1. Introduction
Capsules are closed elastic polymeric membranes, formed by cross-linking proteins to polysaccharides, and encapsulating a liquid droplet core (Lévy & Edwards-Lévy Reference Lévy and Edwards-Lévy1996; Edwards-Lévy & Lévy Reference Edwards-Lévy and Lévy1999). Their typical diameter spans from a few nanometres to a few millimetres. Capsules are primarily used as controlled delivery systems of active substances with practical applications gearing around pharmaceutical industry (Donbrow Reference Donbrow1991; De Cock et al. Reference De Cock, De Koker, De Geest, Grooten, Vervaet, Remon, Sukhorukov and Antipina2010), food processing (Sagis et al. Reference Sagis, de Ruiter, Miranda, de Ruiter, Schroën, van Aelst, Kieft, Boom and van der Linden2008), cosmetics (Miyazawa et al. Reference Miyazawa, Yajima, Kaneda and Yanaki2000), household products such as paints (Suryanarayana, Rao & Kumar Reference Suryanarayana, Rao and Kumar2008), while showing promising applications and future perspectives in areas such as thermal energy storage (Sarı, Alkan & Karaipekli Reference Sarı, Alkan and Karaipekli2010), and injectable scaffolds for soft tissue regeneration (Munarin et al. Reference Munarin, Petrini, Fare and Tanzi2010). The release mechanisms of the active agents cover time scales going from a few seconds to days and can occur either via capsule burst or through slow and prolonged diffusion (Neubauer, Poehlmann & Fery Reference Neubauer, Poehlmann and Fery2014). Capsules with a membrane made of polysiloxane will burst under continuous elongation (Walter, Rehage & Leonhard Reference Walter, Rehage and Leonhard2001; Koleva & Rehage Reference Koleva and Rehage2012), similar to droplets, whereas membranes formed with pure human serum albumin (HSA) or HSA–alginate can sustain very large deformations without rupture (Carin et al. Reference Carin, Barthès-Biesel, Edwards-Lévy, Postel and Andrei2003; de Loubens et al. Reference de Loubens, Deschamps, Boedec and Leonetti2015) making them a good model for mimicking biological cells. The mechanical properties of the capsules can be probed by partial aspiration with a micropipette (Hochmuth Reference Hochmuth2000), atomic force microscopy (Fery & Weinkamer Reference Fery and Weinkamer2007), compression tests (Chang & Olbricht Reference Chang and Olbricht1993b; Rachik et al. Reference Rachik, Barthes-Biesel, Carin and Edwards-Levy2006) or in flow conditions by measuring the elongation of the particle and extracting the shear and area dilatation moduli using the appropriate hyperelastic constitutive law (de Loubens et al. Reference de Loubens, Deschamps, Boedec and Leonetti2015).
When subject to external stresses, capsules can exhibit a strain-softening or a strain-hardening behaviour depending on the composition and the fabrication protocol of their membrane. Those behaviours can be well recovered with hyperelastic constitutive laws such as the generalised Hooke, Mooney–Rivlin, neo-Hookean and Skalak laws (de Loubens et al. Reference de Loubens, Deschamps, Boedec and Leonetti2015; Barthès-Biesel Reference Barthès-Biesel2016). The deformation and dynamics of a single capsule under different flow conditions have been thoroughly investigated by many authors: (i) numerically (Navot Reference Navot1998; Ramanujan & Pozrikidis Reference Ramanujan and Pozrikidis1998; Lac et al. Reference Lac, Barthès-Biesel, Pelekasis and Tsamopoulos2004; Li & Sarkar Reference Li and Sarkar2008; Bagchi & Kalluri Reference Bagchi and Kalluri2009; Dodson & Dimitrakopoulos Reference Dodson and Dimitrakopoulos2009; Farutin, Biben & Misbah Reference Farutin, Biben and Misbah2014; Dupont et al. Reference Dupont, Delahaye, Barthès-Biesel and Salsac2016; Boedec, Leonetti & Jaeger Reference Boedec, Leonetti and Jaeger2017), (ii) analytically (Barthès-Biesel Reference Barthès-Biesel1980; Barthès-Biesel & Rallison Reference Barthès-Biesel and Rallison1981; Vlahovska et al. Reference Vlahovska, Young, Danker and Misbah2011) and (iii) experimentally (Chang & Olbricht Reference Chang and Olbricht1993a,Reference Chang and Olbrichtb; de Loubens et al. Reference de Loubens, Deschamps, Boedec and Leonetti2015, Reference de Loubens, Deschamps, Edwards-Levy and Leonetti2016; Häner, Heil & Juel Reference Häner, Heil and Juel2020). Under a simple shear flow, initially non-spherical capsules can exhibit several complex dynamics including the steady and oscillating tank-treading, tumbling and swinging motions (also called vacillating-breathing in the literature). These dynamics are the result of the interplay between different parameters such as the capillary number, the confinement, and the viscosity contrast between the inner and the suspending fluids (Bagchi & Kalluri Reference Bagchi and Kalluri2009; Vlahovska et al. Reference Vlahovska, Young, Danker and Misbah2011; Walter, Salsac & Barthès-Biesel Reference Walter, Salsac and Barthès-Biesel2011). Conversely, a spherical capsule exhibits only a steady tank-treading motion characterised by a fixed orientation angle with respect to the flow and a steady-state deformed shape while the membrane undergoes a tank-treading motion (Bagchi & Kalluri Reference Bagchi and Kalluri2011; de Loubens et al. Reference de Loubens, Deschamps, Edwards-Levy and Leonetti2016).
Most end-use applications of capsules involve many particle interactions, and often different coupled time scales, with nonlinearity appearing already on the level of the single-particle mechanics. This level of complexity requires a numerical approach to understand the behaviour of suspensions of capsules, the correlation between their microstructure and rheology, and how it compares with well-studied systems such as solid particles and emulsions of drops.
The rheology of a dilute suspension of rigid spheres in an unbounded shear flow has been addressed analytically in the original work of Einstein (Reference Einstein1906, Reference Einstein1911) and extended to the second order by Batchelor & Green (Reference Batchelor and Green1972) to include pair hydrodynamic interactions. Empirical, semi-empirical and analytical models were proposed in the literature to predict the change of the relative viscosity with the volume fraction of rigid sphere suspensions from the dilute to the concentrated regimes (Eilers Reference von Eilers1941; Mooney Reference Mooney1951; Maron & Pierce Reference Maron and Pierce1956; Krieger & Dougherty Reference Krieger and Dougherty1959; Frankel & Acrivos Reference Frankel and Acrivos1967). Experiments and numerical simulations have revealed that a suspension of rigid particles exhibits shear-thinning, Newtonian and shear-thickening behaviour, respectively, as the shear rate is increased. Both normal stress differences, ![]() $N_1$ and
$N_1$ and ![]() $N_2$, have been reported to bear a negative sign with a larger magnitude for
$N_2$, have been reported to bear a negative sign with a larger magnitude for ![]() $N_2$ with respect to
$N_2$ with respect to ![]() $N_1$. The sign of
$N_1$. The sign of ![]() $N_1$ is still subject to discussions because its magnitude is very small. Numerical simulations performed by Sierou & Brady (Reference Sierou and Brady2002) and Gallier et al. (Reference Gallier, Lemaire, Peters and Lobry2014, Reference Gallier, Lemaire, Lobry and Peters2016) have pointed toward the possibility of two distinct physical origins of both normal stress differences. Here
$N_1$ is still subject to discussions because its magnitude is very small. Numerical simulations performed by Sierou & Brady (Reference Sierou and Brady2002) and Gallier et al. (Reference Gallier, Lemaire, Peters and Lobry2014, Reference Gallier, Lemaire, Lobry and Peters2016) have pointed toward the possibility of two distinct physical origins of both normal stress differences. Here ![]() $N_2$ is associated with particle–particle collisions, whereas
$N_2$ is associated with particle–particle collisions, whereas ![]() $N_1$ is mostly of hydrodynamic nature and is affected significantly by the presence or absence of boundaries. Detailed reviews on the rheology of solid particle suspensions can be found in Stickel & Powell (Reference Stickel and Powell2005), Mueller, Llewellin & Mader (Reference Mueller, Llewellin and Mader2010) and Guazzelli & Pouliquen (Reference Guazzelli and Pouliquen2018).
$N_1$ is mostly of hydrodynamic nature and is affected significantly by the presence or absence of boundaries. Detailed reviews on the rheology of solid particle suspensions can be found in Stickel & Powell (Reference Stickel and Powell2005), Mueller, Llewellin & Mader (Reference Mueller, Llewellin and Mader2010) and Guazzelli & Pouliquen (Reference Guazzelli and Pouliquen2018).
Non-Newtonian behaviour in the form of shear-thinning has been reported for emulsions of drops, strain-softening capsules and closed phospholipid bilayer membranes (vesicles). These three classes of deformable particles are characterised by a thin, continuous and impermeable interface encapsulating an internal fluid. However, the interface mechanical properties are different. They all exhibit a negative ![]() $N_2$ and a positive
$N_2$ and a positive ![]() $N_1$, and unlike rigid particles, the magnitude of
$N_1$, and unlike rigid particles, the magnitude of ![]() $N_1$ is larger than
$N_1$ is larger than ![]() $N_2$, which most probably indicates a more dominant role of hydrodynamic interactions as compared with particle–particle collisions (Loewenberg & Hinch Reference Loewenberg and Hinch1996; Clausen, Reasor & Aidun Reference Clausen, Reasor and Aidun2011; Vlahovska & Gracia Reference Vlahovska and Gracia2007; Zhao & Shaqfeh Reference Zhao and Shaqfeh2013; Matsunaga et al. Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016). In analogy to drops, a capillary number, quantifying the shear elastic resistance of the membrane to external stresses, has been widely used in the literature of capsules regardless of the nature of the hyperelastic law and the number of moduli characterising the membrane mechanics. Bagchi & Kalluri (Reference Bagchi and Kalluri2010) have shown that for a single strain-hardening capsule under a simple shear flow, the ratio of the area dilatation to shear elastic moduli, characterising the local inextensibility of the membrane, leads to some atypical effects on the intrinsic viscosity and the shear-thinning behaviour. For a non-dilute suspension of anisotropic strain-hardening capsules, Gross, Krüger & Varnik (Reference Gross, Krüger and Varnik2014) have reported that for small capillary number the effect of the ratio of the area dilatation to shear elastic moduli on the suspension rheology is negligible.
$N_2$, which most probably indicates a more dominant role of hydrodynamic interactions as compared with particle–particle collisions (Loewenberg & Hinch Reference Loewenberg and Hinch1996; Clausen, Reasor & Aidun Reference Clausen, Reasor and Aidun2011; Vlahovska & Gracia Reference Vlahovska and Gracia2007; Zhao & Shaqfeh Reference Zhao and Shaqfeh2013; Matsunaga et al. Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016). In analogy to drops, a capillary number, quantifying the shear elastic resistance of the membrane to external stresses, has been widely used in the literature of capsules regardless of the nature of the hyperelastic law and the number of moduli characterising the membrane mechanics. Bagchi & Kalluri (Reference Bagchi and Kalluri2010) have shown that for a single strain-hardening capsule under a simple shear flow, the ratio of the area dilatation to shear elastic moduli, characterising the local inextensibility of the membrane, leads to some atypical effects on the intrinsic viscosity and the shear-thinning behaviour. For a non-dilute suspension of anisotropic strain-hardening capsules, Gross, Krüger & Varnik (Reference Gross, Krüger and Varnik2014) have reported that for small capillary number the effect of the ratio of the area dilatation to shear elastic moduli on the suspension rheology is negligible.
This paper is devoted to study the dynamics of suspensions of model soft particles with a strain-hardening character, such as certain types of capsules (Carin et al. Reference Carin, Barthès-Biesel, Edwards-Lévy, Postel and Andrei2003), focusing on the role played by a material property of the membrane, namely its inextensibility, on the capsule deformation and on the suspension rheology, at varying the concentration of the dispersed phase and applied shear. To the best of the authors’ knowledge the contribution of the local inextensibility of the membrane to the rheology of semi-dilute and concentrated suspensions of initially spherical strain-hardening capsules has so far not been studied.
The paper is organised as follows. We describe the numerical method in § 2 (benchmarks are provided in appendix A). We then present and discuss our numerical results for the dilute, semi-dilute and concentrated regimes as a function of the parameter ![]() $C$ quantifying the inextensibility of the membrane and the capillary number
$C$ quantifying the inextensibility of the membrane and the capillary number ![]() $Ca$ in § 3. The concluding remarks and further discussions are given in § 4.
$Ca$ in § 3. The concluding remarks and further discussions are given in § 4.
