1. Introduction
Microswimmers are common in nature – they include bacteria, spermatozoa, some algae and synthetic swimmers. In almost all contexts these swimmers interact with boundaries, either biological (e.g. the gut, cell walls) or man-made (e.g. tubes, filters). These interactions have been studied experimentally, numerically and theoretically by many groups. The two main aspects of interaction are hydrodynamic (mediated by the fluid) and steric (direct contact with the boundary); they can have different relative importance depending on the context, but it is widely accepted that both can play a crucial role (Drescher et al. Reference Drescher, Dunkel, Cisneros, Ganguly and Goldstein2011; Kantsler et al. Reference Kantsler, Dunkel, Polin and Goldstein2013; Contino et al. Reference Contino, Lushi, Tuval, Kantsler and Polin2015; Bianchi, Saglimbeni & Leonardo Reference Bianchi, Saglimbeni and Di Leonardo2017).
In the present paper we will be concerned with modelling the steric interaction of a microswimmer with solid surfaces, with an emphasis on the role of the swimmer's shape. For simplicity, the swimmer will be two-dimensional with a fixed shape, although, in principle, the theory could be extended to include a deformable body or flagella. To keep the model tractable, we drop the hydrodynamic interactions when deriving theoretical results, but in § 8 we quantify their importance relative to steric interactions.
1.1. Previous work
Many models have been proposed to mimic the behaviour of microswimmers, with the simplest being the active Brownian particle (ABP) model (Ai et al. Reference Ai, Chen, He, Li and Zheng2013; Redner, Hagan & Baskaran Reference Redner, Hagan and Baskaran2013; Stenhammar et al. Reference Stenhammar, Marenduzzo, Allen and Cates2014; Solon et al. Reference Solon, Fily, Baskaran, Cates, Kafri, Kardar and Tailleur2015; Zöttl & Stark Reference Zöttl and Stark2016; Wagner, Hagan & Baskaran Reference Wagner, Hagan and Baskaran2017) where a particle moves with constant speed and both its swimming direction and spatial position are subject to independent diffusion processes. A more complicated model has the organism moving in a straight line for a random time (run), followed by a random change in direction (tumble); such run-and-tumble models have been investigated both theoretically and numerically (Tailleur & Cates Reference Tailleur and Cates2009; Lambert, Liao & Austin Reference Lambert, Liao and Austin2010; Nash et al. Reference Nash, Adhikari, Tailleur and Cates2010; Costanzo et al. Reference Costanzo, Di Leonardo, Ruocco and Angelani2012; Ezhilan, Pahlavan & Saintillan Reference Ezhilan, Pahlavan and Saintillan2012; Lushi, Goldstein & Shelley Reference Lushi, Goldstein and Shelley2012; Martens et al. Reference Martens, Angelani, Di Leonardo and Bocquet2012; Cates & Tailleur Reference Cates and Tailleur2013; Koumakis, Maggi & Leonardo Reference Koumakis, Maggi and Di Leonardo2014; Lushi, Wioland & Goldstein Reference Lushi, Wioland and Goldstein2014; Molaei et al. Reference Molaei, Barry, Stocker and Sheng2014; Elgeti & Gompper Reference Elgeti and Gompper2015; Ezhilan, Alonso-Matilla & Saintillan Reference Ezhilan, Alonso-Matilla and Saintillan2015; Elgeti & Gompper Reference Elgeti and Gompper2016; Lushi Reference Lushi2016; Razin et al. Reference Razin, Voituriez, Elgeti and Gov2017; Sepúlveda & Soto Reference Sepúlveda and Soto2017; Chen et al. Reference Chen, Wang, Chu, Chen, Sheng and Tsao2018; Lee, Szuttor & Holm Reference Lee, Szuttor and Holm2019). There are also more complex models that incorporate hydrodynamic effects (Fauci & McDonald Reference Fauci and McDonald1995; Saintillan, Shaqfeh & Darve Reference Saintillan, Shaqfeh and Darve2006a, Reference Saintillan, Shaqfeh and Darveb; Saintillan & Shelley Reference Saintillan and Shelley2007, Reference Saintillan and Shelley2008; Crowdy & Or Reference Crowdy and Or2010; Evans & Lauga Reference Evans and Lauga2010; Rusconi et al. Reference Rusconi, Lecuyer, Guglielmini and Stone2010; Saintillan Reference Saintillan2010; Shum, Gaffney & Smith Reference Shum, Gaffney and Smith2010; Crowdy & Samson Reference Crowdy and Samson2011; ten Hagen, Wittkowski & Löwen Reference ten Hagen, Wittkowski and Löwen2011; Costanzo et al. Reference Costanzo, Di Leonardo, Ruocco and Angelani2012; Obuse & Thiffeault Reference Obuse and Thiffeault2012; Saintillan & Shelley Reference Saintillan and Shelley2013; Li & Ardekani Reference Li and Ardekani2014; Lushi et al. Reference Lushi, Wioland and Goldstein2014; Takatori, Yan & Brady Reference Takatori, Yan and Brady2014; Bricard et al. Reference Bricard, Caussin, Savoie, Chikkadi, Shitara, Chepizhko, Peruani, Saintillan and Bartolo2015; Lushi & Vlahovska Reference Lushi and Vlahovska2015; Spagnolie et al. Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015; ten Hagen et al. Reference ten Hagen, Wittkowski, Takagi, Kümmel, Bechinger and Löwen2015; Yeo, Lushi & Vlahovska Reference Yeo, Lushi and Vlahovska2015; Mathijssen et al. Reference Mathijssen, Doostmohammadi, Yeomans and Shendruk2016; Wioland, Lushi & Goldstein Reference Wioland, Lushi and Goldstein2016; Theillard, Matilla & Saintillan Reference Theillard, Matilla and Saintillan2017; Daddi-Moussa-Ider et al. Reference Daddi-Moussa-Ider, Lisicki, Hoell and Löwen2018; Lushi, Goldstein & Shelley Reference Lushi, Goldstein and Shelley2018; Theers et al. Reference Theers, Westphal, Qi, Winkler and Gompper2018; Alonso-Matilla & Saintillan Reference Alonso-Matilla and Saintillan2019; Wagner, Hagan & Baskaran Reference Wagner, Hagan and Baskaran2019). In the present paper we will limit ourselves to the ABP model.
Experiments with microswimmers near boundaries are also plentiful (Rothschild Reference Rothschild1963; Frymier et al. Reference Frymier, Ford, Berg and Cummings1995; Woolley Reference Woolley2003; DiLuzio et al. Reference DiLuzio, Turner, Mayer, Garstecki, Weibel, Berg and Whitesides2005; Lauga Reference Lauga2006; Hill et al. Reference Hill, Kalkanci, McMurry and Koser2007; Berke et al. Reference Berke, Turner, Berg and Lauga2008; Li, Tam & Tanf Reference Li, Tam and Tanf2008; Drescher et al. Reference Drescher, Dunkel, Cisneros, Ganguly and Goldstein2011; Volpe et al. Reference Volpe, Buttinoni, Vogt, Kümmerer and Bechinger2011; Kantsler et al. Reference Kantsler, Dunkel, Polin and Goldstein2013; Kim et al. Reference Kim, Drescher, Park, Bassler and Stone2014; Contino et al. Reference Contino, Lushi, Tuval, Kantsler and Polin2015; Mok, Dunkel & Kantsler Reference Mok, Dunkel and Kantsler2019). As early as 1963, Rothschild measured the density of bull spermatozoa between two glass plates and found accumulation near the plates. Accumulation as well as local alignment and preferred tail rotation were also observed in later experiments (Rothschild Reference Rothschild1963; Woolley Reference Woolley2003; Lauga Reference Lauga2006; Hill et al. Reference Hill, Kalkanci, McMurry and Koser2007; Berke et al. Reference Berke, Turner, Berg and Lauga2008; Li et al. Reference Li, Tam and Tanf2008; Drescher et al. Reference Drescher, Dunkel, Cisneros, Ganguly and Goldstein2011; Li et al. Reference Li, Bensson, Nisimova, Munger, Mahautmr, Tang, Maxey and Brun2011; Volpe et al. Reference Volpe, Buttinoni, Vogt, Kümmerer and Bechinger2011; Denissenko et al. Reference Denissenko, Kanstler, Smith and Kirkman-Brown2012; Kantsler et al. Reference Kantsler, Dunkel, Polin and Goldstein2013; Lefauve & Saintillan Reference Lefauve and Saintillan2014; Contino et al. Reference Contino, Lushi, Tuval, Kantsler and Polin2015). Simulations have shown that either hydrodynamic interactions or steric interactions with thermal fluctuations can lead to accumulation. Later work found that steric effects dominate at walls, while hydrodynamic interactions can play an important role (Drescher et al. Reference Drescher, Dunkel, Cisneros, Ganguly and Goldstein2011; Kantsler et al. Reference Kantsler, Dunkel, Polin and Goldstein2013; Contino et al. Reference Contino, Lushi, Tuval, Kantsler and Polin2015; Bianchi et al. Reference Bianchi, Saglimbeni and Di Leonardo2017).
In principle, the interaction of microswimmers with boundaries requires modelling of both hydrodynamic and steric interactions. Some groups use far-field approximations for hydrodynamic interactions (Katz Reference Katz1974; Katz & Blake Reference Katz and Blake1975; Katz, Blake & Paverifontana Reference Katz, Blake and Paverifontana1975; Hernandez-Ortiz, Stoltz & Graham Reference Hernandez-Ortiz, Stoltz and Graham2005; Saintillan et al. Reference Saintillan, Shaqfeh and Darve2006a; Swan & Brady Reference Swan and Brady2007; Elgeti & Gompper Reference Elgeti and Gompper2009; Crowdy & Or Reference Crowdy and Or2010; Crowdy & Samson Reference Crowdy and Samson2011; Obuse & Thiffeault Reference Obuse and Thiffeault2012; Spagnolie & Lauga Reference Spagnolie and Lauga2012; Lopez & Lauga Reference Lopez and Lauga2014; Schaar, Zöttl & Stark Reference Schaar, Zöttl and Stark2015; Sipos et al. Reference Sipos, Nagy, Di Leonardo and Galajda2015; Spagnolie et al. Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015; ten Hagen et al. Reference ten Hagen, Wittkowski, Takagi, Kümmel, Bechinger and Löwen2015; Kaynan & Yariv Reference Kaynan and Yariv2017; Mirzakhanloo & Alam Reference Mirzakhanloo and Alam2018; Wagner et al. Reference Wagner, Hagan and Baskaran2019), which can be done in several ways: either explicitly with a solution of the Stokes equation, or implicitly through a resistance or mobility matrix. Spagnolie & Lauga (Reference Spagnolie and Lauga2012) used a multipole expansion which in principle can be applied to any swimmer shape, and Takagi et al. (Reference Takagi, Palacci, Braunschweig, Shelley and Zhang2014) solved the Stokes equation in the lubrication limit. Zargar, Najafi & Miri (Reference Zargar, Najafi and Miri2009) solved for the mobility matrix by restricting the swimmer to planar motion near a wall. Despite some simplifications in these models, they are fairly accurate away from boundaries and reproduce observed behaviour (Lauga & Powers Reference Lauga and Powers2009; Marchetti et al. Reference Marchetti, Joanny, Ramaswamy, Liverpool, Prost, Rao and Simha2013; Bechinger et al. Reference Bechinger, Di Leonardo, Löwen, Reichhardt, Volpe and Volpe2016; Zöttl & Stark Reference Zöttl and Stark2016). However, such models remain in general fairly complicated and available theoretical results either ignore the details of swimmers such as shape, or are swimmer dependent.
Steric interactions are often included phenomenologically, such as by using wall potential functions (van Teeffelen & Löwen Reference van Teeffelen and Löwen2008; Wensink & Löwen Reference Wensink and Löwen2008; Hernandez-Ortiz, Underhill & Graham Reference Hernandez-Ortiz, Underhill and Graham2009; Costanzo et al. Reference Costanzo, Di Leonardo, Ruocco and Angelani2012; Kaiser, Wensink & Löwen Reference Kaiser, Wensink and Löwen2012; Chilukuri, Collins & Underhill Reference Chilukuri, Collins and Underhill2014; Mathijssen et al. Reference Mathijssen, Doostmohammadi, Yeomans and Shendruk2016; Tian et al. Reference Tian, Gu, Guo and Chen2017; Caprini & Marconi Reference Caprini and Marconi2018; Daddi-Moussa-Ider et al. Reference Daddi-Moussa-Ider, Lisicki, Hoell and Löwen2018; Sepúlveda & Soto Reference Sepúlveda and Soto2018; Wagner et al. Reference Wagner, Hagan and Baskaran2019). This approach makes it easy to add boundaries to free-space simulations and has low simulation cost. Sepúlveda & Soto (Reference Sepúlveda and Soto2018) used Weeks–Chandler–Anderson functions and a Gaussian potential function for deformable swimmers; they treat the effective wall force as a smooth repulsive force. Hernandez-Ortiz et al. (Reference Hernandez-Ortiz, Underhill and Graham2009) used Gay–Berne functions for steric exclusion of rod-shape swimmers. These models are usually a smooth approximation to the true dynamics, whereas for rigid swimmers the steric effect is by volume exclusion. Moreover, the complicated potential functions make it hard to obtain predictive formulas, although some authors used a simple harmonic potential to represent an elastic boundary, which made the problem tractable (Drescher et al. Reference Drescher, Dunkel, Cisneros, Ganguly and Goldstein2011; Nikola et al. Reference Nikola, Solon, Kafri, Kardar, Tailleur and Voituriez2016; Caprini & Marconi Reference Caprini and Marconi2018).
Another way to model steric interactions is to assign a specific dynamics near the boundary such as reflecting (Li & Tang Reference Li and Tang2009; Koumakis et al. Reference Koumakis, Maggi and Di Leonardo2014; Volpe, Gigan & Volpe Reference Volpe, Gigan and Volpe2014; Paksa et al. Reference Paksa2016; Chen et al. Reference Chen, Wang, Chu, Chen, Sheng and Tsao2018) or vanishing velocity (Sepúlveda & Soto Reference Sepúlveda and Soto2018) boundary conditions, or a repulsive force condition (Spagnolie & Lauga Reference Spagnolie and Lauga2012). Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015) observed that reflecting boundary conditions do not recover the behaviour observed in experiments. Other groups did not try to prescribe any specific law for swimmers at a boundary and focused on statistics such as the invariant density of swimmers (Ezhilan et al. Reference Ezhilan, Pahlavan and Saintillan2012; Yariv & Schnitzer Reference Yariv and Schnitzer2014; Yan & Brady Reference Yan and Brady2015; Malgaretti & Stark Reference Malgaretti and Stark2017) and swim pressure (Caprini & Marconi Reference Caprini and Marconi2018; Chen et al. Reference Chen, Wang, Chu, Chen, Sheng and Tsao2018; Yan & Brady Reference Yan and Brady2015; Speck Reference Speck2020).
When modelling a collection of stochastic swimmers or the statistics of a single swimmer, an ideal approach to include steric interactions is to use no-flux boundary conditions that prevent the organism's body from entering the wall, as described by Nitsche & Brenner (Reference Nitsche and Brenner1990) for passive particles. Most work in the literature using these boundary conditions assumes that swimmers have negligible size or are of spherical shape, so that they can rotate freely at a wall (Burada et al. Reference Burada, Hänggi, Marchesoni, Schmid and Talkner2009; Bearon, Hazel & Thorn Reference Bearon, Hazel and Thorn2011; Bruna & Chapman Reference Bruna and Chapman2013; Yariv & Schnitzer Reference Yariv and Schnitzer2014; Ezhilan & Saintillan Reference Ezhilan and Saintillan2015; Yan & Brady Reference Yan and Brady2015; Alonso-Matilla, Ezhilan & Saintillan Reference Alonso-Matilla, Ezhilan and Saintillan2016; Malgaretti & Stark Reference Malgaretti and Stark2017; Alonso-Matilla & Saintillan Reference Alonso-Matilla and Saintillan2019). Lee (Reference Lee2013) attempted to solve for the invariant density without spatial diffusion for point swimmers in a channel, and managed to find a solution when swimmers had only six swimming directions. Wagner et al. (Reference Wagner, Hagan and Baskaran2017) continued Lee's work and found the invariant density for continuous swimming directions by introducing a wall density function. Schaar et al. (Reference Schaar, Zöttl and Stark2015) later calculated the trapping time at a wall. Ai et al. (Reference Ai, Chen, He, Li and Zheng2013) predicted for point swimmers the optimal swimming speed, the spatial diffusion and the strength of wall potentials for maximal effective diffusion. For spherical or point swimmers, Elgeti & Gompper (Reference Elgeti and Gompper2013) and Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015) investigated asymptotic solutions to the Fokker–Planck equation associated with the ABP model. Elgeti & Gompper (Reference Elgeti and Gompper2016) subsequently found the invariant density for run-and-tumble spherical swimmers.
