Introduction
Among reptile lineages, turtles are of special interest because during their evolutionary history, they underwent several episodes of marine colonization (Evers and Benson, Reference Evers and Benson2019). They are the only reptile lineage that includes extant pelagic marine forms. Some of the earliest known attempts at marine colonization by turtles took place during the Jurassic and involved both stem testudines and panpleurodirans. However, the only marine turtles of this age from Gondwana come from the Vaca Muerta Formation, which outcrops in the Neuquén Basin, central-west Argentina (Gasparini and Fernández, Reference Gasparini, Fernández, Veiga, Spalletti, Howell and Schwarz2005; Gasparini et al., Reference Gasparini, Fernández, de la Fuente, Herrera, Codorniú and Garrido2015; Fernández et al., Reference Fernández, Herrera, Vennari, Campos, de la Fuente, Talevi and Aguirre Urreta2019) and includes records of several Jurassic-Cretaceous tetrapod taxa. In the case of turtles, only two species have been recognized and named, the panpleurodiran Notoemys laticentralis Cattoi and Freiberg, Reference Cattoi and Freiberg1961 and the stem Testudines Neusticemys neuquina (Fernández and de la Fuente, Reference Fernández and de la Fuente1988) (de la Fuente and Fernández, Reference de la Fuente and Fernández1989, Reference de la Fuente and Fernández2011; Fernández and de la Fuente, Reference Fernández and de la Fuente1993, Reference Fernández and de la Fuente1994; Gasparini et al., Reference Gasparini, Spalletti and de la Fuente1997; Lapparent de Broin et al., Reference Lapparent de Broin, de la Fuente and Ferrnández2007). The chronological distributions of these species differ slightly, at least according to present knowledge. Notoemys laticentralis is restricted to the Virgatosphinctes andesensis and Winhauseniceras internisespinosum association biozones (lower–middle Tithonian and upper Tithonian, respectively), whereas Neusticemys neuquina has been found in both aforementioned biozones as well as the Substeuroceras koeneni association Biozone (upper Tithonian–lower Berriasian).
The panpleurodiran platychelyid Notoemys laticentralis is represented by four specimens, whereas 19 specimens have been referred to Neusticemys neuquina. However, Notoemys laticentralis is better understood because its anatomical data and systematics have been updated (e.g., de la Fuente and Iturralde-Vinent, Reference de la Fuente and Iturralde-Vinent2001; Cadena and Gaffney, Reference Cadena and Gaffney2005; Lapparent de Broin et al., Reference Lapparent de Broin, de la Fuente and Ferrnández2007; Cadena and Joyce, Reference Cadena and Joyce2015; López-Conde et al., Reference López-Conde, Sterli, Alvarado-Ortega and Chavarría-Arellano2017). In contrast, knowledge of Neusticemys neuquina is patchy and mostly limited to the original description, alpha taxonomy, and peculiarities of its appendicular skeleton (Fernández and de la Fuente, Reference Fernández and de la Fuente1988, Reference Fernández and de la Fuente1993; de la Fuente, Reference de la Fuente, Gasparini, Coria and Salgado2007; de la Fuente and Fernández, Reference de la Fuente and Fernández2011). In particular, adequately described cranial remains of this turtle have been unavailable heretofore. Here, we report the most complete skull of an adult of Neusticemys neuquina yet discovered, together with an associated cervical vertebra (see Systematic paleontology). Detailed study of the specimen, including computed tomography (CT) scans, has shed new light on the phylogenetic relationships of this species by allowing scoring of previously unknown characters. As a result, we now have a deeper understanding of this key taxon and the evolution of marine adaptations among turtles.
Geological setting
The Neuquén Basin, located on the eastern side of the Andes between 36° and 40°S (Yrigoyen, Reference Yrigoyen1991), is the major source of Tithonian marine reptile fossils in the eastern Pacific. The basin is bounded by two cratonic structures, the San Rafael Block on the northeast and the North Patagonian massif on the southeast; on the west, it is delimited by the Andean volcanic area. This basin is considered to be backarc-retroarc in origin (Digregorio et al., Reference Digregorio, Gulisano, Gutiérrez Pleimling and Minitti1984; Macellari, Reference Macellari1988; Legarreta and Gulisano, Reference Legarreta and Gulisano1989; Legarreta and Uliana, Reference Legarreta, Uliana and McDonald1991). The stratigraphic record (including for the Jurassic beds reported here) is nearly continuous from the Late Triassic to the Cenozoic, and is characterized by infilling continental and marine siliciclastic, carbonate, and evaporite deposits.
An important flooding event (Vaca Muerta Formation, sensu Gasparini et al., Reference Gasparini, Spalletti, Fernández and de la Fuente1999), followed by a gradual swallowing-up cycle (Picún Leufú Formation), is recorded for the Neuquén Basin during the Tithonian. The first lithostratigraphic horizon represents a rapid, well-constrained transgressive episode that extended throughout the Neuquén Basin, resulting in a condensed stratigraphic record with a wide distribution of anoxic conditions in the depositional interface. At that time, the Neuquén Basin was a paleogulf separated from the proto-Pacific by an island arc with numerous gaps, as modeled by Spalleti et al. (Reference Spalletti, Franzese, Matheos and Schwarz2000).
The interval of interest here has been subdivided into three shallowing upward sequences that, due to their thickness and estimated geochronological duration, can be considered as third-order sequences (Spalletti in Gasparini et al., Reference Gasparini, Spalletti, Fernández and de la Fuente1999). The fossiliferous horizons of the lower section of Cerro Lotena are located in the lower Tithonian sequence (V. andesensis Biozone). In the lower section at Cerro Lotena, one specimen of Notoemys laticentralis and six specimens of Neusticemys neuquina have been found (Fernández and de la Fuente, Reference Fernández and de la Fuente1988, Reference Fernández and de la Fuente1994; de la Fuente and Fernández, Reference de la Fuente and Fernández1989; de la Fuente et al., Reference de la Fuente, Sterli and Fernández2014).
According to Leanza and Hugo (Reference Leanza and Hugo1997), as mapped by the Geological Survey (Fig. 1), the Vaca Muerta Formation has a wide distribution in the Picun Leufu area. It is exposed in the southern flange of the Picun Leufu anticline, and continues to the east to the Cerro Cachigüe area. It is also sporadically exposed under the volcanic deposits of Barda Negra. The ammonite content of the Vaca Muerta Formation, where it outcrops in the Cerro Lotena area, has enabled the biozonation of lower and middle Tithonian levels (Leanza, Reference Leanza1980). The lithology of this lithostratigraphic unit is composed of dark bituminous clays, marls, and particularly massive and nodular limestones beds in the lower sections. At the Cerro Lotena locality, the Vaca Muerta Formation has a measured thickness of 129 m (Leanza, Reference Leanza1973). Recently, U-Pb zircon CA-ID-TIMS dating has been used to place the age of the base of the Vaca Muerta Formation in the La Yesera section, just below the V. andesensis Biozone, at ca. 147 Ma (147.122 ± 0.078 Ma; Aguirre-Urreta et al., Reference Aguirre-Urreta, Naipauer, Lescano, López-Martínez, Pujana, Vennari, De Lena, Concheyro and Ramos2019; De Lena et al., Reference De Lena, Otavio, López-Martínez, Lescano, Aguire-Urreta, Concheyro, Vennari, Naipauer, Samankassou, Pimentel, Ramos and Schaltegger2019).
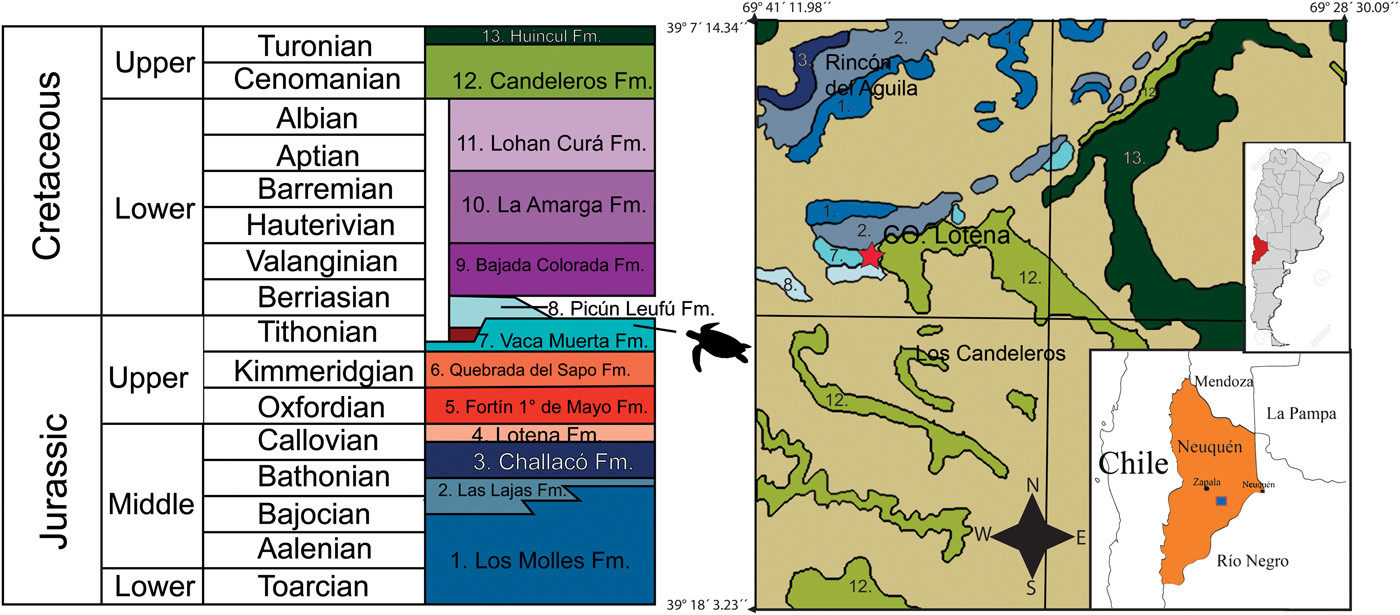
Figure 1. Stratigraphic column and map showing outcrops of Vaca Muerta Formation at Cerro Lotena locality where Neusticemys neuquina was found. Picun Leufu geologic map modified from Leanza and Hugo (Reference Leanza and Hugo1997). The star represents the location where MOZ-PV-064 was discovered and the turtle outline in the stratigraphic column represents the stratigraphic provenance of MOZ-PV-064.
Materials and methods
Material
Specimen MOZ-PV-064 is stored in the Museo Prof. Olsacher at Zapala City in Neuquén Province. It consists of an almost complete skull, an incomplete mandible (missing the anterior part of the symphyseal region), and a single cervical vertebra.
Computed tomography, scanning, and digitalization
Specimen MOZ-PV-064 was scanned in a medical tomograph, model AQUILON 64 TSX 101/E, at the Policlínica de Neuquén medical facility, under a voltage of 140 kV and a current of 149 μA. The raw data files were converted to slices in the software associated with the scanner. A total of 468 slices (voxel size 0.215 mm x 0.215 mm x 0.31 mm) were obtained. Subsequently, bones were segmented using 3D Slicer ver. 4.8.1 (Fedorov et al., Reference Fedorov, Beichel, Kalpathy-Cramer, Finet, Fillion-Robin, Pujol, Bauer, Jennings, Fennessy, Sonka, Buatti, Aylward, Miller, Pieper and Kikinis2012; Kikinis et al., Reference Kikinis, Pieper, Vosburgh and Jolesz2014), the inbuilt tools (painteffect, draweffect) were utilized for editing, and the free module Fast GrowCut (Zhu et al., Reference Zhu, Ivan Kolesov, Gao, Kikinis and Tannenbaum2014) was used to aid editing and segmenting of each individual element. Most of the segmentation was done by hand by delimiting bone limits wherever they were observable in a series of slices in different views, and letting Fast GrowCut incorporate close voxels into the model. Later, three-dimensional (3D) models of the different elements were generated by the same software and used to describe the skull and each bone as isolated, colorized elements. Some bones proved hard to completely segment because in some areas, matrix and bone displayed similar contrast characteristics. However, this caused only minor difficulties (e.g., small bumps on surfaces that were otherwise well segmented). Tomographies and 3D models are provided in the Supplemental datafile ‘CT scan raw files and mesh 3D models.’