2. Numerical method
2.1. Lattice Boltzmann method
The Navier–Stokes equations are recovered in the limit of small Mach and Knudsen numbers by the lattice Boltzmann method (LBM), which is based on the discretisation of the Boltzmann–BGK (Bhatnagar, Gross & Krook Reference Bhatnagar, Gross and Krook1954) equation in time and phase space (Benzi, Succi & Vergassola Reference Benzi, Succi and Vergassola1992; Succi Reference Succi2001; Krüger et al. Reference Krüger, Kusumaatmaja, Kuzmin, Shardt, Silva and Viggen2017). The LBM describes the evolution of the single-particle distribution function ![]() $f_i(\boldsymbol {x},t)$ at a position
$f_i(\boldsymbol {x},t)$ at a position ![]() $\boldsymbol {x}$ and time
$\boldsymbol {x}$ and time ![]() $t$ with a microscopic velocity
$t$ with a microscopic velocity ![]() $\boldsymbol {c}_i$, where
$\boldsymbol {c}_i$, where ![]() $i = 1\ldots Q$, on a regular
$i = 1\ldots Q$, on a regular ![]() $D$-dimensional lattice in discrete time steps
$D$-dimensional lattice in discrete time steps ![]() $\Delta t$. We consider in this work a
$\Delta t$. We consider in this work a ![]() $D3Q19$ model corresponding to a three-dimensional lattice with
$D3Q19$ model corresponding to a three-dimensional lattice with ![]() $Q=19$ velocities. The lattice Boltzmann equation reads
$Q=19$ velocities. The lattice Boltzmann equation reads
with
and
where ![]() $\tau$ is a relaxation time related to the fluid dynamic viscosity by
$\tau$ is a relaxation time related to the fluid dynamic viscosity by ![]() $\mu _0 = \rho _0 c_s^{2}(\tau - {\Delta t}/{2})$ (
$\mu _0 = \rho _0 c_s^{2}(\tau - {\Delta t}/{2})$ (![]() $\rho _0$ being the mean fluid density). Here
$\rho _0$ being the mean fluid density). Here ![]() $c_s=({1}/{\sqrt {3}}) ({\Delta x}/{\Delta t})$ denotes the lattice speed of sound,
$c_s=({1}/{\sqrt {3}}) ({\Delta x}/{\Delta t})$ denotes the lattice speed of sound, ![]() $\Delta x$ is the lattice constant,
$\Delta x$ is the lattice constant, ![]() $\omega _i$ are lattice weights and
$\omega _i$ are lattice weights and ![]() $f_i^{eq}(\boldsymbol {x},t)$ is the equilibrium probability distribution function, depending on the fluid density
$f_i^{eq}(\boldsymbol {x},t)$ is the equilibrium probability distribution function, depending on the fluid density ![]() $\rho$ and velocity
$\rho$ and velocity ![]() $\boldsymbol {u}$ fields as a truncated expansion of the Maxwell–Boltzmann distribution (valid at small Mach number,
$\boldsymbol {u}$ fields as a truncated expansion of the Maxwell–Boltzmann distribution (valid at small Mach number, ![]() $Ma = |\boldsymbol {u}|/c_s \ll 0.1$). Here
$Ma = |\boldsymbol {u}|/c_s \ll 0.1$). Here ![]() $F_i$ on the right-hand side of (2.1) is a source term accounting for any external or internal force and will be used here to incorporate the forces exerted by the membrane on the fluid through the immersed boundary method (IBM). For a D3Q19 LBM, the lattice weights
$F_i$ on the right-hand side of (2.1) is a source term accounting for any external or internal force and will be used here to incorporate the forces exerted by the membrane on the fluid through the immersed boundary method (IBM). For a D3Q19 LBM, the lattice weights ![]() $\omega _i$ read as
$\omega _i$ read as ![]() $1/3$,
$1/3$, ![]() $1/18$ and
$1/18$ and ![]() $1/36$ for
$1/36$ for ![]() $i=1$,
$i=1$, ![]() $i=2,\ldots ,7$ and
$i=2,\ldots ,7$ and ![]() $i=8,\ldots ,19$, respectively. The macroscopic fluid density
$i=8,\ldots ,19$, respectively. The macroscopic fluid density ![]() $\rho$ and velocity
$\rho$ and velocity ![]() $\boldsymbol {u}$ are deduced from the moments of the discrete probability distribution functions as
$\boldsymbol {u}$ are deduced from the moments of the discrete probability distribution functions as
 \begin{equation} \rho = \sum_{i=1}^{19} f_i (\boldsymbol{x},t) \quad \text{and} \quad \boldsymbol{u} = \sum_{i=1}^{19} f_i(\boldsymbol{x},t)\boldsymbol{c}_i / \rho. \end{equation}
\begin{equation} \rho = \sum_{i=1}^{19} f_i (\boldsymbol{x},t) \quad \text{and} \quad \boldsymbol{u} = \sum_{i=1}^{19} f_i(\boldsymbol{x},t)\boldsymbol{c}_i / \rho. \end{equation}For convenience, we set the lattice constant, the time step, the mean fluid density and the relaxation time to unity.
2.2. Membrane model
The capsule is modelled as a two-dimensional hyperelastic thin shell encapsulating an inner fluid and suspended in an outer fluid. The interior and exterior fluids are Newtonian with equal densities and viscosities. The surface of the capsule is discretised into a triangular mesh and endowed with a resistance to in-plane deformations. The membrane Lagrangian variables are defined on a moving curvilinear mesh with coordinates ![]() $(\xi _1,\xi _2)$, freely evolving on the Cartesian mesh on which lies the Eulerian fluid variables. We consider the case of a strain-hardening membrane using the hyperelastic law introduced by Skalak (Reference Skalak1973), where the in-plane elastic deformations are governed by shear and area dilatation resistances. In terms of the deformation invariants,
$(\xi _1,\xi _2)$, freely evolving on the Cartesian mesh on which lies the Eulerian fluid variables. We consider the case of a strain-hardening membrane using the hyperelastic law introduced by Skalak (Reference Skalak1973), where the in-plane elastic deformations are governed by shear and area dilatation resistances. In terms of the deformation invariants, ![]() $I_1=\lambda _1^{2} + \lambda _2^{2} -2$ and
$I_1=\lambda _1^{2} + \lambda _2^{2} -2$ and ![]() $I_2 = \lambda _1^{2} \lambda _2^{2} -1$, where
$I_2 = \lambda _1^{2} \lambda _2^{2} -1$, where ![]() $\lambda _1$ and
$\lambda _1$ and ![]() $\lambda _2$ are the principal stretching ratios on an element of the membrane surface, the strain energy over the surface of the capsule (
$\lambda _2$ are the principal stretching ratios on an element of the membrane surface, the strain energy over the surface of the capsule (![]() $\varOmega _S$) reads as
$\varOmega _S$) reads as
Here, ![]() $G_s$ is the elastic shear modulus and
$G_s$ is the elastic shear modulus and ![]() $C$ is a constant related to the strain-hardening character of the membrane through the scaled area dilatation modulus
$C$ is a constant related to the strain-hardening character of the membrane through the scaled area dilatation modulus ![]() $G_a$ such as
$G_a$ such as ![]() $G_a/G_s= 1 + 2C$. In other words, increasing the value of
$G_a/G_s= 1 + 2C$. In other words, increasing the value of ![]() $C$ enhances the local inextensibility of the membrane (hereafter, we refer to
$C$ enhances the local inextensibility of the membrane (hereafter, we refer to ![]() $C$ as membrane inextensibility) and makes the capsule more strain-hardening. In the small deformation limit,
$C$ as membrane inextensibility) and makes the capsule more strain-hardening. In the small deformation limit, ![]() $C$ and the surface Poisson ratio (
$C$ and the surface Poisson ratio (![]() $\nu _s$) are related by
$\nu _s$) are related by ![]() $C = {\nu _s}/({1-\nu _s})$ (see Barthès-Biesel, Diaz & Dhenin Reference Barthès-Biesel, Diaz and Dhenin2002). The elastic deformations on the surface of the particle are evaluated numerically using a linear finite element method following the approach described in Krüger, Varnik & Raabe (Reference Krüger, Varnik and Raabe2011). In addition to shear elasticity and area dilatation, capsules may also exhibit a resistance to out-of-plane deformations (bending). The existence of a non-negligible bending energy depends on the protocol used to fabricate the capsule and the composition of the encapsulating membrane (de Loubens et al. Reference de Loubens, Deschamps, Georgelin, Charrier, Edwards-Levy and Leonetti2014, Reference de Loubens, Deschamps, Boedec and Leonetti2015; Gubspun et al. Reference Gubspun, Gires, De Loubens, Barthes-Biesel, Deschamps, Georgelin, Leonetti, Leclerc, Edwards-Lévy and Salsac2016). Although it can be of interest to investigate the interplay between the bending and the shear elasticity under different flow conditions, we choose to focus, here, on the role of the area dilatation, which has been somehow overlooked in the existing literature, and we consider, thus, capsules without bending resistance. The volume of the capsules is prescribed using a penalty function that reads as
$C = {\nu _s}/({1-\nu _s})$ (see Barthès-Biesel, Diaz & Dhenin Reference Barthès-Biesel, Diaz and Dhenin2002). The elastic deformations on the surface of the particle are evaluated numerically using a linear finite element method following the approach described in Krüger, Varnik & Raabe (Reference Krüger, Varnik and Raabe2011). In addition to shear elasticity and area dilatation, capsules may also exhibit a resistance to out-of-plane deformations (bending). The existence of a non-negligible bending energy depends on the protocol used to fabricate the capsule and the composition of the encapsulating membrane (de Loubens et al. Reference de Loubens, Deschamps, Georgelin, Charrier, Edwards-Levy and Leonetti2014, Reference de Loubens, Deschamps, Boedec and Leonetti2015; Gubspun et al. Reference Gubspun, Gires, De Loubens, Barthes-Biesel, Deschamps, Georgelin, Leonetti, Leclerc, Edwards-Lévy and Salsac2016). Although it can be of interest to investigate the interplay between the bending and the shear elasticity under different flow conditions, we choose to focus, here, on the role of the area dilatation, which has been somehow overlooked in the existing literature, and we consider, thus, capsules without bending resistance. The volume of the capsules is prescribed using a penalty function that reads as
where ![]() $\kappa _v$ is a modulus that controls the deviation from the reference volume
$\kappa _v$ is a modulus that controls the deviation from the reference volume ![]() $V_0$ corresponding to the stress-free shape. The membrane force is calculated on each membrane node (
$V_0$ corresponding to the stress-free shape. The membrane force is calculated on each membrane node (![]() $\boldsymbol {X}^{m}$) using the principle of virtual work, such that
$\boldsymbol {X}^{m}$) using the principle of virtual work, such that
where ![]() $E = E_s + E_v$ is the membrane total energy.
$E = E_s + E_v$ is the membrane total energy.
To avoid overlap between capsules in relatively dense systems, we introduce a short-range repulsive force intended to mimic the normal component of the lubrication force. The repulsive force acts when the distance between two nodes from different capsules is below the cut-off distance ![]() $\delta _0$, which is set here to the minimum value of
$\delta _0$, which is set here to the minimum value of ![]() $1\Delta x$ corresponding to the fluid resolution limit of the LBM, and vanishes at a node-to-node distance
$1\Delta x$ corresponding to the fluid resolution limit of the LBM, and vanishes at a node-to-node distance ![]() $d_{ij} \geqslant \delta _0$. Its expression is given by
$d_{ij} \geqslant \delta _0$. Its expression is given by
 \begin{equation} \boldsymbol{F}_{rep} = \left\{\begin{array}{@{}ll} \bar{\epsilon}\left[\left(\dfrac{\Delta x}{d_{ij}}\right)^{2} - \left(\dfrac{\Delta x}{\delta_0}\right)^{2}\right] \dfrac{\boldsymbol{d}_{ij}}{d_{ij}} & \text{if} \ d_{ij} < \delta_0 \\ \boldsymbol{0} & \text{if} \ d_{ij} \geqslant \delta_0 \end{array}\right. , \end{equation}
\begin{equation} \boldsymbol{F}_{rep} = \left\{\begin{array}{@{}ll} \bar{\epsilon}\left[\left(\dfrac{\Delta x}{d_{ij}}\right)^{2} - \left(\dfrac{\Delta x}{\delta_0}\right)^{2}\right] \dfrac{\boldsymbol{d}_{ij}}{d_{ij}} & \text{if} \ d_{ij} < \delta_0 \\ \boldsymbol{0} & \text{if} \ d_{ij} \geqslant \delta_0 \end{array}\right. , \end{equation}
where ![]() $\bar {\epsilon }$ is the interaction strength with the dimension of a force. Equation (2.8) was suggested by Glowinski et al. (Reference Glowinski, Pan, Hesla, Joseph and Periaux2001) for suspension of rigid particles, and used by Gross et al. (Reference Gross, Krüger and Varnik2014) to study the rheology of very dense suspensions of red blood cells in a shear flow. Other contact models based for example on an exponential repulsive force or a Lennard–Jones potential can also be used (Buxton et al. Reference Buxton, Verberg, Jasnow and Balazs2005; MacMeccan III Reference MacMeccan2007; Guckenberger & Gekle Reference Guckenberger and Gekle2016). The effect of the short-range repulsive force on the rheology of strain-hardening capsules is discussed in appendix A.4.
