Introduction
Schistosomiasis is a challenging disease worldwide, with at least 92% of the 207 million estimated cases occurring in the African endemic zones (WHO, 2017). Infection with the neglected human schistosome, Schistosoma haematobium (Rinaldi et al., Reference Rinaldi, Okatcha and Popratiloff2011), causes urogenital schistosomiasis, a chronic morbid disease that starts during early life (King, Reference King2010; Shiff et al., Reference Shiff, Naples, Isharwal, Bosompem and Veltri2010; Terer et al., Reference Terer, Bustinduy, Magtanong, Muhoho, Mungai, Muchiri, Kitron, King and Mutuku2013). Control of schistosomiasis is mainly by preventive chemotherapy, whereby school-aged children and other high-risk groups are given praziquantel (PZQ) without previous diagnosis (Utzinger et al., Reference Utzinger, N'Goran, Caffrey and Keiser2011). The extensive use of PZQ in the absence of other alternatives needs careful monitoring to identify early signs of therapeutic failure (Doenhoff et al., Reference Doenhoff, Hagan and Cioli2009). Accordingly, the World Health Organization (WHO) has realized the exigency of detecting new alternatives to PZQ.
Both Plasmodium and schistosome parasites degrade the ingested haemoglobin of the host into amino acids and the potentially toxic haem that is subsequently discarded as haemozoin (Oliveira et al., Reference Oliveira, Kycia and Gomez2005), thus an effective antimalarial drug targeting haem would have a harmful effect against schistosomes (Xiao et al., Reference Xiao, Mei and Jiao2011). Drugs targeting haem are of interest, as free haem does not exist in non-infected individuals (Portela et al., Reference Portela, Boissier, Gourbal, Pradines, Collière, Coslédan, Meunier and Robert2012). Interestingly, the antimalarial drugs artemisinin and its derivatives (artemether, arteether, artesunate, etc.) have shown antischistosomal activities (Utzinger et al., Reference Utzinger, Xiao, Tanner and Keiser2007). Moreover, Xiao et al. (Reference Xiao, Mei and Jiao2011) reported promising in vivo synergistic effects of mefloquine combined with low or moderate effective doses of artemisinins, indicating that artemisinins combined with other antimalarial drugs may have potential application against schistosomiasis.
The WHO has recommended artemisinin-based combination therapies (ACTs, artemisinins + other antimalarial drugs) as the most effective treatment for malaria (WHO, 2016). Furthermore, previous studies have revealed their potent antischistosomal activity (Boulanger et al., Reference Boulanger, Dieng and Cisse2007; Mohamed et al., Reference Mohamed, Mahgoub, Magzoub, Gasim, Eldein, Ahmed and Adam2009; Keiser et al., Reference Keiser, N'Guessan, Adoubryn, Silue, Vounatsou, Hatz, Utzinger and N'Goran2010). The compound naphthoquine phosphate tablet (CO-ArNp), a single oral dose ACT, is composed of artemisinin and naphthoquine phosphate (tetra-aminoquinoline) (Benjamin et al., Reference Benjamin, Moore and Lee2012). The notable in vivo and in vitro effects of the combined artemisinin–naphthoquine phosphate therapy against Schistosoma mansoni have been reported (El-Beshbishi et al., Reference El-Beshbishi, Taman, El-Malky, Azab, El-Hawary and El-Tantawy2013, Reference El-Beshbishi, El Bardicy, Tadros, Ayoub and Taman2015). These results prompted us to examine whether a comparable efficacy can be detected against S. haematobium, in which case the treatment could be used as a broad-spectrum antischistosomal therapy in overlapping schistosomiasis endemic foci.
In this paper, we report for the first time the in vitro activity of artemisinin–naphthoquine phosphate against adult S. haematobium, as well as the morphological and tegumental changes observed by scanning electron microscopy (SEM) as indices of the biological activity of schistosomes (Moraes et al., Reference Moraes, Nascimento, Lopes, Nakano, Yamaguchi, Kato and Kawano2011).