Phylogenetic analysis
To test the phylogenetic relationships of Neusticemys neuquina among turtles, we used the matrix of Evers and Benson (Reference Evers and Benson2019), which was the most up-to-date matrix available at the time that we performed the analysis. We kept all characters and states as defined in that publication. The resulting matrix has 81 taxa and 345 characters (see Supplemental datafile ‘Phylogenetic matrix’). The dataset was analyzed in TNT V1.6 (Goloboff and Catalano, Reference Goloboff and Catalano2016) to conduct parsimony analysis and obtain the most parsimonious trees (MPTs). As in the case of the Evers and Benson (Reference Evers and Benson2019) study, a backbone constraining extant taxa was utilized (Pereira et al., Reference Pereira, Sterli, Moreira and Schrago2017); fossil taxa were left unconstrained to fit wherever they could within the topology. Proganochelys quenstedti Baur, Reference Baur1887 was set as outgroup. Seaching was conducted as a new technologies search (NTS) with default settings, with tree drifting and parsimony ratchet enabled. The initial level of the driven search was set to 30 with 30 hits for minimum length of trees. Using NTS allows using several searching algorithms at the same time. All of the algorithms were selected (sectorial search, ratchet, drift, tree fusing) and the minimum length was set to be hit 30 times. The resulting trees were then subjected to a final round of tree bisection and reconnection (TBR). We did not order any characters during the first run, but a second run was conducted with 30 ordered characters because some of them were considered continuous (Raselli, Reference Raselli2018; Evers and Benson, Reference Evers and Benson2019; characters 7, 14, 18, 34, 44, 67, 76, 79, 90, 93, 94, 103, 107, 123, 130, 131, 138, 142, 147, 205, 210, 217, 248, 253, 291, 304, 325, 339, 340, and 344). The resulting MPTs were then used to generate strict and majority consensus trees; Bremer and jacknife values were calculated to evaluate support of the resulting clades.
Repositories and institutional abbreviations
MACN = Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia,’ Buenos Aires, Argentina; MHNSR = Museo de Historia Natural de San Rafael, San Rafael, Provincia de Mendoza; Argentina; MLP = Museo de la Plata, La Plata, Provincia de Buenos Aires, Argentina; MOZ = Museo Prof. Olsacher de Zapala, Zapala, Provincia de Neuquén, Argentina; OUMNH = Oxford University Museum of Natural History, Oxford, UK.
Systematic paleontology
Testudinata Klein, Reference Klein and Behn1760 (sensu Joyce et al., Reference Joyce, Parham and Gauthier2004)
Angolachelonia Mateus et al., Reference Mateus, Jacobs, Polcyn, Schulp, Vineyard, Buta Neto and Telles Antunes2009
Thalassochelydia Anquetin, Püntener, and Joyce, Reference Püntener, Anquetin and Billon-Brullat2017
Neusticemys Fernández and de la Fuente, Reference Fernández and de la Fuente1993
Type species
Eurysternum? neuquinum Fernández and de la Fuente, Reference Fernández and de la Fuente1988.
Neusticemys neuquina (Fernández and de la Fuente, Reference Fernández and de la Fuente1988)
Figures 2–5, 7, 8
- Reference Fernández and de la Fuente1988
Eurysternum? neuquinum Fernández and de la Fuente, p. 129, pls. 1, 2.
- Reference Fernández and de la Fuente1993
Neusticemys neuquina; Fernández and de la Fuente, p. 284, pl. 1.
- Reference de la Fuente, Gasparini, Coria and Salgado2007
Neusticemys neuquina; de la Fuente, p. 57, fig. 3.2, E–G.
- Reference de la Fuente and Fernández2011
Neusticemys neuquina; de la Fuente and Fernández, p. 15, figs. 1–4.

Figure 2. Neusticemys neuquina from the Titthonian level of Vaca Muerta Formation, photographs and drawings of the skull MOZ-PV-064 in: (1, 2) dorsal view, (3, 4) lateral right view, (5, 6) lateral left view, and (7, 8) anterior view. ane = apertura narina externa; ap = antrum postoticum; cso = crista supraoccipitalis; ct = cavum tympani; fr = frontal; fst = foramen stapedio-temporale; ica = incisura collumellae auris; ju = jugal; mx = maxilla; na = nasal; op = opisthotic; pa = parietal; pf = prefrontal; po = postorbital; pr = prootic; qj = quadratojugal; qu = quadrate; so = supraoccipital; sq = squamaosal.
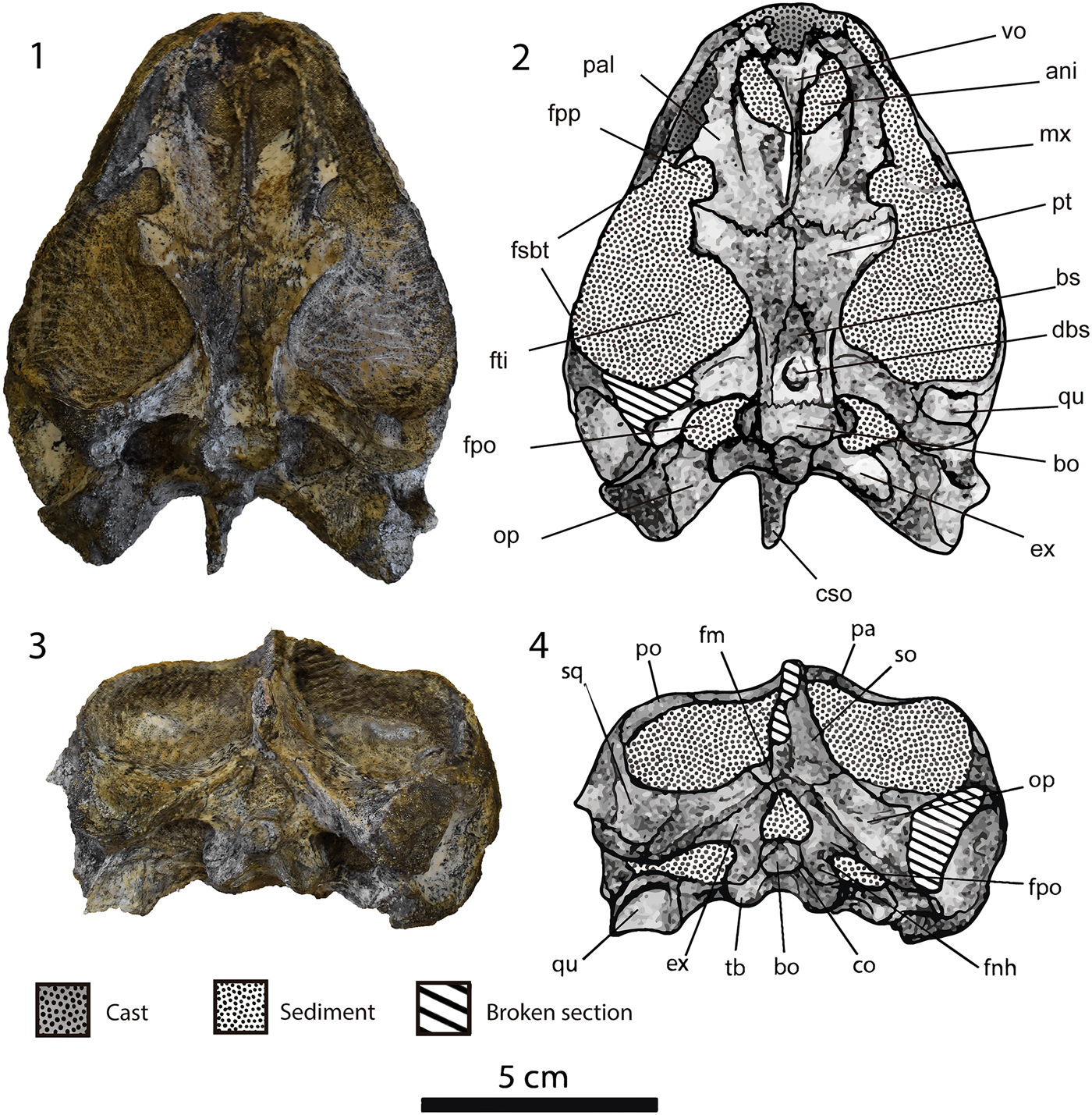
Figure 3. Neusticemys neuquina from Tithonian levels of Vaca Muerta Formation, photographs and drawings of the skull MOZ-PV-064 in: (1, 2) ventral view, and (3, 4) posterior view. ani = apertura narium interna; bo = basioccipital; bs = basisphenoid; co = condylus occipitalis; cso = crista supraoccipitalis; dbs = depression basisphenoidal; ex = exoccipital; fm = foramen magnum; fnh = foramen nervi hypoglossi; fpo = fenestra post-otica; fpp = foramen palatinum posterius; fsbt = fenestra subtemporalis; fti = fossa temporalis inferior; mx = maxilla; op = opisthotic; pa = parietal; pal = palatine; po = postorbital; pt = pterygoid; qu = quadrate; so = supraoccipital; sq = squamaosal; tb = tuberculum basioccipitale; vo = vomer.
Type material
Holotype, MLP 86-III-30-2, posterior third of a carapace, hyo- and hypoplastra, and other postcranial fragments (Fernández and de la Fuente, Reference Fernández and de la Fuente1988). Paratypes MLP 86-III-30-1, anterior part of a carapace, hyo- and hypoplastra, and pelvic girdle; and MOZ-PV-1106, anterior part of a carapace (Fernández and de la Fuente, Reference Fernández and de la Fuente1988).
Emended diagnosis
Neusticemys neuquina belongs to the clade Testudinata because it has a complete carapace and plastron enclosing the pectoral girdle. It is included inside Angolachelonia according to: the posterior orientation of the articular process of the quadrate; the presence of a prominent, ventrally infolding ridge of the posterior surface of the processus articularis of the quadrate; the long interpterygoid contact; and a pterygoid contact with the mandibular articular surfaces of the quadrate. It can be identified as a member of Thalassochelydia by: the anterolateral recess of the anterior surface of the quadrate lateral to the processus trochlearis oticum; the presence of a fossa on the supraoccipital-opisthotic-exoccipital contact area; the foramina anterius caroticus cerebralis found close together, but as independent foramina in the basisphenoid; the presence of the splenial in the mandible; lack of lateral ossifications of the costals, allowing the dorsal exposure of the distal end of ribs and the development of fontanelles only at the most anterior and posterior costals; the presence of rib-free peripherals; two to four vertebral scutes significantly broader than pleurals; and the central articulation of cervical vertebrae not formed. It differs from other Thalassochelydia by possessing a round depression on the ventral surface of the basisphenoid; a relatively larger oval foramen nervi trigemini; reduced and steepened triturating surfaces in both the maxilla and dentary; a flattened and wide carapace of moderate to large size (maximum carapace length 501 mm); a bound keel in the posterior third of the carapace; anterolateral margins of the carapace that are rectilinear and elongated, resulting in an anteroposterior elongation of the carapace; relatively elongated anterior and posterior limbs with flattened carpal and tarsal elements; and an elongated V digit in the pes.
Ocurrence
Cerro Lotena (Zapala, Neuquén Province, Argentina) (Fernández and de la Fuente, Reference Fernández and de la Fuente1988), 39°11′22.35″S, 69°38′29.57″W, lower section of the Vaca Muerta Formation (Weaver, Reference Weaver1931), V. andesensis Biozone, early Tithonian (two-fold division) (Riccardi, Reference Riccardi2015; Vennari, Reference Vennari2016).