$\bar {\epsilon }$ is the interaction strength with the dimension of a force. Equation (2.8) was suggested by Glowinski et al. (Reference Glowinski, Pan, Hesla, Joseph and Periaux2001) for suspension of rigid particles, and used by Gross et al. (Reference Gross, Krüger and Varnik2014) to study the rheology of very dense suspensions of red blood cells in a shear flow. Other contact models based for example on an exponential repulsive force or a Lennard–Jones potential can also be used (Buxton et al. Reference Buxton, Verberg, Jasnow and Balazs2005; MacMeccan III Reference MacMeccan2007; Guckenberger & Gekle Reference Guckenberger and Gekle2016). The effect of the short-range repulsive force on the rheology of strain-hardening capsules is discussed in appendix A.4.
2.3. Membrane dynamics
For the fluid–membrane coupling, we use the IBM (Peskin Reference Peskin2002). In the IBM the Lagrangian massless nodes are interacting with the Eulerian fluid nodes using an interpolation function in a two-way coupling scheme: the Lagrangian membrane forces calculated on the curvilinear mesh are distributed to the surrounding Eulerian fluid nodes on the fixed Cartesian mesh by a smoothed approximation of the delta function, where they enter the discretised lattice Boltzmann equation (2.1) as an external force term. The new fluid velocities are obtained after solving the LBM equation (2.1). The capsules are advected with the fluid velocity, where the velocity of each Lagrangian node on the membrane is interpolated from the surrounding Eulerian fluid node velocity using the same scheme as for the spreading of the forces. The distribution of the membrane forces ![]() $\boldsymbol {F}^{m}$ located at position
$\boldsymbol {F}^{m}$ located at position ![]() $\boldsymbol {X}^{m}(\xi _1,\xi _2,t)$ to the adjacent fluid nodes is given by
$\boldsymbol {X}^{m}(\xi _1,\xi _2,t)$ to the adjacent fluid nodes is given by
where ![]() $\delta$ is a three-dimensional approximation of the Dirac delta function, and
$\delta$ is a three-dimensional approximation of the Dirac delta function, and ![]() $\boldsymbol {f}(\boldsymbol {x},t)$ is the force density acting on the fluid at the Eulerian node
$\boldsymbol {f}(\boldsymbol {x},t)$ is the force density acting on the fluid at the Eulerian node ![]() $\boldsymbol {x}(x_1,x_2,x_3)$. Equation (2.9) is incorporated into (2.1) in a similar manner to an external body force (e.g. gravity) as follows
$\boldsymbol {x}(x_1,x_2,x_3)$. Equation (2.9) is incorporated into (2.1) in a similar manner to an external body force (e.g. gravity) as follows
The velocity of the membrane is obtained from the local Eulerian fluid velocity as
where ![]() $\varOmega _D$ represents the whole fluid domain. Equation (2.11) enforces a no-slip boundary condition on the membrane, although in practice a volume drift is observed with time, and thus the need to use a penalty function on the volume (see (2.6)) or improved IBM schemes (Wu & Shu Reference Wu and Shu2012; Casquero et al. Reference Casquero, Bona-Casas, Toshniwal, Hughes, Gomez and Zhang2020). Note the new fluid velocity
$\varOmega _D$ represents the whole fluid domain. Equation (2.11) enforces a no-slip boundary condition on the membrane, although in practice a volume drift is observed with time, and thus the need to use a penalty function on the volume (see (2.6)) or improved IBM schemes (Wu & Shu Reference Wu and Shu2012; Casquero et al. Reference Casquero, Bona-Casas, Toshniwal, Hughes, Gomez and Zhang2020). Note the new fluid velocity ![]() $\boldsymbol {u}(\boldsymbol {x},t+\Delta t)$ is obtained after solving the discretised lattice Boltzmann equation (2.1), which requires a priori knowledge of the membrane forces. The membrane forces
$\boldsymbol {u}(\boldsymbol {x},t+\Delta t)$ is obtained after solving the discretised lattice Boltzmann equation (2.1), which requires a priori knowledge of the membrane forces. The membrane forces ![]() $\boldsymbol {F}_k^{m}(t)$ are computed before solving (2.1), so to say, with respect to the old position of the membrane nodes
$\boldsymbol {F}_k^{m}(t)$ are computed before solving (2.1), so to say, with respect to the old position of the membrane nodes ![]() $\boldsymbol {X}^{m}(t)$. Thus, the following notation
$\boldsymbol {X}^{m}(t)$. Thus, the following notation ![]() $\boldsymbol {u}(t+\Delta t)$ is used in (2.11) instead of
$\boldsymbol {u}(t+\Delta t)$ is used in (2.11) instead of ![]() $\boldsymbol {u}(t)$. In discrete forms, (2.9) and (2.11) can be rewritten such that
$\boldsymbol {u}(t)$. In discrete forms, (2.9) and (2.11) can be rewritten such that
where ![]() $\sum _k$ runs over the membrane nodes located within the interpolation range of a given fluid node
$\sum _k$ runs over the membrane nodes located within the interpolation range of a given fluid node ![]() $\boldsymbol {x}$, and
$\boldsymbol {x}$, and ![]() $\sum _{\boldsymbol {x}}$ over a cuboidal region centred around a given membrane node
$\sum _{\boldsymbol {x}}$ over a cuboidal region centred around a given membrane node ![]() $\boldsymbol {X}^{m}$. The advection equation of a Lagrangian node on the capsule follows an explicit forward Euler scheme, such that
$\boldsymbol {X}^{m}$. The advection equation of a Lagrangian node on the capsule follows an explicit forward Euler scheme, such that
Let us remark that, in principle, depending on the specific problem, one may need to tune the time step of the numerical integration of the Lagrangian dynamics independently from the lattice Boltzmann time step. For instance, for vanishing values of ![]() $C$, mesh instabilities can arise (owing to the formation of large wrinkles on the capsule surface). We have, therefore, decided to keep
$C$, mesh instabilities can arise (owing to the formation of large wrinkles on the capsule surface). We have, therefore, decided to keep ![]() $C \geqslant 10^{-3}$ such that choosing the time step equal to the lattice Boltzmann
$C \geqslant 10^{-3}$ such that choosing the time step equal to the lattice Boltzmann ![]() $\Delta t$ proved sufficient to prevent such instabilities.
$\Delta t$ proved sufficient to prevent such instabilities.
The Dirac delta function in (2.9) and (2.11) is usually replaced with a smoother interpolation function (![]() $\varphi$) of some shape such as
$\varphi$) of some shape such as ![]() $\delta (\boldsymbol {x}) = \varphi (x_1)\varphi (x_2)\varphi (x_3)$ to avoid jumps in velocities or in the applied forces occurring when the Lagrangian nodes do not coincide with the nodes of the Eulerian grid. Several distribution functions have been used in the IBM literature for a wide range of applications. For detailed reviews on the IBM and its accuracy, we refer the reader to Mittal & Iaccarino (Reference Mittal and Iaccarino2005) and Krüger et al. (Reference Krüger, Kusumaatmaja, Kuzmin, Shardt, Silva and Viggen2017) among other existing works on this topic. In what follows, we use a two-point linear interpolation function as discussed in Krüger (Reference Krüger2012), which is given by
$\delta (\boldsymbol {x}) = \varphi (x_1)\varphi (x_2)\varphi (x_3)$ to avoid jumps in velocities or in the applied forces occurring when the Lagrangian nodes do not coincide with the nodes of the Eulerian grid. Several distribution functions have been used in the IBM literature for a wide range of applications. For detailed reviews on the IBM and its accuracy, we refer the reader to Mittal & Iaccarino (Reference Mittal and Iaccarino2005) and Krüger et al. (Reference Krüger, Kusumaatmaja, Kuzmin, Shardt, Silva and Viggen2017) among other existing works on this topic. In what follows, we use a two-point linear interpolation function as discussed in Krüger (Reference Krüger2012), which is given by
where ![]() $\hat {x}$ can denote
$\hat {x}$ can denote ![]() $x_1$,
$x_1$, ![]() $x_2$ or
$x_2$ or ![]() $x_3$.
$x_3$.
2.4. Simulations details, key parameters and observables
We simulate shear flows, with constant shear rate ![]() $\dot {\gamma }$, in cubic boxes, seeded with
$\dot {\gamma }$, in cubic boxes, seeded with ![]() $N_p$ capsules of radius
$N_p$ capsules of radius ![]() $r$. The computational domain has a side length
$r$. The computational domain has a side length ![]() $L=128\Delta x=16r$ and it is biperiodic along the flow and vorticity directions (
$L=128\Delta x=16r$ and it is biperiodic along the flow and vorticity directions (![]() $x_1$ and
$x_1$ and ![]() $x_2$ directions, respectively). It is bounded, in the
$x_2$ directions, respectively). It is bounded, in the ![]() $x_3$ direction, by two planar walls at which we impose a velocity boundary condition such to generate the driving shear flow as described in Hecht & Harting (Reference Hecht and Harting2010).
$x_3$ direction, by two planar walls at which we impose a velocity boundary condition such to generate the driving shear flow as described in Hecht & Harting (Reference Hecht and Harting2010).
The main control parameters of the problem are the volume fraction of capsules, ![]() $\phi ={4 {\rm \pi}r^{3}}/{3L^{3}}$, the capillary number,
$\phi ={4 {\rm \pi}r^{3}}/{3L^{3}}$, the capillary number, ![]() $Ca$, and the membrane inextensibility,
$Ca$, and the membrane inextensibility, ![]() $C$ (the particle scale Reynolds number being always so small,
$C$ (the particle scale Reynolds number being always so small, ![]() $Re = (\rho _0\dot {\gamma } r^{2})/\mu _0 < 10^{-1}$, that the dynamics can be considered effectively inertia-less). The capillary number, quantifying the relative intensity of viscous and elastic forces, is defined as
$Re = (\rho _0\dot {\gamma } r^{2})/\mu _0 < 10^{-1}$, that the dynamics can be considered effectively inertia-less). The capillary number, quantifying the relative intensity of viscous and elastic forces, is defined as
where ![]() $\tau _{el} = (\mu _0 r) / G_s$ is a time scale associated with the elasticity of the capsule. The value of
$\tau _{el} = (\mu _0 r) / G_s$ is a time scale associated with the elasticity of the capsule. The value of ![]() $C$ depends on the composition of the membrane. For example albumin–alginate capsules have a
$C$ depends on the composition of the membrane. For example albumin–alginate capsules have a ![]() $C$ of the order of unity (Carin et al. Reference Carin, Barthès-Biesel, Edwards-Lévy, Postel and Andrei2003), whereas for red blood cells
$C$ of the order of unity (Carin et al. Reference Carin, Barthès-Biesel, Edwards-Lévy, Postel and Andrei2003), whereas for red blood cells ![]() $C \gg 1$.
$C \gg 1$.
Following Batchelor (Reference Batchelor1970), we evaluate the average particle stress tensor as
 \begin{equation} \varSigma_{ij}^{p} = \frac{1}{N} \sum_{\alpha=1}^{N} n S^{\alpha}_{ij} = -\frac{1}{V_D} \sum_{\alpha=1}^{N} \int_{\varOmega_S} \frac{1}{2}\{F_i^{m,\alpha} X_j^{m,\alpha} + F_{j}^{m,\alpha} X_i^{m,\alpha}\} \,\textrm{d}\varOmega_S^{\alpha}, \end{equation}
\begin{equation} \varSigma_{ij}^{p} = \frac{1}{N} \sum_{\alpha=1}^{N} n S^{\alpha}_{ij} = -\frac{1}{V_D} \sum_{\alpha=1}^{N} \int_{\varOmega_S} \frac{1}{2}\{F_i^{m,\alpha} X_j^{m,\alpha} + F_{j}^{m,\alpha} X_i^{m,\alpha}\} \,\textrm{d}\varOmega_S^{\alpha}, \end{equation}
where ![]() $i$ and
$i$ and ![]() $j$ are indices referring to Cartesian directions,
$j$ are indices referring to Cartesian directions, ![]() $\sum _{\alpha =1}^{N_p}$ is a sum over the number of particles in the averaging volume
$\sum _{\alpha =1}^{N_p}$ is a sum over the number of particles in the averaging volume ![]() $V_D$,
$V_D$, ![]() $n$ the number density and
$n$ the number density and ![]() $\boldsymbol {S}$ the particle stresslet. Here
$\boldsymbol {S}$ the particle stresslet. Here ![]() $\textrm {d}\varOmega _S^{\alpha }$ is the area element centred at
$\textrm {d}\varOmega _S^{\alpha }$ is the area element centred at ![]() $\boldsymbol {X}^{m}$, and
$\boldsymbol {X}^{m}$, and ![]() $\boldsymbol {F}^{m}$ is the surface force density exerted by the membrane on the fluid. The rheology of the system is then assessed in terms of the suspension relative viscosity and normal stress differences. The relative viscosity of the suspension
$\boldsymbol {F}^{m}$ is the surface force density exerted by the membrane on the fluid. The rheology of the system is then assessed in terms of the suspension relative viscosity and normal stress differences. The relative viscosity of the suspension ![]() $\mu _r$ is defined as
$\mu _r$ is defined as
where ![]() $\mu$ is the effective viscosity of the system. The first and second normal stress differences can be deduced from the average particle stress tensor as
$\mu$ is the effective viscosity of the system. The first and second normal stress differences can be deduced from the average particle stress tensor as
The deformation of a capsule in the shear plane can be characterised in terms of the Taylor deformation parameter (Taylor Reference Taylor1934)
where ![]() $r_1$ and
$r_1$ and ![]() $r_3$ are the major and minor principal semi-axes (in the shear plane) of an ellipsoid having the same tensor of inertia as the deformed capsule, and the inclination angle
$r_3$ are the major and minor principal semi-axes (in the shear plane) of an ellipsoid having the same tensor of inertia as the deformed capsule, and the inclination angle ![]() $\theta$, which is the angle the major axis forms with the positive direction of the
$\theta$, which is the angle the major axis forms with the positive direction of the ![]() $x_1$-coordinate (see figure 1). The ellipsoid principal semi-axes are defined as (Ramanujan & Pozrikidis Reference Ramanujan and Pozrikidis1998; Li & Sarkar Reference Li and Sarkar2008; Frijters, Günther & Harting Reference Frijters, Günther and Harting2012; Farutin et al. Reference Farutin, Biben and Misbah2014):
$x_1$-coordinate (see figure 1). The ellipsoid principal semi-axes are defined as (Ramanujan & Pozrikidis Reference Ramanujan and Pozrikidis1998; Li & Sarkar Reference Li and Sarkar2008; Frijters, Günther & Harting Reference Frijters, Günther and Harting2012; Farutin et al. Reference Farutin, Biben and Misbah2014):
where ![]() $I_1$,
$I_1$, ![]() $I_2$ and
$I_2$ and ![]() $I_3$ are the eigenvalues of the tensor of inertia.
