INTRODUCTION
Total body Irradiation (TBI) is a radiotherapeutic technique characterized by implementation of large external beam radiation fields. TBI is routinely implemented in order to ablate bone marrow and/or leukemic cells, and immunosuppress patients prior to receiving a bone marrow transplant.1–Reference Gilson and Taylor4 The first applications of TBI date to the early 20th century, when in 1907 Friedrich Dessauer published the first literature report on the technique.Reference Dessauer5 In the past 100 years, numerous techniques have been developed worldwide to perform TBI utilizing photon beams. However, there exists at present no extant standardized protocol recommendation for TBI execution, as the numbers of treatments have been insufficient to provide datasets to demonstrate statistically significant conclusions.Reference Wheldon and Barrett6
For the purposes of technical reimbursement, TBI is considered as a “special procedure”, owing to the many physical parameters and quality assurance techniques necessary for proper application (e.g., field size, distance and dose rate). Several authors have worked on the elaboration of procedures to improve dose determination and distribution in TBI, leading to the broad standards exemplified in recommendations provided by the American Association of Physicist in Medicine (AAPM).1 Equipment modification,Reference Jones, Rieke, Madsen and Hafermann7 implementation of different X-ray modalities,Reference Vrtar8 technical procedure variations have all been explored in attempts to optimize TBI techniques.Reference Sarfaraz, Yu, Chen and Der9–Reference Hugtenburg, Turner, Baggarley, Pinchin, Oien, Atkinson and Tremewan12 However, most series consistently place the patient in standing upright position. Experimental results, despite best efforts, continue to exhibit significant variation in dose validations for patients.Reference Ban, Sawai, Aoki, Nakagawa and Kusama3 These discrepancies are influenced by patient movement due to fatigue from disease and prior systemic chemotherapy. Many patients have difficulty staying in the treatment position during the prolonged radiation treatment times.
A review of the literature clearly displays the necessity to implement a procedure that takes into account two important aspects: the positioning of the patient to avoid instability and discomfort due to fatigue, and the development of a system that produces a uniformity and accurate dose distribution within the patient.
A lateral total body irradiation (LTBI)—in which the patient is supine and treated with opposed lateral rather than anteroposterior/posterioanterior (AP/PA) beams—affords significant advantages in terms of both positional stability (due to lack of active patient involvement in positional stability) and reproducibility as well as patient comfort. It provides acceptable homogenization of dose within the patient. Consequently, in an effort to explore clinical implementation of LTBI, the specific aims of this study were to present a standardized LTBI technique, the feasibility and dosimetric evaluation LTBI protocol and provide potential future prospective series.
MATERIALS AND METHODS
Study design
A retrospective review was performed on archival data for a series of 145 patients treated with LTBIs from 1999 to 2007 at the Cancer Therapy and Research Center (CTRC) at the University of Texas Health Science Center at San Antonio.
Equipment
Patients were placed into a custom-made methylcrylate “body box” installed on a mobile base (Figure 1), which affords positional adjustment without patient participation during the treatment. Patients were treated using a Clinac 600c linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) with 6 MV X-rays. Thermoluminescent dosimeters (TLDs) of lithium fluoride (Bicron Harshaw TLD-100, Saint-Gobain Industrial Ceramics, Paris, France) for dose verification were used. TLDs were calibrated using a standard polymethyl methylcrylate phantom. TLDs were placed under a 1.5-cm-equivalent tissue material (a 3 × 3 cm square bolus) which was used to produce dose build-up on the skin surface. TLDs were analyzed using a Harshaw-3500 thermoluminescent reader with WinREMS software (Thermo Fisher Scientific Inc., Waltham, MA, USA). Before dose validation, the TLD-100 chips were annealed to 400°C for 1 h, followed by 100°C for 2 hReference Cameron, Suntharalingam and Kenney13 using a Thermolyne 47900 furnace and a Thermolyne Incubator 10200 (Barnstead-Thermolyne Corp., Dubuque, IA, USA) electrical furnace.

Figure 1. Methylcrylate body box installed on a mobile base used for patient simulation and treatment with lateral total body irradiation.
Simulation
Patient simulation was performed in a supine position with hands clasped on upper abdomen (Figure 2). The arms in this position also served as partial shielding to part of the lungs.Reference Pacyna, Darby and Prado14,Reference Kirby, Hanson and Johnston15 The patient’s knees were slightly bent and supported. Patient length along the long axis of the patient was measured and recorded. These lengths included the head, neck, upper chest region (shoulder), lower chest region, abdomen, hip, thigh and calf/ankle region. Back projection of the measured lengths to the tray holder on the head of gantry will be used in building the compensating filters.

