Introduction
Currently, inner ear diseases such as Ménière's disease and delayed endolymphatic hydrops are primarily diagnosed based on medical history, symptoms, physical signs, audiology and vestibular function tests. There is a lack of objective imaging criteria capable of aiding diagnosis.
Increasing numbers of researchers have investigated the use of inner ear magnetic resonance imaging (MRI) after intratympanic gadolinium injection. However, most have focused on the measurement and evaluation of the low-signal area that represents the endolymphatic space in the labyrinth, and have obtained only indirect images of the membranous labyrinth.Reference Zou, Poe, Bjelke and Pyykko1–Reference Fiorino, Pizzini, Beltramello and Barbieri5 Furthermore, the method used has involved complex data quantification and a complicated measurement procedure. Moreover, gadolinium distribution within the ear may vary between different patients or even within the same patient, and gadolinium may not distribute equally across different parts of the labyrinth.
Therefore, we propose a simplified scoring system evaluating the MRI appearance of gadolinium distribution within the perilymphatic space, in order to enable accurate and convenient diagnosis of clinically relevant variation.
Patients and methods
Clinical data
The study analysed a convenience sample of 107 patients who had sought medical help at the otolaryngology department of a provincial Chinese hospital between February and July 2010.
We collected comprehensive clinical data from each patient, including symptoms, physical signs, and hearing and vestibular function test results. The latter tests comprised pure tone audiometry, bithermal caloric tests, and measurement of high stimulus rate auditory brainstem response (ABR) and vestibular-evoked myogenic potential.
A total of 214 ears of the 107 patients were categorised into five groups: no symptoms, Ménière's disease, sudden deafness, delayed endolymphatic hydrops and other disorders. The inclusion criteria for the ‘no symptoms’ group were: (1) absence of subjective symptoms, and of any history of hearing loss, ear congestion or tinnitus; (2) normal results for subjective and objective audiological and vestibular function tests (including pure tone audiometry, acoustic resistance, acoustic emissions, high stimulus rate ABR, bithermal caloric and vestibular-evoked myogenic potential tests); and (3) meeting the above criteria at three-month follow up after MRI examination.
Ménière's disease was diagnosed using the American Academy of Otolaryngology – Head and Neck Surgery Committee on Hearing and Equilibrium guidelines.6
Sudden deafness was confirmed using the 2009 revised S2 guidelines for acute sensorineural hearing loss of the Association of the Scientific Medical Societies of Germany.Reference Klemm, Deutscher and Mösges7
The diagnostic criteria for delayed endolymphatic hydrops were: (1) severe sensorineural deafness or total deafness as the initial presentation; (2) Ménière's disease like vestibular symptoms as the subsequent presentation; (3) no hearing change accompanying vertigo; and (4) exclusion of central nervous system disease.
Patients were included in the ‘other disorders’ group if they failed to meet all of the above four sets of criteria.
The study was approved by the internal review board of the participating hospital, and written, informed consent was obtained from all participants. The study conformed to the specifications of the Helsinki declaration.
Magnetic resonance imaging
Gadolinium diethylenetriaminepenta-acetic acid (Gd-DTPA) dimeglumine injection solution was used as the contrast medium. In all 107 patients, gadolinium hydrate diluted eightfold with saline was injected through the tympanic membrane using a 23 G needle. After injection, patients were kept with their head rotated 45° contralaterally for 30 minutes.
Twenty-four hours later, three-dimensional fluid-attenuated inversion recovery MRI was performed using a 3-Tesla unit. All MRI examinations were performed using a Verio 3.0T 16 channel head machine (Siemens, Erlangen, Germany).
The following images were obtained.
Routine sagittal Turbo SE T2-weighted images were taken via the internal auditory canal (Time of Repetition = 6000 milliseconds, Time of Echo = 96 milliseconds, slice thickness = 3 mm). This view not only helped exclude intracranial and cerebellopontine angle lesions, but also aided positioning for further high-resolution labyrinth scans.
Routine MRI hydrography of the inner ear (3-dimensional-Sampling Perfection with Application-optimized Contrast using different flip angle Evolutions (3D-SPC)) was performed, and served as a reference for labyrinth anatomy (Time of Repetition = 6000 milliseconds, Time of Echo = 132 milliseconds, spatial resolution = 0.5 × 0.5 × 0.5 mm, isotropic acquisition, scan time = 4 minutes 16 seconds).