1.2. The role of shape
The admissible positions and orientations of a non-spherical swimmer are constrained by the presence of walls; the set of admissible values of the degrees of freedom is the configuration space (Nitsche & Brenner Reference Nitsche and Brenner1990) (see § 2). A natural way to include the shape of a swimmer into a model is thus to impose no-flux boundary conditions on the configuration space itself. Krochak, Olson & Martinez (Reference Krochak, Olson and Martinez2010) and Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015) used steric exclusion with no-flux boundary conditions for rigid fibres and rod-like swimmers, respectively, and simulated the invariant density in a channel in the presence of flow.
In the present paper we propose a framework to incorporate the shape of a swimmer into the boundary conditions of a partial differential equation describing its dynamics. The partial differential equation (PDE) is the Fokker–Planck equation derived from the two-dimensional ABP model with no-flux boundary conditions in configuration space. For simplicity, we ignore hydrodynamic interactions for now. (We will introduce them in § 8.) The configuration space is determined by the swimmer's shape and the domain of swimming, which we take to be an infinite channel. We solve explicitly for the invariant density in the limit of small rotational diffusivity. In the same limit, we also solve for the mean reversal time and the longitudinal effective diffusivity of the swimmer. All these quantities are greatly influenced by the shape of the swimmer. In particular the effective diffusivity can become very large when the swimmer tends to align parallel to the channel walls, which can surprisingly occur even for circular swimmers when their centre of rotation does not coincide with the geometric centre (see below). In § 8, we add hydrodynamic interactions into the ABP model, and examine how the cross-sectional invariant density varies with the shape-dependent configuration space.
In the literature so far, authors have often prevented swimmers from entering inside boundaries by applying some ad hoc repulsive force or potential, which models steric interactions. Our main point is that a similar effect can be achieved by a no-flux boundary condition, which is natural in the context of a Fokker–Planck equation. It has the advantage of naturally involving the shape of the organism through the configuration space. As we will, this mathematical elegance allows analytical progress for simple swimmer shapes, at least when neglecting hydrodynamic interactions as a first approximation.
An important observation is in order regarding the ABP model for a finite-size swimmer. The ABP model implicitly assumes that the swimmer rotates about some distinguished fixed point in a co-moving frame. The precise location of this point becomes important when the swimmer has finite size and boundaries are present. For example, figure 1 shows an elliptical swimmer approaching a boundary: the direction of swimming is given by the angle ![]() $\theta$, which is measured counterclockwise from the horizontal, so that
$\theta$, which is measured counterclockwise from the horizontal, so that ![]() $\theta <0$ corresponds to swimming towards the wall. The angle
$\theta <0$ corresponds to swimming towards the wall. The angle ![]() $\theta$ is measured around a point we call the centre of rotation of the swimmer. For a free particle, this corresponds to the centre of hydrodynamic reaction defined by Happel & Brenner (Reference Happel and Brenner1983, p. 174). For an ellipse it would coincide with the geometrical centre. However, the swimmer's propulsion mechanism (e.g. flagella), which is abstracted here since we consider fixed shapes, can displace this centre. Hence, we treat the centre of rotation as a parameter that may be adjusted to model a particular swimmer. To parallel terminology based on the type of propulsion used by a microorganism (Hernandez-Ortiz et al. Reference Hernandez-Ortiz, Stoltz and Graham2005; Saintillan & Shelley Reference Saintillan and Shelley2007; Hernandez-Ortiz et al. Reference Hernandez-Ortiz, Underhill and Graham2009; Saintillan & Shelley Reference Saintillan and Shelley2011), when the centre of rotation is ahead of the geometric centre we will call the swimmer front rotating; when it is behind we call it rear rotating. We assume the centre of rotation is inside a swimmer's body. If the swimmer does not interact with boundaries, then the centre of rotation is not particularly important to the dynamics; but with external boundaries it can influence the tendency of the swimmer to align parallel or perpendicular to a wall, depending on its shape (Lushi, Kantsler & Goldstein Reference Lushi, Kantsler and Goldstein2017). In fact, we will see that even a circular particle can align with a wall if its centre of rotation is behind the geometric centre, despite the absence of hydrodynamic interactions.
$\theta$ is measured around a point we call the centre of rotation of the swimmer. For a free particle, this corresponds to the centre of hydrodynamic reaction defined by Happel & Brenner (Reference Happel and Brenner1983, p. 174). For an ellipse it would coincide with the geometrical centre. However, the swimmer's propulsion mechanism (e.g. flagella), which is abstracted here since we consider fixed shapes, can displace this centre. Hence, we treat the centre of rotation as a parameter that may be adjusted to model a particular swimmer. To parallel terminology based on the type of propulsion used by a microorganism (Hernandez-Ortiz et al. Reference Hernandez-Ortiz, Stoltz and Graham2005; Saintillan & Shelley Reference Saintillan and Shelley2007; Hernandez-Ortiz et al. Reference Hernandez-Ortiz, Underhill and Graham2009; Saintillan & Shelley Reference Saintillan and Shelley2011), when the centre of rotation is ahead of the geometric centre we will call the swimmer front rotating; when it is behind we call it rear rotating. We assume the centre of rotation is inside a swimmer's body. If the swimmer does not interact with boundaries, then the centre of rotation is not particularly important to the dynamics; but with external boundaries it can influence the tendency of the swimmer to align parallel or perpendicular to a wall, depending on its shape (Lushi, Kantsler & Goldstein Reference Lushi, Kantsler and Goldstein2017). In fact, we will see that even a circular particle can align with a wall if its centre of rotation is behind the geometric centre, despite the absence of hydrodynamic interactions.

Figure 1. An elliptical swimmer approaching a wall at a direction ![]() $\theta = -{\rm \pi} /4$. The centre of rotation is not necessarily the geometric centre of the ellipse.
$\theta = -{\rm \pi} /4$. The centre of rotation is not necessarily the geometric centre of the ellipse.
1.3. Outline
In this paper we focus exclusively on a two-dimensional swimmer undergoing steric interactions. In § 2 we describe the configuration space for a swimmer with an arbitrary fixed convex shape, in particular when the swimmer is confined to a channel consisting of two infinite parallel walls. A crucial quantity is the wall distance function, which describes the swimmer's closest point of approach to a wall as a function of the swimmer's orientation. We give explicit examples for needle (rod-like), elliptical, and teardrop-shaped swimmers. The wall distance function can then be used twice to determine the full configuration space in a channel. This configuration space is open if the swimmer can reverse direction in the channel, or closed if the channel is too narrow to do so. We also describe symmetries of the configuration space that follow from symmetries of the swimmer and channel, the most important being the case where a swimmer is left–right symmetric.
In § 3 we describe the stochastic ABP model for our swimmer, and give its corresponding Fokker–Planck equation. This leads to the natural no-flux boundary conditions that we impose at the solid walls. For the infinite channel geometry we average over the lengthwise coordinate. In § 4 we simplify the model by assuming a small rotational diffusivity. This leads to a reduced equation, which is a partial differential equation in one time variable and one angle. The configuration geometry is completely encoded into a single effective angular drift function.
In § 5 we solve for the steady state of the reduced equation, which gives us the invariant probability density of the swimmer, or invariant density for short. The invariant density is strongly dependent on the shape and centre of rotation, as we show with some explicit examples, typically in the limit of rapid swimming. In particular, we show that circular swimmers can align either parallel or perpendicular to the walls, depending on whether they are rear or front rotating, respectively. When a swimmer has broken left–right symmetry, it can undergo a net rotation due to repeated biased interactions with the walls.
We introduce the mean reversal time (MRT) of a swimmer in § 6: the expected time for a swimmer to fully reverse direction in an open channel. This is a generalization of the turnaround time of Holcman & Schuss (Reference Holcman and Schuss2014), who described the expected time for a Brownian needle to reverse direction when its length is slightly shorter than the channel width. For a left–right symmetric swimmer we give a simple integral formula for the MRT. We explicitly compute the MRT in some limits, in particular for a fast swimmer. The MRT in this fast case is exponentially long, since the swimmer sticks to a wall for a very long time before undergoing a large enough random fluctuation that causes reversal.
In § 7 we use a homogenization theory approach to find the longitudinal effective diffusivity ![]() $D_{{eff}}$ for the swimmer in an open channel. In the same reduced limit as above (small rotational diffusion) we give an integral formula for the diffusivity. For a fast swimmer we might expect that the effective diffusivity is related to the MRT: the swimmer makes large excursions and sometimes reverses direction, thereby undergoing an effective random walk for long times. Indeed, we obtain the rigorous bound
$D_{{eff}}$ for the swimmer in an open channel. In the same reduced limit as above (small rotational diffusion) we give an integral formula for the diffusivity. For a fast swimmer we might expect that the effective diffusivity is related to the MRT: the swimmer makes large excursions and sometimes reverses direction, thereby undergoing an effective random walk for long times. Indeed, we obtain the rigorous bound
where ![]() $D_X$ is the diffusivity of the swimmer along the direction of swimming,
$D_X$ is the diffusivity of the swimmer along the direction of swimming, ![]() $\tau _{{rev}}$ is the MRT and
$\tau _{{rev}}$ is the MRT and ![]() $U$ is the swimming speed. The term
$U$ is the swimming speed. The term ![]() $\tfrac 12(\tau _{{rev}}\,U)^2/\tau _{{rev}}$ is equal to the diffusivity for an unbiased random walk with step size
$\tfrac 12(\tau _{{rev}}\,U)^2/\tau _{{rev}}$ is equal to the diffusivity for an unbiased random walk with step size ![]() $\tau _{{rev}} U$ and step time
$\tau _{{rev}} U$ and step time ![]() $\tau _{{rev}}$.
$\tau _{{rev}}$.
In § 8, we incorporate hydrodynamic interactions of the swimmer with walls, using the same interaction terms as in Spagnolie et al. (Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015). We compare the cross-sectional invariant density in a narrow channel for a configuration space corresponding to an ellipsoidal and a near-spherical shape. Finally, we offer some concluding remarks in § 9.
2. Configuration space
In this section we describe how the swimmer's shape interacts with boundaries to create configuration space. We first establish coordinate systems for a convex swimmer (§ 2.1): the fixed laboratory frame and a frame rotating with the swimmer. These are both necessary since the direction of swimming and the magnitude of diffusion are tied to the swimmer's shape, and may be different along different axes (§ 3). We use the term ‘swimmer’ throughout, but our entire formalism applies to passive particles as well, for which ![]() $U=0$. We consider an arbitrary contact point between a swimmer's body and a single wall, and show how to derive the wall distance function for the swimmer. We present a few examples: a needle (one-dimensional segment), an ellipse and a ‘teardrop’ shape. These are all swimmers with a left–right axis of symmetry, but our formalism applies to more general swimmers as well.
$U=0$. We consider an arbitrary contact point between a swimmer's body and a single wall, and show how to derive the wall distance function for the swimmer. We present a few examples: a needle (one-dimensional segment), an ellipse and a ‘teardrop’ shape. These are all swimmers with a left–right axis of symmetry, but our formalism applies to more general swimmers as well.
In § 2.2 we use the wall distance function to obtain the configuration space for a swimmer confined between two infinite, parallel walls. Two very different cases emerge: in the open channel configuration the channel is wide enough to allow the swimmer to completely reverse direction, whereas in the closed configuration the swimmer is unable to do so.
2.1. The wall distance function
The shape of a swimmer is expressed by giving its boundary in parametric form
where ![]() $\boldsymbol {R}_{b}(\varphi )$ is a
$\boldsymbol {R}_{b}(\varphi )$ is a ![]() $2{\rm \pi}$-periodic, piecewise-smooth function (figure 2a). By convention, the swimming direction
$2{\rm \pi}$-periodic, piecewise-smooth function (figure 2a). By convention, the swimming direction ![]() $\boldsymbol {R}_{b}(0)$ is along the positive
$\boldsymbol {R}_{b}(0)$ is along the positive ![]() $X$ axis in the swimmer's co-moving and co-rotating frame. The origin of the
$X$ axis in the swimmer's co-moving and co-rotating frame. The origin of the ![]() $\boldsymbol {R}=(X,Y)$ coordinate system is the centre of rotation of the swimmer. Note that we do not require
$\boldsymbol {R}=(X,Y)$ coordinate system is the centre of rotation of the swimmer. Note that we do not require ![]() $\tan \varphi = Y_{b}(\varphi )/X_{b}(\varphi )$, that is,
$\tan \varphi = Y_{b}(\varphi )/X_{b}(\varphi )$, that is, ![]() $\varphi$ does not necessarily correspond to the polar angle of
$\varphi$ does not necessarily correspond to the polar angle of ![]() $\boldsymbol {R}_{b}(\varphi )$.
$\boldsymbol {R}_{b}(\varphi )$.

Figure 2. Boundary of a convex swimmer in (a) the swimmer's frame, with the swimming direction along the positive ![]() $X$ axis, and (b) the fixed laboratory frame, where the swimming direction makes an angle
$X$ axis, and (b) the fixed laboratory frame, where the swimming direction makes an angle ![]() $\theta$ with the
$\theta$ with the ![]() $x$ axis.
$x$ axis.
In a fixed (laboratory) frame, the boundary of the swimmer is located at (figure 2b)
where ![]() $\boldsymbol {r} = (x,y)$ denotes the centre of rotation of the swimmer and
$\boldsymbol {r} = (x,y)$ denotes the centre of rotation of the swimmer and
is a rotation matrix.
Now take the swimmer to be touching an infinite wall along ![]() $y=0$, as shown in figure 3(a). The contact point
$y=0$, as shown in figure 3(a). The contact point ![]() $W$ between the swimmer and the wall has coordinates
$W$ between the swimmer and the wall has coordinates
We wish to solve for the swimmer's centre of rotation ![]() $\boldsymbol {r} = (x,y)$, which depends only on the convex hull of the swimmer; hence, the swimmer's shape may be assumed convex without loss of generality. We proceed differently depending on whether the contact point
$\boldsymbol {r} = (x,y)$, which depends only on the convex hull of the swimmer; hence, the swimmer's shape may be assumed convex without loss of generality. We proceed differently depending on whether the contact point ![]() $W$ is a corner or a smooth boundary point. (Note that the analysis below can be couched in the language of Legendre transformations and convex analysis, but we opt here for a direct treatment.)
$W$ is a corner or a smooth boundary point. (Note that the analysis below can be couched in the language of Legendre transformations and convex analysis, but we opt here for a direct treatment.)

Figure 3. (a) Convex swimmer touching a horizontal wall at a corner point ![]() $W$. (b,c) Holding
$W$. (b,c) Holding ![]() $W$ fixed, the angle
$W$ fixed, the angle ![]() $\theta$ can vary from the right-tangency angle
$\theta$ can vary from the right-tangency angle ![]() $\theta ^-$ to the left-tangency angle
$\theta ^-$ to the left-tangency angle ![]() $\theta ^+$.
$\theta ^+$.
2.1.1. Corner
Consider first the case where the contact point ![]() $W$ corresponds to a corner of the piecewise-smooth boundary, as in figure 3(a). The parameter
$W$ corresponds to a corner of the piecewise-smooth boundary, as in figure 3(a). The parameter ![]() $\varphi$ has a fixed value for corner
$\varphi$ has a fixed value for corner ![]() $W$. The allowable range of
$W$. The allowable range of ![]() $\theta$ is then determined by the right- and left-tangency values of
$\theta$ is then determined by the right- and left-tangency values of ![]() $\theta$
$\theta$
as depicted in figures 3(b) and 3(c). Here, ![]() $Y_{b}'$ and
$Y_{b}'$ and ![]() $X_{b}'$ are derivatives of
$X_{b}'$ are derivatives of ![]() $Y_{b}$ and
$Y_{b}$ and ![]() $X_{b}$.
$X_{b}$.
For this range of ![]() $\theta$, we can then use (2.4) to deduce the range of
$\theta$, we can then use (2.4) to deduce the range of ![]() $y$ values
$y$ values
We call ![]() $y_*$ the wall distance function. It characterizes the minimum distance from the swimmer's centre of rotation to a horizontal wall, as a function of the swimmer's orientation. Observe that a given corner corresponds to a single
$y_*$ the wall distance function. It characterizes the minimum distance from the swimmer's centre of rotation to a horizontal wall, as a function of the swimmer's orientation. Observe that a given corner corresponds to a single ![]() $\varphi$ value, but a range of
$\varphi$ value, but a range of ![]() $\theta$ values.
$\theta$ values.
