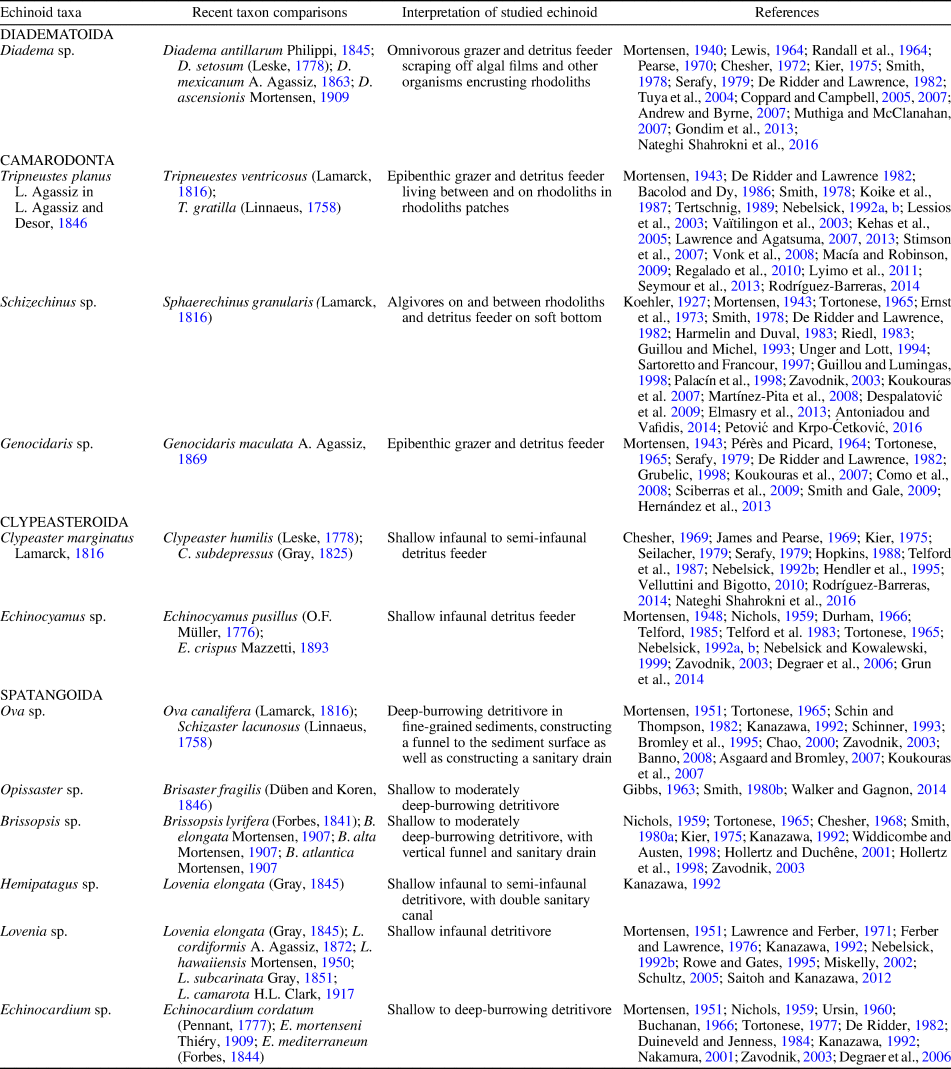
Introduction
Today echinoids form a successful group of marine invertebrates living in a wide range of marine habitats from the equator to the polar seas and from the intertidal zone to abyssal depths and have left an extensive fossil record, dating back to the Ordovician (Pisera, Reference Pisera1994; Smith and Saville, Reference Smith and Savill2001; Kroh and Smith, Reference Kroh and Smith2010; Smith and Kroh, Reference Smith and Kroh2011). The diversity, abundance, and distribution of echinoids depend on numerous factors including, among others, temperature, hydrodynamic regimes, substrate types and complexity, nutrient availability, and distribution of predators (see Ernst et al., Reference Ernst, Hähnel and Seibertz1973; Smith, Reference Smith1984; McClanahan, Reference McClanahan1995, Reference McClanahan1998; Sala and Zabala, Reference Sala and Zabala1996; Guidetti and Mori, Reference Guidetti and Mori2005; Cordeiro et al., Reference Cordeiro, Harborne and Ferreira2014; Labbé-Bellas et al., Reference Labbé-Bellas, Cordeiro, Floeter and Segal2016; Petović and Krpo-Ćetković, Reference Petović and Krpo-Ćetković2016).
Echinoids represent key benthic faunal elements in shallow marine environments. Both regular echinoids, as dominant grazers on hard substrata, and irregular echinoids, as deposit feeders and bioturbators in or on unconsolidated sediments, are prominent in structuring a wide range of marine communities (e.g., Lawrence, Reference Lawrence1975; Carpenter, Reference Carpenter, Gabrié and Salvat1985; Harrold and Pearse, Reference Harrold, Pearse, Jangoux and Lawrence1987; Bak, Reference Bak1990; Widdicombe and Austen, Reference Widdicombe and Austen1998; Lohrer et al., Reference Lohrer, Thrush, Hunt, Hancock and Lundquist2005; Antoniadou and Vafidis, Reference Antoniadou, Vafidis and Withmore2014; Cabanillas-Terán et al., Reference Cabanillas-Terán, Loor-Andrade, Rodríguez-Barreras and Cortés2016).
In general, regular echinoids are more poorly represented than irregular echinoids in the fossil record (Kier, Reference Kier1977; Smith, Reference Smith1984; Greenstein, Reference Greenstein1993b) and usually occur as fragmented remains (e.g., Kier, Reference Kier1977). Beside differences in constructional morphology, this discrepancy is related to differences in paleoecology among regular and irregular forms and taphonomic processes affecting the echinoid test (Kier, Reference Kier1977; Smith, Reference Smith1984; Greenstein, Reference Greenstein1993b; Nebelsick, Reference Nebelsick1996). Regular echinoids diversified as grazers on hard substrata in shallow-water environments that represent areas of active erosion, whereas irregular echinoids diversified as deposit feeders often buried within mobile substrata in areas of active sedimentation where they have higher preservation potential (Smith, Reference Smith1984; Nebelsick, Reference Nebelsick1996). In addition, the poor fossil record of regular echinoids could be related to a taxonomic bias due to the difficulty in the identification of taxa based on fragmentary material (Greenstein, Reference Greenstein and White1993a, Reference Greensteinb).
Herein, an echinoid-rich sedimentary succession from the Miocene of central-western Sardinia (Italy), cropping out along the coast between S'Archittu and Santa Caterina di Pittinuri, is described with the two-fold aim of: (1) reconstructing paleoecological and associated paleoenvironmental conditions, and (2) investigating factors influencing the preservation potential of echinoids and their representation in fossil deposits. This succession includes an abundance of echinoid taxa that can be interpreted with respect to functional morphology and taphonomy. The importance of functional morphological interpretations of skeletal morphologies as well as comparisons to actualistic studies on echinoids for interpreting fossil echinoids have been discussed in detail within an ongoing re-evaluation of the paleoecology and preservation of the rich Miocene echinoid fauna of Sardinia (see Mancosu and Nebelsick, Reference Mancosu and Nebelsick2013, Reference Mancosu and Nebelsick2015, Reference Mancosu and Nebelsick2016, Reference Mancosu and Nebelsick2017a, Reference Mancosu and Nebelsickb; Mancosu et al., Reference Mancosu, Nebelsick, Kroh and Pillola2015).
Geological setting
The development of the Oligo-Miocene volcano-sedimentary succession of Sardinia that is related to the evolution of the present-day Mediterranean area shows a three-fold subdivision: (1) a Chattian to early Burdigalian first cycle, (2) a late Burdigalian to early Serravallian second cycle, and (3) a Serravallian to early Messinian third cycle (Assorgia et al., Reference Assorgia, Barca, Spano, Assorgia, Barca and Spano1997a, Reference Assorgia, Barca, Porcu, Spano, Assorgia, Barca and Spanob, Reference Assorgia, Barca, Mighela, Muntoni, Murgia, Porcu, Rizzo, Rombi, Spano, Assorgia, Barca and Spanoc; Carmignani et al., Reference Carmignani, Oggiano, Funedda, Conti and Pasci2015). This succession is predominately present in the NNW-SSE-oriented Sardinian Basin (Fig. 1.1), which originated during Oligo-Miocene tectonic movements of the Corsica-Sardinia Block (Cherchi and Montandert, Reference Cherchi and Montandert1982; Thomas and Gennesseaux, Reference Thomas and Gennesseaux1986; Carmignani et al., Reference Carmignani, Oggiano, Barca, Conti, Salvadori, Eltrudis, Funedda and Pasci2001; Facenna et al., Reference Facenna, Speranza, D'Ajello Caracciolo, Mattei and Oggiano2002; Speranza et al., Reference Speranza, Villa, Sagnotti, Florindo, Cosentino, Cipollari and Mattei2002).
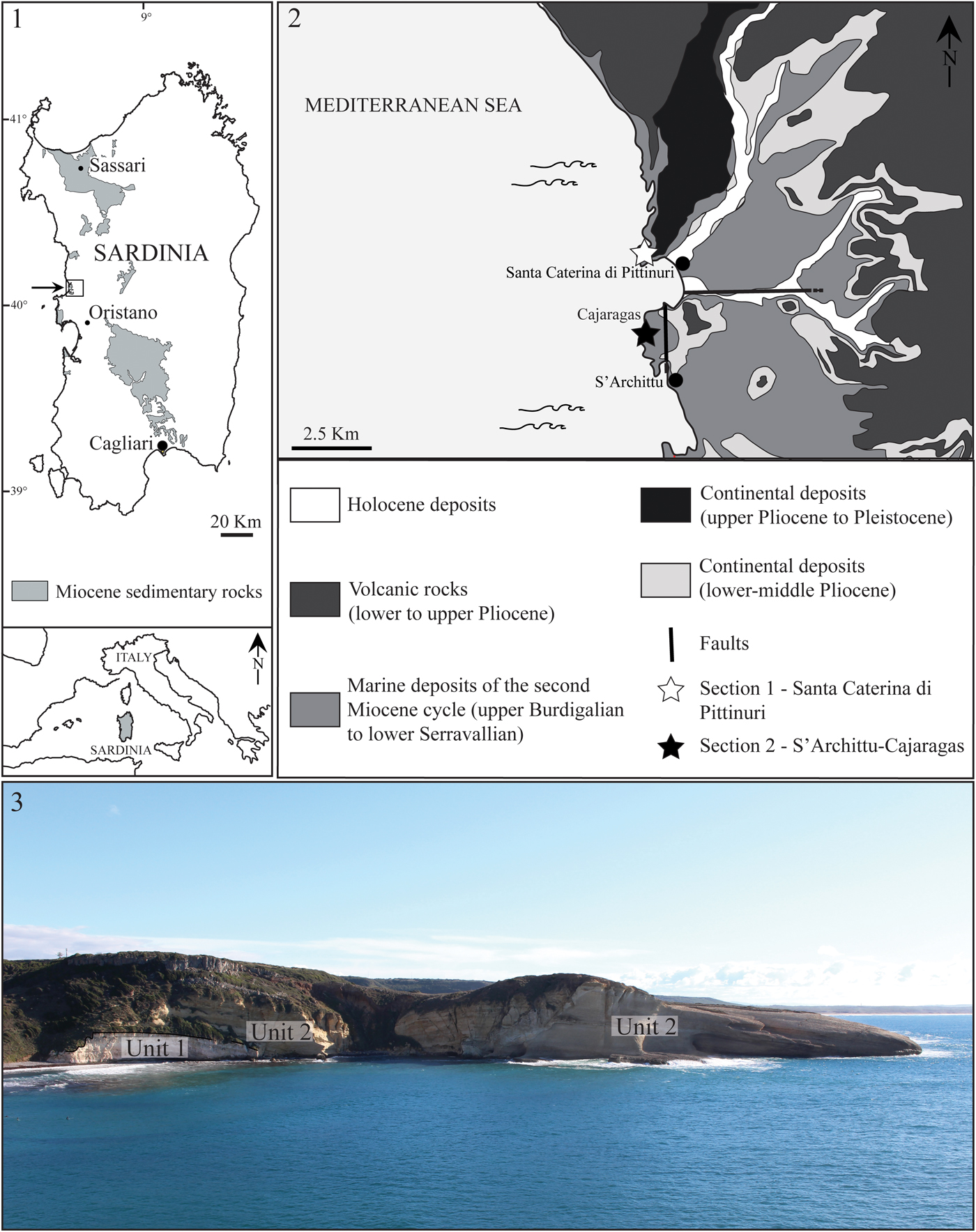
Figure 1. (1) Distribution of Miocene sedimentary rocks in Sardinia; (2) simplified geological map of the southwestern part of the Montiferru area (modified from Carboni et al., Reference Carboni, Lecca and Tilocca2010); (3) panoramic view of the studied sedimentary succession (see Geological setting section for subdivision of Units 1 and 2).