Figure 2. Patient simulation performed in a supine position with hands clasped on upper abdomen and contours marked on the body.
Lateral and anterior linac-portal radiographs were taken of the patient’s chest. Using the lateral radiographs, the physician delineated the portion of the lung that will be blocked. Customizable partial transmission lung blocks were shaped based on the delineations made on films. The anterior radiograph was used to estimate the width of the lungs so that we can correctly calculate the partial transmission lung block while accounting for the low density of the lung.
Dose calculations
In addition to standard linear accelerator quality assurance performance, specific LTBI quality assurance was performed to determine the dose-rate and requisite monitor units (MU) for each session of treatment. The whole body is focused as the target on TBI treatments. However, each region of interest is analyzed separately to account for differences in thickness in order to produce an approximate equivalent dose to the midplane. Variations in body habitus/body thickness were compensated by an array of customized lead filters (Figure 3). The calculation process to determine the thickness of these filters was done at the point of greatest lateral thickness which is usually at the shoulder/upper chest region. The MU number for each treatment was calculated by the following expression:

Figure 3. Variations in body habitus/body thickness compensated by an array of customized lead filters.
where D 0 is the prescribed dose to level of the midline in the patient and ![]() is the dose rate with respect to the area of greatest thickness in the body, obtained through the following relation:
is the dose rate with respect to the area of greatest thickness in the body, obtained through the following relation:
where TMR i represents the tissue-maximum ratio calculated considering distance to the midline, ISL is the inverse square law factor for the treatment distance in TBI, OAF is the off-axis factor, SF is the spoiler factor, TF is the tray factor and S P and S C are the scatter factors for phantom and collimator. The Output corresponds to the output rate in the linear accelerator obtained in the calibration process to the depth of maximum dose (d Max) and is equal to 1.0 cGy/MU. The dose rate to the thickest anatomical site in the supine position, usually the shoulders, is calculated for a 40 × 40 sq cm beam, at 350 cm source to midplane distance with a beam spoiler and tray factor. Using this dose rate, the MU needed to deliver the midplane dose of 1 Gy from one beam is determined. Once the requisite MU for the treatment was calculated, the dose rate to other sites (head and neck, chest, umbilicus, etc.) is calculated. The dose rates at the different treatment sites are higher due to less attenuation. Midplane dose rates at each site are matched to that of the thickest midplane site by the use of lead-compensating filters. The lead thickness was determined using the following expression:
where the linear attenuation coefficient (μ) for the treatment unit is 0.517/cm for the 6 MV beam, Di is the dose rate at the midplane of the thickest site. Dm is the dose rate at a specific site and x m is the thickness of the lead filter for a specific site m.
The formula used to predict the midplane dose based on skin TLD measurements is as follows:
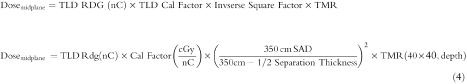
Surface dose is measured with 2–3 TLDs batched with similar sensitivities. The average error between measurements is less than 3%. A calibration factor is obtained by irradiating 2–3 TLDs with 200 cGy. The reading of the TLDs is in nC. The TLD calibration factor is in cGy/nC. The inverse square factor considers the reduction in dose due to increase distance from the point of measurement (skin surface) to patient’s midplane. The depth to midplane is half the separation thickness. The TMR data are unique for TBI. It is obtained for a 40 × 40 sq cm field, 350 SSD (source-skin distance), with a 1.2 cm acrylic beam spoiler and tray at the head of the gantry. The TMR data are unique for the specific machine and treatment setup.
Dose homogeneity can be achieved for small patients as required in AAPM Report No. 17 (Task Group 29). TMR measurements were made in a solid water phantom using a plane parallel chamber. Measurements were made under TBI setup conditions (extended SSD of 350 cm, 1.2 cm spoiler, tray and large 40 × 40 cm field size). Based on these TMR measurements, dose uniformity within 10% can be achieved for patients with a separation thickness of less than 45 cm.
Treatment technique
Patients were placed in the body box with hands clasped on upper abdomen, knees touching and feet together during the entire treatment. Lasers projected down from the ceiling at 350 cm SSD were used to position the patient and body box at the required distance from the linac. The gantry was rotated at 90 degrees. The collimator was rotated at 225 degrees with a maximum field size (40 cm × 40 cm) (Figure 4). The light field was used to verify that the patient and the body box were within the field.

Figure 4. Standardized rotations of 90° and 45° for gantry and collimator, respectively, and a maximum field size (40 cm × 40 cm) at extended distance during treatment.