Example 2.1 (needle swimmer)
As a simple example, take
This is the needle swimmer with centre of rotation displaced by ![]() $X_{{rot}}$, with
$X_{{rot}}$, with ![]() $\lvert X _{{rot}}\rvert \le \ell /2$. It consists of a one-dimensional segment of length
$\lvert X _{{rot}}\rvert \le \ell /2$. It consists of a one-dimensional segment of length ![]() $\ell$, with degenerate ‘corners’ at
$\ell$, with degenerate ‘corners’ at ![]() $\varphi _1=0$ and
$\varphi _1=0$ and ![]() $\varphi _2={\rm \pi}$. At
$\varphi _2={\rm \pi}$. At ![]() $\varphi =\varphi _1=0$, we have
$\varphi =\varphi _1=0$, we have ![]() $-{\rm \pi} \le \theta \le 0$, so from (2.6)
$-{\rm \pi} \le \theta \le 0$, so from (2.6) ![]() $y_*(\theta ) = -\sin \theta \,X_{b}(\varphi _1) = -\sin \theta (\tfrac 12\ell - X_{{rot}})$. At
$y_*(\theta ) = -\sin \theta \,X_{b}(\varphi _1) = -\sin \theta (\tfrac 12\ell - X_{{rot}})$. At ![]() $\varphi =\varphi _2={\rm \pi}$, we have
$\varphi =\varphi _2={\rm \pi}$, we have ![]() $0 \le \theta \le {\rm \pi}$, so from (2.6)
$0 \le \theta \le {\rm \pi}$, so from (2.6) ![]() $y_*(\theta ) = -\sin \theta \,X_{b}(\varphi _2) = -\sin \theta (-\tfrac 12\ell - X_{{rot}})$. We can combine these cases by writing
$y_*(\theta ) = -\sin \theta \,X_{b}(\varphi _2) = -\sin \theta (-\tfrac 12\ell - X_{{rot}})$. We can combine these cases by writing
Only needle positions with ![]() $y \ge y_*(\theta )$ are allowed; see figures 4(a) and 4(b) for a plot. For the case
$y \ge y_*(\theta )$ are allowed; see figures 4(a) and 4(b) for a plot. For the case ![]() $X_{{rot}}=0$, this type of swimmer and configuration space was investigated by Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015).
$X_{{rot}}=0$, this type of swimmer and configuration space was investigated by Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015).

Figure 4. The wall distance function ![]() $y_*(\theta )$ for three different swimmers: (a,b) needle of length
$y_*(\theta )$ for three different swimmers: (a,b) needle of length ![]() $\ell =2a=1$; (c,d) ellipse of length
$\ell =2a=1$; (c,d) ellipse of length ![]() $\ell =2a=1$ and width
$\ell =2a=1$ and width ![]() $2b=1/2$; (e,f) teardrop-shaped swimmer of size
$2b=1/2$; (e,f) teardrop-shaped swimmer of size ![]() $1$ by
$1$ by ![]() $1$. The inset shows the swimmer shape and centre of rotation, with swimming direction to the right. The right column has centre of rotation displaced to
$1$. The inset shows the swimmer shape and centre of rotation, with swimming direction to the right. The right column has centre of rotation displaced to ![]() $X_{{rot}}=-1/4$.
$X_{{rot}}=-1/4$.
We will typically use ![]() $\ell$ to denote the maximum diameter of a swimmer, which controls whether or not it can reverse direction for a given channel width (§ 2.2). The parameter
$\ell$ to denote the maximum diameter of a swimmer, which controls whether or not it can reverse direction for a given channel width (§ 2.2). The parameter ![]() $X_{{rot}}$ controls the position of the centre of rotation: for
$X_{{rot}}$ controls the position of the centre of rotation: for ![]() $X_{{rot}} > 0$ it is closer to the front, and for
$X_{{rot}} > 0$ it is closer to the front, and for ![]() $X_{{rot}} < 0$ it is towards the rear. We refer to these cases as front rotating and rear rotating, respectively.
$X_{{rot}} < 0$ it is towards the rear. We refer to these cases as front rotating and rear rotating, respectively.
2.1.2. Smooth boundary point
When the contact point ![]() $W$ is at a smooth boundary point, given
$W$ is at a smooth boundary point, given ![]() $\theta$ we wish to solve for
$\theta$ we wish to solve for ![]() $(x,y)$ and
$(x,y)$ and ![]() $\varphi$. Two equations come from (2.4), but we need a third, which stems from requiring that the tangent to the swimmer,
$\varphi$. Two equations come from (2.4), but we need a third, which stems from requiring that the tangent to the swimmer,
is horizontal at ![]() $W$
$W$
or
We can solve (2.11) for ![]() $\varphi = \varphi _*(\theta )$, which we then use in (2.4) to obtain
$\varphi = \varphi _*(\theta )$, which we then use in (2.4) to obtain ![]() $\boldsymbol {r} = \boldsymbol {r}_*(\theta )$ at the contact point
$\boldsymbol {r} = \boldsymbol {r}_*(\theta )$ at the contact point
Equation (2.11) can have more than one solution, but we keep the one that leads to a non-negative wall distance function,
Example 2.2 (elliptical swimmer)
An ellipse-shaped swimmer with semi-axes ![]() $a$ and
$a$ and ![]() $b$ can be parameterized as
$b$ can be parameterized as
with ![]() $\lvert X _{{rot}}\rvert \le a$. Here,
$\lvert X _{{rot}}\rvert \le a$. Here, ![]() $a$ and
$a$ and ![]() $b$ are the semi-axes along and perpendicular to the swimming direction, respectively. The tangency condition (2.11) is then
$b$ are the semi-axes along and perpendicular to the swimming direction, respectively. The tangency condition (2.11) is then ![]() $\cot \varphi _*(\theta ) = (a/b)\tan \theta$. After inserting in (2.13) and selecting the non-negative solution we obtain
$\cot \varphi _*(\theta ) = (a/b)\tan \theta$. After inserting in (2.13) and selecting the non-negative solution we obtain
This wall distance function is plotted in figures 4(c) and 4(d).
For ![]() $a\ge b$, it is convenient to rewrite (2.15) as
$a\ge b$, it is convenient to rewrite (2.15) as
where ![]() $e$ is the eccentricity. For
$e$ is the eccentricity. For ![]() $b=0$ (
$b=0$ (![]() $e=1$) we recover the needle case (2.8), with
$e=1$) we recover the needle case (2.8), with ![]() $a=\ell /2$. The case
$a=\ell /2$. The case ![]() $e=0$ is a circular swimmer, which for
$e=0$ is a circular swimmer, which for ![]() $X_{{rot}}=0$ has the same dynamics in our model as a point swimmer (Elgeti & Gompper Reference Elgeti and Gompper2013; Lee Reference Lee2013). Note, however, that for
$X_{{rot}}=0$ has the same dynamics in our model as a point swimmer (Elgeti & Gompper Reference Elgeti and Gompper2013; Lee Reference Lee2013). Note, however, that for ![]() $X_{{rot}}\ne 0$ even a circular swimmer can exhibit alignment with the walls (see example 5.2).
$X_{{rot}}\ne 0$ even a circular swimmer can exhibit alignment with the walls (see example 5.2).
2.1.3. General shapes
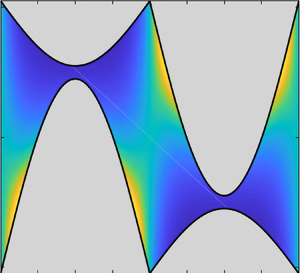
The convex hull for a general swimmer will consist of a combination of smooth parts separated by corners, as for the ‘teardrop’ swimmer depicted in figure 2. The wall distance function ![]() $y_*(\theta )$ cannot be found analytically in general, but is easy to compute numerically. The simplest approach is to discretize the convex hull as a polygon, and then apply the formulas in § 2.1.1 to every corner.
$y_*(\theta )$ cannot be found analytically in general, but is easy to compute numerically. The simplest approach is to discretize the convex hull as a polygon, and then apply the formulas in § 2.1.1 to every corner.
Example 2.3 (teardrop swimmer)
The ‘teardrop’ swimmer depicted in figure 2 is parameterized by
with ![]() $\lvert X _{{rot}}\rvert \le a$. This shape has a smooth boundary except for one corner at
$\lvert X _{{rot}}\rvert \le a$. This shape has a smooth boundary except for one corner at ![]() $\varphi = \varphi _1 = {\rm \pi}$. The wall distance function can be obtained analytically but is a bit cumbersome; we plot it in figures 4(e) and 4(f). Unlike the previous examples, the wall distance function for the teardrop swimmer has a local minimum at
$\varphi = \varphi _1 = {\rm \pi}$. The wall distance function can be obtained analytically but is a bit cumbersome; we plot it in figures 4(e) and 4(f). Unlike the previous examples, the wall distance function for the teardrop swimmer has a local minimum at ![]() $\theta =-{\rm \pi} /2$, rather than a maximum. This value of
$\theta =-{\rm \pi} /2$, rather than a maximum. This value of ![]() $\theta$ corresponds to swimming towards the wall, and the minimum suggests that this shape has a tendency to align perpendicular to the wall, rather than parallel. (This is similar to the triangular swimmer in Lushi et al. Reference Lushi, Kantsler and Goldstein2017.) In the presence of diffusion, the depth of the local minimum is a measure of how long a swimmer gets stuck in that position before fluctuating out. See also example 5.2 for another, simpler model swimmer that aligns perpendicular to the wall.
$\theta$ corresponds to swimming towards the wall, and the minimum suggests that this shape has a tendency to align perpendicular to the wall, rather than parallel. (This is similar to the triangular swimmer in Lushi et al. Reference Lushi, Kantsler and Goldstein2017.) In the presence of diffusion, the depth of the local minimum is a measure of how long a swimmer gets stuck in that position before fluctuating out. See also example 5.2 for another, simpler model swimmer that aligns perpendicular to the wall.
All the examples discussed thus far involve left–right-symmetric swimmers, which satisfy ![]() $(X_{b}(\varphi ),Y_{b}(\varphi )) = (X_{b}(-\varphi ),-Y_{b}(-\varphi ))$. For this class of swimmers, the wall distance function has the symmetry
$(X_{b}(\varphi ),Y_{b}(\varphi )) = (X_{b}(-\varphi ),-Y_{b}(-\varphi ))$. For this class of swimmers, the wall distance function has the symmetry
which is evident in figure 4.
2.2. Channel geometry
So far we have considered a two-dimensional swimmer above a single infinite horizontal wall. In a channel geometry, the swimmer is confined between two parallel infinite walls, at ![]() $y=\pm L/2$. Luckily, we do not need to derive a separate wall distance function for the top wall: we can deduce it by symmetry. The centre of rotation of a swimmer with wall distance function
$y=\pm L/2$. Luckily, we do not need to derive a separate wall distance function for the top wall: we can deduce it by symmetry. The centre of rotation of a swimmer with wall distance function ![]() $y_*(\theta )$ will have its
$y_*(\theta )$ will have its ![]() $y$ coordinate in the range
$y$ coordinate in the range
where
This means that ![]() $\zeta _\pm$ are related by the channel symmetry
$\zeta _\pm$ are related by the channel symmetry
The ![]() $x$ coordinate of the centre of rotation is unconstrained and can be any real number, but the domain for the swimming angle
$x$ coordinate of the centre of rotation is unconstrained and can be any real number, but the domain for the swimming angle ![]() $\theta$ can either be
$\theta$ can either be ![]() $[-{\rm \pi} ,{\rm \pi} ]$ or a union of disjoint intervals. This depends on whether
$[-{\rm \pi} ,{\rm \pi} ]$ or a union of disjoint intervals. This depends on whether ![]() $\zeta _-(\theta ) < \zeta _+(\theta )$ for all
$\zeta _-(\theta ) < \zeta _+(\theta )$ for all ![]() $\theta \in [-{\rm \pi} ,{\rm \pi} ]$, or
$\theta \in [-{\rm \pi} ,{\rm \pi} ]$, or ![]() $\zeta _+(\theta ) = \zeta _-(\theta )$ for some
$\zeta _+(\theta ) = \zeta _-(\theta )$ for some ![]() $\theta$. We call these two cases the open channel and the closed channel, respectively.
$\theta$. We call these two cases the open channel and the closed channel, respectively.
2.2.1. Open channel
In the simplest case, we have
In this case the swimmer can fully reverse direction in the channel. The full configuration space for the swimmer's centre of rotation is then
periodic in the ![]() $\theta$ direction. A typical example for this configuration space is depicted in figure 5(a).
$\theta$ direction. A typical example for this configuration space is depicted in figure 5(a).

Figure 5. Configuration space for the needle in figure 4(b) of length ![]() $\ell =2a=1$ in (a) an open channel of width
$\ell =2a=1$ in (a) an open channel of width ![]() $L = 1.05$; (b) a closed channel of width
$L = 1.05$; (b) a closed channel of width ![]() $L = 0.95$. (
$L = 0.95$. (![]() $x$ direction not shown.)
$x$ direction not shown.)
2.2.2. Closed channel
Another possibility is that ![]() $\zeta _+(\theta _i)=\zeta _-(\theta _i)$ for some set of points
$\zeta _+(\theta _i)=\zeta _-(\theta _i)$ for some set of points ![]() $\{\theta _i\}$. This breaks up
$\{\theta _i\}$. This breaks up ![]() $[-{\rm \pi} ,{\rm \pi} ]$ into inadmissible intervals where
$[-{\rm \pi} ,{\rm \pi} ]$ into inadmissible intervals where ![]() $\zeta _-(\theta ) > \zeta _+(\theta )$, and
$\zeta _-(\theta ) > \zeta _+(\theta )$, and ![]() $N$ disjoint admissible intervals
$N$ disjoint admissible intervals
The relevant interval is determined by the initial orientation of the swimmer. The motion of the swimmer then takes place in the configuration space
which is not periodic in the ![]() $\theta$ direction. A typical example for this configuration space is depicted in figure 5(b). Note that the condition
$\theta$ direction. A typical example for this configuration space is depicted in figure 5(b). Note that the condition ![]() $\zeta _+(\theta _i)=\zeta _-(\theta _i)$ together with the channel symmetry (2.21) implies that
$\zeta _+(\theta _i)=\zeta _-(\theta _i)$ together with the channel symmetry (2.21) implies that ![]() $\zeta _+(\theta _i + {\rm \pi})=\zeta _-(\theta _i + {\rm \pi})$.
$\zeta _+(\theta _i + {\rm \pi})=\zeta _-(\theta _i + {\rm \pi})$.
3. Stochastic model
Now that we have established that the domain of motion for our swimmer is described by the configuration space of § 2, we describe the stochastic model for the swimmer's motion, the ABP model. For simplicity, we neglect hydrodynamic interactions; we will include them in § 8.
3.1. Derivation from the stochastic differential equation
In the ABP model, the Brownian swimmer obeys the stochastic equation
in its own rotating reference frame. (We omitted any intrinsic swimmer rotation for simplicity, though this would not change the derivation appreciably. We will see that a net rotation can still emerge when the swimmer is not left–right symmetric.) In terms of absolute ![]() $x$ and
$x$ and ![]() $y$ coordinates, this becomes an Itô stochastic equation
$y$ coordinates, this becomes an Itô stochastic equation
For now we take ![]() $U$,
$U$, ![]() $D_X$,
$D_X$, ![]() $D_Y$ and
$D_Y$ and ![]() $D_\theta$ to be general functions of
$D_\theta$ to be general functions of ![]() $(x,y,\theta ,t)$. The corresponding Fokker–Planck equation for the probability density
$(x,y,\theta ,t)$. The corresponding Fokker–Planck equation for the probability density ![]() $p(x,y,\theta ,t)$ is then
$p(x,y,\theta ,t)$ is then
where ![]() , and the drift vector and diffusion tensor are respectively
, and the drift vector and diffusion tensor are respectively
 \begin{equation} \boldsymbol{U} = \begin{pmatrix} U\cos\theta \\ U\sin\theta \end{pmatrix}, \quad \boldsymbol{\mathsf{D}} = \begin{pmatrix} D_X\cos^2\theta + D_Y\sin^2\theta & \tfrac{1}{2}(D_X-D_Y)\sin2\theta \\ \tfrac{1}{2}(D_X-D_Y)\sin2\theta & D_X\sin^2\theta + D_Y\cos^2\theta \\ \end{pmatrix}. \end{equation}
\begin{equation} \boldsymbol{U} = \begin{pmatrix} U\cos\theta \\ U\sin\theta \end{pmatrix}, \quad \boldsymbol{\mathsf{D}} = \begin{pmatrix} D_X\cos^2\theta + D_Y\sin^2\theta & \tfrac{1}{2}(D_X-D_Y)\sin2\theta \\ \tfrac{1}{2}(D_X-D_Y)\sin2\theta & D_X\sin^2\theta + D_Y\cos^2\theta \\ \end{pmatrix}. \end{equation}See Kurzthaler, Leitmann & Franosch (Reference Kurzthaler, Leitmann and Franosch2016) and Kurzthaler & Franosch (Reference Kurzthaler and Franosch2017) for the intermediate scattering function for (3.3) in the absence of boundaries and with constant parameters.
For any fixed volume ![]() $V$ we have
$V$ we have
where ![]() $\partial V$ is the boundary of
$\partial V$ is the boundary of ![]() $V$, and the flux vector is
$V$, and the flux vector is
Thus, on the impermeable parts of the boundary we require the no-flux condition
where ![]() $\boldsymbol {n}$ is normal to the boundary.
$\boldsymbol {n}$ is normal to the boundary.