The studied sedimentary succession is located in the southwestern part of the Montiferru area (central-western Sardinia) (Fig. 1.1–1.3) along the coast between the small villages of S'Archittu and Santa Caterina di Pittinuri, and belongs to the second sedimentary cycle (Assorgia et al., Reference Assorgia, Barca, Mighela, Muntoni, Murgia, Porcu, Rizzo, Rombi, Spano, Assorgia, Barca and Spano1997c; Carboni et al., Reference Carboni, Lecca and Tilocca2010). In the Montiferru area, the Miocene volcano-sedimentary sequence starts with andesitic lavas and pyroclastic deposits of rhyolitic and dacitic composition (Assorgia et al., Reference Assorgia, Barca, Mighela, Muntoni, Murgia, Porcu, Rizzo, Rombi, Spano, Assorgia, Barca and Spano1997c; Bottero et al., Reference Bottero, Carboni and Pala2002 and references therein) dated by the K-Ar method to19–16 Ma and 17–13 Ma, respectively (e.g., Assorgia et al., Reference Assorgia, Barca, Spano, Assorgia, Barca and Spano1997a, Reference Assorgia, Barca, Mighela, Muntoni, Murgia, Porcu, Rizzo, Rombi, Spano, Assorgia, Barca and Spanoc and references therein). These deposits lie immediately beneath or are intercalated with a sedimentary succession that consists of heterometric conglomerates, epiclastites, and volcanoclastic deposits of fluviolacustrine origin (e.g., Assorgia et al., Reference Assorgia, Barca, Mighela, Muntoni, Murgia, Porcu, Rizzo, Rombi, Spano, Assorgia, Barca and Spano1997c; Mighela et al., Reference Mighela, Muntoni, Assorgia, Porcu, Spano, Assorgia, Barca and Spano1997) followed by a thick marine sedimentary sequence ranging from late Burdigalian to early Serravallian in age based on their stratigraphic position and macrofossil content (Comaschi Caria, Reference Comaschi Caria1951; Assorgia et al., Reference Assorgia, Barca, Mighela, Muntoni, Murgia, Porcu, Rizzo, Rombi, Spano, Assorgia, Barca and Spano1997c). This sequence consists of calcareous sandstones with abundant macrofossils, mainly pectinids, e.g., Gigantopecten nodosiformis (Pusch, Reference Pusch1837), and echinoids (Clypeaster spp.), passing upward to fine-grained calcarenites, marls, and limestones dominated by spatangoid echinoids. Lower-middle Miocene sedimentary rocks are unconformably overlain by subaerial, fluviodeltaic sandstones and conglomerates intercalated with Pliocene to lower Pleistocene trachytic and phonolitic lava flows (Beccaluva et al., Reference Beccaluva, Maciotta and Venturelli1974; Assorgia et al., Reference Assorgia, Barca, Mighela, Muntoni, Murgia, Porcu, Rizzo, Rombi, Spano, Assorgia, Barca and Spano1997c; Carboni et al., Reference Carboni, Lecca and Tilocca2010). As noted by Mighela et al. (Reference Mighela, Muntoni, Assorgia, Porcu, Spano, Assorgia, Barca and Spano1997), the tectonosedimentary development and the stratigraphic framework of the Montiferru area is comparable in part to that of the well-known Logudoro and Porto Torres basins (northern Sardinia) as described by Mazzei and Oggiano (Reference Mazzei and Oggiano1990) and Funedda et al. (Reference Funedda, Oggiano and Pasci2000, Reference Funedda, Oggiano, Pascucci and Pascucci2003).
The Miocene sedimentary sequence cropping out along the coast between Santa Caterina di Pittinuri and S'Archittu consists at the base of coralline algal grainstones to rudstones (Fig. 1.3, Unit 1) passing upward to very fine-grained lithologies (calcareous sandstones, mudstone, wackestones, and packstones) of Unit 2 (Fig. 1.3) that contains the echinoid assemblages studied herein. Fossil content is dominated by echinoid remains that occur throughout the sedimentary sequence and have been described in part by Comaschi Caria (Reference Comaschi Caria1951, Reference Comaschi Caria1972).
Materials and methods
Paleontological, taphonomic, and sedimentological analyses were conducted in the field and laboratory. Identification of carbonate rocks follows Embry and Klovan (Reference Embry and Klovan1971) and Lokier and Al Junaibi (Reference Lokier and Al Junaibi2016).
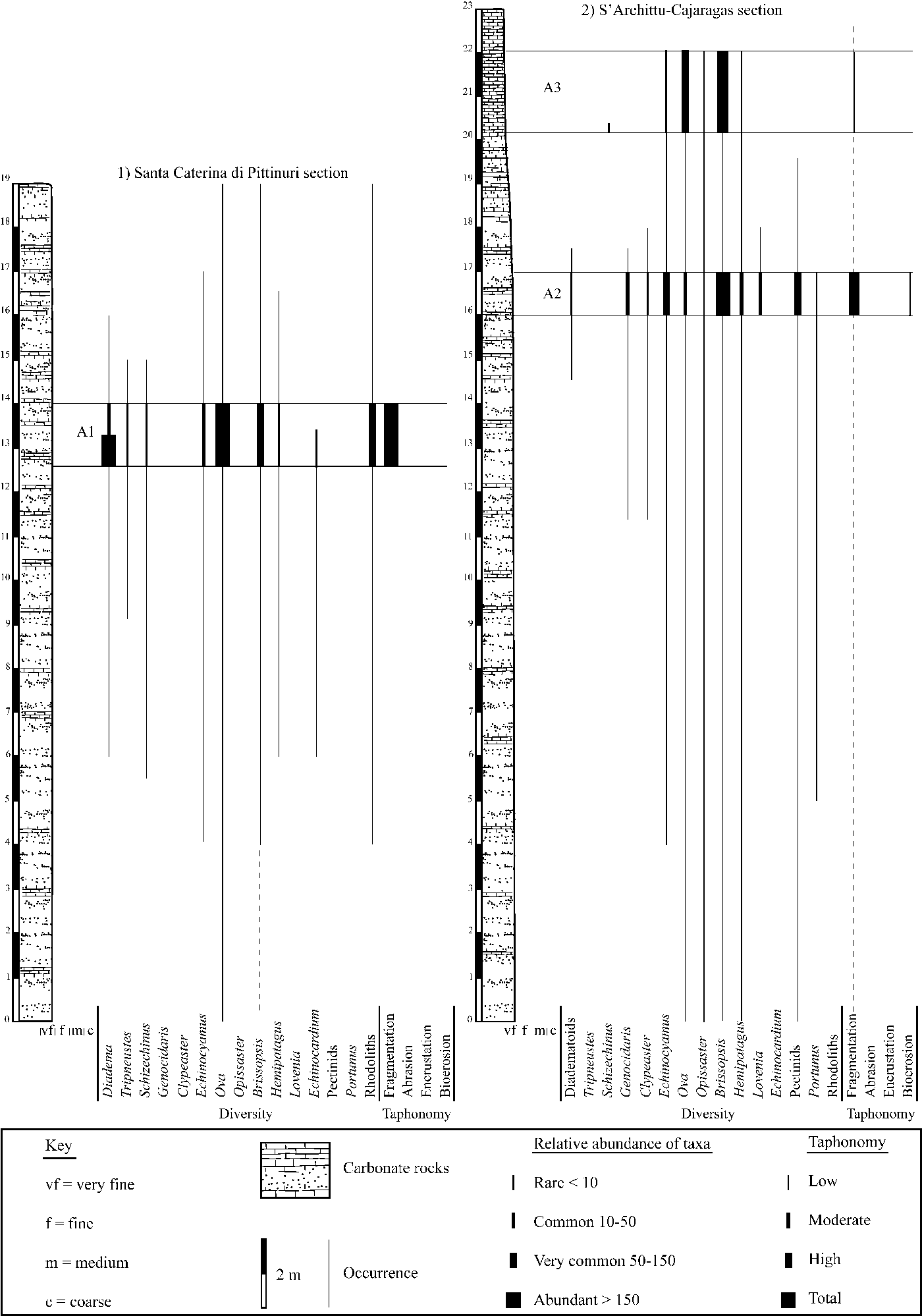
Two stratigraphic sections within the marine sedimentary sequence were measured in which echinoid remains are common throughout (Fig. 2.1, 2.2). These sections include three assemblages from beds that are particularly well exposed and characterized by a large number of echinoid remains. The first echinoid assemblage was found within the sedimentary succession east to Santa Caterina di Pittinuri (40°06′27”N, 08°29′11″E; Fig. 2.1). The second and third echinoid assemblages studied herein were found nearby within the sedimentary sequence cropping out between S'Archittu (40°05′47″N, 08°29′13″E) and Punta Cajaragas (40°05′58″N, 08°29′17″E) (Fig. 2.2). These beds were investigated in detail with respect to relative abundance of echinoid and other taxa, test orientation, as well as taphonomic and sedimentological features. Field determinations include abundance, orientation, preservation, and packing fabric fragments (following Kidwell and Holland, Reference Kidwell and Holland1991). Numerous complete and fragmented echinoid tests were systematically collected throughout the succession in 2017 and 2018. Many test fragments and spines could be attributed to specific echinoid taxa due the presence of characteristic surface characters and their excellent preservation. Taphonomic attributes observed in the field included the degree of fragmentation and orientation with respect to the bedding planes. The modes of life of the Recent analogous taxa of the fossil echinoids recognized in the present study were tabulated and compared with respect to their Recent depth distribution. The combined analysis of sedimentary characteristics, the functional-morphological interpretation of echinoids (and other bioclastic components), and taphonomic interpretation of attributes allowed for a detailed interpretation of paleoenvironment. Finally, the studied material was directly compared to previously investigated echinoids from fossil sublittoral environments. Taxonomic classification at and above genus level follows Kroh and Smith (Reference Kroh and Smith2010) and Smith and Kroh (Reference Smith and Kroh2011). Although some echinoid taxa could be assigned to a species level, rigorous taxonomic revision is beyond the scope of this study. Descriptive terminology of the echinoid test follows Smith (Reference Smith1978, Reference Smith1980b) and Smith and Kroh (Reference Smith and Kroh2011).
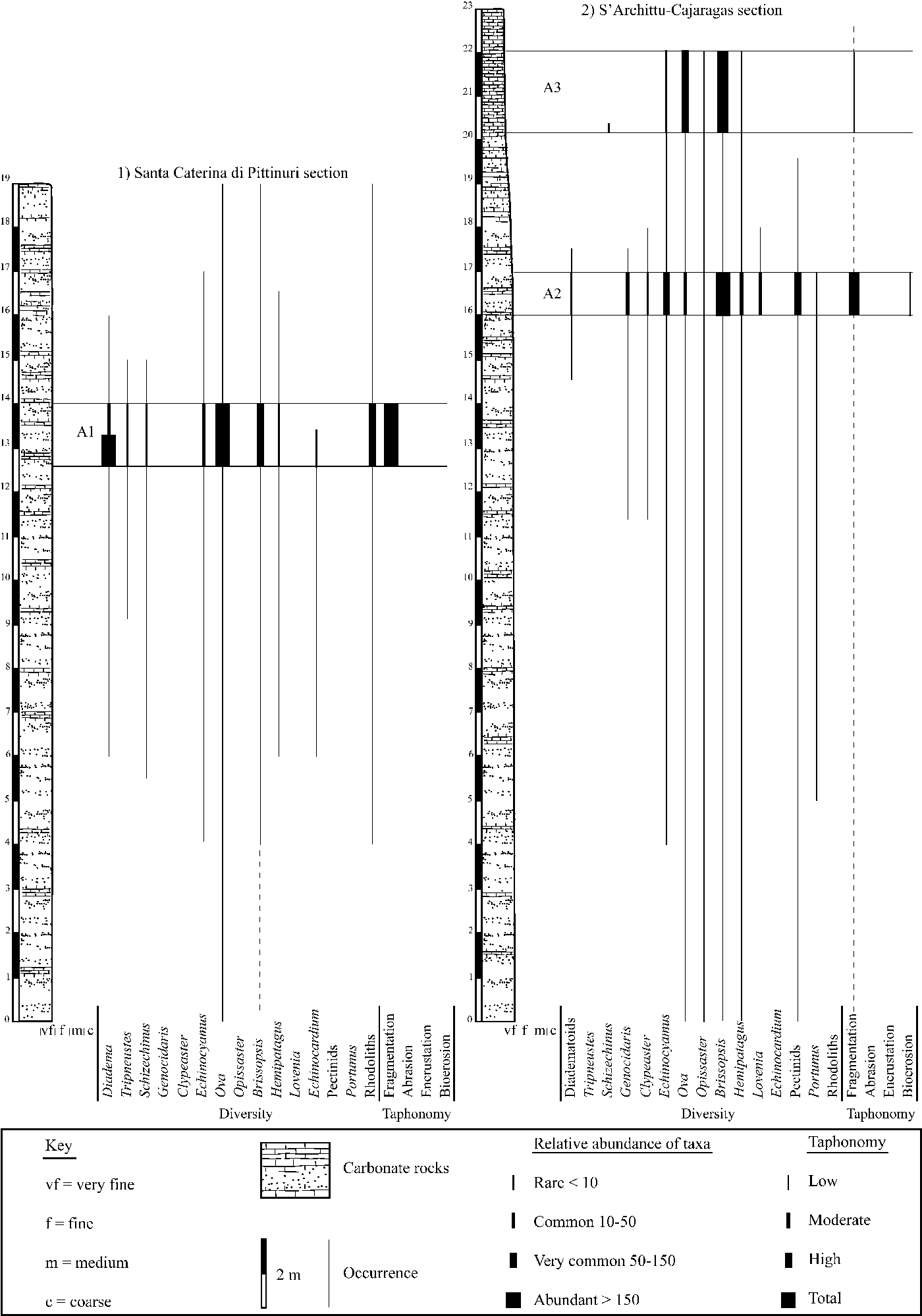
Figure 2. Stratigraphic sections of (1) Santa Caterina di Pittinuri and (2) S'Archittu-Cajaragas, with occurrence, relative abundance, and taphonomic signatures of recognized echinoids (at genus levels) and associated macrofauna and flora within the assemblages studied herein.