Descriptions and comparisons: identification as Neusticemys neuquina.—Neusticemys neuquina was originally diagnosed based on postcranial features (Fernández and de la Fuente, Reference Fernández and de la Fuente1988, Reference Fernández and de la Fuente1993; de la Fuente, Reference de la Fuente, Gasparini, Coria and Salgado2007). However, several years later, two specimens (MACN-PV-105 and MHNSR-PV-1195) with skull and associated postcranial remains, including diagnostic features, were recovered in the Vaca Muerta Formation (de la Fuente et al., Reference de la Fuente, Sterli and Fernández2014; Fernández et al., Reference Fernández, Herrera, Vennari, Campos, de la Fuente, Talevi and Aguirre Urreta2019).
Comparison of the postcranial material associated with MACN-PV-105 and MHNSR-PV-1195, together with the holotype MLP 86-III-30-2 (Fernández and de la Fuente, Reference Fernández and de la Fuente1988) and previously described specimens MLP 92-IV-10-1 and MOZ-PV-6094 (Fernández and de la Fuente, Reference Fernández and de la Fuente1993; de la Fuente, Reference de la Fuente, Gasparini, Coria and Salgado2007; de la Fuente and Fernández, Reference de la Fuente and Fernández2011), allows assignment of the Vaca Muerta material to Neusticemys neuquina. MOZ-PV-064 and the Vaca Muerta skulls share: relatively well-developed temporal emargination; large orbits; an infolding ridge on the posterior surface of the quadrate below the incisura columella auris and over the mandibular condyle of the quadrate; a keeled dorsal surface of the squamosal; a constriction of the pterygoid plate at the base of the quadrate process of the pterygoid; and a depression on the basisphenoid ventral surface. Because MHNSR-PV-1195 is more complete than MACN-PV-105 (a juvenile), the former will be emphasized in comparisons. Similarly sized MHNSR-PV-1195 and MOZ-PV-064 share: possession of a deep pterygoid fossa where the foramen posterius canalis carotici interni is located; a sharp, steep ridge in the lateral margin of the pterygoids that runs from the posterior end of the pterygoid to the basioccipital; and an open foramen palatinum posterius.
General observations
Four skulls and mandibles have been identified as remains of Neusticemys neuquina: three adults (MOZ-PV-064, 151, MHNSR-PV-1195) and one juvenile (MACN-PV-105). MOZ-PV-064 is the most complete; it preserves most of the skull bones and most of the posterior mandibular elements, lacking only the two premaxillae, and most of the right quadratojugal, right squamosal, and right postorbital. The right quadrate lacks the area articularis mandibularis. The skull is filled with sediments that proved hard to remove, so the CT scans were instrumental in clarifying the morphology of hidden parts. General skull shape is rectangular in lateral aspect and trapezoidal in dorsal and ventral aspects. The skull has a large orbit that occupies slightly less than half of the total length of the skull and a well-developed temporal emargination. The orbits are located laterally and have a laterodorsal orientation allowing dorsal exposure of the jugal and maxilla. The temporal emargination is such that it does not prevent the exposure of the foramen stapedio-temporale in dorsal view.
Nasals
Nasals are present in Neusticemys neuquina (Fig. 2.1–2.8), as in Plesiochelys etalloni (Pictet and Humbert, Reference Pictet and Humbert1857) and other thalassochelydians (Gaffney, Reference Gaffney1975a, Reference Gaffney1976; Rieppel, Reference Rieppel1980). The nasals are in contact for their whole length along the midline and have a subtriangular shape (Fig. 2.1, 2.2). They contact the prefrontal posteriorly (Fig. 2.1, 2.2) and the maxilla lateroventrally (Fig. 2.3, 2.4). In Portlandemys gracilis Anquetin, Püntener, and Billon-Bruyat, Reference Anquetin, Püntener and Billon-Bruyat2015 and Jurassichelon oleronensis Pérez-Garcia, Reference Pérez-Garcia2015, the nasals contact the frontal posteromedially (Gaffney, Reference Gaffney1976; Rieppel, Reference Rieppel1980; Anquetin et al., Reference Anquetin, Püntener and Billon-Bruyat2015; Evers and Benson, Reference Evers and Benson2019) by means of an anterior process of the latter that prevents the medial joining of the prefrontals. Other thalassochelydians, e.g., Plesiochelys etalloni, Solnhofia parsonsi (Gaffney, Reference Gaffney1975b), and Portlandemys mcdowelli (Gaffney, Reference Gaffney1975a), present the condition seen in Neusticemys neuquina in which the nasals do not meet the frontal. In Plesiochelys planiceps Owen, Reference Owen1842, it is possible that there was nasofrontal contact, but because the nasals are missing in the only known specimen (OUMNH J1582), this possibility cannot be assessed.
Prefrontal
The prefrontal is composed of a horizontal plate dorsally and a vertical plate ventrally, as is common in turtles (Fig. 2.1–2.8) The vertical plate of the prefrontal in Neusticemys neuquina forms the anterolateral margin of the foramen orbito-nasale. The dorsal plates of the prefrontals contact each other in the midline, and the nasals prevent contact between prefrontals and the apertura narina externa (Fig. 2.1, 2.2, 2.7, 2.8). The prefrontal contacts are medially with the other prefrontal, anteriorly with the nasals, posteriorly with the frontals (Fig. 2.1, 2.2), anterolaterally with the maxilla (Fig. 2.1–2.8), ventrally with the vomer, and ventrolateraly with the palatines. As discussed above, Neusticemys neuquina is comparable with Plesiochelys etallloni, Solnhofia parsonsi, and Portlandemys mcdowelli in which the prefrontals meet medially and prevent the type of frontal-nasal contact seen in Portlandemys gracilis and Jurassichelon olenorensis. In all thalassochelydians including Neusticemys neuquina, frontal and prefrontal have a medial constriction over the orbits at the suture level.
Frontal
The frontal in Neusticemys neuquina is a plate-like element that has a roughly trapezoidal outline (Fig. 2.1, 2.2), being wider posteriorly than anteriorly and not fused with the other frontal. Ventrally, the frontal forms a moderately deep parasagittal ridge, which forms a medial, ventrally open trough that connects the anterior margin of the cavum cranni posteriorly with the fissura ethmoidalis anteriorly (sulcus olfactorius). The frontal participates on the dorsal margin of the orbit and contacts anteriorly with the prefrontal, posteriorly with the parietal, lateroposteriorly with the postorbital, and medially with the other frontal (Fig. 2.1, 2.2). The morphology of both frontals has a trapezoidal outline, whereas on Plesiochelys etalloni the outline is more rectangular (Gaffney, Reference Gaffney1976, fig 8).
Parietal
The parietal is formed by two bone plates that are perpendicular to one another, a horizontal dorsal plate and a ventral parasagittal one (Fig. 2.1–2.8). The dorsal plate has a transverse suture with the frontal and an anteromedially trending one with the postorbital, which starts converging medially almost at the midline of the parietal's total length (Fig. 2.1, 2.2). The dorsal plate contacts the frontal anteriorly, the postorbitals laterally, the supraoccipital posteroventrally, and the other parietal medially. The parasagittal plate of the parietal descends at an acute angle from the anterior end of the parietal to its ventral contact with the epipterygoid and pterygoid. Posteriorly, it is less steepened and contacts the supraoccipital and prootic along its descending arm. The parasagittal plate forms the dorsal margin of the foramen nervi trigemini and seems to prevent the participation of the prootic in the foraminal margin, as is the case in most Thalassochelydia. However, this condition cannot be assessed fully because our CT scan lacked sufficient resolution to determine bone limits in this section. The foramen nervi trigemini is proportionally larger than in other known thalassochelydians, and reaches farther ventrally due to its a very elongated oval outline. Temporal emargination resembles that of Plesiochelys spp. and Portlandemys spp., in which the foramen stapedio-temporale is exposed in dorsal view. In Jurassichelon oleronensis, the foramen is not fully exposed (Rieppel, Reference Rieppel1980); in Solnhofia parsonsi, this condition has not been observed due to preservation biases (Parsons and Williams, Reference Parsons and Williams1961; Gaffney, Reference Gaffney1976; Joyce, Reference Joyce2000), although it has been argued that it is present but hidden (Parsons and Williams, Reference Parsons and Williams1961; Evers and Benson, Reference Evers and Benson2019).
Postorbital
The postorbital is a plate-like bone that roofs most of the posterolateral portion of the skull. In MOZ-PV-064, the postorbital is preserved almost completely on the left side and has a roughly subrectangular shape (Fig. 2.1–2.6). It is limited by the orbital fenestra anteriorly, the frontal anterodorsally, the jugal anteroventrally, the parietal medially, the quadratojugal posteroventrally, and the squamosal and temporal emargination posteriorly (Fig. 2.1–2.8). The arrangement of postorbitals is the same as in Solnhofia parsonsi, Plesiochelys spp., and Portlandemys spp. In Jurassichelon oleronensis, the postorbital does not participate in the temporal emargination because parietal-squamosal contact prevents its exposure.
Jugal
The jugal is a slender, relatively long element that is exposed on the lateral margin of the skull (Fig. 2.3–2.6). It contacts the maxilla anteriorly, the postorbital dorsally, and the quadratojugal posteriorly. It is limited anteriorly by the orbit and ventrally by the maxilla, cheek emargination, and quadratojugal. The jugal lacks the medially directed jugal process, leaving an open foramen palatinum posterius (Fig. 3.1, 3.2). Jugal morphology is proportionally similar to those of Plesiochelys spp. and Jurassichelon oleronensis. In Portlandemys mcdowelli, Portlandemys gracilis, and Solnhofia parsonsi, the foramen palatinum posterius is closed by a medial process of the jugal.
Quadratojugal
The quadratojugal is a triradiate bone with an anterior branch that contacts the jugal anteriorly and the postorbital dorsally; a posterodorsal branch that contacts the squamosal posteriorly, the quadrate ventromedialy, and the postorbital dorsally; and a narrower posteroventral branch that contacts the quadrate posteromedially (Fig. 2.5, 2.6). The quadratojugal is not exposed on the temporal emargination (Fig. 2.1, 2.2), but it constitutes part of the dorsal and posterior margin of the cheek emargination (Fig. 2.5, 2.6). The quadratojugal is only known in some thalassochelydian turtles (i.e., Plesiochelys etalloni, Plesiochelys bigleri Püntener et al., Reference Püntener, Anquetin and Billon-Brullat2017, Portlandemys gracilis, and Jurassichelon oleronensis). In Plesiochelys etalloni, the posteroventral branch of the quadratojugal almosts reaches the area condylus mandibularis, whereas in Neusticemys neuquina, Plesiochelys bigleri, J. oleronensis, and Portlandemys gracilis, it does not reach that far. In thalassochelydian turtles including Neusticemys neuquina, the quadratojugal displays similar robustness with the exception of J. oleronensis in which this element seems to be slender (Rieppel, Reference Rieppel1980, fig. 3; Anquetin et al., Reference Anquetin, Püntener and Joyce2017, fig.1).
Squamosal
The squamosal in Neusticemys neuquina is roughly cone-shaped, forming a well-developed antrum postoticum (Fig. 2.1, 2.2, 2.5, 2.6) as is typical in thalassochelydian turtles (Anquetin et al., Reference Anquetin, Püntener and Joyce2017), with the exception of Plesiochelys bigleri in which the antrum postoticum is formed only by the quadrate (Püntener et al., Reference Püntener, Anquetin and Billon-Brullat2017). In Neusticemys neuquina, the squamosal is gently sloped dorsally with a strong dorsal keel (Fig. 2.5, 2.6), but this keel is rougher than the one found in Plesiochelys bigleri (see Püntener et al., Reference Püntener, Anquetin and Billon-Brullat2017). In Neusticemys neuquina the squamosal also forms the posterodorsal enclosure of the cavum tympani. The squamosal contacts the postorbital anterodorsally, the quadrate anteroventromedially, the quadratojugal anteriorly, and the opisthotic posteromedially. The overall morphology of the squamosal of Neusticemys neuquina is similar to those of most thalassochelydian turtles with the exception of Plesiochelys bigleri for the reasons mentioned above.
