Introduction
The genus Macrolaimus Maupas, 1900 represents nine valid species and also includes four species inquirendae (see Abolafia & Peña-Santiago, Reference Abolafia and Peña-Santiago2014). Specimens of these species are characterized by the cheilostome–gymnostome proportions, postvulval uterine sac length of females, morphology of male and female tail tips, and the morphology of the spicules and the gubernaculum. Nonetheless, some of these species have been poorly described, since the morphological description of some diagnostic characters, which could facilitate their correct identification, are not detailed enough.
In Europe, four species have been described, namely M. canadensis Sanwal, 1960, M. crucis Maupas, 1900, M. ruehmi Andrássy, 1966 and M. somniorum Andrássy, 1984. The latter species had, however, not been illustrated by Andrássy (Reference Andrássy1984). Hence, in the present study, two of these species, M. canadensis and M. ruehmi, are redescribed according to specimens obtained from areas where natural vegetation was growing in the Czech Republic, Corsica (France) and Bosnia and Herzegovina. In addition, new data for M. crucis, identified from natural vegetation in the southern Iberian Peninsula of Spain, are provided.
Materials and methods
Sampling and nematode extraction
Specimens of M. canadensis and M. ruehmi were extracted from bark samples that were obtained from several areas of natural vegetation in central Europe. Additionally, new specimens of M. crucis were extracted from coastal sandy soil that was obtained in the southern area of the Iberian Peninsula.
Nematodes obtained from all locations were extracted from either bark or soil samples using a modified Baermann funnel technique (Baermann, Reference Baermann1917) and fixed in a 4% formalin solution. Specimens obtained from the Czech Republic, Corsica and Bosnia and Herzegovina were transferred to pure glycerine according to the protocol of the De Grisse (Reference De Grisse1969), and those obtained from Spain using the method of Siddiqi (Reference Siddiqi1964). Specimens, representative of each species, were then permanently mounted on glass microscope slides to enable species identification using light microscopy (LM), while others were prepared for scanning electron microscopy (SEM).
Light microscopy (LM)
Measurements of various structures and organs of all mounted specimens were recorded using a Leitz Laborlux S microscope (Leitz, Wetzlar, Germany), after which the De Man's indices (De Man, Reference De Man1880) were calculated for each specimen. Drawings of the bodies and body structures of individual specimens were made using a drawing tube (camera lucida) attached to a Leitz Laborlux S microscope. Pictures of the bodies and structures of studied specimens were taken with a Nikon Eclipse 80i light microscope (Nikon, Tokio, Japan) furnished with differential interference contrast optics (DIC) and a Nikon Digital Sight DS-U1 camera. Micrographs were edited using Adobe® Photoshop® CS.
Scanning electron microscopy (SEM)
Specimens of each species, preserved in glycerine, were also selected for observation using SEM according to the protocol of Abolafia (Reference Abolafia2015). Nematodes were hydrated in distilled water, dehydrated in a graded ethanol–acetone series, critical-point dried, coated with gold and observed with a Zeiss Merlin microscope (5 kV) (Zeiss, Oberkochen, Germany).
The terminology used for the morphology of stoma and spicules follows the proposals by De Ley et al. (Reference De Ley, van de Velde, Mounport, Baujard and Coomans1995) and Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2017), respectively.
Results
Macrolaimus canadensis Sanwal, 1960
Material examined
Twenty-seven females and 17 males, originating from the Czech Republic.
Description
Measurements
See table 1.
Table 1. Morphometrics of M. canadensis Sanwal, 1960. Measurements in micrometres (μm) and in the form: mean ± standard deviation (range) where appropriate.

References: 1, present paper; 2, Rahm (Reference Rahm1928, Reference Rahm1929); 3, Fuchs (Reference Fuchs1938); 4, Sanwal (Reference Sanwal1960); 5, Laumond & Carle (Reference Laumond and Carle1971); 6, Massey (Reference Massey1974).
a = body length/body diameter; b = body length/pharynx length; c = body length/tail length; c′ = tail length/anal body diameter; V = (distance from anterior region to vulva/body length) × 100.
*Measurement taken from drawings. ** Measurement taken from other measurements. – Character absent. ? Unknown measurement.