$I_3$ are the eigenvalues of the tensor of inertia.

Figure 1. (a) Schematic of a single capsule in a shear flow showing the initial (dashed lines) and the typical ellipsoidal steady-state shapes; ![]() $r$ is the radius of the unstressed sphere,
$r$ is the radius of the unstressed sphere, ![]() $r_1$ and
$r_1$ and ![]() $r_3$ are the major and minor semi-axes in the shear plane (
$r_3$ are the major and minor semi-axes in the shear plane (![]() $r_2$ is that in the vorticity direction, not shown) and
$r_2$ is that in the vorticity direction, not shown) and ![]() $\theta$ is the inclination angle. (b,c) Numerically computed steady-state shapes for different values of
$\theta$ is the inclination angle. (b,c) Numerically computed steady-state shapes for different values of ![]() $C$ and
$C$ and ![]() $Ca$ namely in the
$Ca$ namely in the ![]() $x_3x_1$-plane (defined between the shear gradient and flow directions) and
$x_3x_1$-plane (defined between the shear gradient and flow directions) and ![]() $x_2x_1$-plane (defined between the vorticity and the flow directions).
$x_2x_1$-plane (defined between the vorticity and the flow directions).
The rheology and microstructure of suspensions of strain-hardening capsules up to a volume fraction of ![]() $0.5$ are studied for capillary numbers ranging from
$0.5$ are studied for capillary numbers ranging from ![]() $Ca=0.1$ to
$Ca=0.1$ to ![]() $1$. The number of particles is varied from
$1$. The number of particles is varied from ![]() $1$ to
$1$ to ![]() $500$, corresponding to
$500$, corresponding to ![]() $\phi \approx 0.001$ and
$\phi \approx 0.001$ and ![]() $\phi \approx 0.5$, respectively. Each particle is discretised with
$\phi \approx 0.5$, respectively. Each particle is discretised with ![]() $1280$ triangles and
$1280$ triangles and ![]() $642$ nodes, and initialised as a sphere with a radius
$642$ nodes, and initialised as a sphere with a radius ![]() $r=8\Delta x$. When the distance between two neighbouring particles is below
$r=8\Delta x$. When the distance between two neighbouring particles is below ![]() $1\Delta x$, a repulsive force acts on the surface of the two particles with an interaction strength chosen as
$1\Delta x$, a repulsive force acts on the surface of the two particles with an interaction strength chosen as ![]() $\bar {\epsilon } \approx 10^{2} G_s r$. The simulations are initialised with the capsules distributed randomly in the domain with an initial radius
$\bar {\epsilon } \approx 10^{2} G_s r$. The simulations are initialised with the capsules distributed randomly in the domain with an initial radius ![]() $r_0 < r$. The radius of each capsule is then increased in time with a fixed growth rate until reaching the desired size. The relative error on the capsule's volume defined as
$r_0 < r$. The radius of each capsule is then increased in time with a fixed growth rate until reaching the desired size. The relative error on the capsule's volume defined as ![]() $\epsilon _V = |V-V_0|/V_0$ is below
$\epsilon _V = |V-V_0|/V_0$ is below ![]() $0.03\,\%$ in all simulations. For suspensions, the measured quantities are obtained from an average over the number of particles and over time. In terms of strain units (
$0.03\,\%$ in all simulations. For suspensions, the measured quantities are obtained from an average over the number of particles and over time. In terms of strain units (![]() $\dot {\gamma }t$), our simulations reach convergence after the first
$\dot {\gamma }t$), our simulations reach convergence after the first ![]() $5$–
$5$–![]() $7\dot {\gamma }t$. The time average is performed after the initial transient state defined here as the first
$7\dot {\gamma }t$. The time average is performed after the initial transient state defined here as the first ![]() $10\dot {\gamma }t$. Time histories of the mean deformation and relative viscosity in the dilute and semi-dilute limits, together with the effect of mesh discretisation and finite size effects, are shown in appendix A. Details on the performance of our code can be found in Aouane et al. (Reference Aouane, Xie, Scagliarini and Harting2018).
$10\dot {\gamma }t$. Time histories of the mean deformation and relative viscosity in the dilute and semi-dilute limits, together with the effect of mesh discretisation and finite size effects, are shown in appendix A. Details on the performance of our code can be found in Aouane et al. (Reference Aouane, Xie, Scagliarini and Harting2018).
3. Results
3.1. Behaviour of a single capsule in a shear flow
In order to validate our approach, we first limit our simulations to the case of single initially spherical Skalak capsules. Our simulation domain is bounded by two parallel walls and the confinement is set to ![]() $\chi =2r/L=0.125$, so that the effect of the boundaries can be neglected. A schematic representation of the simulation setup, together with examples of steady state shapes for different
$\chi =2r/L=0.125$, so that the effect of the boundaries can be neglected. A schematic representation of the simulation setup, together with examples of steady state shapes for different ![]() $Ca$ and
$Ca$ and ![]() $C$, are depicted in figure 1. The steady Taylor deformation parameter, the inclination angle, the normal stress difference and the intrinsic viscosity, defined as
$C$, are depicted in figure 1. The steady Taylor deformation parameter, the inclination angle, the normal stress difference and the intrinsic viscosity, defined as
as functions of ![]() $Ca$ (for different values of
$Ca$ (for different values of ![]() $C$) are plotted in figure 2. We compare with numerical results obtained using the boundary element method (Lac et al. Reference Lac, Barthès-Biesel, Pelekasis and Tsamopoulos2004) and the front-tracking method (Bagchi & Kalluri Reference Bagchi and Kalluri2010), showing good agreement.
$C$) are plotted in figure 2. We compare with numerical results obtained using the boundary element method (Lac et al. Reference Lac, Barthès-Biesel, Pelekasis and Tsamopoulos2004) and the front-tracking method (Bagchi & Kalluri Reference Bagchi and Kalluri2010), showing good agreement.

Figure 2. Steady Taylor deformation parameter (a), inclination angle (b), intrinsic viscosity (c), and first normal stress difference (d) of a single capsule under a shear flow. The open and full symbols in (a,b) are for ![]() $C=1$ and
$C=1$ and ![]() $C=10$, respectively, and for
$C=10$, respectively, and for ![]() $C=1$ and
$C=1$ and ![]() $C=50$ in (c,d). BEM denotes the boundary element simulations of Lac et al. (Reference Lac, Barthès-Biesel, Pelekasis and Tsamopoulos2004), and FTM the front-tracking method of Bagchi & Kalluri (Reference Bagchi and Kalluri2010). LBM-IBM are the numerical results obtained using our lattice Boltzmann code. The dashed line in (c) indicates the value of the intrinsic viscosity of a dilute suspension of rigid spheres. The legends for (b,d) are indicated in (a,c), respectively.
$C=50$ in (c,d). BEM denotes the boundary element simulations of Lac et al. (Reference Lac, Barthès-Biesel, Pelekasis and Tsamopoulos2004), and FTM the front-tracking method of Bagchi & Kalluri (Reference Bagchi and Kalluri2010). LBM-IBM are the numerical results obtained using our lattice Boltzmann code. The dashed line in (c) indicates the value of the intrinsic viscosity of a dilute suspension of rigid spheres. The legends for (b,d) are indicated in (a,c), respectively.
We next move to explore systematically the parameter space spanned by ![]() $(Ca,C)$, with
$(Ca,C)$, with ![]() $Ca \in [0.1; 1]$ and
$Ca \in [0.1; 1]$ and ![]() $C \in [1; 7500]$. The corresponding data on steady-state elongation, inclination angle, intrinsic viscosity and first normal stress difference are reported in figure 3. We see, from the symbols in figure 3(c), at fixed
$C \in [1; 7500]$. The corresponding data on steady-state elongation, inclination angle, intrinsic viscosity and first normal stress difference are reported in figure 3. We see, from the symbols in figure 3(c), at fixed ![]() $C$, that
$C$, that ![]() $[\mu ]$ decreases with
$[\mu ]$ decreases with ![]() $Ca$, denoting a shear-thinning character. The latter, in turn, appears to be directly correlated to an increase of the elongation of the capsule (figure 3a) and a decrease of its orientation with respect to the flow direction (figure 3b), similarly to what has been reported for drops, vesicles and strain-softening capsules. Moreover, a clear effect of
$Ca$, denoting a shear-thinning character. The latter, in turn, appears to be directly correlated to an increase of the elongation of the capsule (figure 3a) and a decrease of its orientation with respect to the flow direction (figure 3b), similarly to what has been reported for drops, vesicles and strain-softening capsules. Moreover, a clear effect of ![]() $C$ on the various observables can be detected: as
$C$ on the various observables can be detected: as ![]() $C$ grows, their dependence on
$C$ grows, their dependence on ![]() $Ca$ gets weaker. In particular, the steady Taylor parameter
$Ca$ gets weaker. In particular, the steady Taylor parameter ![]() $D$ suggests that the particle is less and less deformed, i.e. it approaches the limit of a rigid sphere. Correspondingly,
$D$ suggests that the particle is less and less deformed, i.e. it approaches the limit of a rigid sphere. Correspondingly, ![]() $[\mu ]$ varies less and less with
$[\mu ]$ varies less and less with ![]() $Ca$ and eventually the shear-thinning is suppressed. For sufficiently large
$Ca$ and eventually the shear-thinning is suppressed. For sufficiently large ![]() $C$, then, a very dilute suspension of strain-hardening capsules tends to behave rheologically as a suspension of rigid spheres (note also that
$C$, then, a very dilute suspension of strain-hardening capsules tends to behave rheologically as a suspension of rigid spheres (note also that ![]() $N_1$ tends to zero, figure 3d), albeit with an intrinsic viscosity surprisingly slightly larger than the Einstein coefficient
$N_1$ tends to zero, figure 3d), albeit with an intrinsic viscosity surprisingly slightly larger than the Einstein coefficient ![]() $[\mu ]=5/2$ (for
$[\mu ]=5/2$ (for ![]() $C \gg 1$,
$C \gg 1$, ![]() $[\mu ]$ seems to approach the limiting value
$[\mu ]$ seems to approach the limiting value ![]() ${\approx }2.84$; see also Bagchi & Kalluri (Reference Bagchi and Kalluri2010) for comparison).
${\approx }2.84$; see also Bagchi & Kalluri (Reference Bagchi and Kalluri2010) for comparison).

Figure 3. Effect of the membrane area incompressibility on the steady-state Taylor deformation parameter (a), inclination angle (b), intrinsic viscosity (c) and first normal stress difference (d) of a single Skalak capsule under a shear flow for different capillary numbers. The legend is given in (a).
3.2. Suspensions: structure
We investigate the multiparticle case and the dependence of particle shape and suspension rheological properties on the parameters describing the system, namely ![]() $Ca$,
$Ca$, ![]() $C$ and
$C$ and ![]() $\phi$. Examples of steady-state configurations of the suspension are shown in figure 4, for fixed
$\phi$. Examples of steady-state configurations of the suspension are shown in figure 4, for fixed ![]() $Ca=1$,
$Ca=1$, ![]() $\phi =0.5$ and for four different values of
$\phi =0.5$ and for four different values of ![]() $C=0.1,10,150$ and
$C=0.1,10,150$ and ![]() $7500$.
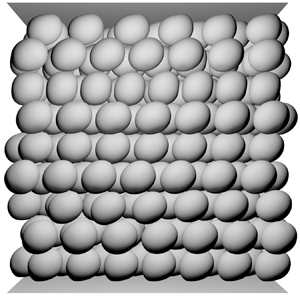
$7500$.