3.2. Infinite channel geometry
The previous section applies to any geometry and general ![]() $\boldsymbol {U}$,
$\boldsymbol {U}$, ![]() $\boldsymbol{\mathsf{D}}$, and
$\boldsymbol{\mathsf{D}}$, and ![]() $D_\theta$, which can be functions of
$D_\theta$, which can be functions of ![]() $(x,y,\theta ,t)$. For our problem, these only depend on
$(x,y,\theta ,t)$. For our problem, these only depend on ![]() $\theta$. In an infinite channel geometry (§ 2.2), which we consider in this paper, we can eliminate the along-channel direction
$\theta$. In an infinite channel geometry (§ 2.2), which we consider in this paper, we can eliminate the along-channel direction ![]() $x$ by defining the marginal probability density
$x$ by defining the marginal probability density
In order for ![]() $\bar{p}$ to be finite,
$\bar{p}$ to be finite, ![]() $p$ has to decay fast enough as
$p$ has to decay fast enough as ![]() $\lvert x \rvert \rightarrow \infty$; we use this assumption to discard some terms after we integrate (3.3) from
$\lvert x \rvert \rightarrow \infty$; we use this assumption to discard some terms after we integrate (3.3) from ![]() $x=-\infty$ to
$x=-\infty$ to ![]() $\infty$, and find an equation for
$\infty$, and find an equation for ![]() $\bar{p}$
$\bar{p}$
where from (3.4) ![]() $D_{yy} = [\boldsymbol{\mathsf{D}}]_{22} = D_X\sin ^2\theta + D_Y\cos ^2\theta$. For the rest of the paper we take
$D_{yy} = [\boldsymbol{\mathsf{D}}]_{22} = D_X\sin ^2\theta + D_Y\cos ^2\theta$. For the rest of the paper we take ![]() $U$,
$U$, ![]() $D_X$,
$D_X$, ![]() $D_Y$ and
$D_Y$ and ![]() $D_\theta$ to be constants, so that (3.9) simplifies to
$D_\theta$ to be constants, so that (3.9) simplifies to
(Note that, instead of defining ![]() $\bar{p}$ as in (3.8), we could assume that
$\bar{p}$ as in (3.8), we could assume that ![]() $p$ is independent of
$p$ is independent of ![]() $x$, in which case
$x$, in which case ![]() $p$ is a density per unit length that satisfies (3.10).) Equation (3.10) is our main focus. The corresponding flux vector (3.6) reduces to
$p$ is a density per unit length that satisfies (3.10).) Equation (3.10) is our main focus. The corresponding flux vector (3.6) reduces to
For the channel geometry, the domain can be characterized by ![]() $\zeta _-(\theta ) < y < \zeta _+(\theta )$, so the normal vector is
$\zeta _-(\theta ) < y < \zeta _+(\theta )$, so the normal vector is
The no-flux boundary conditions on (3.10) comes from (3.7)
For convenience, we gather together the main (3.10) and its no-flux boundary condition (3.13) for an infinite channel geometry
As discussed in § 2, the domain in ![]() $\theta$ is
$\theta$ is ![]() $[-{\rm \pi} ,{\rm \pi} ]$ (periodic) for
$[-{\rm \pi} ,{\rm \pi} ]$ (periodic) for ![]() $\zeta _-(\theta ) < \zeta _+(\theta )$, which means the swimmer can fully reverse direction in the channel (open-channel configuration, figure 5a). If
$\zeta _-(\theta ) < \zeta _+(\theta )$, which means the swimmer can fully reverse direction in the channel (open-channel configuration, figure 5a). If ![]() $\zeta _-(\theta ) \le \zeta _+(\theta )$, the domain ‘pinches off’ whenever
$\zeta _-(\theta ) \le \zeta _+(\theta )$, the domain ‘pinches off’ whenever ![]() $\zeta _-(\theta ) = \zeta _+(\theta )$, and consists of two or more disconnected pieces (closed-channel configuration, figure 5b).
$\zeta _-(\theta ) = \zeta _+(\theta )$, and consists of two or more disconnected pieces (closed-channel configuration, figure 5b).
4. Reduced equation
Equation (3.14) is a challenging equation to solve, in particular because of the complicated boundary shape. We can dramatically simplify the problem by assuming that the diffusivity ![]() $D_\theta$ is small, and carrying out an expansion in powers of
$D_\theta$ is small, and carrying out an expansion in powers of ![]() $\varepsilon =D_\theta$. We call this the small-
$\varepsilon =D_\theta$. We call this the small-![]() $D_\theta$ or reduced limit. The reduced form of (3.14), given by (4.17), will enable us to solve for the invariant density for a swimmer in § 5, as well as many other quantities of interest such as a swimmer's mean reversal time (§ 6) and its effective diffusivity along the channel (§ 7).
$D_\theta$ or reduced limit. The reduced form of (3.14), given by (4.17), will enable us to solve for the invariant density for a swimmer in § 5, as well as many other quantities of interest such as a swimmer's mean reversal time (§ 6) and its effective diffusivity along the channel (§ 7).
Take (3.14) and write ![]() $D_\theta =\varepsilon$:
$D_\theta =\varepsilon$:
where we also defined a slow time ![]() $T = \varepsilon t$,
$T = \varepsilon t$, ![]() $\partial _t \rightarrow \varepsilon \,\partial _T$. We write the regular expansion
$\partial _t \rightarrow \varepsilon \,\partial _T$. We write the regular expansion
and proceed to solve for ![]() $\bar{p}_i$ order by order.
$\bar{p}_i$ order by order.
At order ![]() $\varepsilon ^0$, (4.1) is
$\varepsilon ^0$, (4.1) is
with solution
where ![]() $Q(\theta ,T)$ is as-yet undetermined.
$Q(\theta ,T)$ is as-yet undetermined.
At order ![]() $\varepsilon ^1$, (4.1) is
$\varepsilon ^1$, (4.1) is
Integrate (4.5a) from ![]() $y=\zeta _-$ to
$y=\zeta _-$ to ![]() $\zeta _+$ and use the boundary conditions (4.5b) to get, on the left,
$\zeta _+$ and use the boundary conditions (4.5b) to get, on the left,
 \begin{align}
&\int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
(U\sin\theta\,\partial_y\bar{p}_1 -
D_{yy}\,\partial_y^2\bar{p}_1)\,{\mathrm{d}}y\notag\\ &\quad= \left
[U\sin\theta\,\bar{p}_1 -
D_{yy}(\theta)\,\partial_y\bar{p}_1 \right
]_{\zeta_-(\theta)}^{\zeta_+(\theta)} \nonumber\\
&\quad={-}\zeta_+'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_+(\theta))
+
\zeta_-'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_-(\theta)).
\end{align}
\begin{align}
&\int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
(U\sin\theta\,\partial_y\bar{p}_1 -
D_{yy}\,\partial_y^2\bar{p}_1)\,{\mathrm{d}}y\notag\\ &\quad= \left
[U\sin\theta\,\bar{p}_1 -
D_{yy}(\theta)\,\partial_y\bar{p}_1 \right
]_{\zeta_-(\theta)}^{\zeta_+(\theta)} \nonumber\\
&\quad={-}\zeta_+'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_+(\theta))
+
\zeta_-'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_-(\theta)).
\end{align}
On the right, the integral of the term ![]() $\partial _\theta ^2\bar{p}_0$ is
$\partial _\theta ^2\bar{p}_0$ is
 \begin{align} \int_{\zeta_-(\theta)}^{\zeta_+(\theta)}\partial_\theta^2\bar{p}_0\,{\mathrm{d}}y = \partial_\theta \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \partial_\theta\bar{p}_0\,{\mathrm{d}}y -\zeta_+'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_+(\theta)) + \zeta_-'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_-(\theta)). \end{align}
\begin{align} \int_{\zeta_-(\theta)}^{\zeta_+(\theta)}\partial_\theta^2\bar{p}_0\,{\mathrm{d}}y = \partial_\theta \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \partial_\theta\bar{p}_0\,{\mathrm{d}}y -\zeta_+'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_+(\theta)) + \zeta_-'(\theta)\,\partial_\theta\bar{p}_0(\theta,\zeta_-(\theta)). \end{align}Combining the last two equations, we obtain
 \begin{equation} \partial_T \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0\,{\mathrm{d}}y = \partial_\theta \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \partial_\theta\bar{p}_0\,{\mathrm{d}}y . \end{equation}
\begin{equation} \partial_T \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0\,{\mathrm{d}}y = \partial_\theta \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \partial_\theta\bar{p}_0\,{\mathrm{d}}y . \end{equation}
We can then carry out the ![]() $y$ integral on the right of (4.8) after using (4.4), to get
$y$ integral on the right of (4.8) after using (4.4), to get
 \begin{equation} \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \left ( \partial_\theta Q + \sigma'(\theta)Q\,y \right )\mathrm{e}^{\sigma(\theta)\,y}\,{\mathrm{d}}y = w(\theta)\,\partial_\theta Q - \nu(\theta) Q , \end{equation}
\begin{equation} \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \left ( \partial_\theta Q + \sigma'(\theta)Q\,y \right )\mathrm{e}^{\sigma(\theta)\,y}\,{\mathrm{d}}y = w(\theta)\,\partial_\theta Q - \nu(\theta) Q , \end{equation}where we defined the weight
 \begin{equation} w(\theta) =
\int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
\mathrm{e}^{\sigma(\theta)\,y} \,{\mathrm{d}}y \nonumber\\
= \left (\exp({\sigma(\theta)\,\zeta_+(\theta)}) -
\exp({\sigma(\theta)\,\zeta_-(\theta)}) \right
)/\sigma(\theta),
\end{equation}
\begin{equation} w(\theta) =
\int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
\mathrm{e}^{\sigma(\theta)\,y} \,{\mathrm{d}}y \nonumber\\
= \left (\exp({\sigma(\theta)\,\zeta_+(\theta)}) -
\exp({\sigma(\theta)\,\zeta_-(\theta)}) \right
)/\sigma(\theta),
\end{equation}
and the drift
 \begin{align} \nu(\theta) &={-}
\sigma'(\theta) \int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
y\,\mathrm{e}^{\sigma(\theta)\,y} \,{\mathrm{d}}y
\nonumber\\ &={-}
\frac{\sigma'(\theta)}{\sigma(\theta)}\left ( \left
[y\,\mathrm{e}^{\sigma(\theta)\,y}\right
]_{\zeta_-(\theta)}^{\zeta_+(\theta)} -
\int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
\mathrm{e}^{\sigma(\theta)\,y} \,{\mathrm{d}}y \right )
\nonumber\\ &= \frac{\sigma'(\theta)}{\sigma(\theta)}\left
(w(\theta) -
\exp({\sigma(\theta)\,\zeta_+(\theta)})\,\zeta_+(\theta) +
\exp({\sigma(\theta)\,\zeta_-(\theta)})\,\zeta_-(\theta)
\right ). \end{align}
\begin{align} \nu(\theta) &={-}
\sigma'(\theta) \int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
y\,\mathrm{e}^{\sigma(\theta)\,y} \,{\mathrm{d}}y
\nonumber\\ &={-}
\frac{\sigma'(\theta)}{\sigma(\theta)}\left ( \left
[y\,\mathrm{e}^{\sigma(\theta)\,y}\right
]_{\zeta_-(\theta)}^{\zeta_+(\theta)} -
\int_{\zeta_-(\theta)}^{\zeta_+(\theta)}
\mathrm{e}^{\sigma(\theta)\,y} \,{\mathrm{d}}y \right )
\nonumber\\ &= \frac{\sigma'(\theta)}{\sigma(\theta)}\left
(w(\theta) -
\exp({\sigma(\theta)\,\zeta_+(\theta)})\,\zeta_+(\theta) +
\exp({\sigma(\theta)\,\zeta_-(\theta)})\,\zeta_-(\theta)
\right ). \end{align}
Note that ![]() $w(\theta ) > 0$ if
$w(\theta ) > 0$ if ![]() $\zeta _+(\theta ) > \zeta _-(\theta )$, and
$\zeta _+(\theta ) > \zeta _-(\theta )$, and ![]() $w(\theta ) = 0$ if and only if
$w(\theta ) = 0$ if and only if ![]() $\zeta _+(\theta ) = \zeta _-(\theta )$. Thus,
$\zeta _+(\theta ) = \zeta _-(\theta )$. Thus, ![]() $w(\theta )$ only vanishes when the domain ‘pinches off’, as described in § 2.2.2. Despite the apparent singularity, the weight
$w(\theta )$ only vanishes when the domain ‘pinches off’, as described in § 2.2.2. Despite the apparent singularity, the weight ![]() $w$ is non-singular when
$w$ is non-singular when ![]() $\sigma$ is small
$\sigma$ is small
Another convenient form for the drift ![]() $\nu$ is
$\nu$ is
with
The function ![]() $\nu$ appears singular as
$\nu$ appears singular as ![]() $\varDelta \rightarrow 0$, but the limit exists
$\varDelta \rightarrow 0$, but the limit exists
This expression is valid whether ![]() $\varDelta$ vanishes owing to
$\varDelta$ vanishes owing to ![]() $\sigma (\theta )=0$ or
$\sigma (\theta )=0$ or ![]() $\zeta _+(\theta ) = \zeta _-(\theta )$.
$\zeta _+(\theta ) = \zeta _-(\theta )$.
Doing the ![]() $y$ integral on the left of (4.8), we finally obtain the reduced equation
$y$ integral on the left of (4.8), we finally obtain the reduced equation
The reduced equation is a ![]() $(1+1)$-dimensional drift-diffusion PDE that captures the time evolution of the marginal probability density
$(1+1)$-dimensional drift-diffusion PDE that captures the time evolution of the marginal probability density
 \begin{equation} P(\theta,T) = \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0(\theta,y,T)\,{\mathrm{d}}y = w(\theta)\,Q(\theta,T). \end{equation}
\begin{equation} P(\theta,T) = \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0(\theta,y,T)\,{\mathrm{d}}y = w(\theta)\,Q(\theta,T). \end{equation}
The weight function ![]() $w(\theta )$ and drift
$w(\theta )$ and drift ![]() $\nu (\theta )$ encode the effect of the shape of the configuration space.
$\nu (\theta )$ encode the effect of the shape of the configuration space.
We can transform (4.15) into an equation for ![]() $P$
$P$
with
An explicit form for ![]() $\mu$ in terms of
$\mu$ in terms of ![]() $\varDelta$ in (4.13) is
$\varDelta$ in (4.13) is
Although (4.17) is slightly nicer than (4.15), it has the disadvantage that it requires a derivative ![]() $w'(\theta )$ in
$w'(\theta )$ in ![]() $\mu (\theta )$, which can cause problems for non-smooth swimmer shapes.
$\mu (\theta )$, which can cause problems for non-smooth swimmer shapes.
Example 4.1 ( $\mu (\theta )$ for elliptical and needle swimmers)
$\mu (\theta )$ for elliptical and needle swimmers)
For the elliptical swimmer described by (2.16), we have
with ![]() $\varDelta (\theta ) = \tfrac 12\sigma (\theta )(L - 2a\sqrt {1 - e^2\cos ^2\theta })$. Note that
$\varDelta (\theta ) = \tfrac 12\sigma (\theta )(L - 2a\sqrt {1 - e^2\cos ^2\theta })$. Note that ![]() $\mu$ vanishes when
$\mu$ vanishes when ![]() $e=0$ (circular or point swimmer). The needle is the limit of (4.20) as
$e=0$ (circular or point swimmer). The needle is the limit of (4.20) as ![]() $e\rightarrow 1$
$e\rightarrow 1$
These are plotted in figure 6. Note that ![]() $\mu _{{needle}}(\theta )$ is discontinuous at
$\mu _{{needle}}(\theta )$ is discontinuous at ![]() $\theta =0$, due to the singular derivative of
$\theta =0$, due to the singular derivative of ![]() $w$ in (4.18).
$w$ in (4.18).
5. Invariant density
A natural quantity to compute from the reduced equation (4.15) is the invariant density for the swimmer. This is the time-independent solution ![]() $Q(\theta ,T) = \mathcal {Q}(\theta )$ to (4.15)
$Q(\theta ,T) = \mathcal {Q}(\theta )$ to (4.15)
The value of ![]() $\mathcal {Q}$ is unique for a periodic domain
$\mathcal {Q}$ is unique for a periodic domain ![]() $\varOmega$ or a single component
$\varOmega$ or a single component ![]() $\varOmega _i$; see § 2.2. Note that
$\varOmega _i$; see § 2.2. Note that ![]() $\mathcal {Q}$ (and hence the invariant density) is independent of
$\mathcal {Q}$ (and hence the invariant density) is independent of ![]() $D_\theta$ at leading order.
$D_\theta$ at leading order.
To find the invariant density, we first integrate (5.1) once to get
The solution to (5.2) then can be written
where
and ![]() $\theta ^{{L}}$ is the left-most domain limit (
$\theta ^{{L}}$ is the left-most domain limit (![]() $\theta ^{{L}}=-{\rm \pi}$ for
$\theta ^{{L}}=-{\rm \pi}$ for ![]() $\varOmega$ and
$\varOmega$ and ![]() $\theta ^{{L}}=\theta ^{{L}}_i$ for
$\theta ^{{L}}=\theta ^{{L}}_i$ for ![]() $\varOmega _i$; see § 2.2). The integrand in (
$\varOmega _i$; see § 2.2). The integrand in (
) appears singular as ![]() $\varDelta \rightarrow 0$, but the limit exists as we saw in (4.14).