Premaxilla
The premaxilla in MOZ-PV-064 is preserved as a cast (Figs. 2.1–2.8, 3.1, 3.2). It is limited by the apertura narina externa dorsally and the maxilla laterally. The cast replicates the dorsal surface of the ventral plate of the premaxilla; it preserves the posteroventral suture with the vomer (Fig. 3.1, 3.2). The foramen prepalatinum is not preserved. Compared to other adult and juvenile specimens of Neusticemys neuquina (MOZ-PV-5804, MHNSR-PV-1195, MOZ-PV-6094, MACN-PV-105), the snout was presumably acute, as commonly seen in thalassochelydian turtles with the exception of Plesiochelys etalloni, in which the snout is somewhat stouter.
Maxilla
The maxilla in Neusticemys neuquina, as in most turtles, is a combination of a ventrohorizontal and dorsovertical bone plates. The dorsovertical plate is exposed in lateral view and forms the lateral wall of the apertura narina externa. The dorsovertical plate meets the premaxilla medially, the jugal posterodorsally, and the prefrontal dorsally (Fig. 2.1–2.8). The ventrohorizontal plate forms the triturating surface (Fig. 3.1, 3.2). In the case of Neusticemys neuquina, this triturating surface is not as developed as in other thalassochelydian turtles, e.g., Jurassichelon oleronensis (see Rieppel, Reference Rieppel1980), and seems to be more steepened than the one preserved in Plesiochelys etalloni and Plesiochelys planiceps. It also appears to lack or exhibit the reduced medial ridge that is developed on the aforementioned species (Gaffney, Reference Gaffney1975a). This plate contacts the premaxilla and the vomer anteromedially and the palatines medially (Fig. 3.1, 3.2). However, the plate leaves an open foramen palatinum posterius to the fenestra subtemporalis due to the lack of a medially directed process of the maxilla.
Vomer
The vomer of Neusticemys neuquina has a nail-like shape with a bulkier anterior end and an acute posterior one. It contacts the premaxilla anteriorly, the maxilla anterolaterally, the prefrontal anterodorsally, the palatine dorsolaterally, and the pterygoid posteriorly (Fig. 3.1, 3.2). The vomer separates the palatines preventing them from joining medially. The anterior contact of the vomer with the maxilla and premaxilla is Y-shaped. The vomer also does not participate in the triturating surface or the foramen orbito-nasale as in Jurassichelon oleronensis, Plesiochelys spp., and Portlandemys spp. In J. oleronensis, there is no contact between the pterygoid and the vomer, whereas in other thalassochelydians, this contact is present. Solnhofia parsonsi exhibits a special condition among thalassochelydians because of the development of a secondary palate, resulting in a palatal mosaic that differs from those of other thalassochelydian turtles.
Palatine
The palatine of Neusticemys neuquina is a plate-like element that contacts the vomer ventrolaterally along its entire length (Fig. 3.1–3.2). A dorsal anterior contact with the prefrontal is not now identifiable but might have been present. Posteriorly the palatine contacts the pterygoid along a convex suture. Anterolaterally, it contacts the maxilla. They form the mediolateral and dorsal margins of the foramen palatinum posterius and the lateromedial margin of the orbito-nasal foramen. The palatines also form most of the floor of the orbital capsule. In Neusticemys neuquina, as in other thalassochelydians without a secondary palate, e.g., Plesiochelys etalloni, Plesiochelys bigleri, Portlandemys mcdowelli, and Portlandemys gracilis, the vomer separates the palatines medially and contacts the pterygoids, whereas in Jurassichelon oleronensis, the palatines are partially separated in the anterior portion by the vomer only. The palatines in MOZ-PV-064 are more developed anterolaterally than in other thalassochelydians, due to a narrower triturating surface mostly confined to a labial ridge.
Pterygoid
The pterygoid ventral surface shape in Neusticemys neuquina is similar to those of plesiochelyids and some extant and extinct cryptodires (e.g., Chelydra serpentina Linnaeus, Reference Linnaeus1758), exhibiting a medial constriction as well as expansion at both the anterior and posterior ends (Fig. 3.1, 3.2). The pterygoid is a complex bone that contacts a great number of skull bones. In ventral view, the pterygoid contacts the palatine anteriorly, the vomer anteromedially, the other pterygoid medially, the basisphenoid and basioccipital posteromedially, and the quadrate posterolaterally (Fig. 3.1, 3.2). The pterygoid contacts the epipterygoid and the parietal close to its midline dorsolaterally, and the opisthotic and prootic posterodorsally. The quadrate and pterygoid form the fenestra postotica and the pterygoid does not contact the exoccipital. This condition can also be observed in plesiochelyid turtles but not in protostegids in which contact between these elements is present.
The processus pterygoideous externus is well developed with a very robust end. It meets neither the maxilla nor the jugal, in a condition similar to that exhibited by Plesiochelys planiceps, leaving an open foramen palatinum posterius. The crista pterygoidea is as developed as in plesiochelyids, and their overall morphology is similar and comparable. Ventrally, each pterygoid has a ridge that runs from the posterolateral margin of the processus pterygoideus externus to the level of the basioccipital contact where it runs to the basioccipital tuberosities (Fig. 3.1, 3.2). This ridge seems to be more developed in Neusticemys neuquina than in Plesiochelys planiceps, but it is missing in Plesiochelys etalloni. This feature is also recognized in Portlandemys mcdowelli, Portlandemys gracilis, Solnhofia parsonsi, and Jurassichelon oleronensis, in which these ridges are present and well developed.
The pterygoid floors most of the cavum acustico-jugulare, but leaves a good portion of it exposed in ventral view. This flooring is achieved by the means of the posterior process of the pterygoid, which is proportionally similar in size to those of Plesiochelys planiceps, Plesiochelys etalloni, Solnhofia parsonsi, and Jurassichelon oleronensis.
A deep and well-developed pterygoid fossa can be found on the ventral surface of the skull of Neusticemys neuquina. This deep pterygoid fossa is similar to equivalent fossae in Portlandemys mcdowelli, Portlandemys gracilis, and Jurassichelon oleronensis. In Plesiochelys planiceps and Plesiochelys bigleri, the fossa is shallower; in Plesiochelys etalloni and Solnhofia parsonsi, it is absent.
In Neusticemys neuquina, the foramen posterius canalis carotici interni opens ventromedially into the pterygoid fossa, and the foramen is formed only by the pterygoid. In Portlandemys mcdowelli and Portlandemys gracilis, this foramen is also formed only by the pterygoid, whereas in Plesiochelys etalloni, Plesiochelys planiceps, Plesiochelys bigleri, and Jurassichelon oleronensis, it is formed by both the pterygoid and the basisphenoid. The carotid system in Neusticemys neuquina features a ventrally covered split between the carotid subbranches. This condition is different from the one found in Plesiochelys etalloni and Plesiochelys bigleri, in which the split is ventrally exposed (Raselli and Anquetin, Reference Raselli and Anquetin2019). In contrast, Plesiochelys planiceps, Solnhofia parsonsi, and J. oleronensis have a ventrally covered split.
Epipterygoid
The epipterygoid is a laminar bone that contacts the pterygoid posteroventrolaterally and the parietal dorsally. It has a roughly triangular outline, and seems to contribute to part of the foramen nervi trigemini. Its overall morphology does not seem to differ from the epipterygoids of other thalassochelydians.
Basisphenoid
The basisphenoid of Neusticemys neuquina has an elongated triangular outline in ventral view (Fig. 3.1, 3.2). It is slightly longer than the length of the medial suture between the pterygoids. It contacts the pterygoids anterolaterally and the basioccipital posteriorly. Contacts with the opisthotic and prootic are not clearly defined, but it might be an artifact of CT scan data resolution and interpretation rather than a lack of contact between these elements. A distinct blind depression is recognized on the ventral surface of the basisphenoid, close to the basioccipital suture. This depression is relatively deep, occupying a quarter of the total length of the basisphenoid and almost all of the maximum width of the basisphenoid. This blind depression has a subcircular outline, and has not been identified in other thalassochelydian turtles.
The two foramen posterius canalis carotici interni cerebralis are located close to the suture between the basisphenoid and the pterygoid, and they are restricted to the pterygoid. They are located on the mediolateral walls of the pterygoid fossa just below the higher point of the pterygoid medial ridge. The foramen anterius canalis carotici interni cerebralis are found close together on the anterior end of the dorsum sellae and the posterior end of the sella turcica. This condition is shared by all thalassochelydian turtles.
In turtles, it is frequently seen that the basisphenoid is subdivided into two distinct regions separated by the dorsum sellae. In Neusticemys neuquina, the anteriormost region has the sella turcica and the rostrum basisphenoidale and is shorter than the posterior one, a condition shared with other thalassochelydians. The dorsum sellae on Neusticemys neuquina is tall and high and has a medial ridge with a slight anterodorsal projection. Well-defined ridges connect the dorsum sellae with the clinoid processes. The prootic foramen in Neusticemys neuquina is open, leaving the clinoid processes free, as in most thalassochelydians with the exception of Plesiochelys etalloni in which it is closed.
The rostrum basisphenoidale seems to be long, reaching the vomer and palatine suture of the pterygoid.
Prootic
The prootic in Neusticemys neuquina is similar in its overall morphology to those of thalassochelydians. Its contacts are: anterodorsally with the parietal, dorsally with the supraoccipital, posteriorly with the opisthotic, anterolaterally with the quadrate, lateroventrally with the pterygoid, and ventromedially with the basisphenoid. This element is well exposed on its dorsal and anterior surfaces. As in most thalassochelydians, the prootic seems to be excluded from the foramen nervi trigemini by a ventral process of the parietal. The prootic does not have any kind of ossification for the lateral semicircular canal, a condition shared with Plesiochelys etalloni and Plesiochelys planiceps but not with Jurassichelon oleronensis or Portlandemys mcdowelli (see Evers and Benson, Reference Evers and Benson2019, appendices S1, S4). Solnhofia parsonsi is coded by Evers and Benson (Reference Evers and Benson2019) as ambiguous for this character. The prootic ventral process is short and lacks an extensive posterior contact with the pterygoid, as in most thalassochelydians. A recess is present on the posterior surface of the prootic anterodorsolateral to the fenestra ovalis, as noted by Evers and Benson (Reference Evers and Benson2019, appendix S1). The prootic and opisthotic do not contact ventrally, thus preventing ventral enclosure of the fenestra ovalis.
Opisthotic
The opisthotic of Neusticemys neuquina is similar in shape to the opisthotic of other thalassochelydian turtles, enclosing the posterior half of the fenestra ovalis and having a long paroccipital process. It contacts the prootic anteriorly, the quadrate anterolaterally, the exoccipital posteromedially, the supraoccipital dorsally, and the squamosal posterodorsolaterally (Figs. 2.1, 2.2, 3.1–3.4). The processus interfenestralis is developed into a ventrally directed excrescence in a fashion similar to those of Plesiochelys etalloni, Plesiochelys planiceps, Portlandemys mcdowelli, and Solnhofia parsonsi. As a bony feature, this process seems not to have reached any basicranial elements in Neusticemys neuquina, although in Plesiochelys planiceps and Solnhofia parsonsi, it apparently did with the help of an inferred cartilaginous element. The perilymphatic fenestra is contained only by interfenestralis process of the ophistotic, and is similar in size to that of Plesiochelys planiceps. The paroccipital process of the opisthotic has a dorsoventrally convex surface, as is the case in most thalassochelydians (Solnhofia parsonsi differs in that this process has a slight dorsoventral concavity).