Figure 4. Steady-state configurations for ![]() $\phi =0.5$,
$\phi =0.5$, ![]() $Ca=1$ and (a)
$Ca=1$ and (a) ![]() $C=0.1$, (b)
$C=0.1$, (b) ![]() $C=10$, (c)
$C=10$, (c) ![]() $C=1.5 \times 10^{2}$ and (d)
$C=1.5 \times 10^{2}$ and (d) ![]() $C=7.5 \times 10^{3}$.
$C=7.5 \times 10^{3}$.
In this subsection we focus on particle morphologies, characterised in terms of the Taylor deformation parameter and the semi-axes of the equivalent ellipsoid, whereas in the next we study the rheological properties of the suspensions, highlighting their relations with the structure. Analogously to the single-particle case, the mean Taylor parameter ![]() $\langle D \rangle$ of the suspension decreases with increasing
$\langle D \rangle$ of the suspension decreases with increasing ![]() $C$ (figure 5a), with a steepest descent for
$C$ (figure 5a), with a steepest descent for ![]() $1 < C < 10^{3}$, which confirms that capsules become less deformable and tend to resemble rigid particles. We recall, though, that such a parameter contains information only on two of the three semi-axes of the equivalent ellipsoid. To get a deeper insight, then, we present all three of them separately in figure 5(b–d). Interestingly, a non-monotonic behaviour is found for
$1 < C < 10^{3}$, which confirms that capsules become less deformable and tend to resemble rigid particles. We recall, though, that such a parameter contains information only on two of the three semi-axes of the equivalent ellipsoid. To get a deeper insight, then, we present all three of them separately in figure 5(b–d). Interestingly, a non-monotonic behaviour is found for ![]() $\langle r_1 \rangle$ and
$\langle r_1 \rangle$ and ![]() $\langle r_2 \rangle$ (the semi-axes in the flow and vorticity directions, respectively) for
$\langle r_2 \rangle$ (the semi-axes in the flow and vorticity directions, respectively) for ![]() $0.1<C<10$. In particular, for decreasing
$0.1<C<10$. In particular, for decreasing ![]() $C<10$,
$C<10$, ![]() $\langle r_2 \rangle$, whose direction is orthogonal to the elongational one, grows. Moreover,
$\langle r_2 \rangle$, whose direction is orthogonal to the elongational one, grows. Moreover, ![]() $\langle r_2 \rangle$ always remains larger than
$\langle r_2 \rangle$ always remains larger than ![]() $\langle r_3 \rangle$, indicating that particles, on average, are not spheroids (eventually, for very large
$\langle r_3 \rangle$, indicating that particles, on average, are not spheroids (eventually, for very large ![]() $C$ particles approach the undeformable limit and the quasi-spherical shape,
$C$ particles approach the undeformable limit and the quasi-spherical shape, ![]() $\langle r_i \rangle \rightarrow r$ for
$\langle r_i \rangle \rightarrow r$ for ![]() $i=1,2,3$, is recovered). In this sense, capsules display a lower level of symmetry than droplets, which is to be attributed to the nonlinear elastic characteristics of the membrane. The behaviour, in fact, persists across the various volume fractions explored, even for the lowest
$i=1,2,3$, is recovered). In this sense, capsules display a lower level of symmetry than droplets, which is to be attributed to the nonlinear elastic characteristics of the membrane. The behaviour, in fact, persists across the various volume fractions explored, even for the lowest ![]() $\phi$ (corresponding to the single-particle case), suggesting that the effect originates from the properties of the single-particle stress tensor. The spread of the mean principal semi-axes, mostly of
$\phi$ (corresponding to the single-particle case), suggesting that the effect originates from the properties of the single-particle stress tensor. The spread of the mean principal semi-axes, mostly of ![]() $\langle r_1 \rangle$, is such that the product
$\langle r_1 \rangle$, is such that the product ![]() $\prod _{i=1}^{3} \langle r_i \rangle$ varies with the volume fraction. Such dependence is related to the variances of the distributions of the principal semi-axes (given that the mean volume of capsules,
$\prod _{i=1}^{3} \langle r_i \rangle$ varies with the volume fraction. Such dependence is related to the variances of the distributions of the principal semi-axes (given that the mean volume of capsules, ![]() $\langle \prod _{i=1}^{3} r_i \rangle$ is conserved) and reflects, therefore, the spread in sizes, which decreases as
$\langle \prod _{i=1}^{3} r_i \rangle$ is conserved) and reflects, therefore, the spread in sizes, which decreases as ![]() $C$ increases, because the capsules become more and more rigid (in other words, the distribution tends to become sharply peaked around
$C$ increases, because the capsules become more and more rigid (in other words, the distribution tends to become sharply peaked around ![]() $r_1 \sim r_2 \sim r_3 \sim r$).
$r_1 \sim r_2 \sim r_3 \sim r$).

Figure 5. (a) Steady-state Taylor deformation parameter. (b)–(d) Steady-state mean semi-axes of the capsules normalised by the reference radius as function of ![]() $C$ for different values of
$C$ for different values of ![]() $\phi$. The legend is indicated in (a). Here
$\phi$. The legend is indicated in (a). Here ![]() $Ca=1$.
$Ca=1$.
Next, we consider how the particle deformation depends on the applied load, for given material properties. It is tempting, first, to investigate how the peculiar behaviour for small/moderate ![]() $C$ shows up across different shear values. In figure 6(a–c) we report the variation of
$C$ shows up across different shear values. In figure 6(a–c) we report the variation of ![]() $r_i$ for single capsules with
$r_i$ for single capsules with ![]() $C=0.1,1$ and
$C=0.1,1$ and ![]() $10$, respectively, as a function of
$10$, respectively, as a function of ![]() $Ca$. As
$Ca$. As ![]() $Ca$ increases, the capsules become, obviously, more elongated in the extensional flow direction (
$Ca$ increases, the capsules become, obviously, more elongated in the extensional flow direction (![]() $r_1$ grows), but the two minor semi-axes display opposite trends, depending on the value of
$r_1$ grows), but the two minor semi-axes display opposite trends, depending on the value of ![]() $C$: whereas
$C$: whereas ![]() $r_3$, as expected, decreases (for all
$r_3$, as expected, decreases (for all ![]() $C$),
$C$), ![]() $r_2$ (that aligned with the vorticity direction) grows for
$r_2$ (that aligned with the vorticity direction) grows for ![]() $C<1$. For the smallest
$C<1$. For the smallest ![]() $C$ considered,
$C$ considered, ![]() $C=0.1$, we need, therefore, to find an equivalent breadth,
$C=0.1$, we need, therefore, to find an equivalent breadth, ![]() $r_{\perp }^{{{(eq)}}}$ quantifying the degree of shrinkage or expansion of the capsule in the equatorial plane. We define this
$r_{\perp }^{{{(eq)}}}$ quantifying the degree of shrinkage or expansion of the capsule in the equatorial plane. We define this ![]() $r_{\perp }^{{{(eq)}}}$ as the ratio of the length of the membrane cross-section (an ellipse) over
$r_{\perp }^{{{(eq)}}}$ as the ratio of the length of the membrane cross-section (an ellipse) over ![]() $2{\rm \pi}$ (such that it would be precisely the minor axis, if the capsule were a prolate spheroid), i.e.
$2{\rm \pi}$ (such that it would be precisely the minor axis, if the capsule were a prolate spheroid), i.e. ![]() $r_{\perp }^{{{(eq)}}} = {4 r_2 {\mathcal {E}}(\epsilon (r_2,r_3))}/{2 {\rm \pi}}$, where
$r_{\perp }^{{{(eq)}}} = {4 r_2 {\mathcal {E}}(\epsilon (r_2,r_3))}/{2 {\rm \pi}}$, where ![]() ${\mathcal {E}}(x)$ is the complete elliptic integral of the second kind (Abramowitz & Stegun Reference Abramowitz and Stegun2012) and
${\mathcal {E}}(x)$ is the complete elliptic integral of the second kind (Abramowitz & Stegun Reference Abramowitz and Stegun2012) and ![]() $\epsilon (r_2,r_3) = \sqrt {1-({r_3}/{r_2})^{2}}$ is the eccentricity of the ellipse. In figure 6(d), we plot the transversal deformation, represented by
$\epsilon (r_2,r_3) = \sqrt {1-({r_3}/{r_2})^{2}}$ is the eccentricity of the ellipse. In figure 6(d), we plot the transversal deformation, represented by ![]() $r_{\perp }^{{{(eq)}}}$ versus the elongation,
$r_{\perp }^{{{(eq)}}}$ versus the elongation, ![]() $r_{||} \equiv r_1$, both normalised by the rest radius
$r_{||} \equiv r_1$, both normalised by the rest radius ![]() $r$, for the capsule with
$r$, for the capsule with ![]() $C=0.1$. It can be seen that, although
$C=0.1$. It can be seen that, although ![]() $r_{\perp }^{{{(eq)}}}/r$ never exceeds
$r_{\perp }^{{{(eq)}}}/r$ never exceeds ![]() $1$, i.e. overall the membrane cross-section shrinks with respect to the equilibrium shape, there is a range of
$1$, i.e. overall the membrane cross-section shrinks with respect to the equilibrium shape, there is a range of ![]() $Ca$ for which it expands as the capsule is elongated. This is an intriguing behaviour, in fact the opposite of the slope of the curve in figure 6(d),
$Ca$ for which it expands as the capsule is elongated. This is an intriguing behaviour, in fact the opposite of the slope of the curve in figure 6(d), ![]() $\tilde {\nu }_s = -{\textrm {d} r_{\perp }^{{{(eq)}}}}/{\textrm {d} r_{||}}$, can be interpreted as a local Poisson's ratio, which is negative for
$\tilde {\nu }_s = -{\textrm {d} r_{\perp }^{{{(eq)}}}}/{\textrm {d} r_{||}}$, can be interpreted as a local Poisson's ratio, which is negative for ![]() $1.3 r \lesssim r_{||} \lesssim 1.7 r$. This observation hints at a sort of local (in shear) ‘auxeticity’ (Evans et al. Reference Evans, Nkansah, Hutchinson and Rogers1991) of membranes obeying a Skalak-type constitutive law with low values of the membrane inextensibility parameter.
$1.3 r \lesssim r_{||} \lesssim 1.7 r$. This observation hints at a sort of local (in shear) ‘auxeticity’ (Evans et al. Reference Evans, Nkansah, Hutchinson and Rogers1991) of membranes obeying a Skalak-type constitutive law with low values of the membrane inextensibility parameter.

Figure 6. (a)–(c) Steady-state semi-axes (normalised by the reference radius of the initially spherical stress-free capsule) as a function of ![]() $Ca$ for capsules with
$Ca$ for capsules with ![]() $C=0.1,1$ and
$C=0.1,1$ and ![]() $10$, respectively (the legend is indicated in (a)). (d) Transversal deformation versus elongation (normalised by the reference radius) for capsules with
$10$, respectively (the legend is indicated in (a)). (d) Transversal deformation versus elongation (normalised by the reference radius) for capsules with ![]() $C=0.1$, and
$C=0.1$, and ![]() $Ca \in [0.1; 7.5]$. Open symbols refer to
$Ca \in [0.1; 7.5]$. Open symbols refer to ![]() $\phi =0.1$ (semi-dilute suspension), whereas full symbols to
$\phi =0.1$ (semi-dilute suspension), whereas full symbols to ![]() $\phi =0.001$ (dilute suspension).
$\phi =0.001$ (dilute suspension).