Materials and methods
Compounds and reagents
Compound naphthoquine phosphate tablets (artemisinin : naphthoquine phosphate at a ratio of 3 : 1) were a gift from Xiamen WinWord Industrial Co., Ltd (Xiamen, China). Praziquantel was obtained from Sigma-Aldrich (USA). All cell culture grade reagents were obtained from Gibco (Thermo Fisher Scientific, USA) except dimethyl sulfoxide (DMSO), which was obtained from CARLO ERBA Reagents (France). Foetal bovine serum (FBS) was obtained from Lonza Biologics (Switzerland).
Animals and parasites
The life cycle of S. haematobium Egyptian strain was maintained in Bulinus truncatus and male Syrian golden hamsters (Mesocricetus auratus) at the Schistosome Biological Supply Centre, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt. Briefly, 60–80 g golden hamsters were infected by percutaneous route with 350–400 freshly shed cercariae of S. haematobium. The hamsters were kept in controlled environmental conditions (20–22°C, 12/12 h dark/light cycle), with Rodent Blox and water ad libitum.
After 12 weeks of infection, adult S. haematobium worms were collected from golden hamsters by aseptic perfusion of the portal and mesenteric veins, with sterile saline–sodium citrate (Duvall & DeWitt, Reference Duvall and DeWitt1967). Worms were then transferred to Roswell Park Memorial Institute medium (RPMI-1640) and washed several times. Viable, contractile worms showing intact tegument, as observed by light microscopy, were used in this study.
In vitro artemisinin–naphthoquine phosphate treatment of Schistosoma haematobium
Adult S. haematobium worms were maintained in sterile, flat-bottom Corning Costar 24-well plates (Sigma-Aldrich), using 3–4 pairs of worms per well. The culture medium in each well contained 2 ml RPMI-1640 supplemented with L-glutamine + 20% FBS (heat-inactivated) + 160 μg/ml gentamycin + 300 IU/ml penicillin + 300 μg/ml streptomycin (Ramirez et al., Reference Ramirez, Bickle, Yousif, Mouries and Nwaka2007). Schistosoma haematobium cultures were incubated with CO-ArNp at concentrations of 1–40 μg/ml. Pairs of S. haematobium adults were cultured in medium without and medium with 0.8% DMSO (the highest concentration of solvent used) as negative controls, or exposed to 10 μg/ml PZQ as positive controls. All assays were performed at least twice over two separate studies. Cultures were incubated at 37°C in 5% CO2 atmosphere for 72 h. Worms were observed, under a stereomicroscope every 24 h for changes in general appearance, motor activity and mortality rate.
Treatment was considered lethal when no worm motility was observed during 2 minutes of examination, after agitation of the plate to ensure high parasitic visibility. Accordingly, the mortality rates, LC50 and LC90 of CO-ArNp and PZQ against S. haematobium adult worms were calculated by using the statistical program SPSS v. 20 (IBM, Armonk, USA).
Scanning electron microscopic examination
A total of eight pairs of S. haematobium worms exposed to 20 μg/ml CO-ArNp for 48 h, as well as unexposed worms (negative controls), were sampled and washed twice with PBS, then fixed in 2.5% glutaraldehyde, examined and photographed using SEM (Inspect S, FEI Company, Holland) (Shaw & Erasmus, Reference Shaw and Erasmus1987).
Results and discussion
Effect of artemisinin–naphthoquine phosphate on the viability of Schistosoma haematobium adult worms
Over the 72 h, all S. haematobium worms cultured in RPMI-1640 drug-free medium revealed distinctive wavy as well as peristaltic motion, with maintenance of male–female mating. Worms incubated with PZQ showed immediate muscle contraction, without separation of worms, and all worms were dead 48 h after incubation. Interestingly, incubation of worms with the different concentrations (1–40 μg/ml) of CO-ArNp led to dissociation of S. haematobium pairs and their irreversible debilitation within 24 h; also, all schistosomes displayed only minimal activity, with slow convulsions and abnormal contractions, which were more prominent in female worms that died earlier than males, while male worms appeared shrunken and markedly more coiled than females, and all worms were dead after incubation with 20 and 30 μg/ml for 72 and 48 h, respectively (fig. 1). The LC50 and LC90 of CO-ArNp recorded 72 h post incubation were 11.1 μg/ml and 18.5 μg/ml, while those of PZQ were 0.2 μg/ml and 0.3 μg/ml, respectively, as shown in table 1.