Repository and institutional abbreviation.—Samples are stored in the Museo di Geologia e Paleontologia Domenico Lovisato, Università di Cagliari (MDLCA), under registration numbers MDLCA 23648–23655. Specimens figured herein without registration numbers currently remain in situ.
Results
Facies description and echinoid diversity
Assemblage 1 occurs within pale yellow to white, very fine-grained wacke- to packstones that are intensely bioturbated by large, branched, Thalassinoides-like burrows. This assemblage is dominated by spatangoid echinoids with the schizasterid Ova Gray, Reference Gray1825 and subordinately the brissopsid Brissopsis L. Agassiz, Reference Agassiz1840 (Fig. 3.1), along with rare test remains of the loveniid Hemipatagus Desor, Reference Desor1858 and the echinocardiid Echinocardium Gray, Reference Gray1825 (Fig. 3.2). Among irregular echinoids, the minute clypeasteroid Echinocyamus van Phelsum, Reference van Phelsum1774 is commonly found. Diadematid echinoid remains also occur abundantly (Fig. 3.3, 3.4). These can occur as articulated test elements (Fig. 3.3A) and isolated ambulacral and interambulacral plates (Fig. 3.4A) and associated spines (Fig. 3.3B, 3.4B), which can be present as long segments and fragments. Isolated Aristotle's lantern elements ascribed to these diadematids consist of large hemipyramids, rotulae, and grooved teeth. The regular toxopneustid echinoids Tripneustes L. Agassiz, Reference Agassiz and Agassiz1841 (Fig. 3.5) and Schizechinus Pomel, Reference Pomel1869 (Fig. 3.6) are also present. Other major biotic constituents are common coralline algae rhodoliths (Fig. 4.1) present in discrete layers. These rhodoliths range from 2–13 cm in maximum length, and are dominated by subspherical shapes with a few highly spherical, although more flattened examples also present. Growth forms are dominated by the presence of encrusting thalli and low protuberances. Encrustation by densely packed barnacles reaching heights of ca. 1 cm is very common. Rhodoliths also show bioerosion consisting of small Trypanites Mägdefrau, Reference Mägdefrau1932 and rare Gastrochaenolites Leymerie, Reference Leymerie1842. Further biotic remains consist of rare pectinids and internal bivalve molds. Bioturbation is present, with Thalassinoides-like burrows generally to 2 cm in diameter (Fig. 4.2).
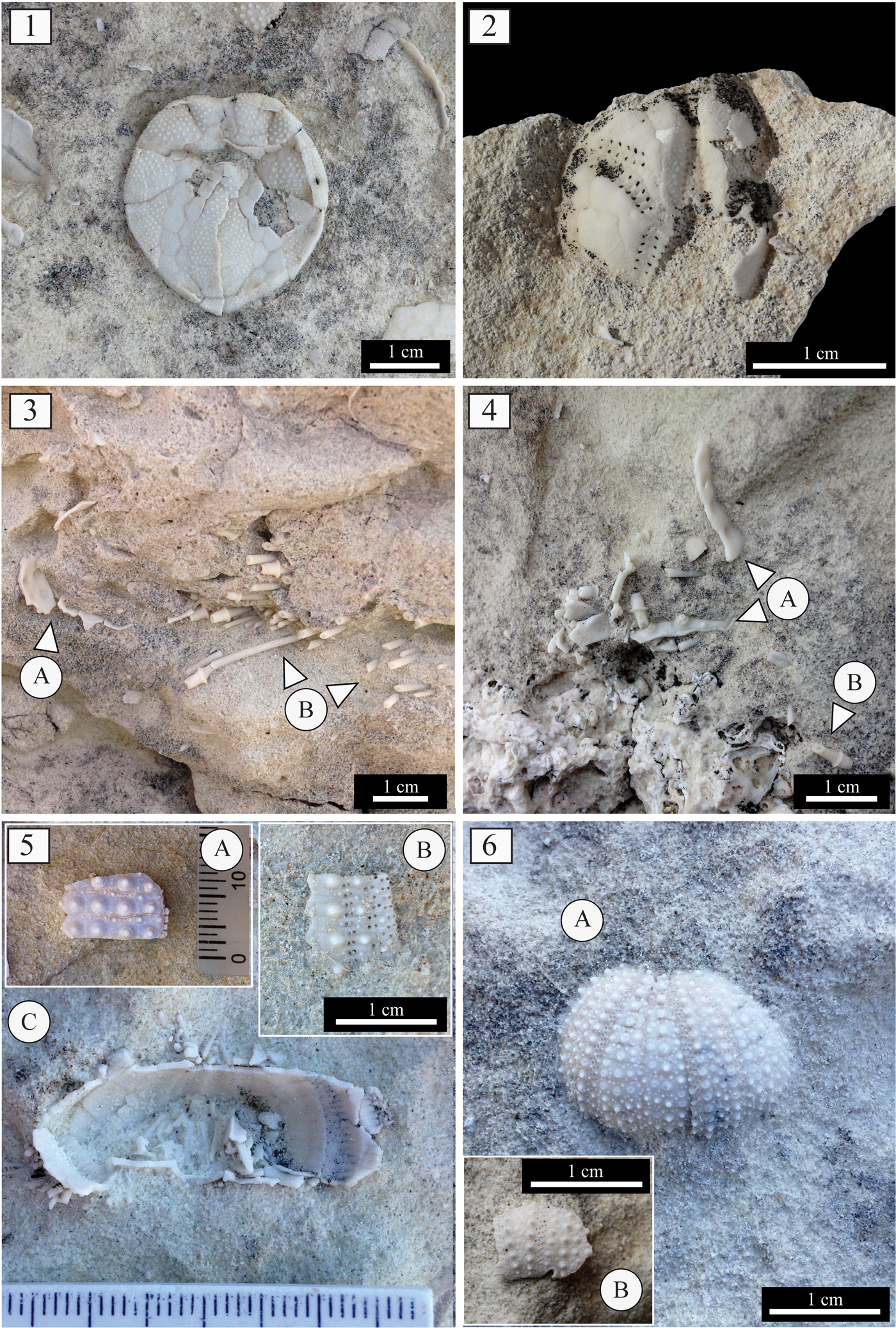
Figure 3. Assemblage 1: (1) Brissopsis in overturned position and spatangoid fragments; (2) Echinocardium (MDLCA 23648); (3, 4) test (A) and spine remains (B) of Diadema; (5) remains of Tripneustes interambulacral (A; MDLCA 23649) and (B) ambulacral (B; MDLCA 23650) plates, Aristotle's lantern (C), and spines (MDLCA 23651); (6) remains of Schizechinus complete test (A; MDLCA 23652) and test fragment (B; MDLCA 23653).

Figure 4. (1) Rhodoliths from Assemblage 1 with encrusting barnacles; (2) detail of the sedimentary succession of Santa Caterina di Pittinuri showing Thalassinoides-like burrows.
Assemblage 2 occurs within highly bioturbated, pale yellow, very fine-grained wacke- to packstones. This assemblage is dominated by the spatangoid Brissopsis (Fig. 5.1A) and the minute clypeasteroid Echinocyamus (Fig. 5.1B). Also present among spatangoids are Ova (Fig. 5.1C), Opissaster Pomel, Reference Pomel1883, and the loveniids Lovenia Desor in L. Agassiz and Desor, Reference Agassiz and Desor1847 and Hemipatagus (Fig. 5.2). The clypeasteroid Clypeaster marginatus Lamarck, Reference Lamarck1816 also occurs (Fig. 5.3). Among regular echinoids, test remains of the minute trigonocidarid Genocidaris A. Agassiz, Reference Agassiz1869 (Fig. 5.4) occur frequently. Small test and spine fragments of diadematid echinoids were found sporadically along with large hemipyramids ascribed to these echinoids. Other major biotic constituents are ossicles of asterozoans, the epitoniid gastropod Cirsotrema Mörch, Reference Mörch1852, the smooth and thin-shelled pectinid bivalve Amusium Röding, Reference Röding1798, remains of the portuniid crab Portunus Weber, Reference Weber1795 often with articulated chelipeds, and isolated barnacles. Internal molds of bivalves and gastropods were also found. The accompanying microfauna includes nodosariid foraminiferans. The fine-grained carbonate deposits are intensely bioturbated by large, branched Thalassinoides-like burrows that reach a diameter of 4 cm. These burrows are often filled by coarse biogenic material consisting predominately of spatangoid test fragments and bivalve shell remains (Fig. 5.5). Complete tests of Echinocyamus and Genocidaris can be also found within these burrows.
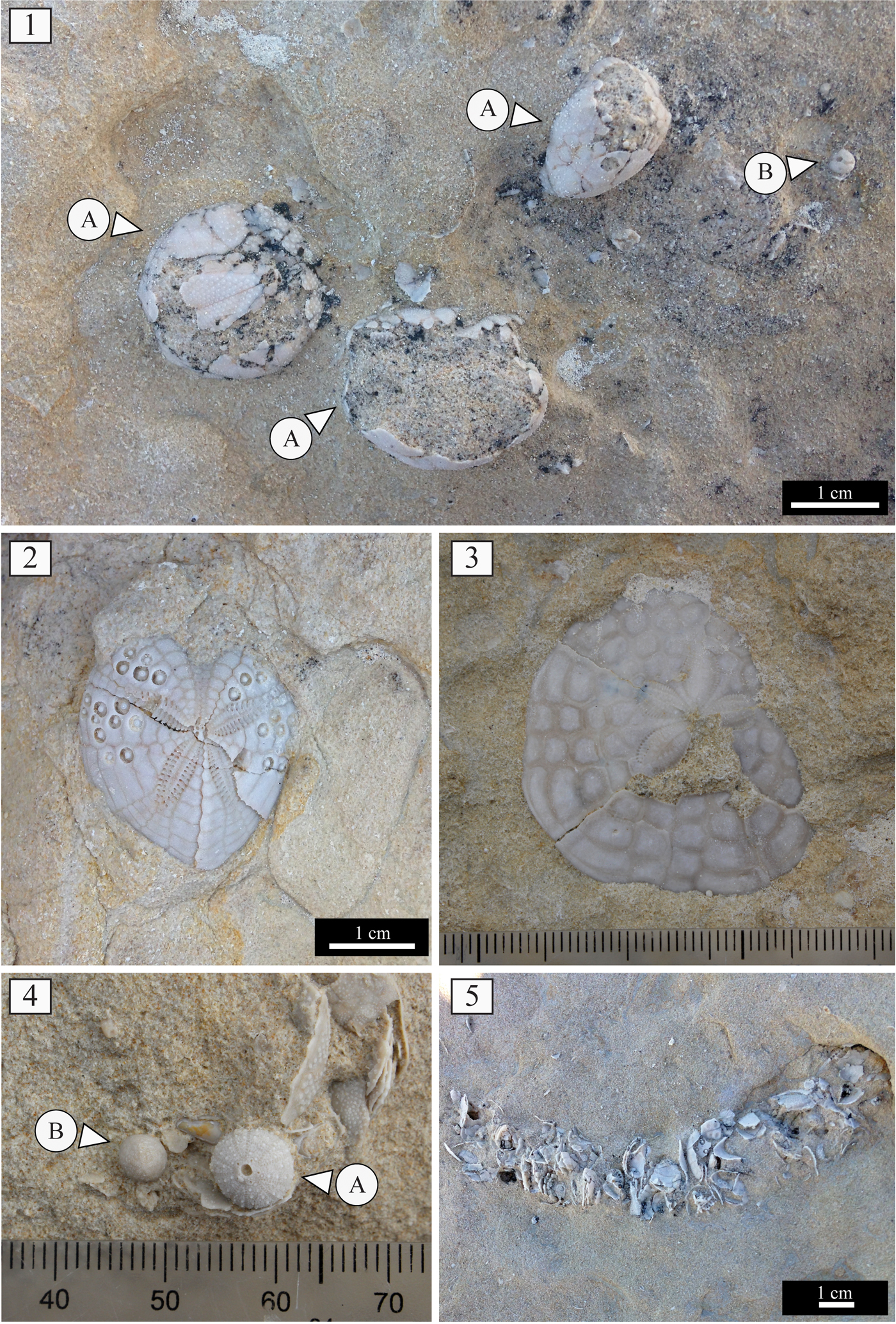
Figure 5. Assemblage 2: (1) spatangoid remains (A) and Echinocyamus (B) in fine-grained sediments within the sedimentary sequence of S'Archittu; (2) Hemipatagus (MDLCA 23654); (3) test remains of Clypeaster marginatus; (4) remains of Genocidaris (A) and Echinocyamus (B) (MDLCA 23655); (5) Thalassinoides-like burrows partially filled with fragments of echinoids and bivalves.