Adults
See figs 1–3. Moderately slender nematodes of small size, 1.03–1.48 mm long. Body cylindrical, tapering towards both extremities, more towards the posterior end. Habitus curved ventrad after fixation, to form an open ‘C’ shape. Cuticle c. 1 μm thick, with very fine annuli, c. 1 μm wide, frequently appearing divided somewhere in its outline, hardly discernible with LM but readily visible with SEM. Lateral field covers 10–11% of body diameter, consisting of two wings (three longitudinal incisures) with transverse incomplete striations, starting at level of anterior part of corpus and ending at tail tip. Lip region convex, continuous with the adjacent body, with the oral region strongly elevated or protruding; lips fused, developing six curved, triangular liplets covering the oral aperture; anterior sensillae arranged in two circles, with six conoid labial setae c. two annuli long and projecting forwards and located at the margin of the oral field, and four (two subdorsal and two subventral) very small cephalic papillae situated beside the labial setae. Amphidial aperture small, oval, located at base of lateral lips. Stoma 1.3–2.2 times longer than wide and 0.6–1.0 times as long as lip-region diameter, heavily sclerotized, subdivided into cheilo-, gymno- and stegostom; cheilostom c. 50% of the stoma length, having cheilorhabdia strongly sclerotized, convergent (curved) and thinner anteriorly; gymnostom very short, c. one-third of the cheilostom length, with arched gymnorhabdia; buccal cavity (=cheilo-gymnostom) 1.4 times longer than wide; stegostom one-half of the cheilostom length and slightly longer than the gymnostom, with funnel-shaped lumen, enveloped by the anterior end of pharynx, lacking visible rhabdia; dorsal pharyngeal gland opening at base of stegostom. Pharynx cephaloboid; pharyngeal corpus cylindrical, 1.7–3.0 times longer than isthmus, with procorpus and metacorpus similar in width; isthmus narrower than corpus and clearly delimited from it; basal bulb nearly pyriform with strongly developed valvular apparatus located at its posterior half; pharyngeal gland nuclei obscure. Cardia short and subcylindrical. Nerve ring at 57–78% of neck length, encircling pharynx at level of anterior half of isthmus. Secretory–excretory gland cell located ventrally to isthmus; excretory pore located posterior to nerve ring, at 67–85% of neck length, at level of isthmus. Deirid located posterior to nerve ring, inside the lateral field, at 78% of neck length, five or six annuli posterior to excretory pore, not clearly visible with LM.