Both entrance and exit doses were made with TLDs and a calibrated plane parallel Roos chamber. Midplane doses in a solid water phantom have been made for various thicknesses with both detectors. These measurements and midplane dose calculations are verified annually. For dose validation, only entrance skin dose measurements for each field are made. For in vivo dose verification, TLDs were placed at the centre of the defined regions of interest (Table 1) on patient’s skin. TLD dose validation derived from skin dose measurements were obtained for the first treatment fraction to ensure correct delivery of the prescribed dose to midplane.
Table 1. Description of the anatomical points of interest where TLD were placed

TLD = thermoluminescent dosimeter.
Differential thickness compensators were built with thin sheets of lead (Figure 3). An array of these lead sheets of varying thicknesses were placed in the beam for each region of interest to attenuate the beam to deliver the prescribed dose to the patient’s midplane. TLD measurements validated the construction of the lead compensators for appropriateness of placement and thickness. Treatments were typically delivered in two sessions daily with 6 h intervals over three consecutive days for a total midplane dose of 12 Gy. Each lateral field, right and left, delivered 100 cGy to midplane with a dose rate between 10 and 15 cGy/min. The midplane dose to all sites is 100 cGy for each field. However, the midplane dose to the chest when lung blocks are used is 0.5 cGy. Different protocols may have varying requirements for lung dose and total prescribed dose to the midplane.