$\varDelta \rightarrow 0$, but the limit exists as we saw in (4.14).
Next we need to determine the constants ![]() $c_1$ and
$c_1$ and ![]() $c_2$. Normalization of
$c_2$. Normalization of ![]() determines
determines ![]() $c_1$, but
$c_1$, but ![]() $c_2$ depends on whether we have an open or closed-channel configuration space (§ 2.2). We treat these two cases separately.
$c_2$ depends on whether we have an open or closed-channel configuration space (§ 2.2). We treat these two cases separately.
5.1. Open channel
For the open-channel configuration space as described in § 2.2.1, ![]() $w(\theta )$ and
$w(\theta )$ and ![]() $\nu (\theta )$ are
$\nu (\theta )$ are ![]() $2{\rm \pi}$-periodic. The boundary condition on
$2{\rm \pi}$-periodic. The boundary condition on ![]() $\mathcal {Q}(\theta )$ is that it be periodic as well. Choosing
$\mathcal {Q}(\theta )$ is that it be periodic as well. Choosing ![]() $\theta ^{{L}}=-{\rm \pi}$ in (5.4), we have
$\theta ^{{L}}=-{\rm \pi}$ in (5.4), we have
We solve for ![]() $c_2$ in (5.5) to obtain
$c_2$ in (5.5) to obtain
and
The constant ![]() $c_1$ is chosen to enforce the normalization of
$c_1$ is chosen to enforce the normalization of ![]() $\mathcal {P} = w\mathcal {Q}$
$\mathcal {P} = w\mathcal {Q}$
 \begin{equation} \int_{-{\rm \pi}}^{\rm \pi}\int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0(\theta,y)\,{\mathrm{d}}y\,{\mathrm{d}}\theta = \int_{-{\rm \pi}}^{\rm \pi} \mathcal{P}(\theta)\,{\mathrm{d}}\theta = 1. \end{equation}
\begin{equation} \int_{-{\rm \pi}}^{\rm \pi}\int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0(\theta,y)\,{\mathrm{d}}y\,{\mathrm{d}}\theta = \int_{-{\rm \pi}}^{\rm \pi} \mathcal{P}(\theta)\,{\mathrm{d}}\theta = 1. \end{equation} If ![]() $\varPhi (\theta )$ happens to be
$\varPhi (\theta )$ happens to be ![]() $2{\rm \pi}$-periodic, then we have
$2{\rm \pi}$-periodic, then we have ![]() $\varPhi ({\rm \pi} )=0$, so
$\varPhi ({\rm \pi} )=0$, so ![]() $c_2=0$ and
$c_2=0$ and
The invariant probability density in this case satisfies detailed balance (Pavliotis Reference Pavliotis2014). In fact ![]() $\varPhi (\theta )$ is periodic for the very important case of a left–right symmetric swimmer, for then we have
$\varPhi (\theta )$ is periodic for the very important case of a left–right symmetric swimmer, for then we have ![]() $\zeta _+(\theta ) = -\zeta _-(-\theta )$, which follows from symmetries (2.18) and (2.21). This leads to
$\zeta _+(\theta ) = -\zeta _-(-\theta )$, which follows from symmetries (2.18) and (2.21). This leads to ![]() $\varDelta (-\theta ) = -\varDelta (\theta )$ and the integrand of (5.4) is odd in
$\varDelta (-\theta ) = -\varDelta (\theta )$ and the integrand of (5.4) is odd in ![]() $\theta$. Choosing
$\theta$. Choosing ![]() $\theta ^{{L}}=-{\rm \pi}$ then gives
$\theta ^{{L}}=-{\rm \pi}$ then gives ![]() $\varPhi (-{\rm \pi} )=\varPhi ({\rm \pi} )=0$, i.e.
$\varPhi (-{\rm \pi} )=\varPhi ({\rm \pi} )=0$, i.e. ![]() $\varPhi$ is periodic.
$\varPhi$ is periodic.
From ![]() $\mathcal {Q}$, we can reconstruct the full invariant density from (4.4) as
$\mathcal {Q}$, we can reconstruct the full invariant density from (4.4) as ![]() $\bar{p}_0(\theta ,y) = \mathcal {Q}(\theta )\,\mathrm {e}^{\sigma (\theta )y}$, with
$\bar{p}_0(\theta ,y) = \mathcal {Q}(\theta )\,\mathrm {e}^{\sigma (\theta )y}$, with ![]() $\sigma (\theta ) = U\sin \theta /D_{yy}(\theta )$. The exponential term reflects the accumulation near both walls, as observed in experiments and simulations. The thickness of the boundary layer is
$\sigma (\theta ) = U\sin \theta /D_{yy}(\theta )$. The exponential term reflects the accumulation near both walls, as observed in experiments and simulations. The thickness of the boundary layer is ![]() $D_{yy}/U\sin \theta$, which agrees qualitatively with the results for a spherical swimmer in Elgeti & Gompper (Reference Elgeti and Gompper2013) and Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015). A typical invariant density in an open-channel configuration is shown in figure 7(a) for a needle swimmer. The marginal invariant probability density
$D_{yy}/U\sin \theta$, which agrees qualitatively with the results for a spherical swimmer in Elgeti & Gompper (Reference Elgeti and Gompper2013) and Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015). A typical invariant density in an open-channel configuration is shown in figure 7(a) for a needle swimmer. The marginal invariant probability density ![]() $\mathcal {P}(\theta )$ is plotted in figure 8(a) for elliptical swimmers with different velocities
$\mathcal {P}(\theta )$ is plotted in figure 8(a) for elliptical swimmers with different velocities ![]() $U$ and centres of rotation
$U$ and centres of rotation ![]() $X_{{rot}}$. From figure 7, the invariant marginal density in
$X_{{rot}}$. From figure 7, the invariant marginal density in ![]() $y$ peaks near both walls, but not exactly at the walls, in accordance with the simulations in the appendix of Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015).
$y$ peaks near both walls, but not exactly at the walls, in accordance with the simulations in the appendix of Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015).

Figure 7. Invariant density ![]() $\bar{p}_0 = \mathcal {Q}(\theta )\,\mathrm {e}^{\sigma (\theta )\,y}$ for
$\bar{p}_0 = \mathcal {Q}(\theta )\,\mathrm {e}^{\sigma (\theta )\,y}$ for ![]() $U=1$ and
$U=1$ and ![]() $D_X=D_Y=0.1$ for the needle in figure 4(b) of length
$D_X=D_Y=0.1$ for the needle in figure 4(b) of length ![]() $\ell =2a=1$ in (a) an open channel of width
$\ell =2a=1$ in (a) an open channel of width ![]() $L = 1.05$; (b) a closed channel of width
$L = 1.05$; (b) a closed channel of width ![]() $L = 0.95$, for the domain
$L = 0.95$, for the domain ![]() $\varOmega _1$ in figure 5(b).
$\varOmega _1$ in figure 5(b).

Figure 8. For an ellipse with ![]() $a=2b=1/2$,
$a=2b=1/2$, ![]() $D_X=D_Y=0.1$,
$D_X=D_Y=0.1$, ![]() $D_\theta =0.01$,
$D_\theta =0.01$, ![]() $U=1$, in a channel of width
$U=1$, in a channel of width ![]() $L=1.2$: (a) marginal invariant probability density
$L=1.2$: (a) marginal invariant probability density ![]() $\mathcal {P}(\theta )$; (b)
$\mathcal {P}(\theta )$; (b) ![]() $1/\mathcal {P}$, normalized to unit area (see (6.8) for definition of
$1/\mathcal {P}$, normalized to unit area (see (6.8) for definition of ![]() $\tau _{{rev}}$).
$\tau _{{rev}}$).
What is the meaning of non-zero ![]() $c_2$? It represents an average rotational drift of the needle's stochastic angle
$c_2$? It represents an average rotational drift of the needle's stochastic angle ![]() $\theta (t)$. To see this, note that in the equilibrium state we have the expectation
$\theta (t)$. To see this, note that in the equilibrium state we have the expectation
since ![]() $\mu \,\mathcal {P} - \mathcal {P}' = c_2$ and
$\mu \,\mathcal {P} - \mathcal {P}' = c_2$ and ![]() $\mathcal {P}(\theta )$ is periodic. Hence, the average rate of angular rotation of the swimmer is
$\mathcal {P}(\theta )$ is periodic. Hence, the average rate of angular rotation of the swimmer is ![]() $\omega = 2{\rm \pi} c_2$. From (5.4), the periodic average of
$\omega = 2{\rm \pi} c_2$. From (5.4), the periodic average of ![]() $\mu (\theta )$ is
$\mu (\theta )$ is
which is zero if and only if ![]() $c_2=0$ ((5.6)).
$c_2=0$ ((5.6)).
Example 5.1 (invariant density for fast needle swimmer)
It is in general quite challenging to get closed-form solutions for the invariant density of a swimmer, but it can be done in the large-![]() $U$ limit. From (5.4) and (4.18) we have
$U$ limit. From (5.4) and (4.18) we have
and so the leading-order invariant marginal density ![]() $\mathcal {P}=c_1w\,\mathrm {e}^\varPhi$ for a left–right-symmetric swimmer is
$\mathcal {P}=c_1w\,\mathrm {e}^\varPhi$ for a left–right-symmetric swimmer is
 \begin{equation} \mathcal{P}(\theta) = \bar{c}_1\,\exp\left (\int_{-{\rm \pi}}^\theta \mu(\vartheta)\,{\mathrm{d}}\vartheta\right ), \end{equation}
\begin{equation} \mathcal{P}(\theta) = \bar{c}_1\,\exp\left (\int_{-{\rm \pi}}^\theta \mu(\vartheta)\,{\mathrm{d}}\vartheta\right ), \end{equation}
with ![]() $\bar{c} _1$ a normalization constant. For large
$\bar{c} _1$ a normalization constant. For large ![]() $U$, the constant
$U$, the constant ![]() $\bar{c} _1$ can be determined by approximating the normalization integral using the maxima of
$\bar{c} _1$ can be determined by approximating the normalization integral using the maxima of ![]() $\mu$.
$\mu$.
We illustrate this here for the needle swimmer of examples 2.1 and 4.1. For large ![]() $U$, we can approximate
$U$, we can approximate ![]() $\coth \varDelta \approx \textrm {sgn}(\theta )$ for
$\coth \varDelta \approx \textrm {sgn}(\theta )$ for ![]() $\mu = \mu _{{needle}}$ in (4.21), and we have
$\mu = \mu _{{needle}}$ in (4.21), and we have
with ![]() $\sigma$ defined in (4.4), and
$\sigma$ defined in (4.4), and ![]() $a = \ell /2$ the needle half-length. Note that the channel width
$a = \ell /2$ the needle half-length. Note that the channel width ![]() $L$ does not appear in (5.14) at leading order in large
$L$ does not appear in (5.14) at leading order in large ![]() $U$: the needle spends most of its time stuck to one of the walls, so the channel width is not important. We can integrate (5.14) and use the result in (5.13) to find
$U$: the needle spends most of its time stuck to one of the walls, so the channel width is not important. We can integrate (5.14) and use the result in (5.13) to find
with
We can see that ‘large ![]() $U$’ in non-dimensional terms means large
$U$’ in non-dimensional terms means large ![]() $\beta$, which is a Péclet number that accounts for the position of the centre of rotation:
$\beta$, which is a Péclet number that accounts for the position of the centre of rotation: ![]() $\beta$ is maximized when the centre of rotation is at the rear (
$\beta$ is maximized when the centre of rotation is at the rear (![]() $X_{{rot}}=-a$), which is a rear-rotating swimmer. We can now use Laplace's method to find the normalization constant
$X_{{rot}}=-a$), which is a rear-rotating swimmer. We can now use Laplace's method to find the normalization constant ![]() $\bar{c} _1$. The maxima of the argument of the exponential in (5.15) correspond to the zeros of
$\bar{c} _1$. The maxima of the argument of the exponential in (5.15) correspond to the zeros of ![]() $\mu$ at
$\mu$ at ![]() $\theta =0$ and
$\theta =0$ and ![]() ${\rm \pi}$ (with
${\rm \pi}$ (with ![]() $-a \le X_{{rot}} < a$). We thus find
$-a \le X_{{rot}} < a$). We thus find
In the limit ![]() $\alpha =1$ (equal diffusivities), (5.17) simplifies to
$\alpha =1$ (equal diffusivities), (5.17) simplifies to
Note that the limit ![]() $\alpha \rightarrow 0$ in (5.17) is well defined and gives
$\alpha \rightarrow 0$ in (5.17) is well defined and gives ![]() $\mathcal {P}(\theta ) = \sqrt {\beta /4{\rm \pi} }\, \lvert \cos \theta \rvert ^{2\beta }$. However, the limit
$\mathcal {P}(\theta ) = \sqrt {\beta /4{\rm \pi} }\, \lvert \cos \theta \rvert ^{2\beta }$. However, the limit ![]() $\alpha \rightarrow \infty$ gives an improperly normalized density, indicating that the limits
$\alpha \rightarrow \infty$ gives an improperly normalized density, indicating that the limits ![]() $\beta$,
$\beta$, ![]() $\alpha \rightarrow \infty$ do not commute. (The quartic term in a Taylor series expansion of the
$\alpha \rightarrow \infty$ do not commute. (The quartic term in a Taylor series expansion of the ![]() $\log$ in (5.15) has coefficient proportional to
$\log$ in (5.15) has coefficient proportional to ![]() $\alpha$, and cannot be neglected when applying Laplace's method.)
$\alpha$, and cannot be neglected when applying Laplace's method.)
In figure 9 we compare a numerical solution for ![]() $\mathcal {P}$ to the large-
$\mathcal {P}$ to the large-![]() $U$ form (5.17).
$U$ form (5.17).

Figure 9. For a needle with ![]() $\ell =1$,
$\ell =1$, ![]() $U=8$,
$U=8$, ![]() $D_X=0.1$,
$D_X=0.1$, ![]() $D_Y=1$,
$D_Y=1$, ![]() $D_\theta =0.01$, in a channel of width
$D_\theta =0.01$, in a channel of width ![]() $L=1.2$ (
$L=1.2$ (![]() $\beta =3.6$): (a) marginal invariant probability density
$\beta =3.6$): (a) marginal invariant probability density ![]() $\mathcal {P}(\theta )$ (solid) and large-
$\mathcal {P}(\theta )$ (solid) and large-![]() $U$ form (5.17) (dashed). Notice the discrepancy near
$U$ form (5.17) (dashed). Notice the discrepancy near ![]() $\theta =0,{\rm \pi}$, which goes away for large
$\theta =0,{\rm \pi}$, which goes away for large ![]() $U$. (b) Value of
$U$. (b) Value of ![]() $1/\mathcal {P}$, normalized to unit area, for the same parameters.
$1/\mathcal {P}$, normalized to unit area, for the same parameters.
There is some discrepancy near ![]() $\theta =0,{\rm \pi}$, which comes from the approximation (5.14) breaking down near those points, but the difference vanishes as
$\theta =0,{\rm \pi}$, which comes from the approximation (5.14) breaking down near those points, but the difference vanishes as ![]() $U$ gets larger. We also plot
$U$ gets larger. We also plot ![]() $1/\mathcal {P}$, which we shall need in § 6, for which the approximation is uniformly much better.
$1/\mathcal {P}$, which we shall need in § 6, for which the approximation is uniformly much better.
Example 5.2 (invariant density for fast circular swimmer)
When the swimmer is perfectly circular, putting ![]() $e=0$ in (4.20) gives
$e=0$ in (4.20) gives ![]() $\mu (\theta ) = X_{{rot}}\,\sigma (\theta )\cos \theta$, which is the same as (5.14) with
$\mu (\theta ) = X_{{rot}}\,\sigma (\theta )\cos \theta$, which is the same as (5.14) with ![]() $a=0$ in the previous example, except that here this expression is exact. The invariant density thus has the form (5.15), with
$a=0$ in the previous example, except that here this expression is exact. The invariant density thus has the form (5.15), with ![]() $\beta = -UX_{{rot}}/2D_Y$, which can have either sign. For
$\beta = -UX_{{rot}}/2D_Y$, which can have either sign. For ![]() $\beta \gg 1$ we recover (5.17) and (5.18) – the circular swimmer tends to align parallel to the wall. For
$\beta \gg 1$ we recover (5.17) and (5.18) – the circular swimmer tends to align parallel to the wall. For ![]() $-\beta \gg 1$ the maxima of
$-\beta \gg 1$ the maxima of ![]() $\int \mu \,{\mathrm {d}}\theta$ switch from
$\int \mu \,{\mathrm {d}}\theta$ switch from ![]() $\{0,{\rm \pi} \}$ to
$\{0,{\rm \pi} \}$ to ![]() $\pm {\rm \pi}/2$, and we get instead of (5.17)
$\pm {\rm \pi}/2$, and we get instead of (5.17)
which for ![]() $\alpha =1$ simplifies to
$\alpha =1$ simplifies to
Comparing the latter to (5.18), we can see that fast front-rotating circular swimmers (![]() $X_{{rot}}>0$) collect at
$X_{{rot}}>0$) collect at ![]() $\theta =\pm {\rm \pi}/2$, swimming towards the wall, rather than aligning parallel to the wall. The limit
$\theta =\pm {\rm \pi}/2$, swimming towards the wall, rather than aligning parallel to the wall. The limit ![]() $\alpha \rightarrow 0$ in 5.19 gives
$\alpha \rightarrow 0$ in 5.19 gives ![]() $\mathcal {P}(\theta ) = \tfrac 12\left (\delta (\theta - {\rm \pi}/2) + \delta (\theta + {\rm \pi}/2)\right )$.