Assemblage 3 occurs within a highly bioturbated, whitish mud- to wackestone and is dominated by the spatangoid Brissopsis (Fig. 6.1) along with subordinate Ova (Fig. 6.2, 6.3). Sporadically present are the irregular echinoids Opissaster, Hemipatagus, and Echinocyamus and the regular echinoid Schizechinus.
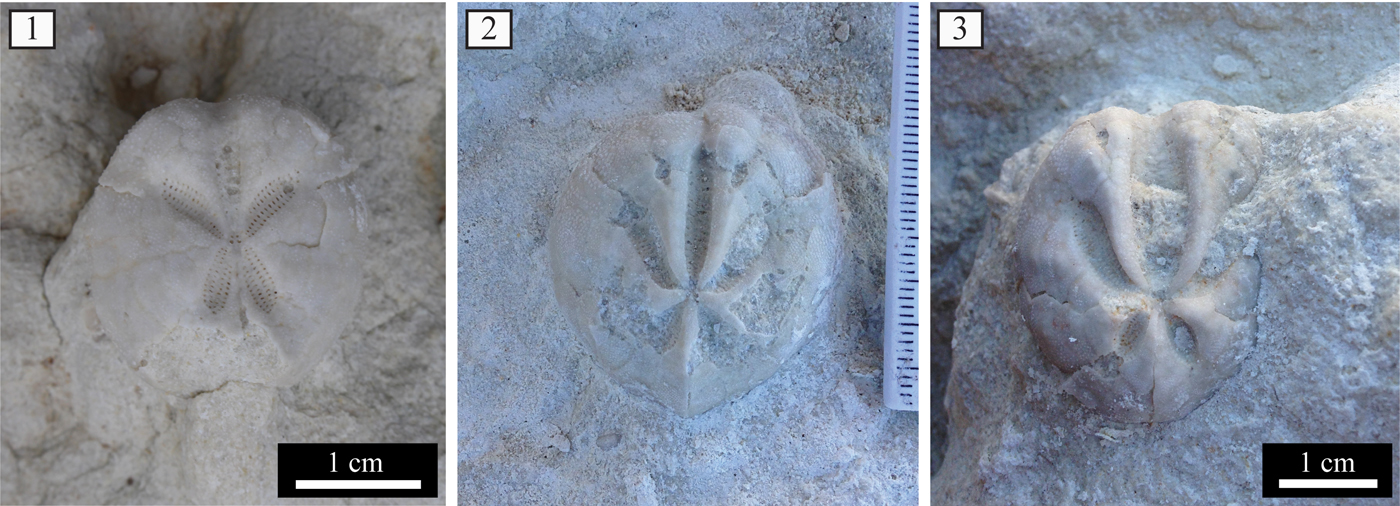
Figure 6. Assemblage 3: (1) Brissopsis; (2) Ova morphotype 1; (3) Ova morphotype 2.
Taphonomy
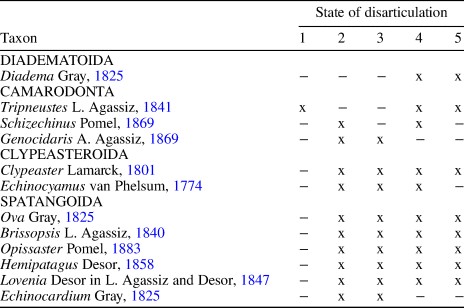
The taphonomic attributes of different echinoid taxa are summarized in Table 1. Echinoids are present as complete specimens as well as variously sized test fragments ranging from half tests to single isolated plates. Both inter- and intraplate fragmentation are present. Evidence of abrasion is lacking because echinoid tests and their fragments are very well preserved. Encrustation of the echinoids was not observed. Bioerosion is present as Oichnus-like circular drillholes on Echinocyamus and the spatangoids.
Table 1. Taphonomic attributes of the various echinoid taxa recognized within the assemblages studied herein. 1 = whole test with spines; 2 = whole test without spines; 3 = quarter to half tests; 4 = larger fragments of articulated ambulacral/interambulacral plates still sutured together; 5 = isolated plates, spine fragments.
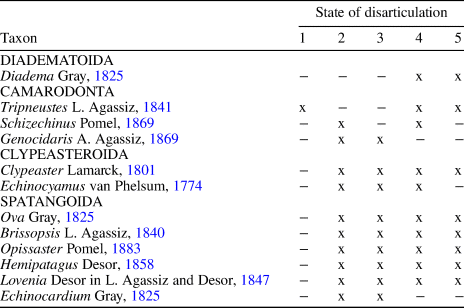
Among regular forms, diadematid echinoids occur commonly as isolated interambulacral and ambulacral plates, Aristotle's lantern elements, and spine fragments; partially preserved tests with associated spines were also found in Assemblage 1 (Fig. 3.3, 3.4). Tripneustes occurs almost exclusively as test fragments, which consist of several ambulacral and interambulacral plates still sutured together (Figs. 3.5A, B) and spine fragments. A single Tripneustes test with spines attached and Aristotle's lantern elements present was found in situ (Figs. 3.5C). Schizechinus occurs as complete tests lacking both spines and the apical system (Fig. 3.6A), and rarely as test fragments (Fig. 3.6B). In contrast, the minute Genocidaris is present mainly as complete tests lacking spines, some of which still retain the apical system (Fig. 5.4).
Clypeaster marginatus is present as complete tests but is mostly represented by pie-shaped portions of tests and smaller fragments. Fragments can be readily recognized due to the small, evenly distributed, sunken tubercles on the surface as well as presence of an internal support structure in the interior of the test. The clypeasteroid echinoid Echinocyamus is present mainly as complete tests, with fragmented material again showing internal supports.
Spatangoid echinoids are especially common and are present in all states of preservation from complete specimens to fragmented materials. The remnants of these echinoids also dominate the infillings of Thalassinoides-like burrows that can also include complete Echinocyamus and very rarely small specimens of complete spatangoids.
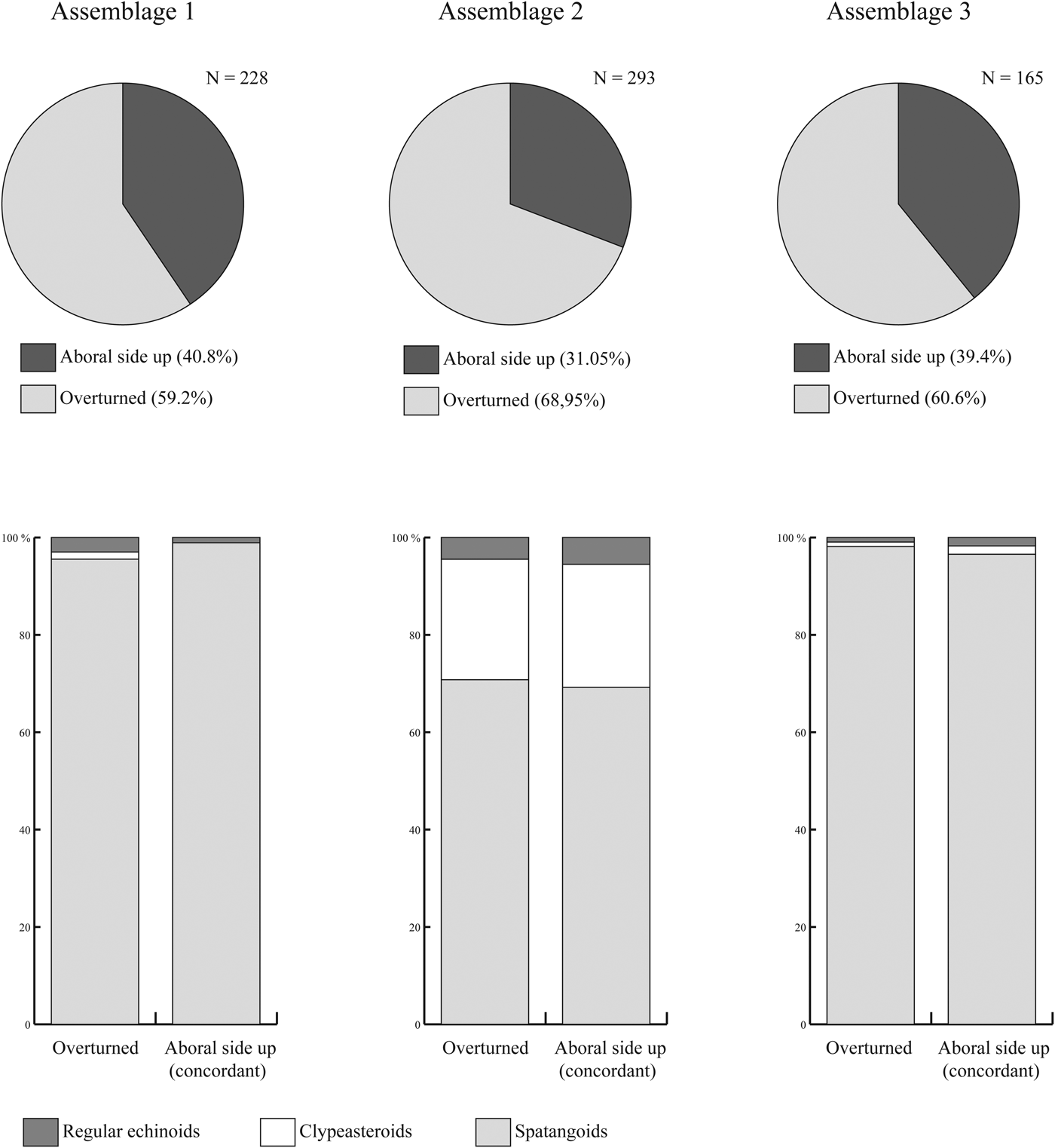
The echinoid remains are not homogeneously distributed within the deposit. In Assemblages 1 and 2, echinoid remains range from densely to loosely packed and are dispersed with complete tests reaching densities of 15 individuals/m2 on exposed rock surfaces. In Assemblage 3, echinoid remains range from loosely packed to dispersed. The echinoids show no preferred orientation both in plan view and cross section. Both complete specimens and fragments show orientations ranging from concordant to perpendicular to the bedding plane. In all three assemblages, complete specimens oriented aboral side up and concordant to the bedding plane are less common than oblique and overturned specimens (Fig. 7).