Isotropic 3-dimensional-Sampling Perfection with Application-optimized Contrast using different flip angle Evolutions (3D-SPC) inversion recovery fluid-attenuated inversion recovery was also performed (Time of Repetition = 6000 milliseconds, Time of Echo = 388 milliseconds, Time of Inversion (TI) = 2100 milliseconds, scan time = 5 minutes 32 seconds, spatial resolution = 0.7 × 0.7 × 0.7 mm).
Image post-processing and scoring
Routine hydrography images were processed with three-dimensional, multiplanar reconstruction and maximum intensity projection, using the Syngo suite, as shown in Figure 1a. A high-signal area suggested the existence of fluid in the small space of the membranous labyrinth, primarily perilymph encased in the perilymphatic space.

Fig. 1 Magnetic resonance imaging (MRI) scans for a 41-year-old man diagnosed with right-sided Ménière's disease. (a) Routine MRI hydrography image, maximum intensity projection, showing symmetrical signal for bilateral labyrinth structures, without abnormality. (b) 3-dimensional-Sampling Perfection with Application-optimized Contrast using different flip angle Evolutions (3D-SPC) inversion recovery fluid-attenuated inversion recovery image, maximum intensity projection reconstruction, showing narrowed right cochlear canal and significantly low signal in the vestibule (arrow = dilated saccule). (c) Thin-section original image from scan (b); arrow indicates dilated saccule. RFP = right-foot-posterior
Figures 1b and 1c show 3-dimensional-Sampling Perfection with Application-optimized Contrast using different flip angle Evolutions (3D-SPC) inversion recovery fluid-attenuated inversion recovery images, in which high-signal areas represent gadolinium in the perilymphatic space.
Using labyrinth hydrography as the anatomical reference, gadolinium distribution in the labyrinth was quantitatively scored by two radiologists in an independent, double-blinded manner. The scoring criteria are shown in Table I. A score of three in the vestibular aspect reflected a low-signal saccule image found medial to the vestibule below the horizontal semicircular canal, accompanied by a normal image of the vestibule above the horizontal semicircular canal, together with a dumbbell-shaped utricle.
Table I Scoring criteria for inner ear 3D-SPC-FLAIR images*

Data represent scores awarded.
* Time of Inversion (TI) = 2100 milliseconds. †On 3D-SPC-FLAIR images. ‡Absence of high-signal contrast medium. **Failure to show high-signal image of entire cochlear canal, or high-signal image of cochlear canal limited to tympanic or vestibular scale, or interrupted high-signal images of semicircular canals, or incomplete high-signal image of vestibule. §All labyrinth structures completely visible. 3D-SPC-FLAIR = 3-dimensional-Sampling Perfection with Application-optimized Contrast using different flip angle Evolutions (3D-SPC) fluid-attenuated inversion recovery magnetic resonance imaging
Statistical analysis
The Statistical Package for the Social Sciences version 17.0 software program (SPSS Inc, Chicago, Illinois, USA) was used to conduct multiple independent-samples non-parametric tests, Bayesian discriminant analysis, multivariate logistic regression and receiver operating characteristic curve analysis. A p value of less than 0.05 was considered statistically significant.
Results
Magnetic resonance imaging
All MRI examinations of the 214 ears were performed within 24 hours of transtympanic injection of 1:8 diluted gadolinium diethylenetriaminepenta-acetic acid.
Of these 214 ears, 206 showed gadolinium within the perilymphatic space after the first injection, and one ear showed the same after the second injection. The remaining seven ears failed to show gadolinium within the labyrinth. The overall success rate was 96.7 per cent.
We analysed the clinical data and radiological scores from a total of 207 ears, excluding the seven ears without a contrast-enhanced image. The results are shown in Table II.
Table II Labyrinth component scores by group
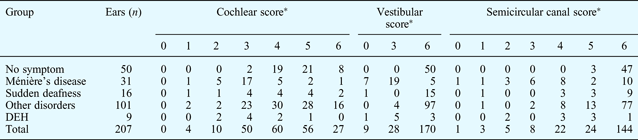
*p = 0.00, between-group differences (non-parametric test). DEH = delayed endolymphatic hydrops
Multiple independent-samples non-parametric tests
We found statistically significant differences for cochlear, vestibular and semicircular canal scores, comparing the five clinical groups.
After rank transformation, Dunnett's t-test was conducted, using the ‘no symptom’ group as the control against which all other groups were compared. The results are shown in Table III.