Fig. 1. Macrolaimus canadensis Sanwal, 1960: (A) neck; (B) female genital system; (C) stoma; (D) male posterior end; (E) entire male; (F) entire female; (G) vaginal region; (H) female posterior end.

Fig. 2. Macrolaimus canadensis Sanwal, 1960 (light microscopy): (A) stoma; (B) neck (arrow pointing the excretory pore); (C) female reproductive system; (D) female posterior end (arrow pointing to the phasmid); (E) lateral field; (F) vaginal region; (G) male posterior end (arrows pointing to the genital papillae); (H) spicules and gubernaculum.
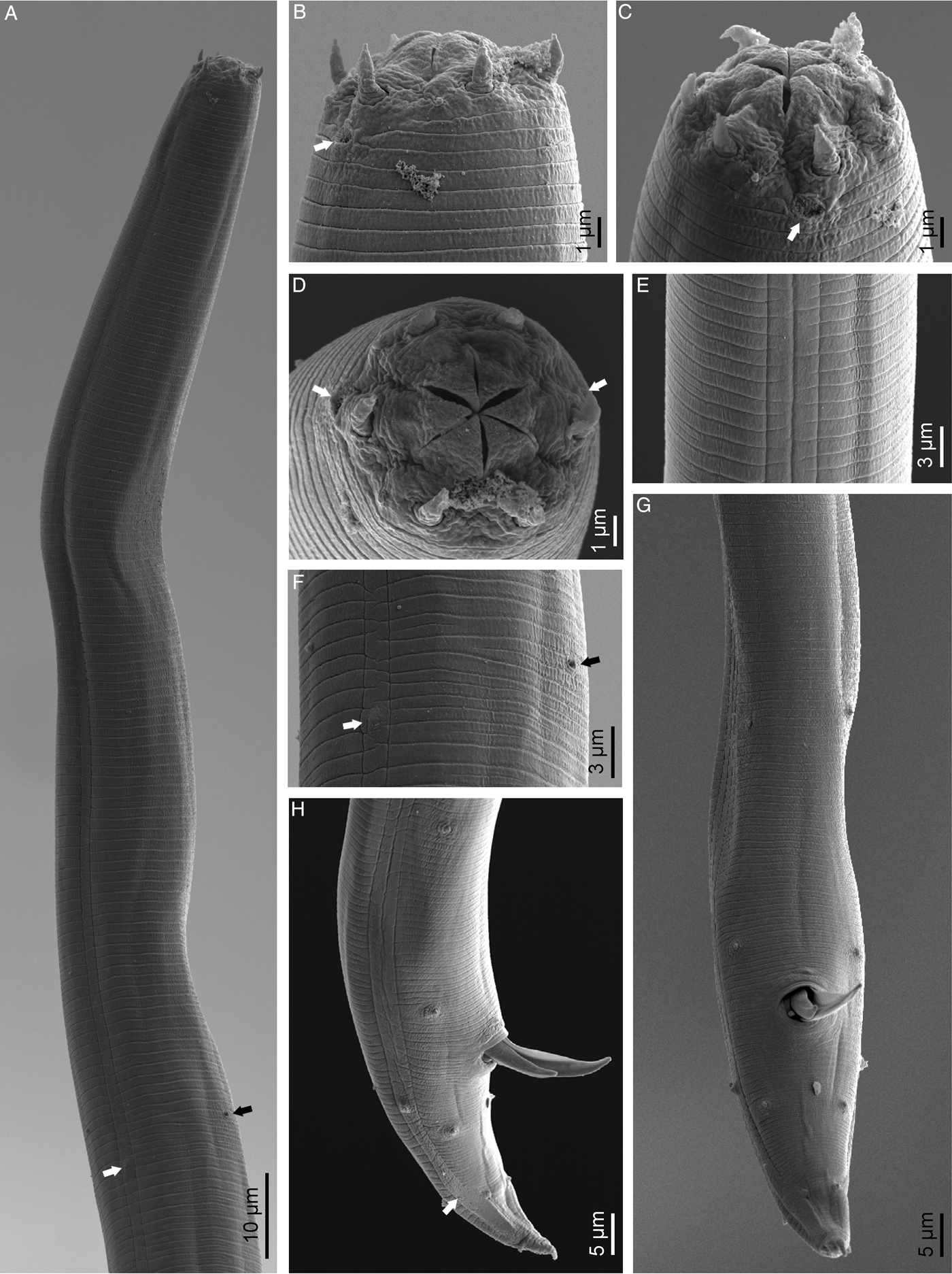
Fig. 3. Macrolaimus canadensis Sanwal, 1960 (scanning electron microscopy): (A) anterior end (black arrow pointing to the excretory pore, white arrow pointing to the right deirid); (B, C, D) lip region in subventral, lateral and frontal views, respectively (arrows pointing to the amphids); (E) lateral field; (F) deirid (white arrow) and excretory pore (black arrow); (G, H) male posterior end in ventral and lateral views, respectively (arrow pointing to the phasmid).
Female
Reproductive system monodelphic–prodelphic. Ovary reflexed near to oviduct junction, very long, surpassing the level of the vulva and reaching the posterior part of intestine; oocytes first situated in two or more rows, then in only one row. Oviduct narrow and short; no distinct narrowing is observed at ovary-oviduct junction. Uterus located on right-hand side of intestine, with two sections separated by a weak internal narrowing: the distal part is long, 5–6 times longer than the body width, tubular, with thick walls, whereas the proximal part (ovejector) is short and swollen and with thin walls. Uterine eggs were absent in the specimens examined. Postvulval uterine sac present, 1.1–1.8 times longer than the body width at its level. Vagina occupying one-third (c. 33–40%) of body diameter, perceptibly bent forward. Vulva a transverse slit, with distinctly protruded lips, especially the anterior one that forms a vulval cone; advulval cuticle lacking differentiations. Rectum 1.3–2.2 times longer than anal body diameter, its anterior part swollen with walls including gland-like cells; anal lips not prominent. Tail conical, slightly ventrad curved, tapering very gradually and ending in an acute dorsad-bent mucro. Phasmids located at posterior half of tail, at 57–69% of tail length from anus.