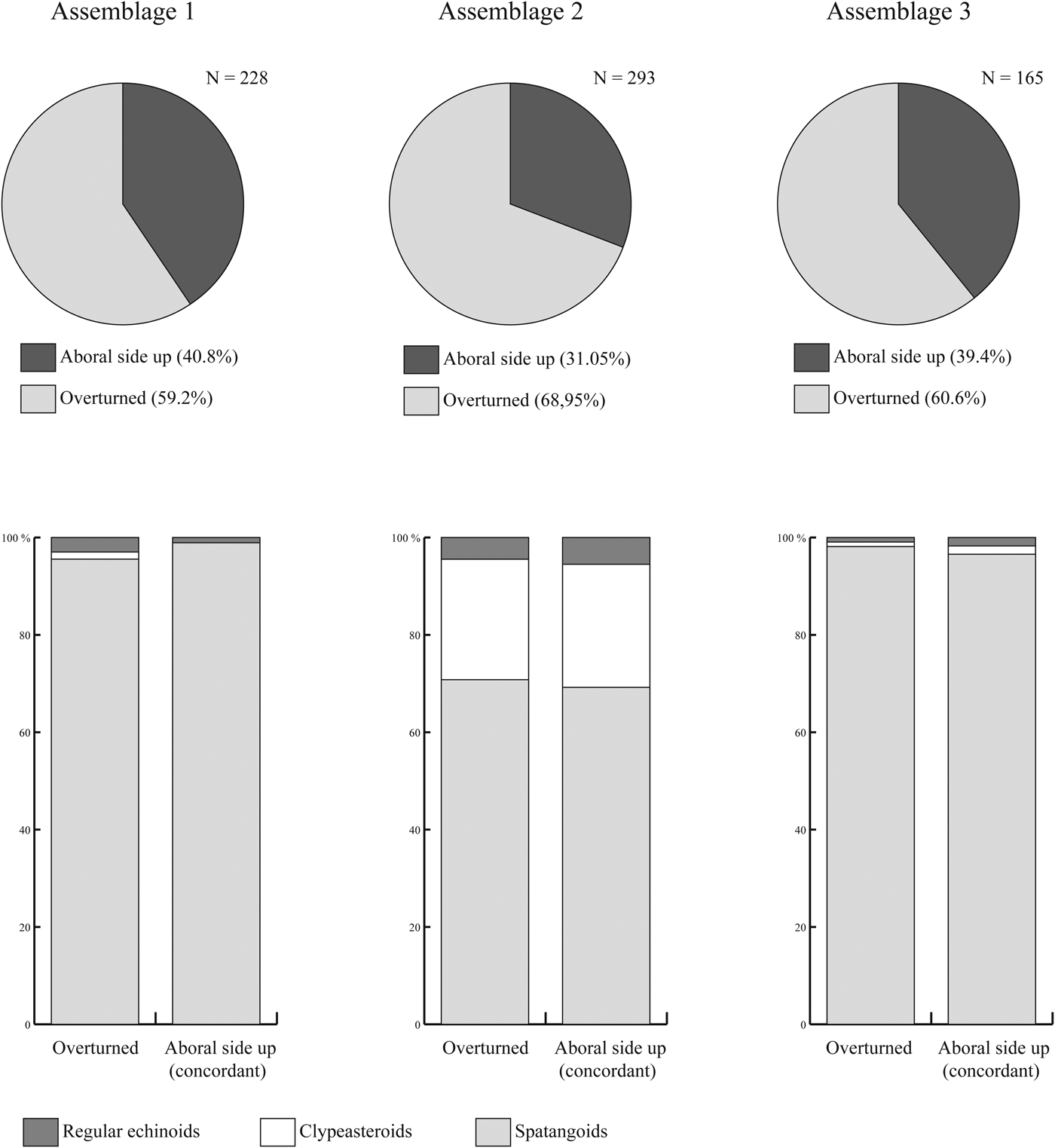
Figure 7. Orientation data of complete echinoid specimens within the assemblages studied herein. N = number of counted specimens.
Discussion
Functional morphology of echinoid tests and actualistic comparisons
The interpretations of life styles, functional morphological aspects, and actualistic comparisons of many of these echinoid taxa have been reviewed in previous papers dealing with the Miocene echinoids of Sardinia (see Mancosu and Nebelsick Reference Mancosu and Nebelsick2013, Reference Mancosu and Nebelsick2015, Reference Mancosu and Nebelsick2016, Reference Mancosu and Nebelsick2017a, Reference Mancosu and Nebelsickb; Mancosu et al., Reference Mancosu, Nebelsick, Kroh and Pillola2015) as summarized in Table 2. Newly discussed taxa (see below) include the diadematoid Diadema Gray, Reference Gray1825, the camarodont Schizechinus, the spatangoid Echinocardium (recorded for the first time from the Miocene of Sardinia), and two morphotypes of Ova.
Table 2. Palaeoecological interpretation of the echinoid taxa recognized herein, with comparisons with Recent analogs.

Diadematid echinoid remains occur abundantly in Assemblage 1 and sporadically in Assemblage 2. Diadema and Centrostephanus Peters, Reference Peters1855 were reported from the Miocene of Sardinia based on spine fragments (Cotteau, Reference Cotteau1895; Lambert, Reference Lambert1907); however, as previously discussed (e.g., by Kroh, Reference Kroh2005 and Donovan et al., Reference Donovan, Renema, Pinnington and Veltkamp2011), subfamilial classification of diadematid echinoids based on spines and test fragments is problematic. The discovered remains can be assigned to the genus Diadema based on the presence of trigeminate ambulacral plates bearing a single large tubercle, with pore-pairs of P2 type in a single series that widen adorally to form a phyllode with pore-pairs of P3 type, interambulacral plates containing up to four subequal, perforated, crenulate tubercles, and hollow and verticillate spines showing clearly asymmetrical distinct bases.
Diadema is interpreted herein as living epifaunally within coralline algal beds as indicated by the presence of oral P3 type isopores. These are partitioned isopores surrounded by a broad attachment area for the rectractor muscle of the tube feet and are present in shallow-water species living on rocks or reef structure, in crevices, or beneath boulders (Smith, Reference Smith1978). Diadematids, e.g., Diadema and Centrostephanus, are epifaunal regular echinoids that inhabit mostly protected littoral and sublittoral environments (Mortensen, Reference Mortensen1940). Diadema is among the most ecologically important echinoids in tropical oceans (Andrew and Byrne, Reference Andrew, Byrne and Lawrence2007; Muthiga and McClanahan, Reference Muthiga, McClanahan and Lawrence2007 and references therein) and has only been recently observed in the shallow water of the Mediterranean Sea, representing an invasive Lessepsian migrant from the Red Sea (Yokes and Galil, Reference Yokes and Galil2006; Nader and El Indary, Reference Nader and El Indary2011; Bronstein et al., Reference Bronstein, Georgopoulou and Kroh2017). Species of Diadema, e.g., D. antillarum Philippi, Reference Philippi1845, D. setosum (Leske, Reference Leske1778), D. mexicanum A. Agassiz, Reference Agassiz1863, and D. ascensionis Mortensen, Reference Mortensen and von Drygalski1909, occupy diverse habitats from shallow water to a depth of 400 m, although they are most abundant in littoral areas, on rock and sandy substrata, coral reefs, mangrove roots, and seagrass beds (Randall et al., Reference Randall, Schroeder and Starck1964; Chesher, Reference Chesher1972; Kier, Reference Kier1975; Smith, Reference Smith1978; Serafy, Reference Serafy1979; Coppard and Campbell, Reference Coppard and Campbell2005, Reference Coppard and Campbell2007; Lessios, Reference Lessios2005; Muthiga and McClanahan, Reference Muthiga, McClanahan and Lawrence2007; Gondim et al., Reference Gondim, Dias and Christoffersen2013; Nateghi Shahrokni et al., Reference Nateghi Shahrokni, Fatemi, Nabavi and Vosoughi2016). They are mainly omnivorous grazers and detritus feeders, scraping algal films off hard substrata and feeding on seagrasses, foraminiferans, crustaceans, and small organisms found on the sea floor (Mortensen, Reference Mortensen1940; Lewis, Reference Lewis1964; Randall et al., Reference Randall, Schroeder and Starck1964; Pearse, Reference Pearse1970; Serafy, Reference Serafy1979; De Ridder and Lawrence, Reference De Ridder, Lawrence, Jangoux and Lawrence1982). Diadema, as many other diadematids, is highly light sensitive, often foraging at night and remaining hidden in rocky crevices and holes during the day (Mortensen, Reference Mortensen1940; Tuya et al., Reference Tuya, Martin and Luque2004; Andrew and Byrne, Reference Andrew, Byrne and Lawrence2007).
The toxopneustid Schizechinus from Assemblages 1 and 3, which was described by Cotteau (Reference Cotteau1895) and Comaschi Caria (Reference Comaschi Caria1951) as Psammechinus calarensis Cotteau, Reference Cotteau1895, is a small to medium-sized echinoid interpreted herein as living in low to moderate energy environments as suggested by the presence of oral P2 isopores (Smith, Reference Smith1978). Schizechinus is exclusively known from fossils and occurs commonly in carbonate and less commonly in siliciclastic shallow-water sediments in Miocene sedimentary successions of the Mediterranean and central Paratethys (see Challis, Reference Challis1980; Schmid et al., Reference Schmid, Harzhauser and Kroh2001; Kroh, Reference Kroh2005).
Schizechinus is closely similar to the extant toxopneustid Sphaerechinus Desor, Reference Desor1856, a monotypic genus living in the Mediterranean and eastern Atlantic Ocean. Sphaerechinus granularis (Lamarck, Reference Lamarck1816) occurs from the littoral zone to depths of 120 m on a wide variety of substrata, including mud and fine- to coarse-grained sands, rocky bottoms, seagrass, and algal meadows, and also in coarse-grained, coralline-algae-dominated sediments (e.g., Koehler, Reference Koehler1927; Mortensen, Reference Mortensen1943; Tortonese, Reference Tortonese1965; Ernst et al., Reference Ernst, Hähnel and Seibertz1973; Smith, Reference Smith1978; Harmelin and Duval, Reference Harmelin and Duval1983; Riedl, Reference Riedl1983; Guillou and Michel, Reference Guillou and Michel1993; Unger and Lott, Reference Unger, Lott, David, Guille, Feral and Roux1994; Sartoretto and Francour, Reference Sartoretto and Francour1997; Palacín et al., Reference Palacín, Turon, Ballesteros, Giribert and López1998; Zavodnik, Reference Zavodnik2003; Koukouras et al., Reference Koukouras, Sinis, Bobori, Savas and Miltiadis-Spyridon2007; Despalatović et al., Reference Despalatović, Grubelić, Piccinetti, Cvitović, Antolić, Žuljević and Nikolić2009; Antoniadou and Vafidis, Reference Antoniadou, Vafidis and Withmore2014; Petović and Krpo-Ćetković, Reference Petović and Krpo-Ćetković2016; Sievers and Nebelsick, Reference Sievers and Nebelsick2018). Sphaerechinus is mainly herbivorous, feeding on seagrass, encrusting coralline algae, and soft algae. It also selectively consumes detritus when living in soft-bottom environments (De Ridder and Lawrence, Reference De Ridder, Lawrence, Jangoux and Lawrence1982; Guillou and Lumingas, Reference Guillou and Lumingas1998; Martínez-Pita et al., Reference Martínez-Pita, Sánchez-España and García2008; Elmasry et al., Reference Elmasry, Omar, Abdel Razek and El-Magd2013).
The echinocardiid Echinocardium sp. from Assemblage 1 represents the first report of this genus in the Miocene of Sardinia. Its globular test (sensu Kanazawa, Reference Kanazawa1992) with a keeled plastron, the presence of nonconjugated, partitioned isopores for funnel-building tube feet in ambulacrum III, together with an inner fasciole, allowed this spatangoid echinoid to burrow deeply in fine-grained sediments. The presence of minute pores within the shield-shaped subanal fasciole indicates that Echinocardium sp. was possibly able to construct and maintain a single sanitary drain, as reported, e.g., by Nichols (Reference Nichols1959) for extant species of Echinocardium, e.g., E. cordatum (Pennant, Reference Pennant1777), E. pennatifidum Norman, Reference Norman1869, and E. flavescens (O.F. Müller, Reference Müller1776).
Extant species of Echinocardium are infaunal deposit feeders that inhabit a wide range of environments from intertidal to midshelf burrowing in different types of sediments, mostly fine sands to mud, predominantly in temperate regions (Mortensen, Reference Mortensen1951; Nichols, Reference Nichols1959; Buchanan, Reference Buchanan1966; Tortonese, Reference Tortonese1977; De Ridder, Reference De Ridder and Lawrence1982; Duineveld and Jenness, Reference Duineveld and Jenness1984; Kanazawa, Reference Kanazawa1992; Nakamura, Reference Nakamura2001; Zavodnik, Reference Zavodnik2003). Field studies on E. cordatum show that this spatangoid inhabits both littoral and offshore environments burrowing at depths from a few to ~ 20 cm deep in sandy and silty sediments. Ursin (Reference Ursin1960) and Buchanan (Reference Buchanan1966) documented populations of E. cordatum from North Sea coasts occurring offshore at depths of 30–40 m, dispersed in large discrete patches at maximum densities of 40 individuals/m2. Higher densities of Echinocardium (to 200 individuals/m2) were reported from Seto Inland Sea, Japan (Nakamura, Reference Nakamura2001) and from the Belgian continental shelf (Degraer et al., Reference Degraer, Wittoeck, Appeltans, Cooreman, Deprez, Hillewaert, Hostens, Mees, Vanden Berghe and Vincx2006).
Two morphotypes of the schizasterid Ova were identified within the studied assemblages (see Fig. 6.2, 6.3). Morphotype 1 has a test with a subcircular outline and a relatively narrow and shallow ambulacrum III. Morphotype 2 differs in having a test with a more depressed wedge-shaped profile, slightly elongated outline, and larger and deeper ambulacrum III with a greater number of partitioned isopores. Both Ova morphotypes co-occur within Assemblages 2 and 3; morphotype 1 has not been recognized within Assemblage 1.