RESULTS
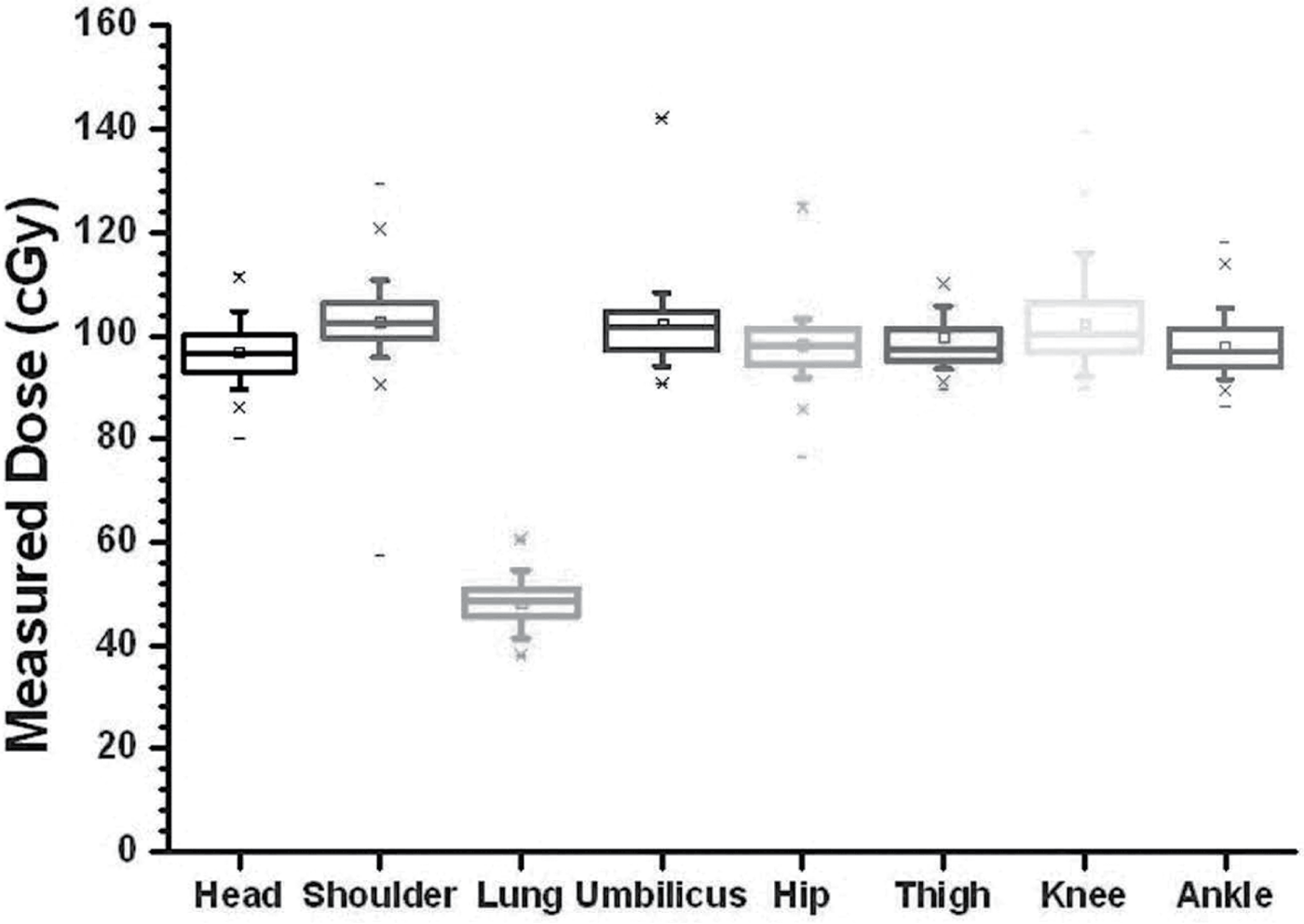
A series of 145 patients with a variety of diagnoses were treated using the LTBI technique (Table 2). The entrance doses in each of the body regions were measured with TLD-100s and used to determine the midplane dose at region of interest. Figure 5 shows the calibration curve for the dosimeters used. A standard error of less than 1% was found. Standard deviation for the difference between calculated and measured dose for 145 patients treated in the CTRC are shown in Figure 6. The calculated dose to midplane is the same as the prescribed dose to patient’s midplane. Each TLD measurement represented a certain region of interest delivered by a right or left lateral field. Skin dose measurements were corrected for attenuation and inverse square law to predict the expected midplane dose of 100 cGy for each region of interest. The average head and neck midplane dose measurements were 98.3 cGy, generally smaller than the expected 100 cGy. Lower chest, abdomen and knee measurements were also generally lower with values of 1.3%, 2.1% and 2.0% of standard deviation, respectively. Upper chest/shoulder, hip and calf/ankle measurements were higher, with values of 2.6%, 1.8% and 2.0% in standard deviations, respectively.

Figure 5. The calibration curve for the thermoluminescent dosimeters used for dose verification.
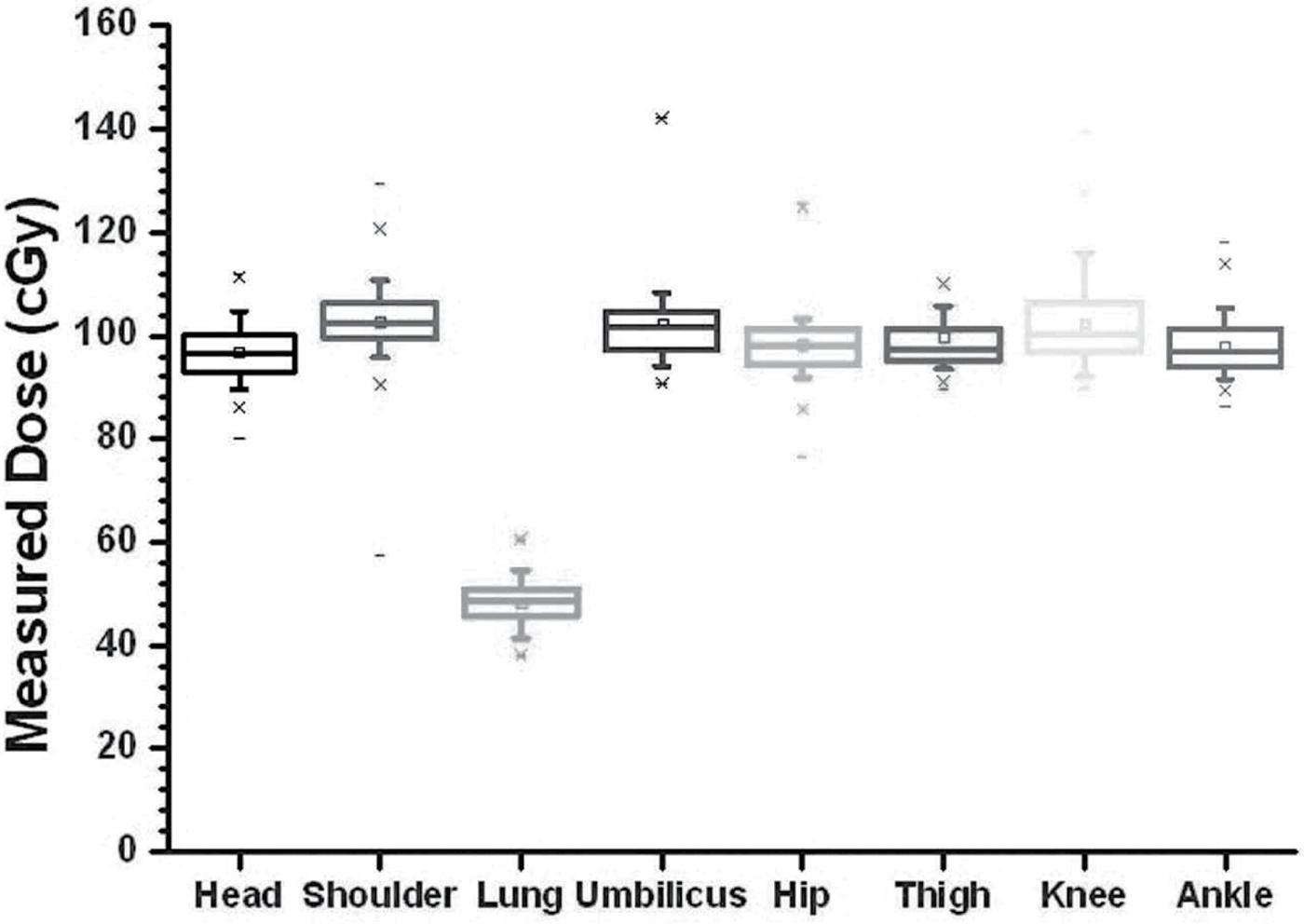
Figure 6. Standard deviation for calculated versus measured dose in regions of head and neck (H/N), shoulder, lung, umbilicus, hip, thigh, knee and ankle.
