognitive rehabilitation not only improves cognitive functions and everyday life activity, but does so by promoting adaptive changes in brain activity via neuroplasticity. For example, Chiaravalloti et al. have shown that the cognitive and behavioral improvements observed using the mSMT behavioral intervention was associated with increased activity in several brain networks, increased resting state functional connectivity between the hippocampus and other brain structures, and that these effects were maintained at long-term follow-up. There are now dozens of research studies showing similar adaptive plasticity changes following various types and forms of cognitive rehabilitation in persons with MS (for a review, see Chiaravalloti, Genova, & DeLuca, Reference Chiaravalloti, Genova and DeLuca2015). How cognitive reserve, that is, effects of early life experiences, moderates the outcomes of cognitive rehabilitation has not received adequate attention as yet.
Finally, there have been mixed, but generally negative, results of pharmacological interventions to improve memory in MS (Krupp et al., Reference Krupp, Christodoulou, Melville, Scherl, MacAllister and Elkins2004, Reference Krupp, Christodoulou, Melville, Scherl, Pai, Muenz and Wishart2011; Morrow et al., Reference Morrow, Smerbeck, Patrick, Cookfair, Weinstock-Guttman and Benedict2013). Several recent studies have focused on exercise as a means to improve cognition in persons with MS. While promising, definitive effects of exercise on cognition awaits further research with improved methodological designs (Sandroff, Motl, Scudder, & DeLuca, Reference Sandroff, Motl, Scudder and DeLuca2016). To date, cognitively based interventions remain the most efficacious approach to treating cognitive impairment in persons with MS.
FUTURE DIRECTIONS
Much progress has been made in understanding the neuropsychological aspects of MS over the past three decades The authors have defined several areas of inquiry that may define the field over the next decade.
Improved Detection of Cognitive Dysfunction in the MS Clinic
Despite the existence of brief, reliable cognitive tests like the SDMT, neuropsychological assessments are not uniformly applied in MS clinics. Referrals for assessment are typically generated by patient or caregiver reports of cognitive symptoms or the need to document impairment for disability claims. Unfortunately, self-reported cognitive deficits exhibit weak correlation with neuropsychological testing (Carone, Benedict, Munschauer, Fishman, & Weinstock-Guttman, Reference Carone, Benedict, Munschauer, Fishman and Weinstock-Guttman2005; O’Brien et al., Reference O’Brien, Gaudino-Goering, Shawaryn, Komaroff, Moore and DeLuca2007) and healthcare professionals, without the benefit of neuropsychological testing, are also inaccurate in detecting the often subtle cognitive deficits associated with MS (Romero, Shammi, & Feinstein, Reference Romero, Shammi and Feinstein2015).
That said, perspectives are changing (Langdon, Reference Langdon2016), and we predict that MS clinics will increasingly use brief measures like the SDMT for screening purposes to detect subtle progression of dementia or transient declines associated with MS relapses. Computerized cognitive tests that are patient self-administered are an attractive option for routine clinic visits (for examples, see (Charvet, Shaw, Frontario, Langdon, & Krupp, Reference Charvet, Shaw, Frontario, Langdon and Krupp2017; Rao et al., Reference Rao, Losinski, Mourany, Schindler, Mamone, Reece and Alberts2017). Another model involves telemedicine in which cognitive tests are administered via telephone or the Internet; for an example, see Barcellos et al. (Reference Barcellos, Bellesis, Shen, Shao, Chinn, Frndak and Benedict2017). Comparing routine tests to baseline measures might identify “cognitive” exacerbations, monitor disease progression, and the baseline measures themselves may be used to screen patients for the need for more comprehensive evaluation. With additional large-scale data collections, research may demonstrate that the prevalence, severity, and longitudinal progression of cognitive dysfunction may be different when we move from convenience sampling to whole clinic populations.
Neuroimaging
Future studies will increasingly use approaches that combine multiple structural (volumetrics, WM and GM lesions, diffusion tensor imaging) and functional techniques (task activation and resting state fMRI) to better understand how MS disrupts the efficiency of interacting brain circuitry. Longitudinal studies using such technologies will capture the transition from adaptive to maladaptive cognitive functions, thus providing a window to understanding how the brain’s “functional reserve” becomes exhausted by this disease.
Pediatric MS
An unresolved question is whether the disease affects the course of adolescent brain development. Specifically, what are the consequences of demyelination and inflammation in the context of ongoing myelination? With the emergence of collaborative worldwide networks of pediatric MS centers, we will see the emergence of controlled longitudinal studies using MRI, cognitive and academic testing, and quality of life assessments on large samples of children and adolescents with MS.
Cognitive Rehabilitation
Future work will focus on providing more class I studies specifically aimed at questions such as proper dosage, time when services should be provided (e.g., early vs. late), examining longer term consequences, and the impact on everyday life activities. The cognitive rehabilitation literature has focused almost exclusively on relapsing-remitting MS patients; future research will examine the effects on progressive MS patients. Ultimately, we believe rehabilitation of cognitive functions in persons with MS will likely be a combination of behavioral (cognitive and exercise) and pharmacological approaches. But given the current lack of evidence of pharmacological and exercise approaches, cognitive rehabilitation is the current intervention of choice.
ACKNOWLEDGMENTS
The authors contributed equally to this review; the authorship order was determined alphabetically. Conflicts of Interest: Dr. Benedict has received honoraria, royalties or consulting fees from Abbvie, Biogen, EMD Serono, Genentech, Genzyme, Novartis, Psychological Assessment resources, Roche, Sanofi, Consortium of MS Centers, International Neuropsychological Society and research funding from the National Multiple Sclerosis Society, Biogen, Accorda, Genzyme, and Novartis. Dr. DeLuca has received honoraria, royalties or consulting fees from Biogen, EMD Serono, Consortium of Multiple Sclerosis Centers, International Neuropsychological Society and research funding from the National Institutes of Health, U.S. Department of Defense, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers, and Biogen. Dr. Enzinger has received funding for travel and speaker honoraria from Biogen, Bayer Schering Pharma, Merck Serono, Novartis, Shire, Genzyme and Teva Pharmaceutical Industries Ltd./sanofi-aventis; received research support from Merck Serono, Biogen, and Teva Pharmaceutical Industries Ltd./sanofi-aventis; and serves on scientific advisory boards for Bayer Schering Pharma, Biogen, Merck Serono, Novartis, Roche and Teva Pharmaceutical Industries Ltd./sanofi-aventis. Dr. Geurts has received honoraria, research grants and/or consulting fees from Biogen, Genzyme, Novartis, the Dutch MS Research Foundation, National MS Society, Canadian MS Society, and the Dutch Royal Society of the Arts & Sciences. M Dr. Krupp has received honoraria, royalties or consulting fees from Abbvie, Eisai, Biogen, Merck, Novartis, Pfizer, Redhill pharmaceuticals, Reata, Sanofi, Gerson Lehman, and research funding from the U.S. Department of Defense, National Multiple Sclerosis Society, Novartis, Biogen and the Lourie Foundation. Dr. Rao has received received royalties from the Cleveland Clinic for licensing Multiple Sclerosis Performance Test-related technology, which includes the Processing Speed Test; honoraria, royalties or consulting fees from Biogen, Genzyme, Novartis, American Psychological Association, International Neuropsychological Society; and research funding from the National Institutes of Health, U.S. Department of Defense, National Multiple Sclerosis Society, CHDI Foundation, Biogen, and Novartis.