Both Ova morphotypes are interpreted here to have burrowed deeply in fine-grained sediments. Morphotype 1, however, owing to its more wedge-shaped profile, deeper and wider frontal ambulacrum with more numerous well-developed partitioned isopores for funnel-building tube feet, posteriorly located apical system, keeled posterior interambulacrum, long and curved anterior-paired petals, shorter posterior-paired petals, as well as peripetalous and lateroanal fascioles possibly buried deeper than morphotype 2. In both forms, the aboral tuberculation is fine, uniform, and dense indicating the presence of a dense canopy of spines enabling burrowing within fine-grained substrata with the spines used to support the top of the burrow and maintain a space for water circulation (e.g., Gale and Smith, Reference Gale and Smith1982; Kanazawa, Reference Kanazawa1992). The presence of a lateroanal fasciole and partitioned isopores in the subanal region enabled the construction of sanitary drains.
Most extant species of the genus Ova and the closely related genus Schizaster include shallow and deeper burrowing forms and inhabit inner neritic environments shallower than 100 m depth (Mortensen, Reference Mortensen1951). Ova canalifera (Lamarck, Reference Lamarck1816) from the Mediterranean is known to live buried in fine-grained sediments to 20 cm deep, with maximum abundances between 20 and 70 m depth (Tortonese, Reference Tortonese1965; Schinner, Reference Schinner1993; Bromley et al., Reference Bromley, Jensen and Asgaard1995; Zavodnik, Reference Zavodnik2003; Koukouras et al., Reference Koukouras, Sinis, Bobori, Savas and Miltiadis-Spyridon2007). This echinoid constructs both a respiratory funnel and a subanal sanitary drain (Schinner, Reference Schinner1993; Asgaard and Bromley, Reference Asgaard, Bromley, Bromley, Buatois, Mángano, Genise and Melchor2007). Schizaster lacunosus (Linnaeus, Reference Linnaeus1758) is a deposit feeder that occurs buried in fine-grained sediments at 5–90 m depth (Mortensen, Reference Mortensen1951; Schin and Thompson, Reference Schin and Thompson1982; Kanazawa, Reference Kanazawa1992; Chao, Reference Chao2000; Banno, Reference Banno2008). Schizaster floridiensis (Kier and Grant, Reference Kier and Grant1965) from the Caribbean Sea lives at water depths of 9–65 m (Rodríguez-Barreras, Reference Rodríguez-Barreras2014) burrowing in mud and sand bottoms to 25 cm below the sediment surface (Chesher, Reference Chesher1966). The distribution of Ova seems to be primarily controlled by the availability of a suitable soft substratum consisting of silts to fine-grained sands within which this echinoid burrows. If such suitable substrata are present, these echinoids can occur both in protected shallow water as well as in deeper environments.
Paleoenvironmental reconstruction of the echinoid assemblages
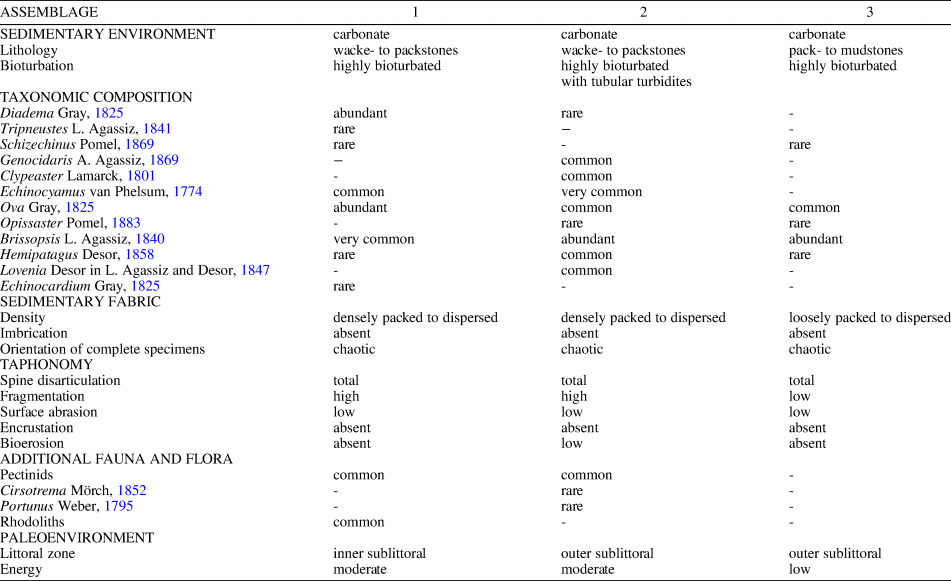
The Miocene echinoid fauna found within the studied sedimentary succession, which is dominated by irregular echinoids (mainly spatangoids) as well as associated fauna and flora, lithology, and sedimentary features, points to relatively deep, sublittoral environments (Figs. 8, 9). The echinoid assemblages are interpreted as autochthonous to parautochthonous. Although taphonomic signatures, e.g., the state of disarticulation and fragmentation and orientation with respect to the bedding plane, clearly show that echinoid remains are not preserved in life positions; they are exquisitely preserved with respect to surface details, including tuberculation, ambulacral pore-pairs, and fascioles, and were not transported for any appreciable distance before final burial. The preservation of a large number of complete tests lacking spines and showing no evidence of encrustation indicates short surface-residence times on the sediment/water interface before being buried in the sediment. Differences among the three studied assemblages with respect to echinoid diversity, the relative abundance of taxa, and the associated fauna, flora, and trace fossils can be detected (see Table 3, Fig. 9).
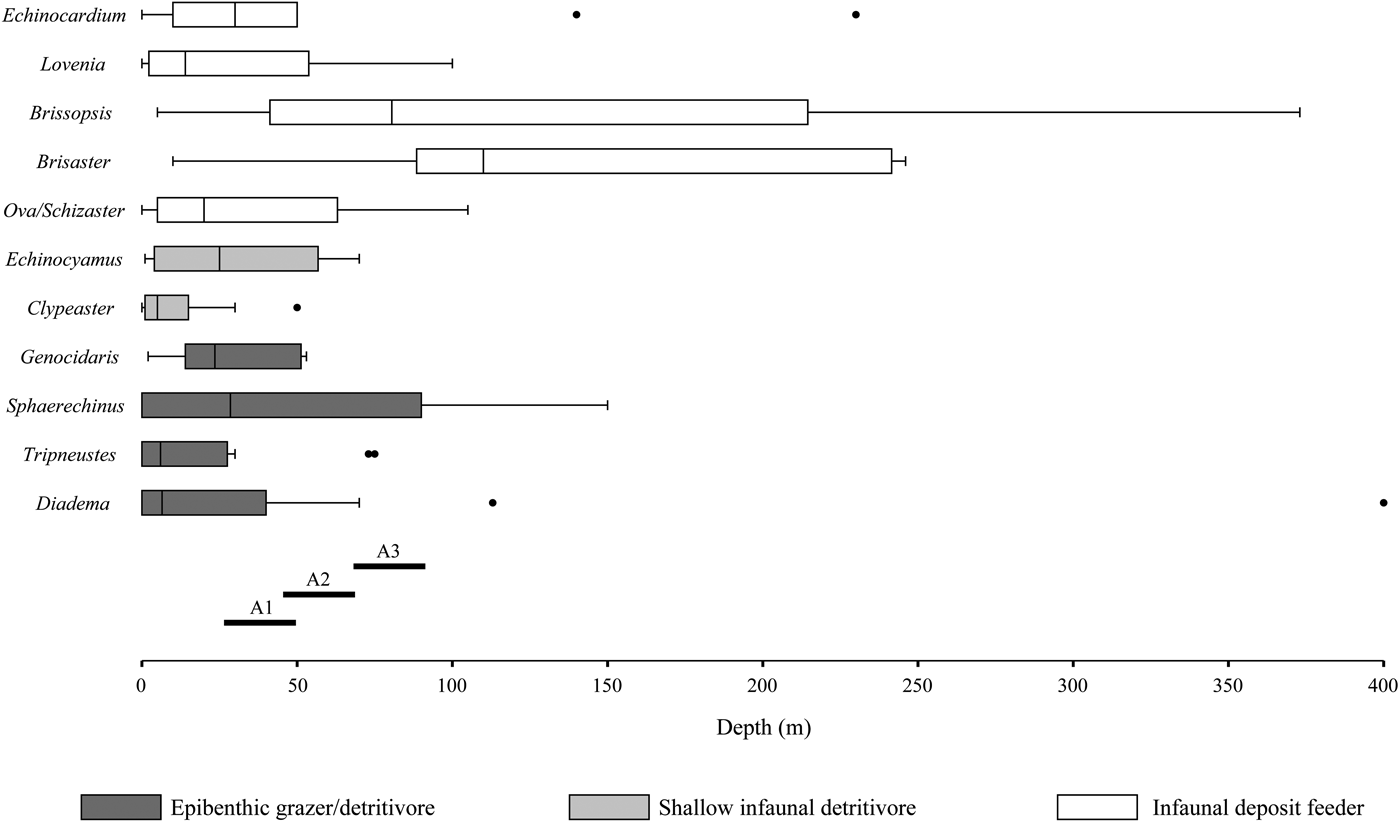
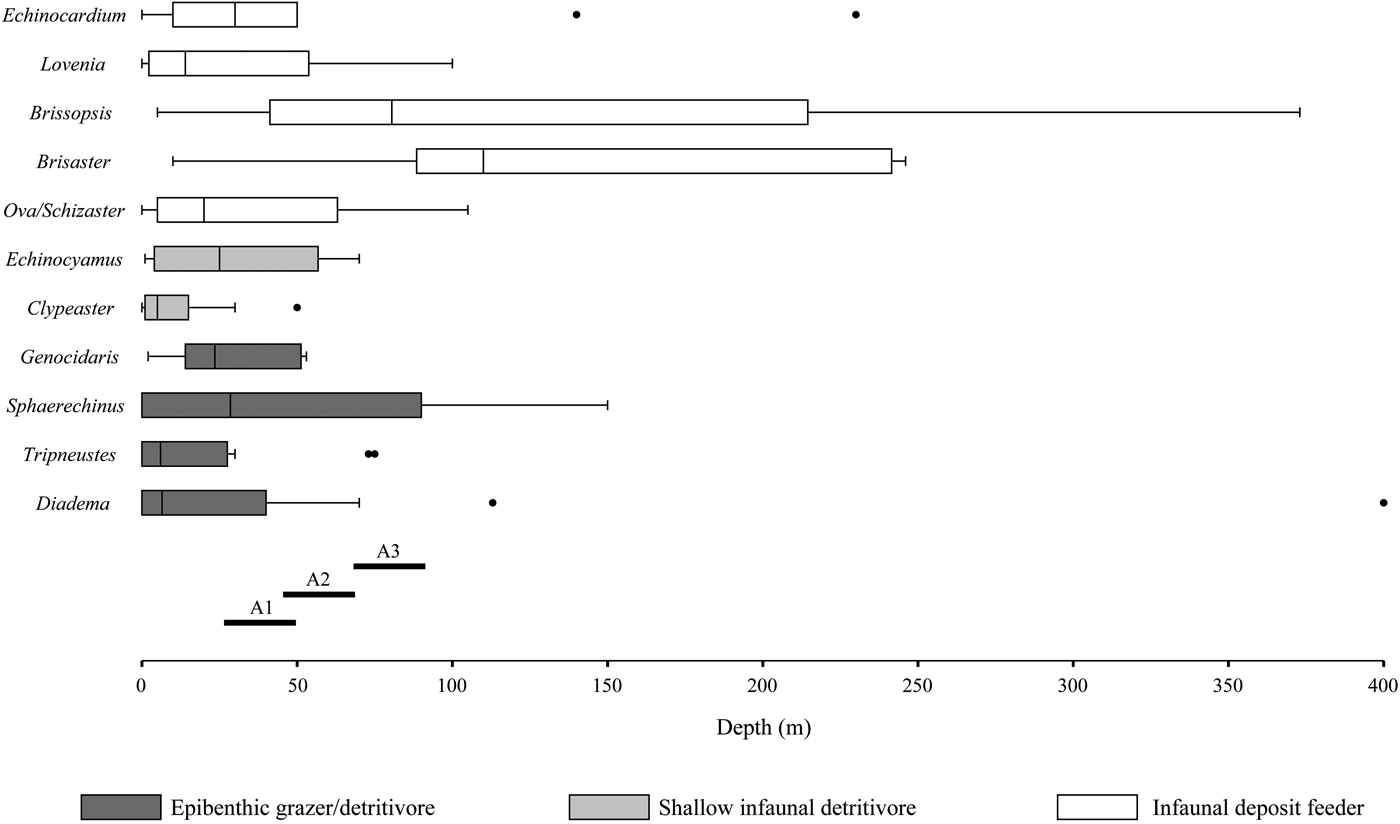
Figure 8. Bathymetric distributions and modes of life of the Recent analogous taxa of the fossil echinoids recognized in the present study with interpreted depths for the three assemblages described herein. Each box plot represents 25% and 75% quartile of all values, Q1 and Q3, respectively. Black line inside box represents the median. Whiskers drawn from Q1 and Q3 to the largest values < 1.5 times the interquartile range (Q1–Q3). Outliers indicated by black dots. A1 = Assemblage 1; A2 = Assemblage 2; A3 = Assemblage 3.