Quadrate
As in most turtles, the quadrate of Neusticemys neuquina forms the cavum tympani, the anterolateral wall of the middle ear, the processus articularis, and the condylus mandibularis. It contacts the pterygoid ventromedially, the opisthotic posteromedially, the prootic anteromedially, the quadratojugal anterolaterally, and the squamosal posterodorsally (Figs. 2.3–2.6, 3.1–3.4). The impression of the cavum tympani is kidney-shaped in lateral view (Fig. 2.3–2.6), and seems to have been as deep as those of Plesiochelys etalloni and Plesiochelys planiceps. Posteroventrally, the cavum exhibits the incisura columella auris, which opens posteriorly as in plesiochelyids and other thalassochelydians. Posterodorsally, the cavum tympani opens to the antrum postoticum formed by the squamosal. In occipital view, ventral to the incisura columella auris, and dorsal to the condylus mandibularis, the quadrate has a distinct ridge that has been proposed to be associated with the M. depressor mandibulae or the M. pterygoideus portio ventralis (Gaffney, Reference Gaffney1976). The condylus mandibularis has a rectangular shape, with its main axis transverse to the main axis of the skull (Fig. 3.1, 3.2). The condylus has two distinct surfaces, lateromedially oriented at an obtuse angle. The lateral surface is concave, whereas the medial one is slightly convex. On the anterior surface and close to the prootic suture, a large and well-developed processus trochelaris oticum can be found, forming a well-defined muscular scar. This process is limited to the medial part of the otic chamber and exhibits a deep recess laterally. The cranial articular process of the quadrate has a strong posterior inclination similar to those seen in Plesiochelys etalloni, Plesiochelys bigleri, and Plesiochelys planiceps.
Basioccipital
The basioccipital in Neusticemys neuquina is a robust element that forms the posteriormost region of the skull floor, with a width/length ratio close to 1.5 in MOZ-PV-064. It contacts the basisphenoid anteriorly, the exoccipital laterodorsally, and the pterygoid anterolaterally, and forms the basal third of the condylus occipitalis (Fig. 3.1–3.4). The basioccipital has two well-developed tuberosities (basi-tuberes) on its ventral surface; they are posteroventrally oriented and are connected to the ridges on the pterygoid. These tuberosities delimit a trough that is deep posteriorly on the basioccipital and the basisphenoid, becoming shallow anteriorly on the pterygoid. The development of these tuberosities resembles that of Plesiochelys etalloni, whereas in other thalassochelydians, e.g., Plesiochelys bigleri, Plesiochelys planiceps, Portlandemys gracilis, and Portlandemys mcdowelli, they are not as developed. The basioccipital prevents the exposure of the interfenestralis process in ventral view, as is typical in thalassochelydians.
Exoccipital
The exoccipital of Neusticemys neuquina is a roughly triangular element that contacts the basioccipital ventrally, the opisthotic anterolaterally, and the supraoccipital dorsally (Fig. 3.3, 3.4). There are two foramina nervi hypoglossi in each exoccipital, but not a foramen jugulare posterius separate from the fenestra postotica. Neusticemys neuquina shares this condition with other thalassocheldyians and extant chelonioids. The foramina nervi hypoglossi are not exposed ventrally because an extension of the basioccipital covers them. The exoccipital participates in the condylus occipitalis and in the adult forms the entire ventral margin of the foramen magnum, seemingly preventing any contribution from the basioccipital. However, in the juvenile specimen of Neusticemys neuquina (MACN-PV-105), the basioccipital might have had some dorsal exposure in the margin. The condition seen in MOZ-PV-064 is shared by most thalassochelydians, with the exception of Plesiochelys bigleri and Solnhofia parsonsi in which the basioccipital does participate in defining the margin of the foramen magnum at least in the adult stage (the only one known for these taxa).
Supraoccipital
The supraoccipital of Neusticemys neuquina is, as in most turtles, a bone that can be divided into two distinct regions, the posterior crista supraoccipitalis and an anteroventral region that contributes on the cavum labyrinthicum and cavum cranii. It contacts the exoccipital posteroventrolaterally, the opisthotic ventrolaterally, the prootic anteroventrolaterally, and the parietal anterodorsally (Figs. 2.1–2.6, 3.3, 3.4). The crista supraoccipitalis extends posteriorly almost to the level of the squamosals, but most of its dorsal portion is not preserved. In occipital view, a shallow fossa can be identified on the lateral surface of the supraoccipital, dorsal to the contact of exoccipital, opisthotic, and supraoccipital.
Mandible
In MOZ-PV-064, the postdentary elements are adequately preserved, the dentary and splenial less so. The latter's preserved portions are mostly represented by a cast or covered by sediments (Fig. 4.1–4.10). In dorsal view, the mandible is U-shaped, but this is an artifact of preservation preparation: its anterior region is formed by sediments and a cast which does not represent the true symphysis. However, in other specimens (MOZ-PV-5804, MOZ-PV-6094), the symphysis is preserved. In these specimens, the true shape of the mandible is V-shaped as is typical of thalassochelydians. The mandibular rami meet at an angle close to 40°. No ramal triturating surface is preserved in MOZ-PV-064, but it is in MHNSR-PV-1195. In the latter, the surface is not as developed as in Plesiochelys etalloni, or most other thalassochelydians for that matter, and it is very steepened, although not reaching the extreme condition seen in Dermochelys coriacea Vandelli, Reference Vandelli1761. In lateral view, the mandible of Neusticemys neuquina is taller than that of Portlandemys gracilis.

Figure 4. Neusticemys neuquina from Tithonian levels of Vaca Muerta Formation, photographs and drawings of the lower jaw MOZ-PV-064 in: (1, 2) dorsal view, (3, 4) ventral view, (5, 6) right lateral view, (7, 8) left lateral view, and (9, 10) left visceral view. ang = angular; art = articular; cor = coronoid; den = dentary; fdm = foramen dentofaciale majus; fic = foramen intermandibularis caudalis; fme = fossa meckelii; fna = foramen nervi auriculotemporalis; pra = prearticular; sp = splenial; sur = surangular.
Dentary
Only the posterior portion of the dentary is preserved in MOZ-PV-064. It preserves the foramen dentofacile majus and the contact with the postdentary elements (Fig. 4.1–4.8). The foramen dentofaciale majus is large and comparable to those of other thalassochelydians. The dentary contacts the splenial medially, the surangular posterolaterally, the coronoid posterodorsally, and the angular ventrally. In MOZ-PV-6094, the dentary forms the anterior end of the mandible including the mandibular symphysis, which seems to have been short (< 30% of the total mandibular length). The dentaries are fused midsagitally and there is no symphyseal hook. The main feature of the dentary of Neusticemys neuquina, as seen in MHNSR-PV-1195, is the lack of a well-developed triturating surface. There is a well-defined labial ridge, but the lingual ridge seems to have been absent or poorly developed, leaving a very steepened triturating surface.
Splenial
Only the left splenial can be identified, situated on the left ramus of the mandible. It is a sheet-like bone and poorly preserved, only the posteroventral portion being present (Fig. 4.9, 4.10). The only recognizable contacts are posteriorly with the prearticular and ventrally with the angular. The topological position of the splenial seems to be in agreement with the known position of this element in other thalassochelydians.
Angular
The angular in MOZ-PV-064 is well preserved on both rami of the mandible. It is an elongated element that forms the posteroventral portion of the mandible (Fig. 4.3–4.10). It contacts the surangular dorsolaterally, the prearticular dorsomedially, and the splenial dorsally. The angular does not seem to have reached the mandibular symphysis, as it does in Portlandemys gracilis (see Anquetin et al., Reference Anquetin, Püntener and Billon-Bruyat2015), but it extends farther anteriorly in this species than its homolog in Plesiochelys etalloni (see Gaffney, Reference Gaffney1976, fig. 48).
Surangular
The surangular is a sheet-like bone that forms the posterolateral wall of the mandible, most of the lateral surface of the area articularis, and the lateral side of the fossa meckelii (Fig. 4.1–4.8). It contacts the articular posteromedially, the dentary anteriorly, the angular ventrally, and the coronoid dorsomedially. The surangular in Neusticemys neuquina is relatively taller than the surangular found in most thalassochelydians. A single foramen auriculotemporalis opens on the lateral wall of the surangular, close to the area articularis.
Coronoid
Most of the coronoid is preserved, although the processus coronoideus is broken at its base and its anteriormost portion is broken and missing on both rami (Fig. 4.9, 4.10). It contacts the prearticular posteroventraly, the surangular posterolaterally, and the dentary anterodorsolaterally. It forms the anteromedial margin of the dorsal opening of the fossa meckelii. Because the anterior portion is not preserved, it is not possible to identify its participation in bounding either the foramen intermandibularis or the unnamed foramen found in all thalassochelydians (see Evers and Benson, Reference Evers and Benson2019).
Articular
The articular of Neusticemys neuquina is a roughly subrectangular element that respectively contacts the surangular and prearticular laterally and medially (Fig. 4.1, 4.2, 4.5–4.10). Together with the prearticular and surangular, it forms the area articularis mandibularis, as in Plesiochelys etalloni. The contributions of the prearticular and surangular are relatively larger than in other species, e.g., Toxochelys latiremis Cope, Reference Cope1873. The area articularis mandibularis displays two distinct and slightly concave areas separated by a medial ridge. The lateral surface of the area mandibularis is formed principally by the surangular, whereas the medial one is formed mainly by the articular and to a lesser degree by the prearticular.
Prearticular
The prearticular in Neusticemys neuquina is a plate-like element that is quite comparable to those of most thalassochelydians. It composes the posteromedial wall of the mandibular ramus and a small portion of the medial surface of the area articularis mandibularis (Fig. 4.1, 4.2, 4.9, 4.10). Posteriorly, it contacts the articular, ventrally the angular, dorsally the coronoid, and anteriorly the splenial. The fossa meckelii is large and well defined. The prearticular forms the medial margin of the fossa and seems to have made a greater participation to its outline than the surangular. The foramen intermandibularis caudalis can be identified in the contact surface between prearticular and angular on the posterior end of the prearticular.
Cervical vertebra
The right half of a cervical vertebra (Fig. 5.1–5.12) preserves most of the neural spine and neural arch, both postzygapophyses, most of the right portion of the centrum, and part of the right transverse process. The centrum preserves the right side and posterior condyle; the left side and anterior articular surface are missing. The posterior condyle is relatively deep and seems to have been taller than wide. The neural spine is high and bears two posteroventrally oriented postzygapophyses. The neural spine and neural arch are slightly higher than the centrum. A strongly developed transverse process is located at the anterior portion of the base of the neural arch. The centrum is strongly ventrally keeled.

Figure 5. Neusticemys neuquina from Tithonian levels of Vaca Muerta Formation, photographs and drawings of the cervical vertebra MOZ-PV-064 in: (1, 7) right lateral view, (2, 8) left lateral view, (3, 9) anterior view, (4, 10) posterior view, (5, 11) ventral view, and (6, 12) posterior view fob = foreign object; nc = neural crest; prz = prezygapophysis; tp = transverse process; vc = ventral crest.
Material
MOZ-PV-064, an almost complete skull with lower jaw and a cervical vertebra.
Remarks
The most complete cranial remains referred to Neusticemys neuquina are described, allowing us to emend and expand the diagnosis of this species previously, mostly known from postcranial materials. The new description allowed comparison with other turtles of the Thalassochelydia clade.
Results of the phylogenetic analyses
Forty MPTs were recovered from the initial analysis using unordered characters, with a tree length (TL) of 1,542 steps, a consistency index (CI) of 0.259, and a retention index (RI) of 0.65 (see Fig. 6 for the strict consensus). The topological hypothesis of the consensus agrees with that of Evers and Benson (Reference Evers and Benson2019). Neusticemys neuquina was recovered nested inside a monophyletic Thalassochelydia in a polytomy alongside Plesiochelys etalloni, Plesiochelys planiceps, Portlandemys mcdowelli, and Jurassichelon oleronensis. Solnhofia parsonsi was recovered as a sister taxon of this clade. The recently named clade Angolachelonia Mateus et al., Reference Mateus, Jacobs, Polcyn, Schulp, Vineyard, Buta Neto and Telles Antunes2009 (Evers and Benson, Reference Evers and Benson2019) was recovered including Thalassochelydia and the Sandownidae as sister groups. In the majority consensus (50% cut off), the Thalassochelydia polytomy is almost resolved, with Neusticemys neuquina recovered as an early thalassochelydian more derived than Solnhofia parsonsi in 60% of the MPTs (see also Supplemental datafile ‘Appendix 1,’ fig. 2).