Fig. 1. In vitro effect of artemisinin–naphthoquine phosphate at various concentrations on the mortality of adult Schistosoma heamatobium under culture conditions. The data were generated from at least two independent studies. Note that the bars show the range of percentage of dead worms in the experiments, and the intervals on the x-axis are not equal.
Table 1. In vitro schistosomicidal activity of artemisinin–naphthoquine phosphate combination and a reference drug (praziquantel) on adult Schistosoma haematobium worms after three days’ exposure.

In comparison, treatment of S. mansoni worms with 20 and 40 μg/ml of the same drug combination killed all parasites at the same time points, but males were more afflicted and died earlier than females (El-Beshbishi et al., Reference El-Beshbishi, El Bardicy, Tadros, Ayoub and Taman2015). In an in vitro study performed by Eissa et al. (Reference Eissa, El Bardicy and Tadros2011), the authors declared that miltefosine killed all worms after five days of incubation with 10 μg/ml, and the drug was more active on S. haematobium than on S. mansoni adult worms. Indeed, S. haematobium and S. mansoni, two major human schistosomes, have different sensitivities to particular antischistosomal drugs, which may be attributed to specific intrinsic biochemical processes (Camacho & Agnew, Reference Camacho and Agnew1995), and depend on the enzyme(s) involved (Pica-Mattoccia et al., Reference Pica-Mattoccia, Novi and Cioli1997). Schistosoma haematobium adults exposed to 20 μg/ml artemether for 72 h, showed no apparent changes (Xiao et al., Reference Xiao, Chollet, Utzinger, Matile, Mei and Tanner2001). Our results may be attributed to drug–drug interaction between naphthoquine phosphate and artemisinin exerting a more toxic effect on the worms than using artemisinins alone. Artemisinins interact with haemin and other iron systems, producing toxic compounds and free radicals, which cause oxidative damage of the parasite (Xiao et al., Reference Xiao, Chollet, Utzinger, Matile, Mei and Tanner2001). Additionally, it is well known that 4-aminoquinoline derivatives interfere with haemozoin formation, hence the accumulated free haem kills the parasite through oxidative stress (Corrêa Soares et al., Reference Corrêa Soares, Menezes and Vannier-Santos2009).
Ultrastructure analysis of artemisinin–naphthoquine phosphate-induced surface damage in Schistosoma haematobium adult worms
The characteristics of the tegument of untreated worms (fig. 2a, b) were compared with those exposed to CO-ArNp. Male and female worms incubated with 20 μg/ml CO-ArNp for 48 h showed surface membrane ultrastructural damage (figs 3 and 4). Tegumental changes were more prominent and outspread in male than female schistosomes. Generally, all inspected male worms showed extensive oedema of the tegument, with thinning of tegumental ridges. There were also pronounced changes in the tubercles in the form of reduction in size and number, with shortening and loss of spines leaving a smooth surface (fig. 3a). Erosion of the tegument in several areas of the body, cleft formation with exposure of the subtegumental tissue (fig. 3b), exfoliation of the tegument and roughness of the surface (fig. 3c), as well as extreme distortion of the tegumental folds, with concomitant collapse and deformity of the tubercles, were encountered (fig. 3c, d). Moreover, in vitro incubated worms with CO-ArNp showed longitudinal muscle contraction, and the worms were bent, shortened and deformed, with marked corrugation at the anterior end (fig. 3e). Besides, the gynaecophoric canal revealed contraction deformity at the edges and formation of protuberance in the tegument (fig. 3f). Disfigurement of the ventral sucker was also noticed in the form of marked oedema in > 50% of treated worms (fig. 3g), while marked contraction and deformity, with erosion and vesicle formation, were seen in the remaining males, as well as evident shrinkage and wrinkling of the area between the oral and ventral suckers (fig. 3h). Xiao et al. (Reference Xiao, Shen, Chollet, Utzinger and Tanner2000) declared that such damage to the suckers may predispose schistosomes to loss of power to adhere to the vasculature, hence making uptake of nutrients more difficult. In turn, this may affect the worms’ maturity, fecundity and ability to deposit eggs – the major contributory factor to schistosomiasis morbidity.