Table 2. Demographic data of patients

The point-dose measurements behind the 50% partial transmission lung block average 48.6 cGy. Dose variability for all other TLD measurement was found to be less than ±3% for each region of interest, with the maximum dose variation in the lung measurement site.
DISCUSSIONS AND CONCLUSIONS
While TBI treatments have a long history of use, there exists a considerable variation in TBI techniques that are currently being employed. There are scattered reports in the application of lateral beams for TBI treatments.Reference Dutreix and Bridier16–Reference Lancaster, Crosbie and Davis20 However, almost all reports specify utilization of either standing or lateral decubitus positioning for AP/PA beams.Reference Quast21–Reference Su, Shi and Papanikolaou29 While groups have reported utilizing lateral beams in addition to AP/PA beams, an upright position was utilized.Reference Christ30 Likewise, while supine TBI has been proposed using a helical tomotherapy application, this procedure utilizes multiple beam angles, rather than lateral beams alone.Reference Hui, Kapatoes, Fowler, Henderson, Olivera, Manon, Gerbi, Mackie and Welsh31 Both AP/PA TBI and LTBI techniques have been developed to be applied to the same medical prescriptions. The use of one or the other depends on anthropomorphic characteristics and on the type and stage of cancer. However, the application of the technique of lateral beams offers a greater level of accuracy for in vivo dose verification and increased convenience for both the patient and radiotherapy staff. This technique is especially useful for paediatric patients who require anaesthesia during treatment.
Upright positioning of the patient as currently practiced requires a patient support stand.Reference Glasgow, Wang and Stanton32 These stands are necessary in order to maximize positional reproducibility in the context of patient fatigue. As standing for protracted periods may be rather difficult for patients who are often ill and experience fatigue, reduction in patient participation and effort required for the treatment is highly desirable. Dose uniformity to the patient’s midplane can be achieved with the use of custom-lead compensators while maintaining a reproducible and comfortable treatment position.
Accurate in vivo dosimetry measurement to individual reference points after each fraction of treatment enables us to verify the given dose and serves as a quality assurance measure for the manufacturing of the custom compensators and when applicable, the lung transmission blocks. The TLD dosimetry described herein for the LTBI technique has been used in our institution with a calculated uncertainty of +2%, even in zones of the body with heterogeneous thickness and lung shielding blocks. Consequently, in our study of 145 cases, we have shown the accuracy of LTBI technique is well within the recommended guidelines for TBI dose delivery.
Our experience suggests that, in comparison to techniques where the patient is treated standing, a comfortable position is readily achieved and reproduced when treated in the supine position with lateral beams. To our knowledge, this series represents the largest dataset reporting feasibility and dosimetry of a supine LTBI technique. Our data demonstrate that the dosimetry as presented is well within specified guidelines for dose-delivery accuracy in TBI as measured by in vivo dosimetry. Consequently, LTBI may be reliably utilized for TBI as a means of maximizing patient comfort and positional reproducibility without dosimetric compromise.
Acknowledgements
The authors wish to recognize the contributions of the staff at the Cancer Therapy & Research Center for their collaboration in this project. Also, they are grateful to the General Secretary and the Academic Interchanges Direction and Inter-Institutional Relations of the Guanajuato University for their support. Presented at the Tenth Mexican Symposium on Medical Physics, Mexico City, Mexico, June 2009.