Male
Reproductive system monorchic, with testis reflexed ventrad, on right-hand side of intestine. Spicules paired and symmetrical, 6.8–10.7 times longer than wide, curved ventrad with rounded manubrium, conoid calamus, ventrad curved lamina with well-developed proximal dorsal hump and thin velum at ventral side. Gubernaculum deltoid, weakly curved ventrad and acute distally. Gland-like cells are not distinguishable around the rectum. Genital papillae consist of two subventral precloacal pairs and five postcloacal pairs arranged as follows: one lateral pair located at lateral field and slightly posterior to the level of cloacal aperture; two subventral pairs, one of them bigger at the anterior third and another smaller at the posterior third to tail length; and two subdorsal pairs at the posterior half of tail, the anterior one bigger; a midventral papilla is located in front of the anterior lip of cloacal aperture. Tail conoid and tapering gradually and curved ventrad, ending in a short dorsad-bent mucro. Phasmids located at posterior half of tail, at 53–61% from anus.
Other material examined
Macrolaimus canadensis specimens were also found in Corsica (France), being similar to those examined from the Czech Republic. The Corsican specimens could only be differentiated from those of the Czech Republic by the slightly shorter length of their spicules (25 μm vs. 27–32 μm long).
Localities and habitats
Specimens of the species were collected at 16 localities (Babí u Náchoda, Bzenec Přívoz, Čechy pod Kosířem, Černá Říčka, Černá za Bory, Dívčí Hrad, Hodonín, Jevíčko, Město Albrechtice, Orlovice, Proskovice (Ostrava), Ptení, Rovensko, Salajna, Skřípov and Soběšice (Brno)) in the Czech Republic and one locality (Incudine Mount) in Corsica. The species was associated with the bark of Larix decidua Mill., Pinus nigra var. corsicana (Loudon) Hyl., P. sylvestris L. and Pinus sp.
Remarks
This species was previously found in Brazil by Rahm (Reference Rahm1928, Reference Rahm1929), Germany by Fuchs (Reference Fuchs1938), Canada by Sanwal (Reference Sanwal1960), France by Laumond & Carle (Reference Laumond and Carle1971) and the USA by Massey (Reference Massey1974). The specimens examined during this study, originating from the Czech Republic and Corsica, are similar to those from Brazil, Germany, Canada, France and the USA. However, in general, specimens originating from the Czech Republic showed greater ranges of measurement values for the various characteristics (see table 1). This is the first record of the genus and species from the Czech Republic.
Macrolaimus ruehmi Andrássy, 1966
Material examined
Twenty-four females and 19 males, originating from the Czech Republic.
Description
Measurements
See table 2.
Table 2. Morphometrics of M. ruehmi Andrássy, 1966. Measurements in micrometres (μm) and in the form: mean ± standard deviation (range) where appropriate.

References: 1, present paper; 2, Rühm (Reference Rühm1956); 3, Meyl (Reference Meyl1960).
a, b, c, c′, V, see table 1.
*Measurement taken from drawings. **Measurement taken from other measurements. – Character absent. ? Unknown measurement.
Adults
See figs 4–6. Moderately slender nematodes of small size, 0.90–1.59 mm long. Body cylindrical, tapering towards both extremities, more towards the posterior end. Habitus curved ventrad after fixation, to an open ‘C’ shape or sigmoid. Cuticle c. 1 μm thick, with very fine annuli, c. 1 μm wide, sometimes appearing divided somewhere in its outline, hardly discernible with LM, but readily visible with SEM. Lateral field covers 9–15% of body diameter, consisting of two wings (three longitudinal incisures) with transverse incomplete striations, starting at level of anterior part of corpus and ending at tail tip. Lip region convex, continuous with the adjacent body, with the oral region strongly elevated or protruding; lips fused, developing six curved, triangular liplets covering the oral aperture; anterior sensillae arranged in two circles, with six conoid labial setae c. as long as two annuli wide and projecting forward, and located at the margin of the oral field, and four (two subdorsal and two subventral) very small cephalic papillae situated beside the labial setae. Amphidial aperture small, oval, located at base of lateral lips. Stoma 1.7–2.5 times longer than wide and 0.7–1.1 times as long as lip region diameter, heavily sclerotized, subdivided into cheilo-, gymno- and stegostom; cheilostom c. 50% of the stoma length, having cheilorhabdia strongly sclerotized, convergent (curved) and thinner anteriorly; gymnostom long, slightly shorter than the cheilostom, with slightly arched gymnorhabdia having a constriction outside at their middle length; buccal cavity (= cheilo-gymnostom) 1.8 times longer than wide; stegostom one-half of the cheilostom length, with funnel-shaped lumen, enveloped by the anterior end of pharynx, lacking visible rhabdia; dorsal pharyngeal gland opening at base of stegostom. Pharynx cephaloboid: pharyngeal corpus cylindrical, 1.6–2.3 times longer than isthmus, with procorpus and metacorpus similar in width; isthmus narrower and clearly delimited from corpus; basal bulb nearly pyriform, with strongly developed valvular apparatus located at its posterior half; pharyngeal gland nuclei obscure. Cardia short and conoid. Nerve ring at 61–75% of neck length, encircling pharynx at level of anterior half of isthmus. Secretory–excretory gland cell located ventrally to isthmus and basal bulb; excretory pore located posterior to nerve ring, at 66–86% of neck length, at level of isthmus. Hemizonid at level of excretory pore. Deirid located posterior to nerve ring, inside the lateral field, at 71% of neck length, six or seven annuli posterior to excretory pore, inconspicuous under light microscope.