Fig. 2. Normal surface ultrastructure of Schistosoma haematobium adult worms (controls). (a) Normal dorsal tegument of a male worm (×5000); S: spines, t: tubercles, w: wrinkles. (b) Normal tegument of a female worm (×3000); fi: fissures.
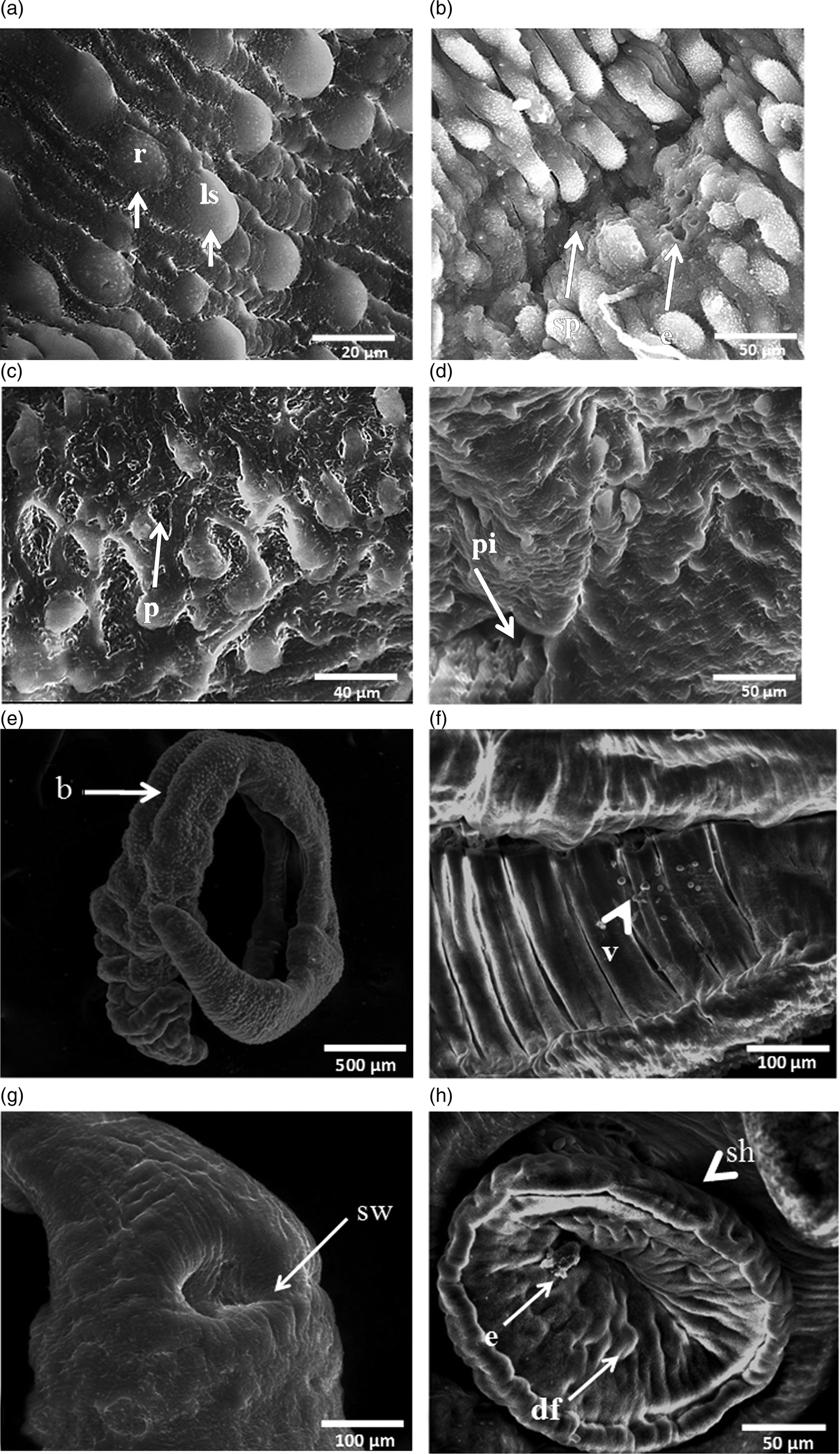
Fig. 3. SEM micrographs of adult Schistosoma haematobium male worms incubated with 20 μg/ml CO-ArNp for 48 h. (a) Tegumentary changes in a male worm, including reduction in size and number of tubercles, and loss of spines (×5000); r: tubercles reduced in size, ls: tubercles showing loss of spines. (b) Tegumental erosion and splitting (×2400); sp: splitting, e: erosion. (c) Tegumental peeling off, with deformity of the tubercles (×3000); p: peeling. (d) Extensive oedema of the tegument, with deep pitting, as well as marked atrophy of the tubercles (×1600); pi: pitting. (e) Male S. haematobium, showing contraction and deformity at the anterior end, with bending at the mid-body region (×270); b: binding. (f) Gynaecophoric canal with small vesicles and contraction deformity at the edges (×1000); v: vesicles. (g) Marked swelling of the ventral sucker and the tegument around it (×1200); sw: swelling. (h) Ventral sucker, showing contraction and deformity, erosion and vesicles, together with shrinkage of the area between suckers (×1600); df: deformity, e: erosion, sh: shrinkage.