$\mathcal {P}(\theta ) = \tfrac 12\left (\delta (\theta - {\rm \pi}/2) + \delta (\theta + {\rm \pi}/2)\right )$.
5.2. Closed channel
For a closed-channel configuration space, as described in § 2.2.2, the domain is given by ![]() $\varOmega _i$ in (2.25), for some fixed
$\varOmega _i$ in (2.25), for some fixed ![]() $i$. Now the boundary condition is that there be no net flux in the
$i$. Now the boundary condition is that there be no net flux in the ![]() $\theta$ direction, so the constant
$\theta$ direction, so the constant ![]() $c_2=0$ in (5.2). The solution for the invariant density is thus
$c_2=0$ in (5.2). The solution for the invariant density is thus
with ![]() $c_1$ obtained by the normalization condition for
$c_1$ obtained by the normalization condition for ![]() $\mathcal {P} = w\mathcal {Q}$
$\mathcal {P} = w\mathcal {Q}$
 \begin{equation} \int_{\theta^{{L}}_i}^{\theta^{{R}}_i} \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0(\theta,y)\,{\mathrm{d}}y\,{\mathrm{d}}\theta = \int_{\theta^{{L}}_i}^{\theta^{{R}}_i} \mathcal{P}(\theta)\,{\mathrm{d}}\theta = 1. \end{equation}
\begin{equation} \int_{\theta^{{L}}_i}^{\theta^{{R}}_i} \int_{\zeta_-(\theta)}^{\zeta_+(\theta)} \bar{p}_0(\theta,y)\,{\mathrm{d}}y\,{\mathrm{d}}\theta = \int_{\theta^{{L}}_i}^{\theta^{{R}}_i} \mathcal{P}(\theta)\,{\mathrm{d}}\theta = 1. \end{equation}A typical invariant density in a closed-channel configuration is shown in figure 7(b) for a needle swimmer.
6. Mean exit times and MRTs
A standard problem in drift-diffusion processes is to compute the mean exit time (MET) of a particle to some exit, also called a first-passage time (Ward & Keller Reference Ward and Keller1993; Redner Reference Redner2001; Holcman & Schuss Reference Holcman and Schuss2014; Kurella et al. Reference Kurella, Tzou, Coombs and Ward2015; Grebenkov Reference Grebenkov2016; Marcotte et al. Reference Marcotte, Doering, Thiffeault and Young2018). Associated with the reduced drift-diffusion equation (4.17) is a reduced equation for the mean exit time ![]() $\tau (\theta )$
$\tau (\theta )$
The left-hand side of (6.1a) is the adjoint of the linear operator in (4.17) (Redner Reference Redner2001; Holcman & Schuss Reference Holcman and Schuss2014; Kurella et al. Reference Kurella, Tzou, Coombs and Ward2015). The solution to (6.1) gives the expected time ![]() $\tau$ for a particle starting at
$\tau$ for a particle starting at ![]() $\theta$ (for any
$\theta$ (for any ![]() $y$) to reach an ‘exit’ at
$y$) to reach an ‘exit’ at ![]() $\theta =\theta ^{{L}}$ or
$\theta =\theta ^{{L}}$ or ![]() $\theta =\theta ^{{R}}$. Note that since
$\theta =\theta ^{{R}}$. Note that since ![]() $T= D_\theta t$ in (4.17) the dimensional MET is
$T= D_\theta t$ in (4.17) the dimensional MET is ![]() $\tau /D_\theta$, which goes to infinity as
$\tau /D_\theta$, which goes to infinity as ![]() $D_\theta \rightarrow 0$, i.e. the exit cannot be reached if
$D_\theta \rightarrow 0$, i.e. the exit cannot be reached if ![]() $D_\theta =0$. The word exit here is interpreted loosely: the MET merely signifies the first time a swimmer's orientation achieves the value
$D_\theta =0$. The word exit here is interpreted loosely: the MET merely signifies the first time a swimmer's orientation achieves the value ![]() $\theta ^{{L}}$ or
$\theta ^{{L}}$ or ![]() $\theta ^{{R}}$, starting from some value
$\theta ^{{R}}$, starting from some value ![]() $\theta$.
$\theta$.
6.1. Solving the MET equation
To solve (6.1), define ![]() ${T} = w\tau '$ which satisfies
${T} = w\tau '$ which satisfies
where ![]() $w(\theta )$ and
$w(\theta )$ and ![]() $\nu (\theta )$ are defined in (4.10). Use the integrating factor
$\nu (\theta )$ are defined in (4.10). Use the integrating factor ![]() $\tilde {c}_1\,\mathrm {e}^{\varPhi (\theta )}$ from (5.4) to get
$\tilde {c}_1\,\mathrm {e}^{\varPhi (\theta )}$ from (5.4) to get

where ![]() $\tilde {\mathcal {P}}(\theta ) = \tilde {c}_1\,w\,\mathrm {e}^{\varPhi }$ is analogous to the invariant density for a closed channel (5.21) and
$\tilde {\mathcal {P}}(\theta ) = \tilde {c}_1\,w\,\mathrm {e}^{\varPhi }$ is analogous to the invariant density for a closed channel (5.21) and ![]() $\mathcal {C}$ is an integration constant. We choose
$\mathcal {C}$ is an integration constant. We choose ![]() $\tilde {c}_1$ so that the normalization condition (5.22) is satisfied:
$\tilde {c}_1$ so that the normalization condition (5.22) is satisfied: ![]() $\tilde {G}(\theta ^{{R}}) = 1$; hence,
$\tilde {G}(\theta ^{{R}}) = 1$; hence, ![]() $\tilde {G}(\theta )$ is the equilibrium probability of finding the swimmer between
$\tilde {G}(\theta )$ is the equilibrium probability of finding the swimmer between ![]() $\theta ^{{L}}$ and
$\theta ^{{L}}$ and ![]() $\theta$, if the channel were closed. The MET
$\theta$, if the channel were closed. The MET ![]() $\tau$ has a unique maximum at
$\tau$ has a unique maximum at ![]() $\theta =\theta _*$ with
$\theta =\theta _*$ with ![]() $\mathcal {C} = \tilde {G}(\theta _*)$.
$\mathcal {C} = \tilde {G}(\theta _*)$.
Now integrate (6.3)
 \begin{equation} \tau(\theta) = \int_{\theta^{{L}}}^{\theta} \frac{1}{\tilde{\mathcal{P}}(\vartheta)} \left ( \mathcal{C} - \tilde{G}(\vartheta)\right ) \,{\mathrm{d}}\vartheta, \end{equation}
\begin{equation} \tau(\theta) = \int_{\theta^{{L}}}^{\theta} \frac{1}{\tilde{\mathcal{P}}(\vartheta)} \left ( \mathcal{C} - \tilde{G}(\vartheta)\right ) \,{\mathrm{d}}\vartheta, \end{equation}
which satisfies the left boundary condition ![]() $\tau (\theta ^{{L}})=0$. The right boundary condition then requires
$\tau (\theta ^{{L}})=0$. The right boundary condition then requires ![]() $\tau (\theta ^{{R}}) = 0$, which fixes the integration constant
$\tau (\theta ^{{R}}) = 0$, which fixes the integration constant
 \begin{equation} \mathcal{C} = \left.\int_{\theta^{{L}}}^{\theta^{{R}}} \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta \right/ \int_{\theta^{{L}}}^{\theta^{{R}}} \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}. \end{equation}
\begin{equation} \mathcal{C} = \left.\int_{\theta^{{L}}}^{\theta^{{R}}} \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta \right/ \int_{\theta^{{L}}}^{\theta^{{R}}} \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}. \end{equation}
Equations (6.4) and (6.5) give the MET for a swimmer starting at ![]() $\theta$ and exiting at either
$\theta$ and exiting at either ![]() $\theta ^{{L}}$ or
$\theta ^{{L}}$ or ![]() $\theta ^{{R}}$. We now focus on a particular version of this mean exit time with a more natural interpretation – the MRT.
$\theta ^{{R}}$. We now focus on a particular version of this mean exit time with a more natural interpretation – the MRT.
6.2. Mean reversal time
The MRT ![]() $\tau _{{rev}}$ (or turnaround time (Holcman & Schuss Reference Holcman and Schuss2014)) is the expected time for a swimmer initially oriented with
$\tau _{{rev}}$ (or turnaround time (Holcman & Schuss Reference Holcman and Schuss2014)) is the expected time for a swimmer initially oriented with ![]() $\theta =0$ to reverse direction to
$\theta =0$ to reverse direction to ![]() $\theta =\pm {\rm \pi}$. It can be obtained from (6.4) by setting
$\theta =\pm {\rm \pi}$. It can be obtained from (6.4) by setting ![]() $-\theta ^{{L}}=\theta ^{{R}}={\rm \pi}$ and
$-\theta ^{{L}}=\theta ^{{R}}={\rm \pi}$ and ![]() $\theta =0$
$\theta =0$
 \begin{equation} \tau_{{rev}} = \tau(0) = \int_{-{\rm \pi}}^0 \frac{1}{\tilde{\mathcal{P}}(\vartheta)} \left ( \mathcal{C} - \tilde{G}(\vartheta)\right ) \,{\mathrm{d}}\vartheta. \end{equation}
\begin{equation} \tau_{{rev}} = \tau(0) = \int_{-{\rm \pi}}^0 \frac{1}{\tilde{\mathcal{P}}(\vartheta)} \left ( \mathcal{C} - \tilde{G}(\vartheta)\right ) \,{\mathrm{d}}\vartheta. \end{equation}
In Appendix A we show how the constant ![]() $\mathcal {C}$ can be eliminated to obtain
$\mathcal {C}$ can be eliminated to obtain
 \begin{equation} \tau_{{rev}}= \frac{\tilde{G}(0)}{1 + \mathrm{e}^{{\rm \pi}\bar{\mu}}} \int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} + \tanh({\rm \pi}\bar{\mu}/2) \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta, \end{equation}
\begin{equation} \tau_{{rev}}= \frac{\tilde{G}(0)}{1 + \mathrm{e}^{{\rm \pi}\bar{\mu}}} \int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} + \tanh({\rm \pi}\bar{\mu}/2) \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta, \end{equation}
where ![]() $\bar {\mu }$ is defined in (5.11). For a left–right-symmetric swimmer,
$\bar {\mu }$ is defined in (5.11). For a left–right-symmetric swimmer, ![]() $\tilde {\mathcal {P}}=\mathcal {P}$,
$\tilde {\mathcal {P}}=\mathcal {P}$, ![]() $\bar {\mu } = 0$ and
$\bar {\mu } = 0$ and ![]() $\tilde {G}(0)=1/2$ by symmetry, so we obtain the compact expression
$\tilde {G}(0)=1/2$ by symmetry, so we obtain the compact expression
Example 6.1 (MRT with diffusion only)
In the absence of swimming (![]() $U=0$), we have
$U=0$), we have ![]() $\nu = 0$ from (4.10b), and
$\nu = 0$ from (4.10b), and ![]() $\tilde {\mathcal {P}} = \mathcal {P} = c_1w$, with
$\tilde {\mathcal {P}} = \mathcal {P} = c_1w$, with ![]() $c_1^{-1} = \int _{-{\rm \pi} }^{\rm \pi} w \,{\mathrm {d}}\theta$. Hence, (6.8) is
$c_1^{-1} = \int _{-{\rm \pi} }^{\rm \pi} w \,{\mathrm {d}}\theta$. Hence, (6.8) is
For a needle with wall distance function (2.8), we get from (2.20)
where ![]() $X_{{rot}}$ drops out: the centre of rotation is immaterial in the absence of swimming. We can then easily compute the integral (6.9) to obtain
$X_{{rot}}$ drops out: the centre of rotation is immaterial in the absence of swimming. We can then easily compute the integral (6.9) to obtain
The ‘narrow exit’ limit corresponds to ![]() $\lambda = 1 - \delta$, with
$\lambda = 1 - \delta$, with ![]() $\delta$ small
$\delta$ small
This is similar but not identical to Holcman & Schuss's result (Holcman & Schuss Reference Holcman and Schuss2014, (5.13))
valid as ![]() $\delta \rightarrow 0$, but otherwise unconstrained. In figure 10, a comparison to a finite-element numerical solution of the full PDE (i.e. without using the reduced equation) shows excellent agreement with our small-
$\delta \rightarrow 0$, but otherwise unconstrained. In figure 10, a comparison to a finite-element numerical solution of the full PDE (i.e. without using the reduced equation) shows excellent agreement with our small-![]() $D_\theta$ form (6.11), but less so with the form (6.13). Possibly there is a parameter regime where (6.13) shows better agreement.
$D_\theta$ form (6.11), but less so with the form (6.13). Possibly there is a parameter regime where (6.13) shows better agreement.

Figure 10. MRT for a needle as a function of ![]() $D_\theta$, for
$D_\theta$, for ![]() $\lambda =0.9$,
$\lambda =0.9$, ![]() $D_Y=1$. The dashed line is (6.11), which is technically valid for small
$D_Y=1$. The dashed line is (6.11), which is technically valid for small ![]() $D_\theta$ but applies over a wide range. The dotted line is from Holcman & Schuss (Reference Holcman and Schuss2014). The solid line is from a finite-element simulation of the full PDE.
$D_\theta$ but applies over a wide range. The dotted line is from Holcman & Schuss (Reference Holcman and Schuss2014). The solid line is from a finite-element simulation of the full PDE.
For an elliptical shape (2.16) with ![]() $U=0$, we have
$U=0$, we have
Then
where ![]() $E$ is a complete elliptic integral of the second kind. We also have
$E$ is a complete elliptic integral of the second kind. We also have
where ![]() $K$ and
$K$ and ![]() $\Pi$ are the complete elliptic integrals of the first and third kinds. Together, the product of (6.15) and (6.16) into (6.9) gives the reversal time for an ellipse. The MRT for a needle is compared to different ellipses in figure 11.
$\Pi$ are the complete elliptic integrals of the first and third kinds. Together, the product of (6.15) and (6.16) into (6.9) gives the reversal time for an ellipse. The MRT for a needle is compared to different ellipses in figure 11.

Figure 11. MRT ![]() $\tau _{{rev}}$ for a needle and different ellipses, as a function of gap size
$\tau _{{rev}}$ for a needle and different ellipses, as a function of gap size ![]() $\delta$. An ellipse reverses more rapidly as it becomes more circular, since it spends less time aligning with the wall.
$\delta$. An ellipse reverses more rapidly as it becomes more circular, since it spends less time aligning with the wall.
6.3. Asymptotics of MRT
The integrand in (6.8) is the inverse of ![]() $\mathcal {P}(\theta ) = c_1 w(\theta )\,\mathrm {e}^{\varPhi (\theta )}$. The integral itself will thus typically be dominated by the minima of
$\mathcal {P}(\theta ) = c_1 w(\theta )\,\mathrm {e}^{\varPhi (\theta )}$. The integral itself will thus typically be dominated by the minima of ![]() $\mathcal {P}$, which correspond to values of
$\mathcal {P}$, which correspond to values of ![]() $\theta$ that the swimmer finds difficult to cross when trying to reverse. This could be because
$\theta$ that the swimmer finds difficult to cross when trying to reverse. This could be because ![]() $\delta = 1-\ell /L$ is small: this is the narrow escape problem discussed by Holcman & Schuss (Reference Holcman and Schuss2014) (see example 6.1). However, for a swimmer the long reversal time is usually due to a swimmer ‘sticking’ to the top or bottom wall for long times.
$\delta = 1-\ell /L$ is small: this is the narrow escape problem discussed by Holcman & Schuss (Reference Holcman and Schuss2014) (see example 6.1). However, for a swimmer the long reversal time is usually due to a swimmer ‘sticking’ to the top or bottom wall for long times.
To approximate the integral in (6.8), we look for minima ![]() $\theta _*$ of
$\theta _*$ of ![]() $\mathcal {P}(\theta )$, where we can approximate
$\mathcal {P}(\theta )$, where we can approximate
This gives us the approximation
This should be multiplied by the number of minima with value ![]() $\mathcal {P}(\theta _*)$ in
$\mathcal {P}(\theta _*)$ in ![]() $0 \le \theta < {\rm \pi}$, should there be more than one. We can easily obtain a more accurate, if messier, approximation by retaining higher-order terms in (6.17).