In figure 7(a) we show the average Taylor deformation parameter as function of ![]() $Ca$, for various
$Ca$, for various ![]() $\phi$. Two sets of data corresponding to
$\phi$. Two sets of data corresponding to ![]() $C=50$ (closed symbols) and
$C=50$ (closed symbols) and ![]() $C=150$ (open symbols) are reported. The deformation grows as
$C=150$ (open symbols) are reported. The deformation grows as ![]() $\langle D \rangle \sim Ca$ for small capillary numbers, as expected, and then sublinearly as the
$\langle D \rangle \sim Ca$ for small capillary numbers, as expected, and then sublinearly as the ![]() $Ca$ increases (eventually we observe a logarithmic dependence
$Ca$ increases (eventually we observe a logarithmic dependence ![]() $\langle D \rangle \sim \log (Ca)$, in agreement with previous numerical Dodson III & Dimitrakopoulos (Reference Dodson and Dimitrakopoulos2010) and experimental Hardeman et al. (Reference Hardeman, Goedhart, Dobbe and Lettinga1994) findings), reflecting the strain-hardening character of the capsules. It can be asked whether one may find a functional form that allows to recast the variability among curves into a single curve-shape parameter,
$\langle D \rangle \sim \log (Ca)$, in agreement with previous numerical Dodson III & Dimitrakopoulos (Reference Dodson and Dimitrakopoulos2010) and experimental Hardeman et al. (Reference Hardeman, Goedhart, Dobbe and Lettinga1994) findings), reflecting the strain-hardening character of the capsules. It can be asked whether one may find a functional form that allows to recast the variability among curves into a single curve-shape parameter, ![]() $Ca^{*}(\phi ,C)$, that is
$Ca^{*}(\phi ,C)$, that is
where ![]() $A$ is a constant prefactor and the function
$A$ is a constant prefactor and the function ![]() $g(x)$ has to be chosen such that it reproduces and connects both behaviours at small and large
$g(x)$ has to be chosen such that it reproduces and connects both behaviours at small and large ![]() $Ca$, that is
$Ca$, that is ![]() $g(x) \sim x$ as
$g(x) \sim x$ as ![]() $x \rightarrow 0$ and
$x \rightarrow 0$ and ![]() $g(x) \sim \log (x)$ for
$g(x) \sim \log (x)$ for ![]() $x \gg 1$. This is indeed possible and we show it in figure 7(a). There, the fits, indicated by the dashed lines, are obtained from (3.2), choosing for the function
$x \gg 1$. This is indeed possible and we show it in figure 7(a). There, the fits, indicated by the dashed lines, are obtained from (3.2), choosing for the function ![]() $g$ the expression
$g$ the expression ![]() $g(x) = \log (1+x)$, with the same
$g(x) = \log (1+x)$, with the same ![]() $A=0.1$ and, from bottom to top,
$A=0.1$ and, from bottom to top, ![]() $Ca^{*} = 0.13$,
$Ca^{*} = 0.13$, ![]() $Ca^{*}=0.07$ and
$Ca^{*}=0.07$ and ![]() $Ca^{*}=0.037$, respectively. For a fixed capillary number,
$Ca^{*}=0.037$, respectively. For a fixed capillary number, ![]() $\langle D \rangle$ increases linearly with the volume fraction
$\langle D \rangle$ increases linearly with the volume fraction ![]() $\phi$, similarly to suspensions of drops and neo-Hookean capsules (Loewenberg & Hinch Reference Loewenberg and Hinch1996; Matsunaga et al. Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016). Conversely, the larger is the membrane inextensibility
$\phi$, similarly to suspensions of drops and neo-Hookean capsules (Loewenberg & Hinch Reference Loewenberg and Hinch1996; Matsunaga et al. Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016). Conversely, the larger is the membrane inextensibility ![]() $C$, the less deformed are the capsules.
$C$, the less deformed are the capsules.

Figure 7. (a) Mean Taylor deformation parameter for a suspension of strain-hardening capsules as a function of ![]() $Ca$, for two values of membrane inextensibility,
$Ca$, for two values of membrane inextensibility, ![]() $C=50$ (filled symbols) and
$C=50$ (filled symbols) and ![]() $C=150$ (open symbols). The dashed lines are fits of the numerical data using (3.2) with
$C=150$ (open symbols). The dashed lines are fits of the numerical data using (3.2) with ![]() $A=0.1$ and (from bottom to top)
$A=0.1$ and (from bottom to top) ![]() $Ca^{*}=0.13$,
$Ca^{*}=0.13$, ![]() $Ca^{*}=0.07$ and
$Ca^{*}=0.07$ and ![]() $Ca^{*}=0.037$, respectively. The arrow indicates a growing volume fraction
$Ca^{*}=0.037$, respectively. The arrow indicates a growing volume fraction ![]() $\phi$. (b) Mean Taylor deformation parameter as a function of the effective capillary number (3.4). The dashed line corresponds to the fitting function (3.2) (
$\phi$. (b) Mean Taylor deformation parameter as a function of the effective capillary number (3.4). The dashed line corresponds to the fitting function (3.2) (![]() $A_{{{eff}}}=0.1$ and
$A_{{{eff}}}=0.1$ and ![]() $Ca_{{{eff}}}^{*}=4 \times 10^{-3}$). Inset: Lin-Log plot of
$Ca_{{{eff}}}^{*}=4 \times 10^{-3}$). Inset: Lin-Log plot of ![]() $\langle D \rangle$ vs
$\langle D \rangle$ vs ![]() $Ca_{{{eff}}}$, highlighting the logarithmic behaviour for
$Ca_{{{eff}}}$, highlighting the logarithmic behaviour for ![]() $Ca_{{{eff}}}>Ca_{{{eff}}}^{*}$. The legend is indicated in (b).
$Ca_{{{eff}}}>Ca_{{{eff}}}^{*}$. The legend is indicated in (b).
Given the self-similar form of (3.2), we would like to find a universal curve for ![]() $\langle D \rangle$, through a proper definition of an effective capillary number
$\langle D \rangle$, through a proper definition of an effective capillary number ![]() $Ca_{{{eff}}}$. The enhancement of deformation with
$Ca_{{{eff}}}$. The enhancement of deformation with ![]() $\phi$ can be interpreted as an effect of larger viscous stresses around the particle, owing to the fact that the effective viscosity of the suspension increases with the volume fraction (this aspect will be discussed in more detail in the next subsection). This suggests that we should replace, in
$\phi$ can be interpreted as an effect of larger viscous stresses around the particle, owing to the fact that the effective viscosity of the suspension increases with the volume fraction (this aspect will be discussed in more detail in the next subsection). This suggests that we should replace, in ![]() $Ca_{{{eff}}}$, the ‘bare’ dynamic viscosity with the effective one,
$Ca_{{{eff}}}$, the ‘bare’ dynamic viscosity with the effective one, ![]() $\mu _0 \rightarrow \mu _{{{eff}}} = \mu _0(1+[\mu ]\phi )$. Here we assume, for simplicity, linearity in
$\mu _0 \rightarrow \mu _{{{eff}}} = \mu _0(1+[\mu ]\phi )$. Here we assume, for simplicity, linearity in ![]() $\phi$ and a constant (with
$\phi$ and a constant (with ![]() $Ca$ and
$Ca$ and ![]() $C$) intrinsic viscosity, equal to its large
$C$) intrinsic viscosity, equal to its large ![]() $C$ limit,
$C$ limit, ![]() $[\mu ] \approx 2.8$ (see § 3.1).
$[\mu ] \approx 2.8$ (see § 3.1).
Furthermore, we note that for a non-zero membrane inextensibility, it is more appropriate to base the capillary number on the Young's modulus instead of the shear modulus (Barthès-Biesel & Rallison Reference Barthès-Biesel and Rallison1981). We propose to replace ![]() $G_s$ with
$G_s$ with ![]() $E_s=({(2+4C)}/{(1+C)})G_s$. However, this might not be sufficient. In fact, the imposed constraint of volume conservation, for a spherical equilibrium shape, effectively entails an extra tension on the surface, because the capsules tend to become essentially undeformable as the area dilatation modulus is increased. This can be accounted for by the substitution
$E_s=({(2+4C)}/{(1+C)})G_s$. However, this might not be sufficient. In fact, the imposed constraint of volume conservation, for a spherical equilibrium shape, effectively entails an extra tension on the surface, because the capsules tend to become essentially undeformable as the area dilatation modulus is increased. This can be accounted for by the substitution
(![]() $\beta$ being a free parameter, which we set hereafter to
$\beta$ being a free parameter, which we set hereafter to ![]() $\alpha =0.07$) in the effective capillary number, which then finally reads
$\alpha =0.07$) in the effective capillary number, which then finally reads
When plotted as a function of ![]() $Ca_{{{eff}}}$, the values of
$Ca_{{{eff}}}$, the values of ![]() $\langle D \rangle$ for different
$\langle D \rangle$ for different ![]() $\phi$ and
$\phi$ and ![]() $C$ collapse onto a single master curve, as shown in figure 7(b). Such a curve can also be fitted using (3.2), with
$C$ collapse onto a single master curve, as shown in figure 7(b). Such a curve can also be fitted using (3.2), with ![]() $A=0.1$ and
$A=0.1$ and ![]() $Ca_{{{eff}}}^{*}=4 \times 10^{-3}$. Note that the existence of a single
$Ca_{{{eff}}}^{*}=4 \times 10^{-3}$. Note that the existence of a single ![]() $Ca_{{{eff}}}^{*}$ capable to fit all data sets upon the rescaling (3.4) is equivalent to saying that the dependence of the curve-shape parameter
$Ca_{{{eff}}}^{*}$ capable to fit all data sets upon the rescaling (3.4) is equivalent to saying that the dependence of the curve-shape parameter ![]() $Ca^{*}$ on
$Ca^{*}$ on ![]() $Ca$ and
$Ca$ and ![]() $C$ is such that
$C$ is such that ![]() $Ca^{*} \propto {G_s^{{{(eff)}}}(C)}/{\mu _{{{eff}}}(\phi )}$.
$Ca^{*} \propto {G_s^{{{(eff)}}}(C)}/{\mu _{{{eff}}}(\phi )}$.
3.3. Suspensions: rheology
We now consider the rheological response of the system by looking at the suspension relative viscosity and normal stress differences. In figure 8, we plot ![]() $N_1$ and
$N_1$ and ![]() $N_2$ (normalised by
$N_2$ (normalised by ![]() $\mu _0 \dot {\gamma }$), for various volume fractions, for
$\mu _0 \dot {\gamma }$), for various volume fractions, for ![]() $Ca=1$ as a function of the membrane inextensibility (figure 8a,b) and for
$Ca=1$ as a function of the membrane inextensibility (figure 8a,b) and for ![]() $C=1$ as a function of the capillary number (figure 8c,d). We see that both (though
$C=1$ as a function of the capillary number (figure 8c,d). We see that both (though ![]() $N_2$ only weakly) show a non-monotonic dependence on
$N_2$ only weakly) show a non-monotonic dependence on ![]() $C$, a behaviour that is enhanced as
$C$, a behaviour that is enhanced as ![]() $\phi$ is increased. In particular,
$\phi$ is increased. In particular, ![]() $N_1$ initially grows with
$N_1$ initially grows with ![]() $C$, reaches a peak at
$C$, reaches a peak at ![]() $C \approx 10$, and then starts to decrease. For emulsions and strain-softening capsules, the magnitude of
$C \approx 10$, and then starts to decrease. For emulsions and strain-softening capsules, the magnitude of ![]() $N_1$ is significantly larger than the magnitude of
$N_1$ is significantly larger than the magnitude of ![]() $N_2$, whereas the opposite is true for suspensions of rigid particles. Here
$N_2$, whereas the opposite is true for suspensions of rigid particles. Here ![]() $N_1$ and
$N_1$ and ![]() $N_2$ are known to be correlated to hydrodynamic interaction, and particle–particle collisions, respectively (Guazzelli & Pouliquen Reference Guazzelli and Pouliquen2018). We find that for strain-hardening capsules
$N_2$ are known to be correlated to hydrodynamic interaction, and particle–particle collisions, respectively (Guazzelli & Pouliquen Reference Guazzelli and Pouliquen2018). We find that for strain-hardening capsules ![]() $N_1$ is positive and grows monotonically with
$N_1$ is positive and grows monotonically with ![]() $Ca$, whereas
$Ca$, whereas ![]() $N_2$ has a negative sign and decreases with
$N_2$ has a negative sign and decreases with ![]() $Ca$ (in qualitative agreement with what found for suspensions of strain-softening capsules Matsunaga et al. Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016). The magnitude of
$Ca$ (in qualitative agreement with what found for suspensions of strain-softening capsules Matsunaga et al. Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016). The magnitude of ![]() $N_1$ is always larger than
$N_1$ is always larger than ![]() $N_2$, but the ratio
$N_2$, but the ratio ![]() $|N_1|/|N_2|$ diminishes with the increase of
$|N_1|/|N_2|$ diminishes with the increase of ![]() $C$. It seems, therefore, in principle possible, by tuning their deformability through
$C$. It seems, therefore, in principle possible, by tuning their deformability through ![]() $C$, to make collections of such soft particles behave rheologically more as solid suspensions or as emulsions and suspensions of strain-softening capsules.
$C$, to make collections of such soft particles behave rheologically more as solid suspensions or as emulsions and suspensions of strain-softening capsules.

Figure 8. Normal stress differences (normalised by the dynamics viscosity of the fluid times the applied shear) as a function of ![]() $C$ for
$C$ for ![]() $Ca=1$ (a,b) and as function of
$Ca=1$ (a,b) and as function of ![]() $Ca$ for
$Ca$ for ![]() $C=1$ (c,d). The legend is shown in (a).
$C=1$ (c,d). The legend is shown in (a).
To delve deeper into this aspect, we study how the dependence of the relative viscosity of the suspension on the capsule volume fraction changes with ![]() $C$. We report in figure 9 the behaviour of
$C$. We report in figure 9 the behaviour of ![]() $\mu _r$ versus
$\mu _r$ versus ![]() $\phi$ for
$\phi$ for ![]() $Ca=0.5$ and various
$Ca=0.5$ and various ![]() $C$. The relative viscosity of a suspension can be expressed as a polynomial function of the volume fraction as
$C$. The relative viscosity of a suspension can be expressed as a polynomial function of the volume fraction as
where ![]() $[\mu ]$ is the intrinsic viscosity and the second-order term accounts for pair hydrodynamic interactions and allows to expand the validity of (3.5) to semi-dilute cases (Batchelor & Green Reference Batchelor and Green1972). All data sets in figure 9 can be reasonably well fitted by a quadratic relation of the type (3.5). As
$[\mu ]$ is the intrinsic viscosity and the second-order term accounts for pair hydrodynamic interactions and allows to expand the validity of (3.5) to semi-dilute cases (Batchelor & Green Reference Batchelor and Green1972). All data sets in figure 9 can be reasonably well fitted by a quadratic relation of the type (3.5). As ![]() $C$ increases, the data tend to approach a limiting curve with
$C$ increases, the data tend to approach a limiting curve with ![]() $[\mu ]=2.84$ and
$[\mu ]=2.84$ and ![]() $K=13.5$ (dashed line), whereas for low
$K=13.5$ (dashed line), whereas for low ![]() $C$ they tend to agree well with the curve (dotted line) for strain-softening particles reported in Matsunaga et al. (Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016).