Table III Dunnett's t-test results*

* ‘No symptom’ group used as control. SCC = semicircular canal; DEH = delayed endolymphatic hydrops
In addition, least significant differences analysis was performed (via rank transformation) to compare the Ménière's disease and delayed endolymphatic hydrops groups in terms of cochlear, vestibular and semicircular canal scores. The p values for these comparisons were as follows: for cochlear scores, 0.880; for vestibular scores, 0.053; and for semicircular canal scores, 0.354. Thus, the p value was greater than 0.05 for all three comparisons, indicating that there were no statistically significant differences between the Ménière's disease and delayed endolymphatic hydrops groups in terms of radiological scores for these three parts of the labyrinth. Therefore, we combined these two clinical groups into one, termed the endolymphatic hydrops group, containing a total of 40 ears.
Bayesian discriminant analysis
The discriminant function was created using the cochlear, vestibular and semicircular canal scores as three variables, and the Y value of each group was calculated. The category with the highest weight was regarded as the discriminant attribute of the group.
In the ‘no symptom’ group, Y = −27.788 + 1.972 × cochlear score + 4.027 × vestibular score + 3.262 × semicircular canal score.
In the endolymphatic hydrops group, Y = −11.454 + 1.275 × cochlear score + 1.461 × vestibular score + 2.855 × semicircular canal score.
In the sudden deafness group, Y = −22.521 + 1.572 × cochlear score + 3.991 × vestibular score + 2.659 × semicircular canal score.
In the ‘other disorder’ group, Y = −25.457 + 1.674 × cochlear score + 4.053 × vestibular score + 3.062 × semicircular canal score.
The accuracy of the models was examined by self- and cross-validation techniques, which both revealed the same results. The accuracy of each model was as follows: no symptom group, 56 per cent; endolymphatic hydrops group, 80 per cent; sudden deafness group, 37.5 per cent; and ‘other disorders’ group, 36.6 per cent. Therefore, the models were more accurate for diagnosing endolymphatic hydrops and less accurate for diagnosing sudden deafness and other disorders.
Logistic regression and receiver operating characteristic curve analysis
We used logistic regression coupled with receiver operating characteristic curve analysis to evaluate our model in terms of the diagnostic power for endolymphatic hydrops. A logistic regression model was established using endolymphatic hydrops confirmed by clinical ‘gold standards’ as the dependent variable (endolymphatic hydrops = 1, no endolymphatic hydrops = 0) and using the cochlear, vestibular and semicircular canal scores as covariates. The model, shown in Table IV, generated a new variable (pre-1) which predicted individual probability: pre-1 = 65.026 − 0.418 × cochlear score − 7.938 × vestibular score − 3.939 × semicircular canal score.
Table IV Logistic regression model*

* Endolymphatic hydrops as dependent variable and labyrinth component scores as independent variables. RC = regression coefficient; SE = standard error; SCC = semicircular canal
A receiver operating characteristic curve was constructed using the new variable, pre-1, as the test variable, as shown in Figure 2a. The area under the curve was 0.989 and the Youden index was 0.96. The cut-off point was 0.3982299, and the value above or equal to it was positive. The sensitivity was 0.975 and the specificity 0.980.

Fig. 2 Receiver operating characteristic curves for (a) the new variable, pre-1, in the endolymphatic hydrops group, and (b) the total perilymphatic space score in the endolymphatic hydrops group. See text for statistical parameters.
The receiver operating characteristic curve for total MRI scores in the endolymphatic hydrops group was obtained, as shown in Figure 2b. The area under the curve was 0.975, the Youden index 0.90, the cut-off point 14.5, the specificity 0.96 and the sensitivity 0.935.
Discussion
A recent Japanese study classified endolymphatic hydrops based on vestibular and cochlear MRI images obtained within 24 hours of intratympanic gadolinium injection.Reference Nakashima, Naganawa, Pyykko, Gibson, Sone and Nakata8 However, we believe that this study was limited by the following: clinician subjectivity; the fact that few temporal bone specimens were used to determine the normal area ratio value; the small number of patients receiving post-gadolinium MRI scanning; and failure to compare classification results with subjects’ clinical data. The researchers diagnosed endolymphatic hydrops by examining the ratio of the area of the membranous labyrinth with endolymph to the area of the entire space occupied by fluid. The area ratio was used, instead of the volume ratio, calculated from an unspecified level of the irregularly shaped inner ear. Furthermore, the researchers studied indirect images that contained no contrast medium, while ignoring gadolinium distribution in the perilymphatic space. In inner ear disorders, membranous and bony labyrinth lesions may be present, resulting in an uneven mixture of gadolinium and perilymph.