$0 \le \theta < {\rm \pi}$, should there be more than one. We can easily obtain a more accurate, if messier, approximation by retaining higher-order terms in (6.17).
Example 6.2 (MRT for a fast needle swimmer)
An ideal example for the asymptotic approximation of ![]() $\tau _{{rev}}$ is the fast needle swimmer of example 5.1. Figure 9(b) clearly shows strong peaks in
$\tau _{{rev}}$ is the fast needle swimmer of example 5.1. Figure 9(b) clearly shows strong peaks in ![]() $1/\mathcal {P}$ at
$1/\mathcal {P}$ at ![]() $\theta _* = \pm {\rm \pi}/2$ even for this modest value of
$\theta _* = \pm {\rm \pi}/2$ even for this modest value of ![]() $U$. Hence, we are justified in expanding around the minimum of (5.17) at
$U$. Hence, we are justified in expanding around the minimum of (5.17) at ![]() $\theta _*={\rm \pi} /2$, which gives
$\theta _*={\rm \pi} /2$, which gives
Recall that ![]() $\alpha = D_X/D_Y$ is the ratio of diffusivities, and
$\alpha = D_X/D_Y$ is the ratio of diffusivities, and ![]() $\beta$ is a large Péclet number defined in (5.16). Inserting these in (6.18), we find
$\beta$ is a large Péclet number defined in (5.16). Inserting these in (6.18), we find
or in the case ![]() $\alpha =1$
$\alpha =1$
This is exponential in the Péclet number ![]() $\beta$, so that the reversal time can become extremely long. Note also that the MRT is independent of the channel width
$\beta$, so that the reversal time can become extremely long. Note also that the MRT is independent of the channel width ![]() $L$ in this limit. For the parameters in figure 9 (
$L$ in this limit. For the parameters in figure 9 (![]() $\beta =3.6$), the numerical MRT is
$\beta =3.6$), the numerical MRT is ![]() $\tau _{{rev}} \approx 1.54\times 10^3$, whereas the approximation (6.20) gives
$\tau _{{rev}} \approx 1.54\times 10^3$, whereas the approximation (6.20) gives ![]() $1.38 \times 10^3$. The approximation is thus reasonably good even for a modest Péclet number
$1.38 \times 10^3$. The approximation is thus reasonably good even for a modest Péclet number ![]() $\beta$.
$\beta$.
7. Effective diffusion along the channel
For the open-channel configuration, as a microswimmer travels down the channel, it will occasionally reverse direction. For long times, we expect these reversals to lead to an effective diffusion process on large scales. One way to capture this limit exactly is to derive an effective diffusion equation using a homogenization approach (Sagues & Horsthemke Reference Sagues and Horsthemke1986; McCarty & Horsthemke Reference McCarty and Horsthemke1988; Childress & Soward Reference Childress and Soward1989; Majda & Kramer Reference Majda and Kramer1999). We proceed to do so for our microswimmer in a channel, and find the effective diffusivity in the same reduced limit as in § 4 (small ![]() $D_\theta$).
$D_\theta$).
7.1. Homogenized equation
Rewrite the Fokker–Planck (3.3) as
where ![]() $\boldsymbol {U} = (u,v)$. In this section we only assume that
$\boldsymbol {U} = (u,v)$. In this section we only assume that ![]() $\boldsymbol{\mathsf{D}}$,
$\boldsymbol{\mathsf{D}}$, ![]() $D_\theta$ and
$D_\theta$ and ![]() $\boldsymbol {U}$ do not depend on
$\boldsymbol {U}$ do not depend on ![]() $x$. (The derivation could easily be extended to allow
$x$. (The derivation could easily be extended to allow ![]() $x$ dependence.) Later (§ 7.2) we will specialize to the forms in (3.4), which are functions of
$x$ dependence.) Later (§ 7.2) we will specialize to the forms in (3.4), which are functions of ![]() $\theta$ only.
$\theta$ only.
We will homogenize in the ![]() $x$ direction only, since the swimmer is confined between walls in the
$x$ direction only, since the swimmer is confined between walls in the ![]() $y$ direction and the
$y$ direction and the ![]() $\theta$ direction is periodic. We introduce a large scale
$\theta$ direction is periodic. We introduce a large scale ![]() $x/\eta$ and long time
$x/\eta$ and long time ![]() $t/\eta ^2$, where
$t/\eta ^2$, where ![]() $\eta$ is a small expansion parameter. After rescaling
$\eta$ is a small expansion parameter. After rescaling ![]() $t \rightarrow t/\eta ^2$ and
$t \rightarrow t/\eta ^2$ and ![]() $x \rightarrow x/\eta$, (7.1) becomes
$x \rightarrow x/\eta$, (7.1) becomes
where we defined the linear operator
The no-flux boundary conditions for (7.2a) are
We expand the probability density ![]() $p$ as a regular series in
$p$ as a regular series in ![]() $\eta$
$\eta$
Define the cell integral of ![]() $f(\theta ,y)$ as
$f(\theta ,y)$ as

To ensure uniqueness at each order, we enforce the cell-integrated probability ![]() $\left \langle p \right \rangle = \left \langle p_0 \right \rangle$, so that
$\left \langle p \right \rangle = \left \langle p_0 \right \rangle$, so that ![]() $\left \langle p_i \right \rangle = 0$ for
$\left \langle p_i \right \rangle = 0$ for ![]() $i>0$.
$i>0$.
Now we collect powers of ![]() $\eta$ in (7.2) with the expansion (7.3). At leading order in
$\eta$ in (7.2) with the expansion (7.3). At leading order in ![]() $\eta$ we have from (7.2a)
$\eta$ we have from (7.2a)
with boundary conditions from (7.2c)
The operator ![]() $\mathcal {L}$ only involves
$\mathcal {L}$ only involves ![]() $(\theta ,y)$, so we can solve (7.5) with
$(\theta ,y)$, so we can solve (7.5) with
where ![]() $\rho (\theta ,y)$ is the cell-normalized
$\rho (\theta ,y)$ is the cell-normalized ![]() $x$-independent invariant density for (7.1), and so
$x$-independent invariant density for (7.1), and so ![]() $\left \langle p_0 \right \rangle = {P}(x,t)$.
$\left \langle p_0 \right \rangle = {P}(x,t)$.
At order ![]() $\eta ^1$, (7.2a) gives
$\eta ^1$, (7.2a) gives
We can solve this by letting
where ![]() $\chi (\theta ,y)$ satisfies the cell problem
$\chi (\theta ,y)$ satisfies the cell problem
with ![]() $\left \langle \chi \right \rangle =0$. The solvability condition for the cell problem (7.9) demands
$\left \langle \chi \right \rangle =0$. The solvability condition for the cell problem (7.9) demands
In our case, the left and right sides of (7.10) vanish separately after using the channel symmetry ![]() $\rho (\theta +{\rm \pi} ,y) = \rho (\theta ,-y)$. On the left, we have
$\rho (\theta +{\rm \pi} ,y) = \rho (\theta ,-y)$. On the left, we have ![]() $u(\theta ) = U\cos \theta$, so
$u(\theta ) = U\cos \theta$, so ![]() $u(\theta +{\rm \pi} ) = -u(\theta )$ and the integral must vanish. On the right, we have
$u(\theta +{\rm \pi} ) = -u(\theta )$ and the integral must vanish. On the right, we have ![]() $D_{xy}(\theta ) = \tfrac 12(D_X-D_Y)\sin 2\theta$ from (3.4), so
$D_{xy}(\theta ) = \tfrac 12(D_X-D_Y)\sin 2\theta$ from (3.4), so ![]() $D_{xy}(\theta +{\rm \pi} ) = D_{xy}(\theta )$ and
$D_{xy}(\theta +{\rm \pi} ) = D_{xy}(\theta )$ and ![]() $\partial _y\rho (\theta +{\rm \pi} ,y) = -(\partial _y\rho )(\theta ,-y)$ and the integral again vanishes. There is thus no ‘ratchet effect’ to cause a net drift, since there is no breaking of the left–right symmetry of the channel. Having patterned walls as in Yariv & Schnitzer (Reference Yariv and Schnitzer2014) and Malgaretti & Stark (Reference Malgaretti and Stark2017) would likely cause such a drift.
$\partial _y\rho (\theta +{\rm \pi} ,y) = -(\partial _y\rho )(\theta ,-y)$ and the integral again vanishes. There is thus no ‘ratchet effect’ to cause a net drift, since there is no breaking of the left–right symmetry of the channel. Having patterned walls as in Yariv & Schnitzer (Reference Yariv and Schnitzer2014) and Malgaretti & Stark (Reference Malgaretti and Stark2017) would likely cause such a drift.
At order ![]() $\eta ^2$, (7.2) gives
$\eta ^2$, (7.2) gives
The solvability condition then yields the effective heat equation
where the effective diffusivity in ![]() $x$ is
$x$ is
The solvability condition (7.10) implies that ![]() $\chi$ only affects the effective diffusivity up to an additive multiple of
$\chi$ only affects the effective diffusivity up to an additive multiple of ![]() $\rho$.
$\rho$.
7.2. Reduced equation limit
We solve the cell problem (7.9) in the same small-![]() $D_\theta$ limit as in § 4. Anticipating that the effective diffusivity should diverge as
$D_\theta$ limit as in § 4. Anticipating that the effective diffusivity should diverge as ![]() $\varepsilon =D_\theta$ becomes smaller, we expand
$\varepsilon =D_\theta$ becomes smaller, we expand
The leading-order ![]() $\varepsilon ^{-1}$ cell problem (7.9) is the same as (4.3), with solution
$\varepsilon ^{-1}$ cell problem (7.9) is the same as (4.3), with solution
At next order ![]() $\varepsilon ^0$ we have the PDE and boundary conditions
$\varepsilon ^0$ we have the PDE and boundary conditions
where we used the invariant density ![]() $\bar{p}_0 = \mathcal {Q}(\theta )\,\mathrm {e}^{\sigma (\theta )y}$. Integrate (7.16a) from
$\bar{p}_0 = \mathcal {Q}(\theta )\,\mathrm {e}^{\sigma (\theta )y}$. Integrate (7.16a) from ![]() $y=\zeta _-$ to
$y=\zeta _-$ to ![]() $\zeta _+$ and use the boundary conditions (7.16b)
$\zeta _+$ and use the boundary conditions (7.16b)
where ![]() $\mathcal {P} = w\mathcal {Q}$, and
$\mathcal {P} = w\mathcal {Q}$, and
with ![]() $\alpha = D_X/D_Y$ as in (5.17). We integrate (7.17) once
$\alpha = D_X/D_Y$ as in (5.17). We integrate (7.17) once
with ![]() $d$ a constant of integration. The solvability condition (7.10) ensures that
$d$ a constant of integration. The solvability condition (7.10) ensures that ![]() $H(\theta )$ is a periodic function of
$H(\theta )$ is a periodic function of ![]() $\theta$. Next use the integrating factor
$\theta$. Next use the integrating factor ![]() $\mathrm {e}^{\varPhi (\theta )}$ from (5.4), with
$\mathrm {e}^{\varPhi (\theta )}$ from (5.4), with ![]() $\theta ^{{L}}=-{\rm \pi}$
$\theta ^{{L}}=-{\rm \pi}$
We integrate again and find
where we used ![]() $F(\theta )$ from (5.4). By rearranging and using (5.7), we find after introducing new constants
$F(\theta )$ from (5.4). By rearranging and using (5.7), we find after introducing new constants
 \begin{equation} {X}(\theta) = \tilde{c}_1\,\mathrm{e}^{\varPhi(\theta)} \left (\int_{-{\rm \pi}}^\theta\frac{H(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta - d_2 F(\theta) \right ) + d_1\mathcal{Q}(\theta), \end{equation}
\begin{equation} {X}(\theta) = \tilde{c}_1\,\mathrm{e}^{\varPhi(\theta)} \left (\int_{-{\rm \pi}}^\theta\frac{H(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta - d_2 F(\theta) \right ) + d_1\mathcal{Q}(\theta), \end{equation}
where we used ![]() $\tilde {\mathcal {P}} = \tilde {c}_1w\,\mathrm {e}^{\varPhi }$ as in § 6.1. The constant
$\tilde {\mathcal {P}} = \tilde {c}_1w\,\mathrm {e}^{\varPhi }$ as in § 6.1. The constant ![]() $d_1$ is adjusted to satisfy
$d_1$ is adjusted to satisfy ![]() $\left \langle \chi \right \rangle =0$ and is immaterial to the effective diffusivity. The constant
$\left \langle \chi \right \rangle =0$ and is immaterial to the effective diffusivity. The constant ![]() $d_2$ is used to enforce periodicity of
$d_2$ is used to enforce periodicity of ![]() ${X}$ and can be eliminated to obtain
${X}$ and can be eliminated to obtain
 \begin{equation} {X}(\theta) = \tilde{c}_1\,\mathrm{e}^{\varPhi(\theta)} \left ( \int_{-{\rm \pi}}^\theta\frac{H(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta - \frac{F(\theta)}{F({\rm \pi})} \int_{-{\rm \pi}}^{\rm \pi}\frac{H(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta \right ) + d_1\mathcal{Q}(\theta). \end{equation}
\begin{equation} {X}(\theta) = \tilde{c}_1\,\mathrm{e}^{\varPhi(\theta)} \left ( \int_{-{\rm \pi}}^\theta\frac{H(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta - \frac{F(\theta)}{F({\rm \pi})} \int_{-{\rm \pi}}^{\rm \pi}\frac{H(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)} \,{\mathrm{d}}\vartheta \right ) + d_1\mathcal{Q}(\theta). \end{equation}In this reduced limit, the effective diffusivity (7.13) is
to leading order in ![]() $D_\theta$. The expected value
$D_\theta$. The expected value ![]() $\mathbb{E}D _{xx}$ is taken over the invariant marginal density
$\mathbb{E}D _{xx}$ is taken over the invariant marginal density ![]() $\mathcal {P}(\theta )$, and
$\mathcal {P}(\theta )$, and ![]() $D_{{enh}}$ is the ‘enhanced’ part of the diffusivity.
$D_{{enh}}$ is the ‘enhanced’ part of the diffusivity.
7.3. Bounding  $D_{{eff}}$ by
$D_{{eff}}$ by  $\tau _{{rev}}$ for a left–right-symmetric swimmer
$\tau _{{rev}}$ for a left–right-symmetric swimmer
Since the expressions are getting a bit complicated, for the sake of brevity we will focus on the left–right-symmetric case for this section. In that case the term involving ![]() $F(\theta )$ vanishes in (7.23), and
$F(\theta )$ vanishes in (7.23), and ![]() $\tilde {c}_1\,\mathrm {e}^{\varPhi }$ becomes
$\tilde {c}_1\,\mathrm {e}^{\varPhi }$ becomes ![]() $\mathcal {Q}$
$\mathcal {Q}$
After restoring in (7.25) the definition of ![]() $H$ from (7.19), it can be shown that the enhanced diffusivity
$H$ from (7.19), it can be shown that the enhanced diffusivity ![]() $D_{{enh}}$ in (7.24) can be written as
$D_{{enh}}$ in (7.24) can be written as
To derive this we used the symmetries ![]() $\varXi (\theta + {\rm \pi}) = \varXi ({\rm \pi} - \theta ) = -\varXi (\theta )$,
$\varXi (\theta + {\rm \pi}) = \varXi ({\rm \pi} - \theta ) = -\varXi (\theta )$, ![]() $\mathcal {P}(\theta + {\rm \pi}) = \mathcal {P}({\rm \pi} - \theta ) = \mathcal {P}(\theta )$, the latter holding for a left–right-symmetric swimmer. Rearranging the inner-most integral in (7.27) gives us
$\mathcal {P}(\theta + {\rm \pi}) = \mathcal {P}({\rm \pi} - \theta ) = \mathcal {P}(\theta )$, the latter holding for a left–right-symmetric swimmer. Rearranging the inner-most integral in (7.27) gives us
where the MRT ![]() $\tau _{{rev}}$ was defined in (6.8). The expected value in (7.27) is taken over the invariant marginal density
$\tau _{{rev}}$ was defined in (6.8). The expected value in (7.27) is taken over the invariant marginal density ![]() $\mathcal {P}(\theta )$, as in (7.24).
$\mathcal {P}(\theta )$, as in (7.24).