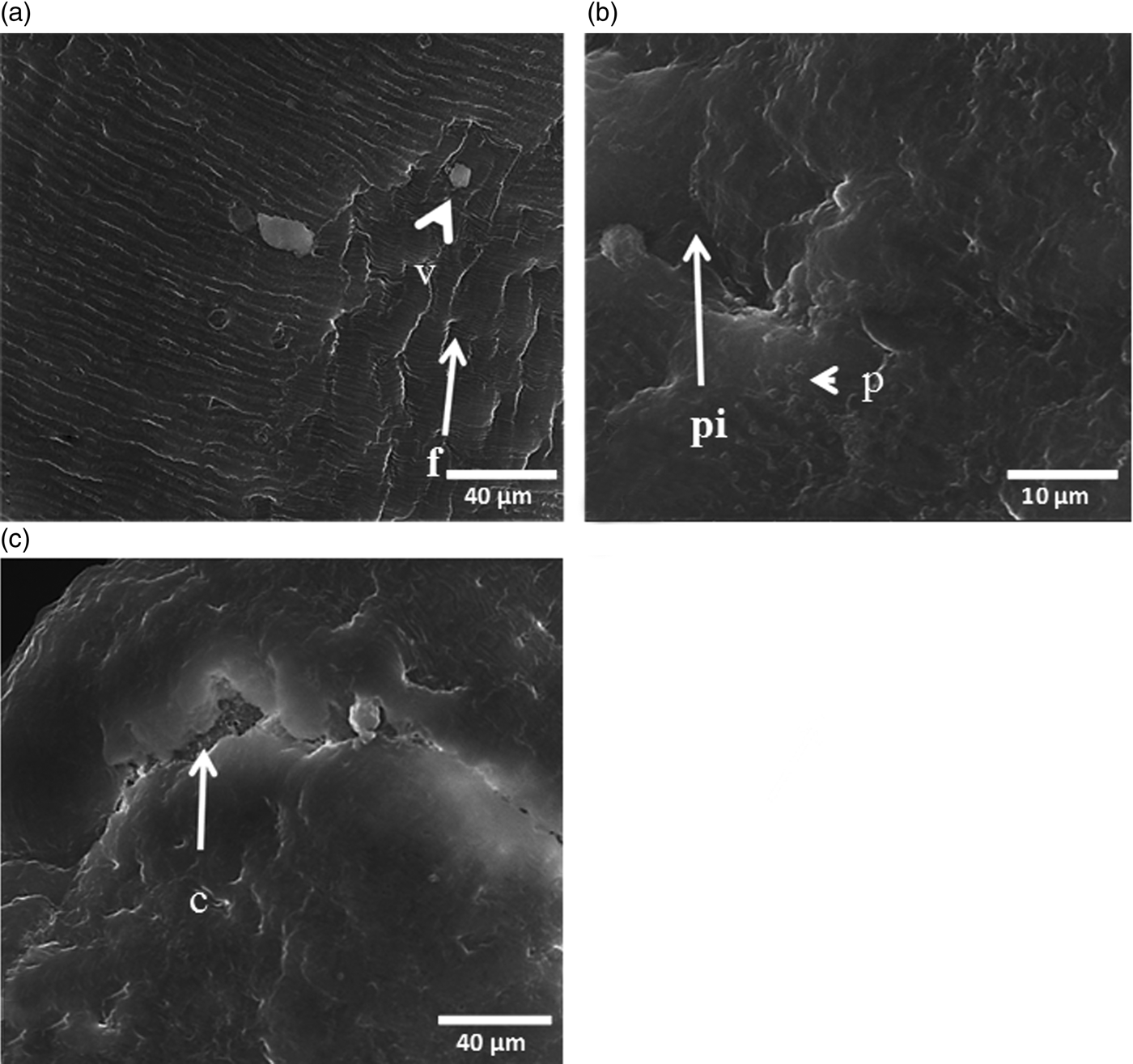
Fig. 4. SEM micrographs of adult Schistosoma haematobium female worms incubated with 20 μg/ml CO-ArNp for 48 h. (a) Tegumental changes in the mid-body, including loss of the normally arranged fissures in some parts, with contraction of the tegument as well as furrows and vesicle formation (×3000); f: furrows, v: vesicle. (b) Marked oedema and pitting of the tegument, as well as peeling off (×1000); pi: pitting, p: peeling. (c) Swollen female tegument with deep crake formation, exposing the subtegumental tissue (×3000); c: crake.
Treatment with the same dose of CO-ArNp and for the same time point revealed less extensive tegumental changes in females, as the damage was localized to the mid-body. Tegumental swelling with absence of normal arranged fissures was evident in all female worms examined; besides, constriction and collapse of the tegument with furrow formation and vesiculation (fig. 4a). In addition, the tegumental surface of some worms (< 50%) was distorted, exfoliated (fig. 4b) and eroded, leaving profound fissures with exposure of the subtegumental tissues (fig. 4c).
The tegument has a pivotal role in protection, immunomodulation, sensation, nutrient ingestion and synthesis of some proteins, osmoregulation, and excretion (Skelly & Wilson, Reference Skelly and Wilson2014). It is noteworthy that alterations in the tegument structure deteriorate its functions and lead to worm death (Shaw & Erasmus, Reference Shaw and Erasmus1987). The results of the present study demonstrated the schistosomicidal capability of CO-ArNp, wherein S. haematobium females revealed faster and higher susceptibility to the combined regimen compared to males, although male worms showed the most prominent and extensive tegumental damage. Corroborating our results, Soliman & Ibrahim (Reference Soliman and Ibrahim2005) reported