Fig. 4. Macrolaimus ruehmi Andrássy, 1966: (A) stoma; (B) female genital system; (C, D) spicule; (E, F) gubernaculum; (G) entire male; (H) entire female; (I) neck; (J) male posterior end; (K, L) female posterior end.

Fig. 5. Macrolaimus ruehmi Andrássy, 1966 (light microscopy): (A) neck (arrow pointing to the excretory pore); (B) stoma; (C) female genital system; (D) male posterior end (white arrows pointing to the genital papillae, black arrow pointing to the phasmid); (E) lateral field; (F) testis; (G) female posterior end (arrow pointing to the phasmid).

Fig. 6. Macrolaimus ruehmi Andrássy, 1966 (scanning electron microscopy): (A) anterior end (black arrow pointing to the excretory pore, white arrow pointing to the right deirid); (B–E) lip region in ventral, subdorsal, lateral and frontal views, respectively (arrows pointing to the amphids); (F) female posterior end (arrow pointing to the phasmid); (G, H) male posterior end in ventral and lateral views, respectively (arrows pointing to the phasmid); (I) deirid (white arrow) and excretory pore (black arrow); (J) lateral field.
Female
Reproductive system monodelphic–prodelphic. Ovary reflexed near to oviduct junction, very long, surpassing the level of the vulva; oocytes first situated in two rows, then in only one row. Oviduct narrow and short; no distinct narrowing is observed at ovary–oviduct junction. Uterus located on right-hand side of intestine, with two sections separated by a weak internal narrowing: the distal part is long, four times longer than the body width, tubular, whereas the proximal part (ovejector) is short and with thin walls. Uterine eggs were absent in the specimens examined. Postvulval uterine sac present, 1.0–1.8 times longer than the body width at its level. Vagina occupying a quarter (29–40%) of body diameter, slightly bent forward. Vulva a transverse slit, with distinctly protruded lips that form a vulval cone; advulval cuticle lacking differentiations. Rectum 1.3–2.2 times longer than anal body diameter, its anterior part with walls including gland-like cells; anal lips not prominent. Tail conical, almost straight but ventrad curved at posterior part, tapering very gradually and ending in a short wart-like mucro. Phasmids posterior, at 53–65% of tail length from anus.
Male
Reproductive system monorchic, with testis reflexed dorsad, on right-hand side of intestine. Spicules paired and symmetrical, 5.0–7.8 times longer than wide, curved ventrad with rounded manubrium, conoid calamus, ventrad curved lamina with well-developed proximal dorsal hump and small velum at ventral side. Gubernaculum deltoid, almost straight and acute distally. Gland-like cells are poorly distinguishable around the rectum. Genital papillae consist of two subventral, precloacal pairs and five postcloacal pairs arranged as follows: one lateral pair located at lateral field and slightly posterior to the level of cloacal aperture; two subventral pairs, one of them bigger at the anterior third and another smaller at the posterior third to tail length; and two subdorsal pairs at the posterior half of tail, the anterior one bigger. A very reduced midventral papilla is located in front of the anterior lip of cloacal aperture. Tail conoid and tapering gradually and curved ventrad, ending in a short conoid mucro. Phasmids located at posterior half of tail, at 56–76% from anus.
Other material examined
The material, representing two females that were obtained from Bosnia and Herzegovina, was similar to the specimens from the Czech Republic. However, these specimens had slightly bigger stoma (1.7–2.5 vs. 2.6–3.0 times longer than wide) than those from the Czech Republic.