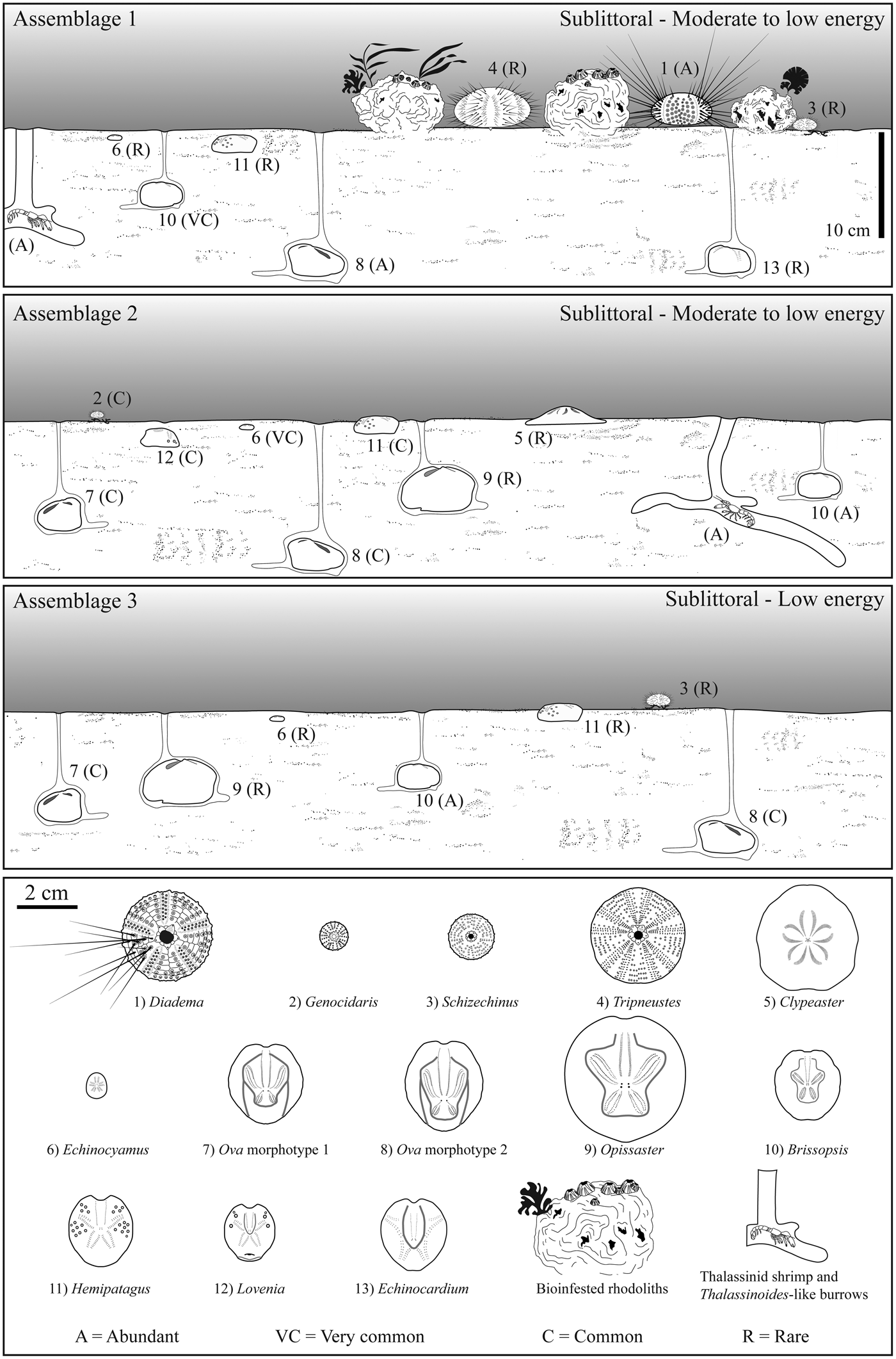
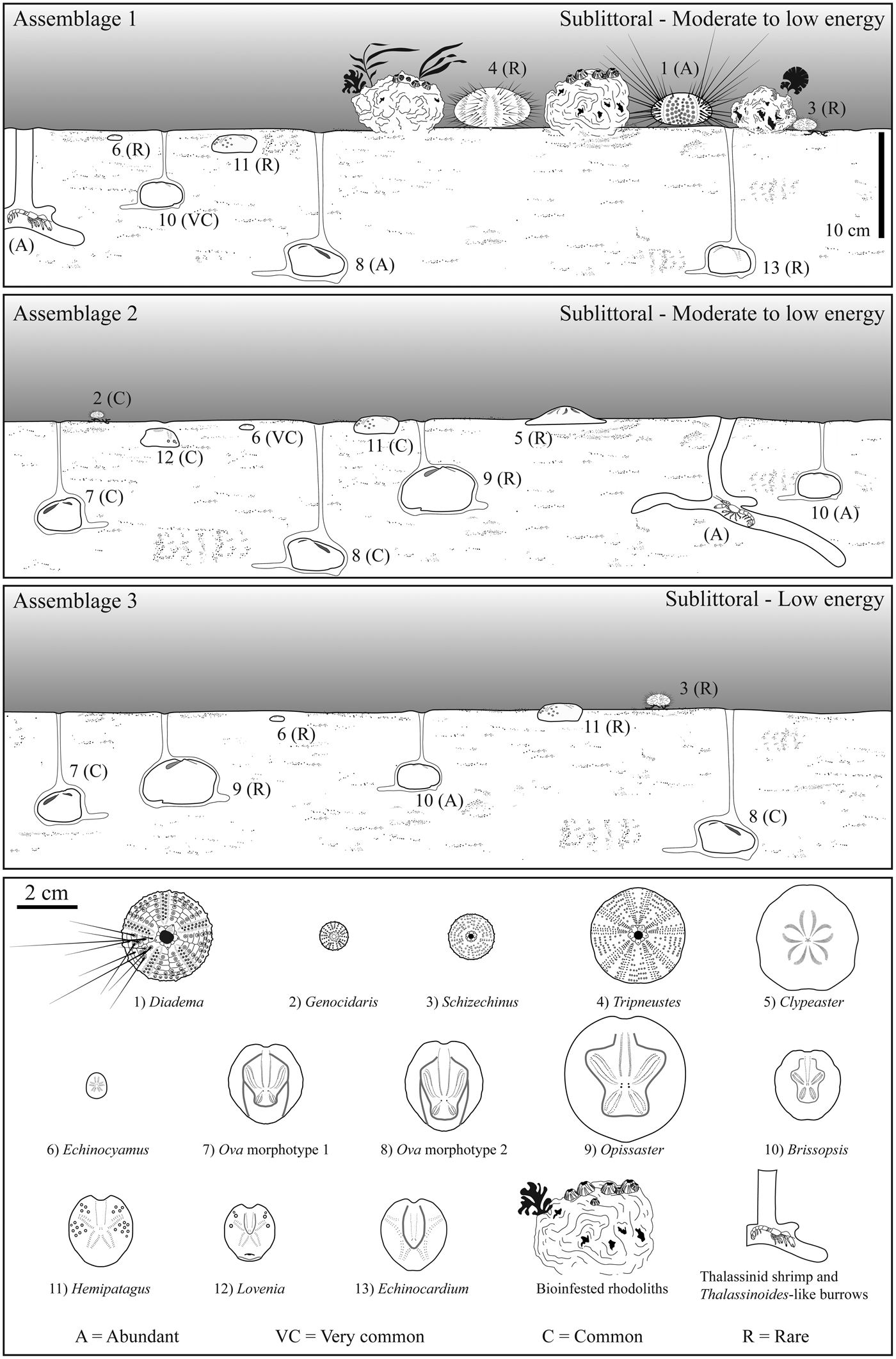
Figure 9. Paleoecological reconstruction of the echinoid assemblages from the investigated levels in the sedimentary successions studied herein. The presence and depths of bioturbation are indicated; depth scale is the same for Assemblage 1, 2, and 3. See text for density and preservation of the various taxa within the assemblages.
Table 3. Summary of taxonomic, sedimentological, and taphonomic features of the echinoid assemblages from Santa Caterina di Pittinuri and S'Archittu-Cajaragas.
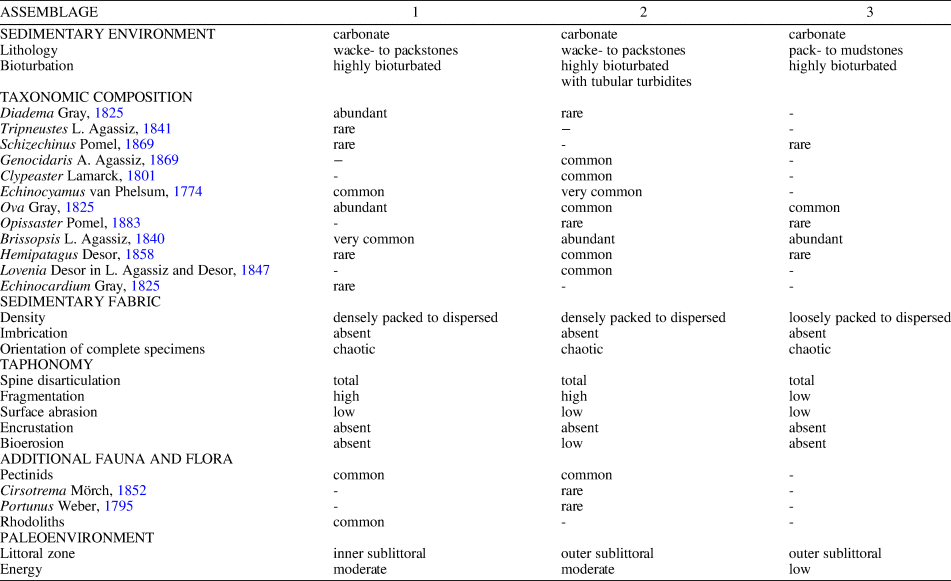
In Assemblage 1, the co-occurrence of the camarodonts Tripneustes and Schizechinus and the diadematoid Diadema among regular forms, the spatangoid echinoids Ova, Brissopsis, Hemipatagus, Echinocardium, and the clypeasteroid Echinocyamus, and the presence of rhodoliths loosely scattered throughout the fine-grained sediments, indicate a highly structured sublittoral environment still within the photic zone, with soft substrata and rhodolith patches.
Rhodolith beds frequently occur today in the mesophotic zone mostly at ~ 40–60 m water depth (Bassi et al., Reference Bassi, Nebelsick, Checconi, Hohenegger and Iryu2009; Foster et al., Reference Foster, Amado-Filho, Kamenos, Riosmena-Rodriguez, Steller, Lang, Marinelli, Roberts and Taylor2013; Basso et al., Reference Basso, Babbini, Kaleb, Bracchi and Falace2016) where there are low, but still sufficient light levels for photosynthesis (Littler et al., Reference Littler, Littler and Hanisak1991; Foster, Reference Foster2001). Rhodolith beds provide three-dimensional hard substrata and support a high diversity and abundance of marine flora and fauna (e.g., Steller et al., Reference Steller, Riosmena-Rodrìguez, Foster and Roberts2003; Pascelli et al., Reference Pascelli, Riul, Riosmena-Rodrìguez, Scherner, Nunes, Hall-Spencer, Oliveira and Horta2013; Teichert, Reference Teichert2014; Horta et al., Reference Horta, Riul, Amado Filho, Gurgel, Berchez, Nunes, Scherner, Pereira, Lotufo, Peres, Sissini, Bastos, Rosa, Munoz, Martins, Gouvêa, Carvalho, Bergstrom, Schubert, Bahia, Rodrigues, Rörig, Barufi and Figueiredo2016; Hernandez-Kantun et al., Reference Hernandez-Kantun, Hall-Spencer, Grall, Adey, Rindi, Maggs, Bárbara, Peña, Riosmena-Rodríguez, Aguirre and Nelson2017, and references therein), including echinoids (James, Reference James2000; Kamenos et al., Reference Kamenos, Moore and Hall-Spencer2004; Gagnon et al., Reference Gagnon, Matheson and Stapleton2012; Gondim et al., Reference Gondim, Dias, Duarte, Riul, Lacouth and Christoffersen2014; Horta et al., Reference Horta, Riul, Amado Filho, Gurgel, Berchez, Nunes, Scherner, Pereira, Lotufo, Peres, Sissini, Bastos, Rosa, Munoz, Martins, Gouvêa, Carvalho, Bergstrom, Schubert, Bahia, Rodrigues, Rörig, Barufi and Figueiredo2016).