Figure 6. Simplified, reduced strict consensus of 40 most parsimonious trees of |TL 1,542 steps (CI 0.259; RI 0.650), as the result of the unordered phylogenetic analyses based on the matrix of Evers and Benson (Reference Evers and Benson2019). Numbers on branches represent Bremmer and Jacknife support values. Taxa not otherwise discussed in the text are: Allaeochelys libyca Havlik, Joyce, and Böme, Reference Havlik, Joyce and Böhme2014; Annemys latiens Sukhanov and Narmandakh, Reference Sukhanov and Narmandakh2006; Annemys levensis Sukhanov and Narmandakh, Reference Sukhanov and Narmandakh2006; Apalone spinifera (LeSueur, Reference LeSueur1827); Arundelemys dardeni Lipka et al., Reference Lipka, Therrien, Weishampel, Jamniczky, Joyce, Colbert and Brinkman2006; Australochelys africanus Gaffney and Kitching, Reference Gaffney and Kitching1994; Baptemys wyomingensis Leidy, Reference Leidy1870; Brachyopsemys tingitana Tong and Meylan, Reference Tong, Meylan, Brinkman, Holroyd and Gardner2013; Carettochelys insculpta Ramsay, Reference Ramsay1887; Chrysemys picta (Schneider, Reference Schneider1783); Chubutemys copelloi Gaffney et al., Reference Gaffney, Rich, Vickers-Rich, Constantine, Vacca and Kool2007; Dermatemys mawii Gray, Reference Gray1847; Eileanchelys waldmanni Anquetin et al., Reference Anquetin, Barrett, Jones, Moore-Fay and Evans2008; Emarginachelys cetacea Whetstone, Reference Whetstone1978; Emys orbicularis (Linnaeus, Reference Linnaeus1758); Eubaena cephalica Hay, Reference Hay1904; Geoclemys hamiltonii (Gray, Reference Gray, Griffith, Pidgeon and Griffith1830); Glyptops plicatulus Cope, Reference Cope1877; Gopherus polyphemus (Daudin, Reference Daudin1802); Kallokibotion bajazidi Nopcsa, Reference Nopcsa1923; Kayentachelys aprix (Gaffney et al., Reference Gaffney, Hutchison, Jenkins and Meeker1987); Kinosternon suburum hippocrepis (Bonnaterre, Reference Bonnaterre1789); Leyvachelys cipadi Cadena, Reference Cadena2015; Lissemys punctata (Bonnaterre, Reference Bonnaterre1789); Macrochelys temmincki (Troost in Harlan, Reference Troost1835); Meiolania platyceps Owen, Reference Owen1886; Pelodiscus sinensis (Wiegmann, Reference Wiegmann1835); Platysternon megacephalum Gray, Reference Gray1831; Pleurosternon bullockii Owen, Reference Owen1842; Sandownia harrisi Meylan et al., Reference Meylan, Moody, Walker and Chapman2000; Xinjiangchelys radiplicaoides (Young and Chow, Reference Young and Chow1953); and X. wusu Rabi et al., Reference Rabi, Zhou, Wings, Ge and Joyce2013.
Adhering to the Evers and Benson (Reference Evers and Benson2019) definition of Thalassochelydia, we include Solnhofia parsonsi as a thalassochelydian. Eight non-ambiguous synapomorphies support Thalassochelydia as monophyletic: (1) the otic process is limited to the medial part of the otic chamber, and there is a deep recess laterally (character 81[1]); (2) there is a fossa on the posterodorsal surface of the floor of the supraoccipital (character 113[1]); (3) the foramina anterior canalis carotici interni are found close together but as independent canals (character 142[1]); (4) the splenial is present (character 185[0]); (5) the costals lack lateral ossification, allowing dorsal exposure of the distal ends of the ribs and the development of fontanelles only in the anterior- and posteriormost costals (character 210[2]); (6) rib-free peripherals are present (character 211[1]); (7) vertebrals 2–4 are significantly broader than pleurals (character 224[1]); and (8) articulation of the cervical vertebrae is not formed because they are either platycoelous or amphicoelous (character 278[0]). If Neusticemys neuquina is placed as an early branching thalassochelydian, two ambiguous synapomorphies can be added as synapomorphies to the definition of Thalassochelydia: the absence of contact between pterygoid and exoccipital (character 96[0]), and the foramina nervi hypoglossi covered in ventral view by an extension of the basioccipital (character 116[2]).
Angolachelonia is supported by eight unambiguous synapomorphies. These synapomorphies are the same as the ones reported for this group by Evers and Benson (Reference Evers and Benson2019) (characters 18[0], 83[1], 84[1], 94[0], 103[1], 181[1], 182[1], and 184[1]) and will not be reported in detail here (for more information on synapomorphies, see Supplemental datafiles ‘Appendix 1,’ fig. 5 and ‘Appendix 2’).
In the ordered character analyses, 986 MPTs were recovered with a TL of 1,574 steps, CI of 0.254, and RI of 0.661. The topology of the strict consensus is very similar to that of Evers and Benson (Reference Evers and Benson2019) (see Supplemental datafile ‘Appendix 1,’ fig. 3) with most clades involved in a polytomy. Neusticemys neuquina was recovered nested inside Thalassochelydia, as in the unordered analyses, but in this analysis Solnhofia parsonsi is not included in the clade. Thalassochelydia was recovered as a polytomy nested inside a greater polytomy involving several clades and taxa. However, in the majority consensus tree (50% cut off; see Supplemental datafile ‘Appendix 1,’ fig. 4) Angolachelonia was recovered in 74% of the MPTs, Solnhofia parsonsi was recovered as a member of Thalassochelydia in 54% of MPTs, and Neusticemys neuquina was recovered in a polytomy alongside Plesiochelys etalloni and Plesiochelys planiceps in 54% of the MPTs. Portlandemys mcdowelli and Jurassichelon oleronensis were recovered as monophyletic in 97% of the MPTs and as the sister group of the Plesiochelys and Neusticemys neuquina clade.
Only two synapomorphies support Thalassochelydia (without Solnhofia parsonsi) as a monophyletic unit: lack of a medial process of the jugal ventral to the orbit (character 27[0]), and involvement of the parabasisphenoid in forming the foramen posterius carotici interni (character 152[0]). When characters were optimized over the majority consensus, some ambiguous synapomorphies were found to support both the previous clade and Thalassochelydia with Solnhofia parsonsi included. The former clade is now defined by four more synapomorphies: (1) wide open nature of squamosal-quadrate contact (character 39[1]); (2) presence of both labial and lingual ridges (character 58[1]); (3) a flat or nearly horizontal contact between vomer and maxilla (character 67[0]); and (4) a prominent tomial ridge of the dentary (character 175[0]). The latter clade (Thalassochelydia + Solnhofia parsonsi) is supported by some of the unambiguous synapomorphies found in the unordered analyses (characters 81[1], 113[1], 185[0], 211[1], 224[1], and 278[0]). However, two were not recovered (characters 142[1] and 210 [2]), and two new synapomorphies were added: moderate quadratojugal emargination (i.e., ‘the margin of the temporal emargination is principally formed by the quadratojugal and jugal, but the maxilla is included on the anterior section of the margin and/or the quadrate is included on the posterior section of the margin [character 34[1]’); and the geniculate ganglion positioned within the facial nerve canal (character 127[1]).
Angolachelonia was not recovered as a clade in the strict consensus. However, it was recovered as monophyletic in 75% of all MPTs. When synapomorphies were optimized over the majority consensus, Angolachelonia was supported by five of the eight synapomorphies that supported it in our unordered analyses as well as that of Evers and Benson (Reference Evers and Benson2019) (characters 83[1], 84[1], 103[1], 181[1], and 184[1]; characters 18[0], 94[0] and 182[1] were not recovered as synapomorphies).
Discussion
Taxonomic assignment of Neusticemys neuquina
Neusticemys neuquina has been traditionally recognized as closely related to other Tethyan Jurassic taxa referred to Eurysternidae or Plesiochelyidae, e.g., Eurysternum wagleri Meyer, Reference Meyer1839, Solnhofia parsonsi, Plesiochelys etalloni, Plesiochelys planiceps, and Portlandemys mcdowelli (see Fernández and de la Fuente, Reference Fernández and de la Fuente1988, Reference Fernández and de la Fuente1993). Recently, most of these taxa, together with Jurassichelon oleronensis, have been recognized as a clade and defined by Anquetin et al. (Reference Anquetin, Püntener and Joyce2017) as Thalassochelydia.
Thalassochelydia is a clade known mainly from the Oxfordian to the Tithonian of eastern Europe and from the Tithonian of Argentina (Anquetin et al., Reference Anquetin, Püntener and Joyce2017). Although some remains from the Lower Cretaceous of Switzerland (Pictet and Campiche Reference Pictet and Campiche1858–1860; Püntener et al., Reference Püntener, Billon-Bruyat, Bocat, Berger and Joyce2014) and Uzbekistan (Nessov and Krasovskaya, Reference Nessov and Krasovskaya1984; Sukhanov, Reference Sukhanov, Benton, Shishkin, Unwin and Kurochkin2000; Karl et al., Reference Karl, Tichy and Valdiserri2012) have been attributed to thalassochelydian turtles, the stratigraphic provenance of the former is a topic of discussion and the latter lacks any diagnostic features; its assignment to Thalassochelydia is problematic (Anquetin et al., Reference Anquetin, Püntener and Joyce2017). Thalassochelydian turtles have a variety of adaptations for a marine lifestyle, e.g., the elongation and flattening of the manus, the development of carapacial and plastral fontanelles in some taxa, and large foramina interorbitalia that could have hosted salt glands (Billon-Bruyat et al., Reference Billon-Bruyat, Lécuyer, Martineau and Mazin2005). These adaptations are not as extreme as the ones developed by chelonioid turtles but are regarded as adaptations to marine environments (Hirayama, Reference Hirayama1994, Reference Hirayama, Calloway and Nicholls1997, Reference Hirayama1998).
The phylogenetic affinities of Thalassochelydia are still uncertain because different studies seem to place them in different positions within turtle phylogeny. Anquetin et al. (Reference Anquetin, Püntener and Joyce2017) regarded them as pan-cryptodiran turtles. However, in that contribution, no phylogenetic analysis was performed; this referral was based on the study by Anquetin et al. (Reference Anquetin, Püntener and Billon-Bruyat2015) in which this clade was recovered as monophyletic. Cadena and Parham (Reference Cadena and Parham2015) did not recover Thalassochelydia as a monophyletic clade: Plesiochelys etalloni was recovered as stem-Testudines, whereas Jurassichelon oleronensis and Solnhofia parsonsi were recovered as pan-Chelonioidea, effectively making Thalassochelydia polyphyletic. More recent studies have placed them as stem-testudines (Evers and Benson, Reference Evers and Benson2019) or even as stem-Pleurodira (Evers et al., Reference Evers, Barret and Benson2019). In this study, Thalassochelydia was recovered in a position similar to that found by Evers and Benson (Reference Evers and Benson2019), as might be expected because we used the same matrix.