Localities and habitats
The species was collected at six localities (Benkov u Střelic, Horka nad Moravou, Hrušky, Ladín, Olomouc and Znojmo) in the Czech Republic and one locality (Banja Luka) in Bosnia and Herzegovina. The specimens were associated with the bark of Betula pendula L., Fraxinus sp., Prunus armeniaca L., Pinus nigra L., P. silvestris L., Quercus sp. and Tilia sp.
Remarks
Specimens of M. ruehmi from the Czech Republic are similar to M. crucis specimens of German populations previously studied by Rühm (Reference Rühm1956) and Meyl (Reference Meyl1960), which have been renamed as M. ruehmi by Andrássy (Reference Andrássy1966). This is the first record of the species from the Czech Republic and Bosnia and Herzegovina.
Macrolaimus crucis Maupas, 1900
Material examined
Eight females and seven males, originating from Spain.
Description
Measurements
See table 3.
Table 3. Morphometrics of M. crucis Maupas, 1900. Measurements in micrometres (μm) and in the form: mean ± standard deviation (range) where appropriate.

References: 1, present paper; 2, Maupas (Reference Maupas1900); 3, Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2014).
1 SEM observations in the present study indicate that this measurement is a mistake.
a, b, c, c′, V, see table 1.
*Measurement taken from drawings. **Measurement taken from other measurements. – Character absent. ? Unknown measurement.
Diagnostic description (additional data from Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2014))
The Spanish specimens of M. crucis (fig. 7) examined during this study are characterized by the body length (0.72-0.86 mm in females and 0.72–0.99 mm in males); lip region 11–15 μm wide and bearing six short, seta-like papillae; stoma 6–11 × 5–9 μm with gymnostom slightly shorter than cheilostom; neck 146–167 μm long; pharyngeal corpus 1.7–2.4 times isthmus length; excretory pore located at isthmus level; female reproductive system monodelphic–prodelphic; postvulval uterine sac absent; female tail conical (50–58 μm, c = 14.3–17.1, c’ = 2.9–3.6) with acute tip bent dorsad; phasmids located at 63–76% of tail length from anus; male tail conical and curved ventrad (47–60 μm, c = 15.1–19.7, c’ = 2.5–3.0) with phasmids located at 57–64% of tail length; spicules 27–34 μm long and gubernaculum 11–13 μm long with slightly fusiform corpus.

Fig. 7. Macrolaimus crucis Maupas, 1900 (male, scanning electron microscopy): (A–C) lip region in subventral, lateral and frontal views, respectively; (D) anterior end (black arrow pointing to the excretory pore, white arrow pointing to the right deirid); (E–G) lateral field at anterior end, deirid level and midbody level, respectively (black arrow pointing to the excretory pore, white arrow pointing to the right deirid); (H) cloacal opening region; (I, J) male posterior end in ventral and lateral views, respectively (arrows pointing to the phasmid).
Localities and habitats
Macrolaimus crucis has been collected from four littoral localities in the southern Iberian Peninsula: 1) sandy soil, close to the beach near Salinas de Cabo de Gata (Almería Province) where Lycium intricatum Boiss., Lygeum spartum (L.) Kunth, Ononis talaverae Devesa & G. López and Thymelaea hirsuta (L.) Endl. grew; (2) dry soil from a rocky area close to the beach near Salobreña (Granada Province) where L. intricatum grew; (3) sandy soil from coastal dunes near Águilas (Murcia Province) and (4) near Cartagena (Murcia Province) where Ammophila arenaria (L.) Link. grew.
Remarks
The specimens examined are similar to those studied by Maupas (Reference Maupas1900) and Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2014). Since the latter authors provided a detailed description of the species from the southern Iberian Peninsula, there is no need to repeat the description here. The SEM study revealed that the phasmid position on the male tail is posterior, not anterior, as was reported previously by the latter Spanish authors, using LM. As a result of the present study, the distribution area of this species in the southern Iberian Peninsula of Spain has been expanded.