Thus, we believe that these authors’ imaging classification criteria for endolymphatic hydrops may have been compromised by a lack of solid scientific grounding. In addition, their study suffered from inadequate research subjects, statistical analysis and quantified data.
• Internationally agreed imaging criteria for endolymphatic hydrops diagnosis are needed
• Previous studies have focused on assessing the endolymphatic space
• This study presents a simplified magnetic resonance imaging (MRI) scoring system assessing gadolinium distribution in the perilymphatic space
• The proposed MRI scoring system is only applicable to Ménière's disease and delayed endolymphatic hydrops
A review of the literature shows that there is little other research investigating the use of MRI scoring systems to diagnose endolymphatic hydrops. We believe that the present study addresses this deficiency.
Any candidate scoring system should be examined for its applicability, feasibility and diagnostic criteria.
In our study, non-parametric testing of data from all the clinical diagnosis groups revealed no differences between the Ménière's disease and delayed endolymphatic hydrops groups in terms of radiological scores for various parts of the labyrinth; this is in line with current pathological knowledge. Hence, the data from both these groups were combined for further analysis.
Bayesian discriminant analysis revealed that our scoring system was most accurate for diagnosing endolymphatic hydrops (80 per cent accuracy), while it was less accurate for other diseases. Therefore, we conducted logistic regression and receiver operating characteristic curve analysis, focusing on endolymphatic hydrops. A diagnostic function was developed, based on the scores for the different labyrinth components, as follows: pre-1 = 65.026 − 0.418 × cochlear score − 7.938 × vestibular score − 3.939 × semicircular canal score. A diagnosis of endolymphatic hydrops can be made if the pre-1 score reaches 0.3982299. Based on the above formula, a vestibular score of three or lower would cause pre-1 to rise higher than the cut-off point, regardless of the semicircular canal and cochlear scores; therefore, endolymphatic hydrops could be diagnosed.
Another diagnostic method comprised receiver operating characteristic curve analysis of the total MRI scores. When the direct sum of all scores from different aspects fell below 14.5, a diagnosis of endolymphatic hydrops could be established.
For both methods, the area under the receiver operating characteristic curve was above 0.9, suggesting high diagnostic accuracy. While the former method determines endolymphatic hydrops by taking into account the varying scores of different labyrinthine components, it is disadvantaged by the complex formula and difficult calculation. By contrast, the latter method adds all labyrinthine component scores together, and hence is easier to perform. However, it demonstrates lower sensitivity and specificity compared with the former method.
Thus, this study has developed a new radiological scoring system for evaluating gadolinium distribution within the perilymphatic space, based on a large number of samples. In addition, our statistical analysis compared endolymphatic hydrops diagnosis based on our MRI scoring system with that based on a clinical gold standard method. The applicability and diagnostic power of our MRI scoring criteria were assessed with reference to components of the inner ear. We developed two sets of diagnostic criteria, one based on the radiological scores of different labyrinthine components, and the other based on the total radiological score of these various components. These methods can be used to diagnose endolymphatic hydrops conveniently and effectively, and in addition could also serve as quantitative indices for clinical disease staging and evaluation of treatment effectiveness.
Our MRI scoring system had a high level of accuracy for diagnosing Ménière's disease and delayed endolymphatic hydrops, but was less accurate for diagnosing sudden deafness and other disorders. Further studies are needed to confirm our results and to explore the application of our endolymphatic hydrops MRI scoring system in cases of sudden deafness and other diseases, in order to expand our system's applications.
Conclusion
We report a convenient and effective method with which to diagnose endolymphatic hydrops, using a system to score the MRI appearance of the perilymphatic space. This system has a high level of diagnostic accuracy for Ménière's disease and delayed endolymphatic hydrops, but not for sudden deafness and other disorders. Although all our patients with sudden deafness and other disorders also suffered from vertigo or hearing disorders, their clinical histories and symptoms failed to support the diagnosis of Ménière's disease or delayed endolymphatic hydrops. Therefore, the MRI scoring system for diagnosing endolymphatic hydrops proposed in this study is only applicable to Ménière's disease and delayed endolymphatic hydrops.
Acknowledgements
We sincerely thank He Bao Chang, Lecturer of the Department of Epidemiology and Health Statistics, School of Public Health, Fujian Medical University, for his great assistance in data analysis.