Originally, Neusticemys neuquina was considered to be related to Thalassochelydia on the basis of some plastral and carapacial features, e.g., retention of carapacial fontanelles in adult stages, lateral and central plastral fontanelles, long and hexagonal neural plates, and a loose connection between the carapace and the plastron. However, many such features are regarded as adaptations correlated with a marine lifestyle, susceptible to convergence and therefore with little phylogenetic significance (Zangerl, Reference Zangerl1980; de la Fuente, Reference de la Fuente, Gasparini, Coria and Salgado2007). When skulls are compared, the similarities between thalassochelydian turtles and Neusticemys neuquina become more apparent. All thalassochelydian turtles exhibit an infolding ridge on the posterior surface of the quadrate, as originally observed for plesiochelyids by Anquetin et al. (Reference Anquetin, Püntener and Billon-Bruyat2015). This feature is also found on sandownids and was considered by Evers and Benson (Reference Evers and Benson2019) to be a synapomorphy of Angolachelonia. Thalassochelydian features seen in Neusticemys neuquina are: a relatively well-developed temporal emargination, large dorsolaterally oriented orbits, the quadrate process of the pterygoid oriented posterolaterally, a relatively shallow antrum postoticum, and a small depression on the posterodorsal wall of the supraoccipital (Fig. 7.1). Additionally, the quadrate bears a well-developed processus trochlearis oticum restricted to the medial area between the quadrate and the parietal, and also has a recessus on its anterior surface lateral to the processus trochlearis oticum (Fig. 7.2); the foramina canalis carotici interni are located close together in the basisphenoid (Fig. 7.3); and the splenial is present in the mandible (Fig. 7.4).
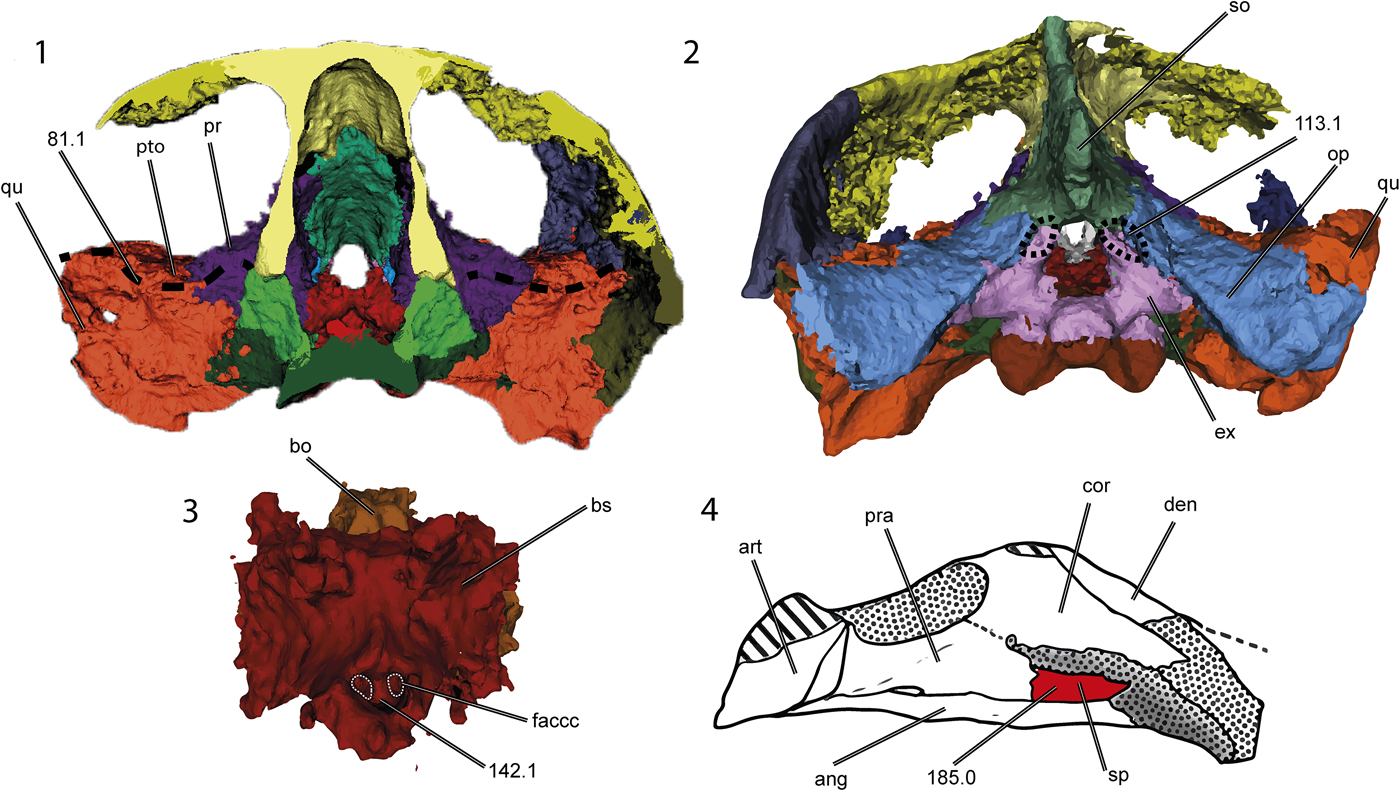
Figure 7. Thalassochelydian synapomorphies on the skull and lower jaw of Neusticemys neuquina (colorized 3D models and drawing): (1) transverse section of the skull, (2) skull in posterior view, (3) basicranial bones, and (4) lower jaw in left visceral view (shading as for Fig. 2). Dashed lines demark the fossa in the ventral surface of the supraoccipital (113.1) showing the lateral recess of the quadrate (81.1) and the foramen anterior canalis carotici cerebralis (142.1); the splenial (185.0) is colorized. ang = angular; art = articular; bo = basioccipital; bs = basisphenoid; cor = coronoid; den = dentary; faccc = foramen anterius canalis caratoci cerebralis; ex = exoccipital; op = opisthotic; pr = prootic; pra = prearticular; pto = processus trochlearis oticum; qu = quadrate; so = supraoccipital; sp = splenial. Not to scale.
Some features that make Neusticemys neuquina unique among Thalassochelydia are: a large, oval foramen nervi trigemini (Fig. 8.1); reduction of the triturating surfaces in both the mandible and maxilla when compared with skulls of other thalassochelydians (only the labial ridge is developed and steepens medially at an acute angle, in a condition that resembles that seen in Dermochelys coriacea) (Fig. 8.2, 8.3); development of a small depression on the ventral surface of the basisphenoid (Fig. 8.4); strong dorsal keeling of the squamosals; anterior development of the palatines due to reduction of the triturating surfaces; and a tall surangular bone.
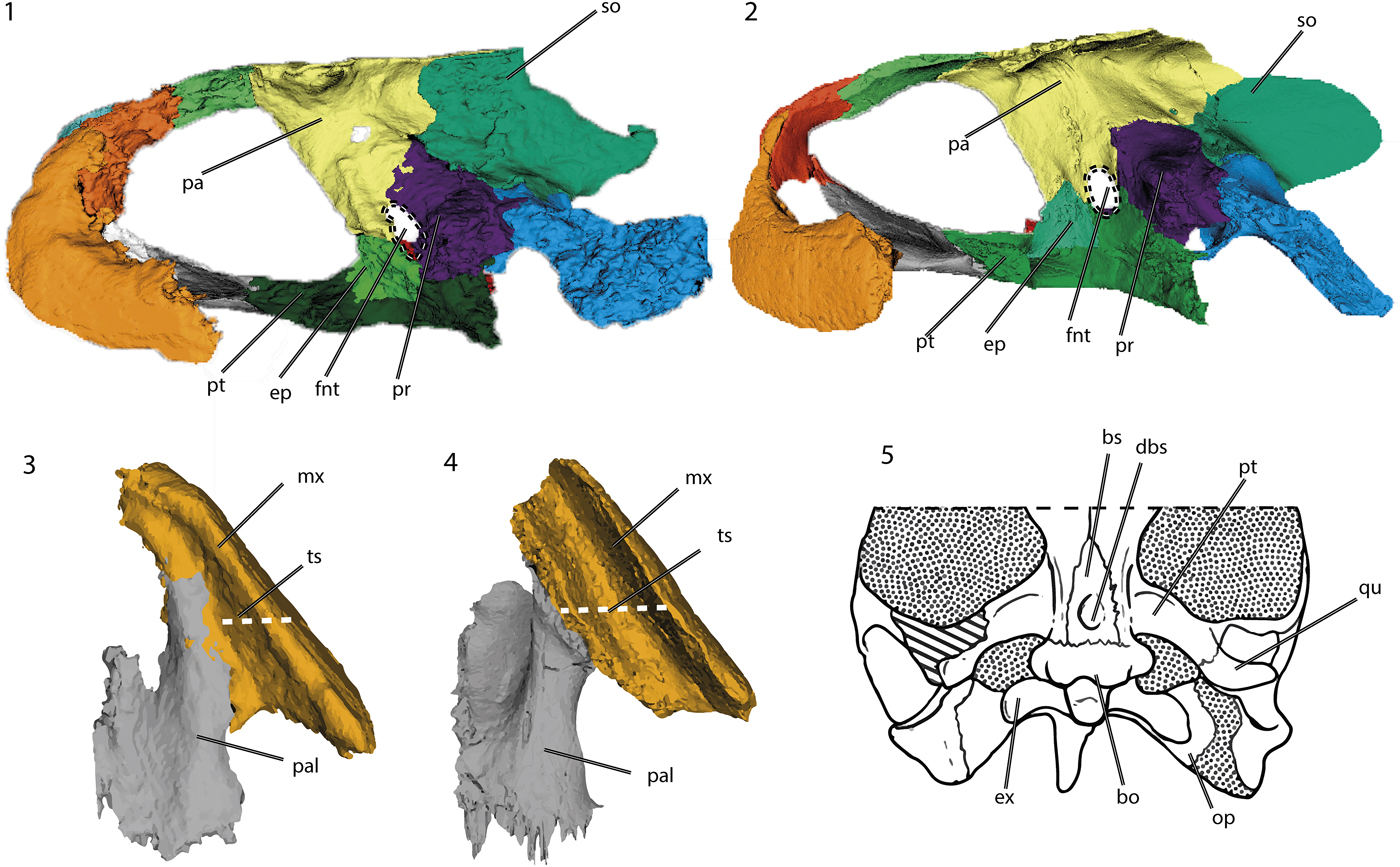
Figure 8. Diagnostic characters of Neusticemys neuquina from Tithonian levels of Vaca Muerta Formation, colorized 3D models and drawing of the skull in: (1, 2) lateral view without the most lateral bones of (1) Neusticemys neuquna and (2) Plesiochelys planiceps (OUMNH J1582); (3, 4) palatal ventral view of (3) Neusticemys neuquina and (4) Plesiochelys planiceps (OUMNH J1582) (dashed white lines compare the relative width of the triturating surface between these taxa), and (5) drawing of Neusticemys neuquina skull in ventral view. bo = basioccipital; bs = basisphenoid; dbs = basisphenoidal depression; ep = epipterygoid; ex = exoccipital; fnt = foramen nervi trigemini; mx = maxilla; op = opisthotic; pa = parietal; pal = palatine; pr = prootic; pt = pterygoid; qu = quadrate; so = supraoccipital; ts = triturating surface. Not to scale. Plesiochelys planiceps (OUMNH J1582) models available at Morphosource (http://www.morphosource.org): Project: Evers & Benson 2018, Turtle CT Data and 3D Models (Evers and Benson, Reference Evers and Benson2019).
Among Thalassochelydia, it is not possible to establish with certainty the position of Neusticemys neuquina vis-à-vis the individual families Eurysternidae, Plesiochelyidae, and Thalassemydidae. Carapacial and plastral features resemble those of eurysternids, whereas the cranial material resembles those of plesiochelyids. However, these apparent cross resemblances might simply reflect a lack of information regarding the skull morphology of eurysternid and thalassemydid turtles, because for the former, only the skulls of eurysternid juveniles (Klein et al., Reference Klein, Schoch and Schweigert2016) and an adult Solnhofia parsonsi (Parsons and Williams, Reference Parsons and Williams1961; Gaffney, Reference Gaffney1975b; Joyce, Reference Joyce2000) are known, and for thalassemydids no skulls have been recorded (Anquetin et al., Reference Anquetin, Püntener and Joyce2017). Solnhofia parsonsi is a bizarre turtle from the Upper Jurassic of Europe: it has a developed secondary palate; a long, narrow snout; and the triturating surface is smooth and broad, lacking any accessory ridges (Gaffney, Reference Gaffney1975b; Joyce, Reference Joyce2000). Its skull is probably highly derived and does not represent conditions in most eurysternids, so until more cranial material is recovered for this family, a real assessment of the eurysternid skull evolution is not possible. It is also noteworthy that in some analyses (Mateus et al., Reference Mateus, Jacobs, Polcyn, Schulp, Vineyard, Buta Neto and Telles Antunes2009; Evers and Benson, Reference Evers and Benson2019), Solnhofia parsonsi appears to be more closely related to sandownids, so its membership in Thalassochelydia might well change in the future.