$C$ they tend to agree well with the curve (dotted line) for strain-softening particles reported in Matsunaga et al. (Reference Matsunaga, Imai, Yamaguchi and Ishikawa2016).

Figure 9. Relative viscosity as function of the volume fraction for several values of ![]() $C$ and
$C$ and ![]() $Ca=0.5$. The curves correspond to (3.5) with
$Ca=0.5$. The curves correspond to (3.5) with ![]() $[\mu ]=1.63$,
$[\mu ]=1.63$, ![]() $K=0.64$ (dotted line) and
$K=0.64$ (dotted line) and ![]() $[\mu ]=2.84$,
$[\mu ]=2.84$, ![]() $K=13.5$ (dashed line), respectively.
$K=13.5$ (dashed line), respectively.
In figure 10(a) we extend the study of the relative viscosity to a range of capillary numbers, ![]() $Ca \in [0.1, 0.5]$, in order to analyse the response of the system to changes in the applied shear. The shear-thinning character of the suspension of capsules can be appreciated: for a given volume fraction, in fact,
$Ca \in [0.1, 0.5]$, in order to analyse the response of the system to changes in the applied shear. The shear-thinning character of the suspension of capsules can be appreciated: for a given volume fraction, in fact, ![]() $\mu _r$ tends to decrease with
$\mu _r$ tends to decrease with ![]() $Ca$ (the larger
$Ca$ (the larger ![]() $\phi$ the more evident is the shear-thinning), but the spread is reduced for larger
$\phi$ the more evident is the shear-thinning), but the spread is reduced for larger ![]() $C$, i.e. the shear-thinning tends to be suppressed, confirming that also for semi-dilute and moderately concentrated regimes, the rheology of suspensions of strain-hardening capsules resemble that of solid suspensions. In order to find a universal behaviour of the relative viscosity across the various shear rates, recently Rosti, Brandt & Mitra (Reference Rosti, Brandt and Mitra2018) introduced, for suspensions of deformable viscoelastic spheres (and Takeishi et al. (Reference Takeishi, Rosti, Imai, Wada and Brandt2019) extended to the case of red blood cells), the notion of a reduced effective volume fraction, calculated using for the particle volume (when they are in the deformed state), that of a sphere with a radius equal to the smallest particle semi-axis
$C$, i.e. the shear-thinning tends to be suppressed, confirming that also for semi-dilute and moderately concentrated regimes, the rheology of suspensions of strain-hardening capsules resemble that of solid suspensions. In order to find a universal behaviour of the relative viscosity across the various shear rates, recently Rosti, Brandt & Mitra (Reference Rosti, Brandt and Mitra2018) introduced, for suspensions of deformable viscoelastic spheres (and Takeishi et al. (Reference Takeishi, Rosti, Imai, Wada and Brandt2019) extended to the case of red blood cells), the notion of a reduced effective volume fraction, calculated using for the particle volume (when they are in the deformed state), that of a sphere with a radius equal to the smallest particle semi-axis ![]() $r_3$, i.e.
$r_3$, i.e. ![]() $\varPhi _{{{eff}}} = \frac {4}{3}{\rm \pi} r_3^{3}$. The rationale behind this approach is that the dynamically ‘active’ direction is the velocity gradient (wall-normal direction) and, because the deformed particles do not tumble and tend to align with the flow direction, then the relevant length is the minor axis. For
$\varPhi _{{{eff}}} = \frac {4}{3}{\rm \pi} r_3^{3}$. The rationale behind this approach is that the dynamically ‘active’ direction is the velocity gradient (wall-normal direction) and, because the deformed particles do not tumble and tend to align with the flow direction, then the relevant length is the minor axis. For ![]() $r_3$, the average value as measured in the simulations was taken. Here, we propose to relate
$r_3$, the average value as measured in the simulations was taken. Here, we propose to relate ![]() $r_3$ to the radius at rest
$r_3$ to the radius at rest ![]() $r$ through the Taylor deformation parameter
$r$ through the Taylor deformation parameter ![]() $D$. To this aim, let us assume the particles to be prolate ellipsoids with
$D$. To this aim, let us assume the particles to be prolate ellipsoids with ![]() $r_1 > r_2 = r_3$ (this is an approximation that allows to close the problem, although we know that the specific relation among the three axes depends on the value of membrane inextensibility
$r_1 > r_2 = r_3$ (this is an approximation that allows to close the problem, although we know that the specific relation among the three axes depends on the value of membrane inextensibility ![]() $C$). Here
$C$). Here ![]() $r_1$ and
$r_1$ and ![]() $r_3$ enter into the expression of
$r_3$ enter into the expression of ![]() $D$ (2.20), that can be inverted as
$D$ (2.20), that can be inverted as
Owing to volume conservation we have ![]() $r^{3} = r_1r_3^{2}$ and therefore
$r^{3} = r_1r_3^{2}$ and therefore ![]() $r^{3} = (({1+D})/({1-D}))r_3^{3}$, which implies
$r^{3} = (({1+D})/({1-D}))r_3^{3}$, which implies
 \begin{equation} r_3 = \left(\frac{1-D}{1+D}\right)^{1/3}r. \end{equation}
\begin{equation} r_3 = \left(\frac{1-D}{1+D}\right)^{1/3}r. \end{equation}
If we now define, as in Rosti et al. (Reference Rosti, Brandt and Mitra2018), the effective volume fraction as ![]() $\varPhi _{{{eff}}} \equiv \frac {4}{3}{\rm \pi} \langle r_3 \rangle ^{3} n$ and assume (3.7) to be valid also for average quantities in the suspension, we obtain
$\varPhi _{{{eff}}} \equiv \frac {4}{3}{\rm \pi} \langle r_3 \rangle ^{3} n$ and assume (3.7) to be valid also for average quantities in the suspension, we obtain
As we learned from the previous section, ![]() $\langle D \rangle$, in turn, depends on
$\langle D \rangle$, in turn, depends on ![]() $\phi$, on the capillary number and on
$\phi$, on the capillary number and on ![]() $C$. If we plug (3.2) inside (3.8), with the rescaled effective capillary number, (3.4), and we plot
$C$. If we plug (3.2) inside (3.8), with the rescaled effective capillary number, (3.4), and we plot ![]() $\mu _r$ as a function of the obtained
$\mu _r$ as a function of the obtained ![]() $\varPhi _{{{eff}}}$, we observe a reasonable overlap of the data, although with some deviations, especially for the largest values of
$\varPhi _{{{eff}}}$, we observe a reasonable overlap of the data, although with some deviations, especially for the largest values of ![]() $C$ (see inset of figure 10a). We ascribe this partial failure to the fact that our strain-hardening capsules are not precisely aligned with the flow direction (see figure 2b). Consequently, the relevant, flow-orthogonal, length is not exactly
$C$ (see inset of figure 10a). We ascribe this partial failure to the fact that our strain-hardening capsules are not precisely aligned with the flow direction (see figure 2b). Consequently, the relevant, flow-orthogonal, length is not exactly ![]() $r_3$, but the vertical semi-axis of the ellipse, resulting as the section of the particle ellipsoid on a plane perpendicular to the flow direction (and crossing its centre). It is easy to show, by geometrical arguments, that such length is
$r_3$, but the vertical semi-axis of the ellipse, resulting as the section of the particle ellipsoid on a plane perpendicular to the flow direction (and crossing its centre). It is easy to show, by geometrical arguments, that such length is ![]() $\ell = r_3 \sqrt {(({1+b^{2}})/({1+({r_3}/{r_1})^{2}b^{2}}) ) }$, where
$\ell = r_3 \sqrt {(({1+b^{2}})/({1+({r_3}/{r_1})^{2}b^{2}}) ) }$, where ![]() $b=\tan (\theta )$, and
$b=\tan (\theta )$, and ![]() $\theta$ is the inclination angle. If we define the effective volume fraction in terms of this length (and recalling that
$\theta$ is the inclination angle. If we define the effective volume fraction in terms of this length (and recalling that ![]() $r_3/r_1 = (1-D)/(1+D)$), equation (3.8) becomes
$r_3/r_1 = (1-D)/(1+D)$), equation (3.8) becomes
 \begin{equation} \varPhi_{{{eff}}} = \frac{1-\langle D \rangle}{1+\langle D \rangle} \left(\frac{1+b^{2}}{1+\left(\dfrac{1-\langle D \rangle}{1+ \langle D \rangle}\right)^{2}b^{2}}\right)^{3/2}\phi. \end{equation}
\begin{equation} \varPhi_{{{eff}}} = \frac{1-\langle D \rangle}{1+\langle D \rangle} \left(\frac{1+b^{2}}{1+\left(\dfrac{1-\langle D \rangle}{1+ \langle D \rangle}\right)^{2}b^{2}}\right)^{3/2}\phi. \end{equation}
The parameter ![]() $b$ itself depends, through
$b$ itself depends, through ![]() $\theta$, on
$\theta$, on ![]() $Ca$ and
$Ca$ and ![]() $C$. However, for simplicity, we consider it here as a fit constant, taking for
$C$. However, for simplicity, we consider it here as a fit constant, taking for ![]() $\theta$ values restricted to the range in figure 2(b). In particular, for
$\theta$ values restricted to the range in figure 2(b). In particular, for ![]() $\theta = {{\rm \pi} }/{5}$ (
$\theta = {{\rm \pi} }/{5}$ (![]() $b\approx 0.726$), we get a nice collapse of all data sets onto a single master curve which can be well fitted, among others, with an Eilers function (Eilers Reference von Eilers1941)
$b\approx 0.726$), we get a nice collapse of all data sets onto a single master curve which can be well fitted, among others, with an Eilers function (Eilers Reference von Eilers1941)
 \begin{equation} \mu_r(\varPhi_{{{eff}}}) = \left[1 + \frac{B\varPhi_{{{eff}}}}{1- \dfrac{\varPhi_{{{eff}}}}{\varPhi_{{{m}}}}}\right]^{2}, \end{equation}
\begin{equation} \mu_r(\varPhi_{{{eff}}}) = \left[1 + \frac{B\varPhi_{{{eff}}}}{1- \dfrac{\varPhi_{{{eff}}}}{\varPhi_{{{m}}}}}\right]^{2}, \end{equation}
with parameters ![]() $B=1.4$ and
$B=1.4$ and ![]() $\varPhi _{{{m}}}=0.7$ (figure 10b). Choosing
$\varPhi _{{{m}}}=0.7$ (figure 10b). Choosing ![]() $B=1.7$ and
$B=1.7$ and ![]() $\varPhi _{{{m}}}=1.2$ also the branch at large
$\varPhi _{{{m}}}=1.2$ also the branch at large ![]() $\varPhi _{{{eff}}}$ can be fitted, but these are somehow not sound values. We argue, instead, that the deviations from the Eilers fit (which occur for the set of data corresponding to the largest
$\varPhi _{{{eff}}}$ can be fitted, but these are somehow not sound values. We argue, instead, that the deviations from the Eilers fit (which occur for the set of data corresponding to the largest ![]() $C=150$ and
$C=150$ and ![]() $\phi =0.4,0.5$) are due to the fact that under these conditions important hydrodynamic correlations emerge which cannot be simply adhered to a reduced volume effect. Let us stress that the relation (3.8), as much as in the approach of Rosti et al. (Reference Rosti, Brandt and Mitra2018), needs an empirical input (the function
$\phi =0.4,0.5$) are due to the fact that under these conditions important hydrodynamic correlations emerge which cannot be simply adhered to a reduced volume effect. Let us stress that the relation (3.8), as much as in the approach of Rosti et al. (Reference Rosti, Brandt and Mitra2018), needs an empirical input (the function ![]() $g(x)$ in (3.2)). However, for small effective capillary number, it is possible to approximate
$g(x)$ in (3.2)). However, for small effective capillary number, it is possible to approximate ![]() $g$ with its linear part, thus providing a closed and explicit expression for
$g$ with its linear part, thus providing a closed and explicit expression for ![]() $\varPhi _{{{eff}}}(Ca,C,\phi )$ and, consequently, an explicit dependence of the relative viscosity
$\varPhi _{{{eff}}}(Ca,C,\phi )$ and, consequently, an explicit dependence of the relative viscosity ![]() $\mu _r$ on
$\mu _r$ on ![]() $Ca$,
$Ca$, ![]() $C$ and
$C$ and ![]() $\phi$.
$\phi$.