Assemblage 2, with the co-occurrence of the spatangoids Ova, Opissaster, Brissopsis, Lovenia, and Hemipatagus, the clypeasteroids Echinocyamus and Clypeaster marginatus, and the camarodont Genocidaris, represents a relatively deep, outer-sublittoral environment with low to moderate water energy and mobile, fine-grained sand substrate. The sediments were heavily affected by Thalassinoides-like burrows presumably produced by thalassinid shrimps that can form large populations in extant littoral and sublittoral environments (see Dworschak, Reference Dworschak2000). Sporadic high-energy events not only led to temporarily exhumation, overturning, and reworking of the echinoids, but also to the infilling of burrows by densely packed echinoid remains. The Thalassinoides-like burrows filled by echinoid test fragments and bivalve shell remains are interpreted as tubular tempestites that represent open tubes produced by burrowing animals subsequently filled with sediments and bioclasts transported by storm-generated currents (Wanless et al., Reference Wanless, Tedesco and Tyrrell1988; Tedesco and Wanless, Reference Tedesco and Wanless1991).
Assemblage 3, with its lower diversity and the dominance of burrowing spatangoid echinoids including Brissopsis and, subordinately, Ova, and the sporadic occurrence of Opissaster, Hemipatagus, Echinocyamus, and Schizechinus represents a slightly deeper and quieter environment with muddy substrate, possibly slightly below normal storm wave base. Depositional environments characterized by fine-grained, carbonate sediments with highly bioturbated internal structures resulting from the activities of infaunal animals, including echinoids and crustacean decapods, occur today in relatively shallow sublittoral settings with low energy conditions and episodic storm events (e.g., Blom and Aslop, Reference Blom and Aslop1988; Scoffin, Reference Scoffin1988; Bentley and Nittrouer, Reference Bentley and Nittrouer2012) and provide an analog for the environments described herein.
Various trophic resources were exploited, as denoted by the co-occurrence of omnivorous and algal-grazing regular echinoids and both shallow- and deeper-burrowing, deposit-feeding irregular echinoids. Niche separation among regular echinoids was reported according to food preferences, type of foraging, morphological adaptations, predation, and water depth (e.g., Keller, Reference Keller1983; McClanahan, Reference McClanahan1988; Jacob et al., Reference Jacob, Terpstra and Brey2003; Coppard and Campbell, Reference Coppard and Campbell2005; Privitera et al., Reference Privitera, Noli, Falugi and Chiantore2008; Bonaviri et al., Reference Bonaviri, Fernández, Fanelli, Badalamenti and Gianguzza2011; Cordeiro et al., Reference Cordeiro, Harborne and Ferreira2014; Cabanillas-Terán et al., Reference Cabanillas-Terán, Loor-Andrade, Rodríguez-Barreras and Cortés2016). A further example of habitat/resource partitioning has been reported for the sea urchins Arbacia lixula (Linnaeus, Reference Linnaeus1758) and Paracentrotus lividus (Lamarck, Reference Lamarck1816), which can coexist even at relatively high densities in the infralittoral zone of the Mediterranean due to nonoverlapping feeding preferences (Régis, Reference Régis1979; Privitera et al., Reference Privitera, Noli, Falugi and Chiantore2008; Bonaviri et al., Reference Bonaviri, Fernández, Fanelli, Badalamenti and Gianguzza2011; Antoniadou and Vafidis, Reference Antoniadou, Vafidis and Withmore2014, and references therein).
The co-occurrence of different deposit-feeding irregular echinoids was observed in all assemblages. Interspecific competition below the sediment-water interface among different burrowing, deposit-feeding echinoids could have been limited by their different burrowing depths, feeding strategies, and food selection leading to infaunal tiering (see discussion by Mancosu and Nebelsick, Reference Mancosu and Nebelsick2017a, Reference Mancosu and Nebelsickb, and references therein). Spatangoid-dominated echinoid assemblages that indicate outer sublittoral environments with low-energy conditions have been reported to occur throughout Miocene deposits of the circum-Mediterranean area (e.g., Néraudeau et al., Reference Néraudeau, Goubert, Lacour and Rouchy2001; Kroh and Nebelsick, Reference Kroh and Nebelsick2003; Mancosu and Nebelsick, Reference Mancosu and Nebelsick2016, Reference Mancosu and Nebelsick2017b). A comparable echinoid fauna as those described herein was recognized in the lower/middle Miocene sedimentary succession of the Porto Torres area, northern Sardinia (see Mancosu and Nebelsick, Reference Mancosu and Nebelsick2017b).
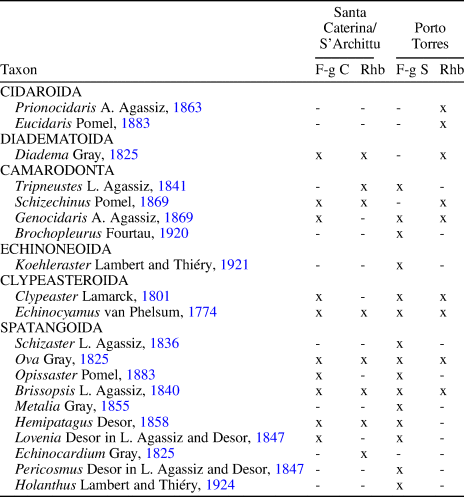
Differences with respect to lithology and echinoid diversity are recognized (see Table 4). In Porto Torres, the fine-grained sandstones, which have a higher terrigenous content than the fine-grained deposits of Santa Caterina-S'Archittu, are also intensely bioturbated by Thalassinoides-like burrows and are likewise associated with intercalated rhodolith beds. This succession contains a higher echinoid diversity, with nine genera of spatangoids, the presence of the echinoneid Koehleraster Lambert and Thiéry, Reference Lambert and Thiéry1921 and clear differences in the regular echinoids associated with the rhodolith beds, with spines and test fragments of the cidaroids Prionocidaris A. Agassiz, Reference Agassiz1863 and Eucidaris Pomel, Reference Pomel1883 along with the remains of Schizechinus and trigonocidarids. Differences in echinoid diversity and composition between Porto Torres and Santa Caterina-S'Archittu could be related to the preference for particular substrata in some echinoid taxa.
Table 4. Comparison between echinoid faunas of Santa Caterina-S'Archittu and Porto Torres. F-g C = fine-grained carbonates; F-g S = fine-grained sandstones; Rhb = rhodolith beds.
In Porto Torres, rhodoliths and accompanying echinoid faunas are associated with tubular tempestites, whereas those in the present study occur with Thalassinoides-like burrows containing surrounding sediment. This could indicate a lack of high storm activity in Assemblage 1, although more studies are needed in this respect on the morphologies and coralline algal diversities within the rhodoliths of the two localities. In both this study and Porto Torres, a general low-energy, moderately deep, sublittoral environment with high rates of bioturbation and episodes of sediment deposition by storms is interpreted.
Preservation potential of echinoids and comparative taphonomy
Paleoecological interpretation can be biased by taphonomic and sedimentological overprinting that affects the preservation of the various echinoid taxa and their representation within the assemblages. The factors leading to the taphonomy of Miocene echinoids has been discussed in detail (see Mancosu and Nebelsick, Reference Mancosu and Nebelsick2013, Reference Mancosu and Nebelsick2015, Reference Mancosu and Nebelsick2016, Reference Mancosu and Nebelsick2017a, Reference Mancosu and Nebelsickb; Mancosu et al., Reference Mancosu, Nebelsick, Kroh and Pillola2015). The results of the present study show that preservation potentials can vary widely among different taxa in sublittoral environments (see Table 1). Regular echinoid preservation displays a taphonomic gradient ranging from intact tests with spines attached to isolated plates and spine fragments. These differences in preservation can be related to differences in skeletal microstructure as well as variations in paleoenvironmental and taphonomic conditions and episodic events. Diadematids, for example, have tests with imbricate or only slightly interlocking plates that tend to disarticulate rapidly when subjected to postmortem transportation and reworking. These echinoids thus show a lower preservation potential than camarodont echinoids (Smith, Reference Smith1984; Greenstein, Reference Greenstein1989, Reference Greenstein1991, Reference Greenstein1992, Reference Greenstein and White1993a, Reference Greensteinb, Reference Greenstein1995; Kidwell and Baumiller, Reference Kidwell and Baumiller1990; see discussion by Mancosu et al., Reference Mancosu, Nebelsick, Kroh and Pillola2015). The occurrence of diadematid test remains and associated spines are interpreted to be the result of a rapid influx of sediments in an otherwise relatively calm background depositional environment.
Taphonomic signatures show that additional factors other than test stability and infaunal mode of life play important roles in the preservation of irregular echinoids. In the interpreted moderately deeper-water environments with low to moderate water energies, the echinoid tests were only sporadically exposed to high water movement and sediment reworking (see discussion by Mancosu and Nebelsick, Reference Mancosu and Nebelsick2017b, and references therein). A further important factor influencing the preservation potential of infaunal echinoids is sediment disturbance due to the pervasive bioturbation by deep-tier thalassinid decapod crustaceans and infaunal echinoids themselves, specifically spatangoids, which are among the most active and widespread bioturbators in extant marine environments, able to rework relatively large volumes of sediment (e.g., Hollertz et al., Reference Hollertz, Sköld and Rosenberg1998; Hollertz and Duchêne, Reference Hollertz and Duchêne2001; Lohrer et al., Reference Lohrer, Thrush, Hunt, Hancock and Lundquist2005; Thompson and Riddle, Reference Thompson and Riddle2005; Gingras et al., Reference Gingras, Pemberton, Dashtgard and Dafoe2008). Bioturbation thus represents a source of echinoid test breakage in quiet sublittoral environments (see discussion by Mancosu and Nebelsick, Reference Mancosu and Nebelsick2017b).
Conclusions
An echinoid-dominated, fine-grained, carbonate sedimentary succession from the middle Miocene of central-western Sardinia has been recognized. Three assemblages have been detected based on echinoid diversity and relative abundance as well as associated fauna and flora, trace fossils, and lithological/sedimentological features. The results of this study allow an outer sublittoral environment at moderate depth, below fair-weather wave base, to be reconstructed. Differences among the assemblages can be related to substrate variation and the availability of food resources.
Assemblage 1 occurs in very fine-grained packstone to wackestone with rhodolith patches and is characterized by the co-occurrence of infaunal deposit feeders, mainly spatangoids and epibenthic grazers, e.g., the diadematid Diadema and the toxopneustids Tripneustes and Schizechinus. This assemblage represents a sheltered environment with structural substrate complexity, including hard substrata, represented by rhodolith patches, and fine-grained soft substrata, where different food resources could be exploited.
Assemblages 2 occurs in very fine-grained packstones to wackestones, highly bioturbated by Thalassinoides-like burrows filled by echinoid and bivalve debris that are interpreted as tubular tempestites. The assemblage is dominated by burrowing deposit feeding spatangoids (Brissopsis, Ova, Opissaster, Lovenia, and Hemipatagus) and, subordinately, clypeasteroids (Echinocyamus and Clypeaster marginatus), with regular echinoids represented by the small trigonocidarid Genocidaris. This assemblage indicates a moderately energetic environment with fine-grained sediments.
Assemblage 3 occurs in mudstone and is largely dominated by the spatangoid Brissopsis and, subordinately, by two different morphotypes of Ova. Associated echinoid taxa, including the spatangoids Opissaster and Hemipatagus, the clypeasteroid Echinocyamus, and the regular echinoid Schizechinus, are rarely encountered. Assemblage 3, with its lower echinoid diversity, points to a deeper-water environment with muddy substrata, low-energy conditions, and limited food resources.
The co-occurrence of different regular and irregular echinoids within each assemblage indicates resource partitioning among both epifaunal regular echinoids and infaunal deposit-feeding irregular forms. These findings of the present study complement those of recent paleoecological investigations on the echinoid fauna of the Miocene of Sardinia and indicate that the diversity pattern of echinoids in sublittoral environments is a reflection of both environmental factors and taphonomic processes that affect preservation of the echinoid taxa. Substrate heterogeneity, including both hard and soft bottoms, low-energy conditions with sporadic episodes of rapid sedimentation, possibly related to storms, and pervasive bioturbation, which is potentially a source of shell breakage, led to the composition and preservation of a highly diversified echinoid fauna.
Acknowledgments
We thank G.L. Pillola and L. Lecca (Università degli Studi di Cagliari) for their help and useful discussions during preparation of this paper. We are grateful to L. Zachos and J.R. Thompson for their comments and suggestions that helped improve the manuscript.