Discussion
The study of these three species provides new and useful information on the morphology of the genus. Regarding the lip region, the morphology of these species agrees well with the results of the studies conducted for M. arboreus Truskova & Eroshenko, 1977 by Shokoohi et al. (Reference Shokoohi, Panahi, Fourie and Abolafia2018), M. crucis by Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2014) and M. richteri Swart & Heyns, 1992 (Swart & Heyns, Reference Swart and Heyns1992). However, some structures of the three species examined during this study are slightly different. For example, the morphology of the postvulval sac is slightly longer than the body diameter of M. canadensis and M. ruehmi vs. very reduced for M. crucis; the number of precloacal papillae, of which two pairs were discernible for M. canadensis and M. ruehmi vs. three pairs for M. crucis; spicules with a dorsal hump being present in M. canadensis and M. ruehmi males; and the gubernacula being deltoid in males of M. canadensis and M. ruehmi vs. slightly fusiform for M. crucis males. Such data confirm that more similarities occur among specimens of M. canadensis and M. ruehmi in terms of their morphology.
Regarding the number of precloacal papillae, specimens of all species of the genus Macrolaimus have four pairs, except those of M. natator Timm, 1960. Specimens of the latter species have three pairs of precloacal papillae (Timm, Reference Timm1960), which coincides with the description of M. crucis that originated from the southern Iberian Peninsula of Spain, as noted in the present study. The number of genital papillae is reduced in some lineages (Osche, Reference Osche1958; Chabaud & Petter, Reference Chabaud and Petter1961). Within the family Chambersiellidae, specimens of the genus Diastolaimus Rahm, 1928, which is the closest to Macrolaimus (Nadler et al., Reference Nadler, De Ley and Mundo-Ocampo2006; Boström et al., Reference Boström, Holovachov and Nadler2011), have different numbers of precloacal papillae: three pairs are present in D. croca (Massey, 1963) Andrássy, 1984 and D. damalis (Massey, 1966) Andrássy, 1984 and four pairs in D. mexicanus Cid del Prado, 2012 (see Massey, Reference Massey1963, Reference Massey1966 and Cid del Prado, Reference Cid del Prado2012, respectively). Furthermore, the only species of the chambersiellid genus Geraldius Sanwal, 1971, G. bakeri (Sanwal, 1957) Sanwal, 1971, has seven pairs of precloacal papillae (see Sanwal, Reference Sanwal1957, Reference Sanwal1971; Holovachov et al., Reference Holovachov, Esquivel and Bongers2003). In addition, the genus Steinernema Travassos, 1927, described by Travassos (Reference Travassos1927a) and renamed later by the same author (Travassos, Reference Travassos1927b) and probable ancestor of Chambersiellidae (Bert et al., Reference Bert, Leliaert, Vierstraete, Vanfleteren and Borgonie2008), exhibits 7–10 pairs of precloacal genital papillae. Thus, according to this, the reduction of the number of genital papillae in Macrolaimus species could be ascribed to an apomorphic condition.
On the other hand, the number of female genital branches becomes reduced, from two (didelphic) to one (monodelphic) in nematode species (Chitwood & Chitwood, Reference Chitwood and Chitwood1950; Lorenzen, Reference Lorenzen1978, Reference Lorenzen1981). In Chambersiellidae, females of the genera Geraldius and Diastolaimus are didelphic (plesiomorphic condition), while those of Macrolaimus are monodelphic (apomorphic condition). Females of the genus Steinernema also have didelphic genital branches.
According to the above-mentioned criteria for the genus Macrolaimus, M. crucis could be an ancestral species while M. canadensis and M. ruehmi could be derived species. Unfortunately, molecular analyses for these species could not be performed to support this interpretation.
Acknowledgements
The authors thank Martin Dančák from the Palacký University Olomouc for identification of algae and lichens from bark samples. Branimir Njezic from the University of Banja Luka is acknowledged for providing bark samples from Bosnia and Herzegovina. SEM pictures were obtained with the assistance of technical staff and equipment of the Centro de Instrumentación Científico-Técnica (CICT), University of Jaén. The English text has been revised by Professor Hendrika Fourie (North-West University, Unit for Environmental Sciences and Management, Potchefstroom, South Africa).
Financial support
The present research was supported financially by the European Social Fund and the state budget of the Czech Republic for the project entitled ‘Indicators of tree vitality (Reg. No. CZ.1.07/2.3.00/20.0265)’, the University of Jaén/Caja Rural Jaén Foundation for the project entitled ‘Filogeografía de nematodos rabdítidos (Nematoda, Rhabditida) en ambientes xerofíticos del sur de la península ibérica’ (UJA2014/03/01), and the research activity ‘EI_RNM2_2017’ of the University of Jaén.
Conflict of interest
None.