Figure 10. (a) Relative viscosity as a function of the volume fraction for different ![]() $Ca$ and
$Ca$ and ![]() $C$. The inset shows
$C$. The inset shows ![]() $\mu _r$ plotted as function of the effective volume fraction as defined in (3.8). The legend of (a) is shown in (b). (b) Relative viscosity as a function of the effective volume fraction (see (3.9)). The dashed line corresponds to a fit of the numerical data using (3.10), with
$\mu _r$ plotted as function of the effective volume fraction as defined in (3.8). The legend of (a) is shown in (b). (b) Relative viscosity as a function of the effective volume fraction (see (3.9)). The dashed line corresponds to a fit of the numerical data using (3.10), with ![]() $B=1.4$ and
$B=1.4$ and ![]() $\varPhi _{{{m}}}=0.7$. Same for the dotted line but with
$\varPhi _{{{m}}}=0.7$. Same for the dotted line but with ![]() $B=1.7$ and
$B=1.7$ and ![]() $\varPhi _{{{m}}}=1.2$
$\varPhi _{{{m}}}=1.2$
4. Conclusions
The rheology of a suspension of strain-hardening capsules is investigated numerically from the dilute to the concentrated regimes in a simple shear flow. We have addressed the role of the membrane inextensibility, ![]() $C$, on the capsule shape and on the suspension rheology, at varying the volume fraction,
$C$, on the capsule shape and on the suspension rheology, at varying the volume fraction, ![]() $\phi$, and the capillary number,
$\phi$, and the capillary number, ![]() $Ca$ (based on the applied shear). Our results indicate that increasing the membrane inextensibility makes the capsules less deformable, and as a consequence the shear-thinning character of the suspension is hindered. We show that, upon a proper definition of an effective
$Ca$ (based on the applied shear). Our results indicate that increasing the membrane inextensibility makes the capsules less deformable, and as a consequence the shear-thinning character of the suspension is hindered. We show that, upon a proper definition of an effective ![]() $C$- and
$C$- and ![]() $\phi$-dependent capillary number, the mean Taylor deformation parameters relative to various data sets collapse onto a single master curve. However, it proved necessary to go beyond the deformation parameter, in order to get a deeper insight on the complex impact the membrane inextensibility has on the full three-dimensional capsule shape. The three principal semi-axes displayed, in fact, a non-monotonic dependence on
$\phi$-dependent capillary number, the mean Taylor deformation parameters relative to various data sets collapse onto a single master curve. However, it proved necessary to go beyond the deformation parameter, in order to get a deeper insight on the complex impact the membrane inextensibility has on the full three-dimensional capsule shape. The three principal semi-axes displayed, in fact, a non-monotonic dependence on ![]() $C$, and for small
$C$, and for small ![]() $C$ (
$C$ (![]() $C=0.1$) and a certain range of
$C=0.1$) and a certain range of ![]() $Ca$, an auxetic behaviour of the capsules was observed. The characteristic shape behaviour was reflected in a non-monotonic dependence of the first normal stress difference with the membrane inextensibility. Finally, the rheological response of the suspension has been analysed also in terms of its relative viscosity. The latter showed a universal behaviour across the various concentrations, shear rates and membrane inextensibilities explored, once an effective volume fraction, taking into account the capsules elongation and orientation, was introduced.
$Ca$, an auxetic behaviour of the capsules was observed. The characteristic shape behaviour was reflected in a non-monotonic dependence of the first normal stress difference with the membrane inextensibility. Finally, the rheological response of the suspension has been analysed also in terms of its relative viscosity. The latter showed a universal behaviour across the various concentrations, shear rates and membrane inextensibilities explored, once an effective volume fraction, taking into account the capsules elongation and orientation, was introduced.
Acknowledgements
We are grateful to M. Léonetti for fruitful discussions and to the referees for their comments and suggestions. The authors gratefully acknowledge the computing time granted by the John von Neumann Institute for Computing (NIC) and provided on the supercomputer JURECA at Jülich Supercomputing Centre (JSC), by the High Performance Computing Center Stuttgart (HLRS) on the Hazel Hen supercomputer and by the Regional Computing Centre Erlangen (RRZE).
Funding
The authors acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG) within the Cluster of Excellence ‘Engineering of Advanced Materials’ (project EXC 315) (Bridge Funding), and within the research unit FOR2688 ‘Instabilities, Bifurcations and Migration in Pulsatile Flows’ (grant number HA4382/8-1). This work was also supported by the Competence Network for Scientific High-Performance Computing in Bavaria (KONWIHR III, project ‘Dynamics of Complex Fluids’) and the European Cooperation in Science and Technology (COST) Action MP1305: ‘Flowing Matter’.
Declaration of interests
The authors report no conflict of interest.
Appendix A
A.1. Particle discretisation and mesh quality
Our membrane is discretised using triangular elements. The spherical capsule results from refining an icosahedron recursively ![]() $N_r$ times. The total number of faces denoted
$N_r$ times. The total number of faces denoted ![]() $N_f$ is defined from the total number of vertices
$N_f$ is defined from the total number of vertices ![]() $N_v$ and the number of recursive refinement such as
$N_v$ and the number of recursive refinement such as ![]() $N_f=2N_v-4$ and
$N_f=2N_v-4$ and ![]() $N_v=2+10 \cdot 4^{N_r}$. In this work, we used
$N_v=2+10 \cdot 4^{N_r}$. In this work, we used ![]() $N_r=3$ leading to
$N_r=3$ leading to ![]() $N_v=642$ and
$N_v=642$ and ![]() $N_f=1280$. To study the effect of the mesh discretisation on the shape and rheology, we varied
$N_f=1280$. To study the effect of the mesh discretisation on the shape and rheology, we varied ![]() $N_r$ from
$N_r$ from ![]() $2$ to
$2$ to ![]() $4$, which corresponds namely to
$4$, which corresponds namely to ![]() $N_f=320$ and
$N_f=320$ and ![]() $N_f=5120$. We set
$N_f=5120$. We set ![]() $C$ to unity to test situations with large deformations.
$C$ to unity to test situations with large deformations.
We report in table 1 some of the relevant quantities measured for different number of faces ![]() $(N_f=320, 1280,5120)$,
$(N_f=320, 1280,5120)$, ![]() $C=1$ and
$C=1$ and ![]() $Ca=0.5$. The standard deviations of the different shape and rheology parameters are negligible for the three meshes, whereas the errors stemming from decreasing the number of faces of the mesh are significantly small.
$Ca=0.5$. The standard deviations of the different shape and rheology parameters are negligible for the three meshes, whereas the errors stemming from decreasing the number of faces of the mesh are significantly small.
Table 1. Steady-state shape and rheology quantities (time averaged) for ![]() $Ca=0.5$ and
$Ca=0.5$ and ![]() $C=1$.
$C=1$.

A.2. Statistically stationary state and time averages
Figure 11 depicts the time evolution of the mean deformation and relative viscosity of a suspension of capsules in the semi-dilute limit with ![]() $\phi =0.3$ for two different values of the membrane inextensibility
$\phi =0.3$ for two different values of the membrane inextensibility ![]() $(C=1,150)$ and a fixed capillary number
$(C=1,150)$ and a fixed capillary number ![]() $(Ca=0.5)$. The time average is performed on the interval where the Taylor deformation index averaged over the number of particles reaches a steady-state value with fluctuations less than
$(Ca=0.5)$. The time average is performed on the interval where the Taylor deformation index averaged over the number of particles reaches a steady-state value with fluctuations less than ![]() $1\,\%$. In term of strain units, we have observed that the transient time spans over the first
$1\,\%$. In term of strain units, we have observed that the transient time spans over the first ![]() $5$ to
$5$ to ![]() $7 \dot {\gamma }t$.
$7 \dot {\gamma }t$.

Figure 11. (a) History of the mean deformation and (b) the relative viscosity of the capsules for a semi-dilute suspension (![]() $\phi =0.3$) and two different membrane inextensibilities
$\phi =0.3$) and two different membrane inextensibilities ![]() $(C=1,150)$.
$(C=1,150)$.
A.3. Grid independency
In order to check that the system size in the periodic directions is large enough to ensure grid independency of the results, we test here the behaviour of two relevant observables, namely the mean Taylor deformation parameter, ![]() $\langle D \rangle$, and the relative viscosity,
$\langle D \rangle$, and the relative viscosity, ![]() $\mu _r$. We performed simulations at changing the box size from
$\mu _r$. We performed simulations at changing the box size from ![]() $8r \times 8r \times 16r$ to
$8r \times 8r \times 16r$ to ![]() $64r \times 64r \times 16r$, for fixed
$64r \times 64r \times 16r$, for fixed ![]() $C=150$,
$C=150$, ![]() $Ca=0.5$,
$Ca=0.5$, ![]() $\phi =0.3$ and wall-to-wall distance
$\phi =0.3$ and wall-to-wall distance ![]() $L=16r$. We have varied the number of particles (
$L=16r$. We have varied the number of particles (![]() $N_p$) to keep a fixed volume fraction. For the sake of comparison, we look at the relative (percentage) errors for the mean Taylor deformation parameter and the relative viscosity, defined as
$N_p$) to keep a fixed volume fraction. For the sake of comparison, we look at the relative (percentage) errors for the mean Taylor deformation parameter and the relative viscosity, defined as
and
 \begin{equation} \epsilon_{\mu_r} = \frac{\mu_r - \mu_r^{(0)}}{\mu_r^{(0)}}, \end{equation}
\begin{equation} \epsilon_{\mu_r} = \frac{\mu_r - \mu_r^{(0)}}{\mu_r^{(0)}}, \end{equation}
respectively (![]() $\langle D \rangle ^{(0)}$ and
$\langle D \rangle ^{(0)}$ and ![]() $\mu _r^{(0)}$ being the values corresponding to the cubic reference case, used throughout the paper). The results are reported in table 2. All relative errors are small and decrease from
$\mu _r^{(0)}$ being the values corresponding to the cubic reference case, used throughout the paper). The results are reported in table 2. All relative errors are small and decrease from ![]() $0.47\,\%$ to
$0.47\,\%$ to ![]() $0.38\,\%$ (for the deformation parameter) and from
$0.38\,\%$ (for the deformation parameter) and from ![]() $3\,\%$ to
$3\,\%$ to ![]() $2\,\%$ (for the relative viscosity), denoting a satisfactory degree of convergence for the resolution employed.
$2\,\%$ (for the relative viscosity), denoting a satisfactory degree of convergence for the resolution employed.
Table 2. Relative errors on ![]() $\langle D \rangle$ and
$\langle D \rangle$ and ![]() $\mu _r$ for different system sizes.
$\mu _r$ for different system sizes.

A.4. Effect of the short-range repulsive force
A well-known limitation of the LB-IBM scheme is the interpolation of the membrane velocity from the surrounding fluid when the distance between two boundary nodes is below the lattice resolution limit that can result in a permanent overlap between neighbouring nodes (Krüger Reference Krüger2012). The probability of such event to occur increases with the volume fraction of the suspension. To prevent such situation a short-range repulsive force must be applied on neighbouring nodes from different membranes when the node-to-node distance (![]() $d_{ij}$) is below one lattice spacing (
$d_{ij}$) is below one lattice spacing (![]() $\Delta x$), whereas it vanishes for
$\Delta x$), whereas it vanishes for ![]() $d_{ij} > \Delta x$ (see (2.8)). Without any short-range repulsion, we do indeed observe crossing particles for
$d_{ij} > \Delta x$ (see (2.8)). Without any short-range repulsion, we do indeed observe crossing particles for ![]() $\phi >0.3$. Such force is, therefore, needed, however its details do not affect the macroscopic behaviour of the suspension, within the range of volume fractions explored in this study. To show this, we report in figure 12 results on the relative viscosity from simulations with
$\phi >0.3$. Such force is, therefore, needed, however its details do not affect the macroscopic behaviour of the suspension, within the range of volume fractions explored in this study. To show this, we report in figure 12 results on the relative viscosity from simulations with ![]() $C=150$,
$C=150$, ![]() $Ca=0.5$ and
$Ca=0.5$ and ![]() $\phi \in [10^{-3}, 0.5]$, for three different values of the force amplitude:
$\phi \in [10^{-3}, 0.5]$, for three different values of the force amplitude: ![]() $\bar {\epsilon }/(G_s r) = 0$ (absence of force),
$\bar {\epsilon }/(G_s r) = 0$ (absence of force), ![]() $\bar {\epsilon }/(G_s r) = 1$ and
$\bar {\epsilon }/(G_s r) = 1$ and ![]() $\bar {\epsilon }/(G_s r) = 10^{2}$ (the value used in all simulations throughout the paper). Only a slight deviation (of less than
$\bar {\epsilon }/(G_s r) = 10^{2}$ (the value used in all simulations throughout the paper). Only a slight deviation (of less than ![]() $10\,\%$) can be detected for
$10\,\%$) can be detected for ![]() $\bar {\epsilon }/(G_s r) = 0$ at
$\bar {\epsilon }/(G_s r) = 0$ at ![]() $\phi =0.3$ (for larger
$\phi =0.3$ (for larger ![]() $\phi$ data are not available because of the occurrence of crossings), otherwise all sets of data basically overlap, within error bars.
$\phi$ data are not available because of the occurrence of crossings), otherwise all sets of data basically overlap, within error bars.

Figure 12. Effect of the short-range repulsive force on the relative viscosity of a suspension of capsules.