that both atorvastatin alone and its combination with medroxyprogesterone acetate administered to experimentally infected hamsters led to more marked tegumental damage in S. haematobium male worms than females. Similarly, Eraky et al. (Reference Eraky, Aly, Selem, El-Kholy and Rashed2016) reported more pronounced tegumental alterations in male worms incubated with phytol than females exposed to the same dose and time period. Importantly, the differences in the severity and extent of morphological changes, and death rates and time, caused by Co-ArNp may be ascribed to the ability of the drug to attack different targets in both genders, based on the structural, physical and biochemical variations between schistosome males and females (Shaw & Erasmus, Reference Shaw and Erasmus1987). Drugs attacking schistosomes in a sex-dependent pattern could control disease morbidity and limit the spread of infection by decreasing the number of eggs released.
Considering the results of this study, artemisinin–naphthoquine phosphate exerts in vitro effects on S. haematobium adult worms, as it affects the morphology, tegumental surface and viability of the exposed worms. Therefore, this work opens up perspectives for future research on this drug in vivo to disclose its mechanism(s) of action.
Acknowledgements
Our sincere gratitude to Prof. Barbara L. Doughty, Texas A & M University, USA, for her enthusiastic editing to improve the phrasing of the revised manuscript.
Conflict of interest
None.
Ethical standards
All study procedures were approved by Mansoura Faculty of Medicine Institutional Research Board (MFM-IRB approval number: R/16.05.38), Mansoura University, Mansoura, Egypt, and Theodor Bilharz Research Institute (TBRI), Imbaba, Giza, Egypt, and complied with the relevant international animal ethics regulations for the care and use of experimental animals.