The postcranium of Neusticemys neuquina, to the degree it is known, might resemble that of eurysternids because of convergent evolution and adaptation to a marine lifestyle. Other turtles have the same carapace features as Neusticemys neuquina as secondary adaptations to the marine realm. Such features usually include the opening of peripheral and plastral fontanelles and the development of flippers or flipper-like limbs (Hirayama, Reference Hirayama1994; Evers et al., Reference Evers, Barret and Benson2019). These features are present in protostegids, dermochelyids, and cheloniids, which implies that such features regularly appear as adaptations to a marine lifestyle and therefore lack unambiguous phylogenetic significance (Zangerl, Reference Zangerl1980). Nevertheless, carapace and plastral features have led some authors (e.g., Lapparent de Broin, Reference Lapparent de Broin2001) to propose Neusticemys neuquina as a protostegid turtle or to place it within the much more inclusive group Eucryptodira (de la Fuente, Reference de la Fuente, Gasparini, Coria and Salgado2007). Protostegidae was a group of highly derived marine turtles that appeared at least as early as the Barremian (Cadena and Parham, Reference Cadena and Parham2015) and were dominant from the Aptian to the Turonian until they started dwindling after the Campanian (Zangerl, Reference Zangerl1953; Hirayama, Reference Hirayama1994). They include some of the biggest turtles ever to have lived, e.g., Archelon ischiurus Wieland, Reference Wieland1896 and Protostega gigas Cope, Reference Cope1871 from the Cretaceous of North America. However, with the new cranial material of Neusticemys neuquina, placement in Protostegidae is unlikely and its position as a thalassochelydian is correspondingly strengthened. The main differences between Neusticemys neuquina and protostegids are: (1) the vomer in Neusticemys neuquina contacts the pterygoid, whereas in protostegid turtles, the vomer does not contact the pterygoids because the palatines meet at their midline (Hirayama, Reference Hirayama1994, Reference Hirayama1998; Raselli, Reference Raselli2018; Evers et al., Reference Evers, Barret and Benson2019); (2) the jugal in Neusticemys neuquina is relatively small and does not reach the quadrate, whereas in protostegid turtles, the jugal is large and extends toward the quadrate, almost reaching it (Hirayama, Reference Hirayama1994, Reference Hirayama1998; Kear and Lee, Reference Kear and Lee2006; Cadena and Parham, Reference Cadena and Parham2015; Raselli, Reference Raselli2018; Evers et al., Reference Evers, Barret and Benson2019); (3) cheek emargination in Neusticemys neuquina is well developed and involves the jugal, the quadratojugal, and the quadrate, whereas protostegids have a straight cheek in which the only bone involved is the jugal (Hirayama, Reference Hirayama1994; Kear and Lee, Reference Kear and Lee2006; Cadena and Parham, Reference Cadena and Parham2015; Evers et al., Reference Evers, Barret and Benson2019); and (4) medial contact of the prefrontals, which never occurs in protostegid turtles (although this last feature is variable among thalassochelydian turtles; for protostegid synapomorphies, see Hirayama, Reference Hirayama1994, Reference Hirayama1998; Cadena and Parham, Reference Cadena and Parham2015; Evers and Benson, Reference Evers and Benson2019).
Phylogenetic comments
In the unordered analyses, Neusticemys neuquina was recovered nested within the Thalassochelydia even when Thalassochelydia was defined in different ways. For example, it remains a thalassochelydian under the definition of Anquetin et al. (Reference Anquetin, Püntener and Joyce2017, p. 329): “all turtles more closely related to Eurysternum wagleri Meyer, Reference Meyer1839, Plesiochelys etalloni (Pictet & Humbert, Reference Pictet and Humbert1857), and Thalassemys hugii Rütimeyer, Reference Rütimeyer1873, than to Pelomedusa subrufa (Bonnaterre, Reference Bonnaterre1789), Testudo graeca Linnaeus, Reference Linnaeus1758, or Protostega gigas (Cope, Reference Cope1871)”, or under Evers and Benson's (Reference Evers and Benson2019, p. 27) definition: “The clade that includes all turtles more closely related to Eurysternum wagleri Meyer, Reference Meyer1839, Plesiochelys etalloni (Pictet & Humbert, Reference Pictet and Humbert1857) and Thalassemys hugii Rütimeyer, Reference Rütimeyer1873, than to Pelomedusa subrufa (Bonnaterre, Reference Bonnaterre1789), Testudo graeca Linnaeus, Reference Linnaeus1758, Protostega gigas (Cope, 1871b), or Sandownia harrisi Meylan et al., Reference Meylan, Moody, Walker and Chapman2000.” Of these two definitions for Thalassochelydia, the latter is less inclusive than the former. The definition by Evers and Benson (Reference Evers and Benson2019) was modified from that of Anquetin et al. (Reference Anquetin, Püntener and Joyce2017) so that Thalassochelydia would not include sandownids and would only include Plesiochelyidae, Eurysternidae, and Thalassemydidae. Neusticemys neuquina cannot be placed, however, into any of the aforementioned thalassochelydian groups because the relationships between them are still uncertain and most of them are underrepresented in phylogenetic matrices.
In 60% of the MPTs of the unordered analysis, Neusticemys neuquina was found to be the sister group of a clade that includes Plesiochelis etalloni, Plesiochelys planiceps, Portlandemys mcdowelli, and Jurassichelon oleronensis. This position also adds two characters as ambiguous synapomorphies for Thalassochelydia because the optimization of characters in this consensus sets them as plesiomorphic for the clade. Hypothesizing Neusticemys neuquina as an early branching member of Thalassochelydia would imply an early diversification and dispersion of thalassochelydian turtles because the provenance of Neusticemys neuquina is the Upper Jurassic of Argentina.
In the ordered analysis (Supplemental Appendix 1, fig. 3), Neusticemys neuquina was recovered inside a polytomy with all of the Plesiochelys spp., Portlandemys mcdowelli, and Jurassichelon oleronensis, supporting its thalassochelydian affinities. However, there are two main differences from the unordered analyses. First, Solnhofia parsonsi is excluded from Thalassochelydia in the strict consensus, and secondly, Angolachelonia was not recovered as a monophyletic clade. The exclusion of Solnhofia parsonsi from Thalassochelydia is due to the two different positions that this taxon adopted in our phylogenetic framework. It can form a monophyletic grouping with Thalassochelydia, as in the unordered analyses, or it can align with Sandownidae. The issues surrounding the disruption of Angolachelonia are a bit less clear and seem to be related to the inclusion of Neusticemys neuquina in the phylogenetic matrix of Evers and Benson (Reference Evers and Benson2019). In the majority of the MPTs (75% as the majority consensus, Supplemental Appendix 1, fig. 4) Angolachelonia was recovered as monophyletic. However, in the other 25% of the MPTs, it was not recovered as monophyletic because both Sandownidae and Thalassochelydia can be found as sister groups of the total group Chelonioidea, making Angolachelonia polyphyletic and in some cases paraphyletic. When Neusticemys neuquina was excluded from the analysis, Angolachelonia was recovered, as is to be expected because we used the same matrix as Evers and Benson (Reference Evers and Benson2019), and they found this same result. At the moment, it is uncertain why the inclusion of this taxon generates these different arrangements; most probably, it is an artifact of mosaic and/or uninformative ones characters (including homoplasies).
The stratigraphic provenance of Neusticemys neuquina is not novel for the clade because Thalassochelydia is known from Upper Jurassic beds of Europe. However, the geographic provenance is unique because Neusticemys neuquina is the only thalassochelydian known from the Southern Hemisphere (and outside of Europe). If the origin of Thalassochelydia occurred within the Jurassic of Europe, and if an early thalassochelydian of the same age as some of the older representatives reached the Gondwanan realm, then the diversification and dispersion of thalassochelydian turtles must have taken place early in the origin of the clade.
The ordered analysis, nevertheless, produced a very different result. In this analysis, the phylogenetic position of Neusticemys neuquina is that of a derived thalassochelydian, probably a plesiochelyid turtle. In this scenario, Neusticemys neuquina might instead represent a late dispersion of the thalassochelydian clades from Europe. Currently, we lack evidence to support one paleobiogeographical hypothesis over the other, and phylogenetic analyses with more thalassochelydian turtle representatives will be needed to clarify the inner relationships of the clade and reconstruct its diversification as well as its dispersion history.
Evers and Benson (Reference Evers and Benson2019) discussed the marine invasion of turtles and found at least five different times when the marine realm has been invaded if the stem turtle Odontochelys semitestacea Li et al., Reference Li, Wu, Rieppel, Wang and Zhao2008 is included. This stem turtle was found in a mixed environment and has been the subject of debate because both its status as a turtle and its paleoecology are controversial. If O. semitestacea is omitted from consideration, Thalassochelydia and the panpleurodira Platychelyidae become the oldest turtle clades to invade marine realms even though they seem to have never achieved a truly pelagic lifestyle. Among the different turtle clades that invaded the marine realm, the only one to fully achieve this lifestyle was the Chelonioidea, which in our study includes members from Protostegidae. Whether they were pelagic or coastal marine forms, Thalassochelydia represents one of the oldest turtle clades that successfully inhabited and diversified in a marine realm.
Conclusions
Neusticemys neuquina is one of the two Upper Jurassic species of Testudinata recorded for the Neuquén Basin. Uncertainty concerning its phylogenetic relationships have persisted since its discovery in the late 1980s. This was a consequence of the lack of cranial remains and ambiguous features in the known specimens of Neusticemys neuquina. The present study has highlighted cranial morphological traits, enabling the inclusion of this species in modern phylogenetic frameworks. The result is the nesting of Neusticemys neuquina within the thalassochelydians and the reaffirmation of initial hypotheses suggesting close relationships between this species and European taxa. Nonetheless, prevailing uncertainties have not been completely cleared up because thalassochelydian interrelationships are still unresolved, and more taxa need to be included to obtain better-resolved phylogenetic hypotheses.
The position of Neusticemys neuquina inside Thalassochelydia is still insecure. But depending on its resolution, it could support either an early dispersal of thalassochelydian turtles during the Late Jurassic or a singular dispersal event once the group was established in Europe. At the moment, the evidence is inconclusive because neither dispersal hypothesis has more support than the other.
Acknowledgments
We thank A. Garrido (MOZ, Zapala) who let us work on the specimen of Neusticemys neuquina described in this paper and also provided us with valuable geological information about the Cerro Lotena area. We thank J. González for making the drawings included in Figures 2–5. We are also grateful to C. Sancho (IANIGLA-Mendoza) who prepared the material, F. Barrios (MOZ, Zapala) for his valuable help in handling the study specimen at Moguillansky clinic in Neuquén city, and L. Marcial Garat for his help inside that facility. We would like to give our thanks to the image department team at the Moguillansky clinic, particularly the technicians in tomography E. González, A. Antiao, and E. Guareschi, the coordinator of the department G. Cuevas, and the director G. Bianchi. We are grateful to E. Vlachos for his help and guidance in figure preparation and edition. We extend our gratitude toward M.V. Rivas for English proofing of the original manuscript, and R. MacPhee for his kind offer and assistance to read and English-proof the original text. We would also extend our thanks to editors H.-D. Sues and J. Kastigar, as well as reviewers S. Evers and E. Cadena, for their insightful comments and corrections which further improved the original manuscript. This work was partially supported by PICTs 2013-0095 (to MSdlF) and 2016-1039 (to MSF), and Universidad Nacional de La Plata Programa de Incentivos.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.kb2107q.