Since ![]() $\varXi (\theta )$ defined by (7.18) is non-negative in
$\varXi (\theta )$ defined by (7.18) is non-negative in ![]() $[0,{\rm \pi} /2]$, (7.27) immediately gives us the bound
$[0,{\rm \pi} /2]$, (7.27) immediately gives us the bound
with
 \begin{equation} \max_{0 \le \theta \le {\rm \pi}/2} \varXi(\theta) = \begin{cases} U, & \alpha \ge 1/2; \\ U/\sqrt{4\alpha(1 - \alpha)}, & \alpha < 1/2. \end{cases} \end{equation}
\begin{equation} \max_{0 \le \theta \le {\rm \pi}/2} \varXi(\theta) = \begin{cases} U, & \alpha \ge 1/2; \\ U/\sqrt{4\alpha(1 - \alpha)}, & \alpha < 1/2. \end{cases} \end{equation}
This useful bound allows us to estimate ![]() $D_{{enh}}$ from
$D_{{enh}}$ from ![]() $\tau _{{rev}}$, which is simpler to compute ((6.8)). The estimate tends to improve the longer the swimmer spends aligned with the walls (figure 12).
$\tau _{{rev}}$, which is simpler to compute ((6.8)). The estimate tends to improve the longer the swimmer spends aligned with the walls (figure 12).

Figure 12. Effective diffusivity from (7.24) for an ellipse with ![]() $a=1$,
$a=1$, ![]() $b=1/2$,
$b=1/2$, ![]() $D_X=D_Y=0.1$,
$D_X=D_Y=0.1$, ![]() $D_\theta =0.01$, in a channel of width
$D_\theta =0.01$, in a channel of width ![]() $L=1.2$. The dashed lines are the bound
$L=1.2$. The dashed lines are the bound ![]() $\tfrac 12U^2\tau _{{rev}}$ from (7.28), with
$\tfrac 12U^2\tau _{{rev}}$ from (7.28), with ![]() $\alpha =1$ and
$\alpha =1$ and ![]() $\tau _{{rev}}$ given by (6.8). The decrease in exponential rate as the centre of rotation
$\tau _{{rev}}$ given by (6.8). The decrease in exponential rate as the centre of rotation ![]() $X_{{rot}}$ is moved forward is similar to the asymptotic form of
$X_{{rot}}$ is moved forward is similar to the asymptotic form of ![]() $\tau _{{rev}}$ for a needle, (6.21), with
$\tau _{{rev}}$ for a needle, (6.21), with ![]() $\beta$ given by (5.16).
$\beta$ given by (5.16).
8. Hydrodynamic interactions
In this section, we model a swimmer as a point singularity (stresslet) and add hydrodynamic interactions with walls to the ABP model (3.2). Our goal is to examine the importance of including shape in addition to hydrodynamic interactions in a narrow channel. Hydrodynamic interactions modify the velocity and vorticity experienced by the swimmer. The swimmer's shape modifies the configuration space, that is, the allowable range of ![]() $\theta$ and
$\theta$ and ![]() $y$ values.
$y$ values.
Since it is challenging to directly compare invariant densities ![]() $\bar{p}(\theta ,y)$ for two different shapes, which have different configuration spaces, we compare instead cross-sectional (marginal) invariant densities
$\bar{p}(\theta ,y)$ for two different shapes, which have different configuration spaces, we compare instead cross-sectional (marginal) invariant densities ![]() $\int _{-{\rm \pi} }^{\rm \pi} \bar{p}(\theta ,y)\,{\mathrm {d}}\theta$ for a narrow open channel.
$\int _{-{\rm \pi} }^{\rm \pi} \bar{p}(\theta ,y)\,{\mathrm {d}}\theta$ for a narrow open channel.
We take the model from Spagnolie et al. (Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015) where the velocity field of an ellipsoidal swimmer with ![]() $X_{{rot}}=0$ is approximated by a stresslet with non-dimensional magnitude
$X_{{rot}}=0$ is approximated by a stresslet with non-dimensional magnitude ![]() $\alpha$. Here
$\alpha$. Here ![]() $\alpha >0$ and
$\alpha >0$ and ![]() $\alpha <0$ represent a pusher and puller, respectively. Using the result in Spagnolie et al. (Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015) and axial symmetry of the ellipsoid, we can obtain the swimmer's translational and rotational velocity in a channel. We assume the motion is confined to the
$\alpha <0$ represent a pusher and puller, respectively. Using the result in Spagnolie et al. (Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015) and axial symmetry of the ellipsoid, we can obtain the swimmer's translational and rotational velocity in a channel. We assume the motion is confined to the ![]() $x$–
$x$–![]() $y$ plane, even though the hydrodynamic interactions are based on an ellipsoid. Assuming an isotropic spatial diffusion (
$y$ plane, even though the hydrodynamic interactions are based on an ellipsoid. Assuming an isotropic spatial diffusion (![]() $D_X=D_Y$), we arrive at the Itô stochastic equation
$D_X=D_Y$), we arrive at the Itô stochastic equation
which is a generalization to (3.2) that includes hydrodynamic interactions. Here, ![]() $\varGamma =(a^2-b^2)/(a^2+b^2)\in [0,1]$, where
$\varGamma =(a^2-b^2)/(a^2+b^2)\in [0,1]$, where ![]() $a$ and
$a$ and ![]() $b$ are the semi-major and semi-minor axes of the ellipsoid. Equation (8.1) is a far-field approximation, but Spagnolie et al. (Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015) have shown that this model is surprisingly accurate when a swimmer is near a wall, so we expect (8.1) to be an adequate model for narrow channel confinement. Since the noise is purely additive, we can easily integrate (8.1) using the explicit Euler method for stochastic differential equations (SDEs). We use an adaptive step size that is decreased as the swimmer approaches boundaries. If a step would take the swimmer inside a boundary, that step is rejected, the step size is decreased and new Gaussian random numbers are generated. This ‘rejection sampling’ approach realizes the no-flux boundary condition at the walls. We integrate over many realizations and for long enough times that the probability density of swimmers closely approximates the invariant density.
$b$ are the semi-major and semi-minor axes of the ellipsoid. Equation (8.1) is a far-field approximation, but Spagnolie et al. (Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015) have shown that this model is surprisingly accurate when a swimmer is near a wall, so we expect (8.1) to be an adequate model for narrow channel confinement. Since the noise is purely additive, we can easily integrate (8.1) using the explicit Euler method for stochastic differential equations (SDEs). We use an adaptive step size that is decreased as the swimmer approaches boundaries. If a step would take the swimmer inside a boundary, that step is rejected, the step size is decreased and new Gaussian random numbers are generated. This ‘rejection sampling’ approach realizes the no-flux boundary condition at the walls. We integrate over many realizations and for long enough times that the probability density of swimmers closely approximates the invariant density.
Spagnolie et al. (Reference Spagnolie, Moreno-Flores, Bartolo and Lauga2015) argue that, without noise, a puller (![]() $\alpha <0$) is repelled by a wall when initially aligned parallel to it; it is stable when aligned perpendicular to the wall. The opposite is true for a pusher (
$\alpha <0$) is repelled by a wall when initially aligned parallel to it; it is stable when aligned perpendicular to the wall. The opposite is true for a pusher (![]() $\alpha >0$). Figure 13 shows cross-sectional invariant densities for various stresslet magnitudes
$\alpha >0$). Figure 13 shows cross-sectional invariant densities for various stresslet magnitudes ![]() $\alpha$. In figure 13(a) (puller), there is a plateau between
$\alpha$. In figure 13(a) (puller), there is a plateau between ![]() $[-0.1,0.1]$ with small peaks, corresponding to the swimmer accumulating predominantly perpendicular to the wall. The plateau and peaks become more pronounced with a larger stresslet amplitude. Figure 13(b) (pusher) shows concentration peaks near both walls, corresponding to parallel alignment, which get closer to the walls as
$[-0.1,0.1]$ with small peaks, corresponding to the swimmer accumulating predominantly perpendicular to the wall. The plateau and peaks become more pronounced with a larger stresslet amplitude. Figure 13(b) (pusher) shows concentration peaks near both walls, corresponding to parallel alignment, which get closer to the walls as ![]() $\alpha$ is increased. Simulations with a wider channel (not shown) agree with past experiments (Berke et al. Reference Berke, Turner, Berg and Lauga2008; Li & Tang Reference Li and Tang2009; Li et al. Reference Li, Bensson, Nisimova, Munger, Mahautmr, Tang, Maxey and Brun2011; Gachelin et al. Reference Gachelin, Miño, Berthet, Lindner, Rousselet and Clément2013) and simulations (Hernandez-Ortiz et al. Reference Hernandez-Ortiz, Stoltz and Graham2005; Nash et al. Reference Nash, Adhikari, Tailleur and Cates2010; Costanzo et al. Reference Costanzo, Di Leonardo, Ruocco and Angelani2012; Chilukuri et al. Reference Chilukuri, Collins and Underhill2014; Alonso-Matilla et al. Reference Alonso-Matilla, Ezhilan and Saintillan2016).
$\alpha$ is increased. Simulations with a wider channel (not shown) agree with past experiments (Berke et al. Reference Berke, Turner, Berg and Lauga2008; Li & Tang Reference Li and Tang2009; Li et al. Reference Li, Bensson, Nisimova, Munger, Mahautmr, Tang, Maxey and Brun2011; Gachelin et al. Reference Gachelin, Miño, Berthet, Lindner, Rousselet and Clément2013) and simulations (Hernandez-Ortiz et al. Reference Hernandez-Ortiz, Stoltz and Graham2005; Nash et al. Reference Nash, Adhikari, Tailleur and Cates2010; Costanzo et al. Reference Costanzo, Di Leonardo, Ruocco and Angelani2012; Chilukuri et al. Reference Chilukuri, Collins and Underhill2014; Alonso-Matilla et al. Reference Alonso-Matilla, Ezhilan and Saintillan2016).

Figure 13. Cross-sectional invariant density ![]() $\int \bar{p}(\theta ,y)\,\mathrm {d} \theta$ with stresslet magnitude (a)
$\int \bar{p}(\theta ,y)\,\mathrm {d} \theta$ with stresslet magnitude (a) ![]() $\alpha <0$ (pullers); (b)
$\alpha <0$ (pullers); (b) ![]() $\alpha >0$ (pushers). The channel has width
$\alpha >0$ (pushers). The channel has width ![]() $L=1.2$ and the swimmer is an ellipsoid with
$L=1.2$ and the swimmer is an ellipsoid with ![]() $a=2b=0.5$,
$a=2b=0.5$, ![]() $X_{{rot}}=0$,
$X_{{rot}}=0$, ![]() $U=1$,
$U=1$, ![]() $D_X=D_Y=0.1$,
$D_X=D_Y=0.1$, ![]() $D_\theta =0.01$,
$D_\theta =0.01$, ![]() $\varGamma =0.6$ in (8.1).
$\varGamma =0.6$ in (8.1).
A simple way to quantify the importance of correctly accounting for the shape of the swimmer is to perform simulations using the configuration space for a spherical swimmer of radius ![]() $0.5$, but retain hydrodynamic interactions for an ellipsoidal swimmer. This artificial situation is not meant to model a real swimmer, but is merely used to confirm that the shape of configuration space plays an important role in the final results. Figure 14 shows the cross-sectional invariant densities for that case, and we can see that a pusher (figure 14b) no longer exhibits parallel accumulation at the walls. The ellipsoidal configuration space is thus responsible for the parallel alignment of a pusher, and must be taken into account. In comparison, we still observe a plateau for a puller, only without small peaks for perpendicular accumulation; a puller's shape plays a lesser role.
$0.5$, but retain hydrodynamic interactions for an ellipsoidal swimmer. This artificial situation is not meant to model a real swimmer, but is merely used to confirm that the shape of configuration space plays an important role in the final results. Figure 14 shows the cross-sectional invariant densities for that case, and we can see that a pusher (figure 14b) no longer exhibits parallel accumulation at the walls. The ellipsoidal configuration space is thus responsible for the parallel alignment of a pusher, and must be taken into account. In comparison, we still observe a plateau for a puller, only without small peaks for perpendicular accumulation; a puller's shape plays a lesser role.
Note that, instead of a spherical swimmer, we used one that is very close to a sphere, to avoid numerical issues with the spherical swimmers sticking to the walls for very long times. The importance of accounting for shape is less pronounced for wider channels, since a swimmer experiences fewer steric wall interactions.
9. Discussion
We used both theory and numerics to analyse the dynamics of finite-sized swimmers in a channel. The shape of swimmers is embedded in their configuration space, which is defined even for non-smooth swimmers via their convex hull. Shape enters solely in the boundary conditions to the Fokker–Planck equation. Ignoring hydrodynamic interactions for simplicity, we derived a reduced equation in the small ![]() $D_\theta$ limit for both open and closed channels, from which we computed the invariant density. For open-channel geometry, we calculated the MRT. We used homogenization techniques and solved the cell problem to find the effective diffusivity. The shape of a swimmer is encoded in the rotational drift term
$D_\theta$ limit for both open and closed channels, from which we computed the invariant density. For open-channel geometry, we calculated the MRT. We used homogenization techniques and solved the cell problem to find the effective diffusivity. The shape of a swimmer is encoded in the rotational drift term ![]() $\mu$ in the reduced equation (4.17), and appears explicitly in the integral solution for the invariant density, MRT, and effective diffusivity. The integral of
$\mu$ in the reduced equation (4.17), and appears explicitly in the integral solution for the invariant density, MRT, and effective diffusivity. The integral of ![]() $\mu$ vanishes for a left–right-symmetric swimmer, and many of our expressions then greatly simplify. In particular the mean angular drift vanishes for such a symmetric swimmer. In § 8, we added hydrodynamic interactions with the walls and discussed the importance of shape. We showed that, in a narrow channel, including the exact shape in configuration space is important for a pusher, but less so for a puller since they tend to be aligned perpendicular the walls.
$\mu$ vanishes for a left–right-symmetric swimmer, and many of our expressions then greatly simplify. In particular the mean angular drift vanishes for such a symmetric swimmer. In § 8, we added hydrodynamic interactions with the walls and discussed the importance of shape. We showed that, in a narrow channel, including the exact shape in configuration space is important for a pusher, but less so for a puller since they tend to be aligned perpendicular the walls.
A particular novelty in our work is to explicitly allow the position of the centre of rotation, ![]() $X_{{rot}}$, to vary. The sign of
$X_{{rot}}$, to vary. The sign of ![]() $X_{{rot}}$ affects the configuration space, as shown in figure 4, and changes a swimmer's tendency to align with walls. When a swimmer's centre of rotation is behind its geometrical centre, it tends to align with a wall; in the opposite case, it tends to stay perpendicular to the wall even for a needle or circular swimmer (examples 5.1 and 5.2). A more systematic study of the role of
$X_{{rot}}$ affects the configuration space, as shown in figure 4, and changes a swimmer's tendency to align with walls. When a swimmer's centre of rotation is behind its geometrical centre, it tends to align with a wall; in the opposite case, it tends to stay perpendicular to the wall even for a needle or circular swimmer (examples 5.1 and 5.2). A more systematic study of the role of ![]() $X_{{rot}}$ for realistic swimmers will be the subject of future work.
$X_{{rot}}$ for realistic swimmers will be the subject of future work.
In our work we focused on two-dimensional swimmers for simplicity and ease of presentation. However, generalizing the formalism to three-dimensional axisymmetric swimmers is straightforward (Nitsche & Brenner Reference Nitsche and Brenner1990). In that case, the wall distance function ![]() $y_*$ is essentially the same as in two dimensions, and all that changes is that the
$y_*$ is essentially the same as in two dimensions, and all that changes is that the ![]() $\partial _\theta ^2$ operator must be replaced by the three-dimensional surface Laplacian. The dynamics for three-dimensional axisymmetric swimmers will thus be similar to two-dimensional left–right-symmetric swimmers, with some quantitative changes. In particular, following almost the same calculations, we found the invariant density is given by a formula similar to (5.21), and when applied to spherical swimmers with
$\partial _\theta ^2$ operator must be replaced by the three-dimensional surface Laplacian. The dynamics for three-dimensional axisymmetric swimmers will thus be similar to two-dimensional left–right-symmetric swimmers, with some quantitative changes. In particular, following almost the same calculations, we found the invariant density is given by a formula similar to (5.21), and when applied to spherical swimmers with ![]() $X_{{rot}}=0$ it agrees with the results in Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015) and Elgeti & Gompper (Reference Elgeti and Gompper2013). A Fully three-dimensional swimmer that is not axisymmetric will require considerably more work to implement: the configuration space has an extra dimension due to the extra degrees of freedom. Of course, this will potentially also make the dynamics richer, and will allow for interesting effects such as chirality.
$X_{{rot}}=0$ it agrees with the results in Ezhilan & Saintillan (Reference Ezhilan and Saintillan2015) and Elgeti & Gompper (Reference Elgeti and Gompper2013). A Fully three-dimensional swimmer that is not axisymmetric will require considerably more work to implement: the configuration space has an extra dimension due to the extra degrees of freedom. Of course, this will potentially also make the dynamics richer, and will allow for interesting effects such as chirality.
Another challenging direction is to find asymptotic results when adding hydrodynamic interactions with boundaries. It is also possible to include variable swimmer shapes, which would allow the inclusion of flagella, by letting the configuration space itself be time dependent. Yet another potential avenue is to treat the interaction of multiple swimmers, in a manner analogous to Burada et al. (Reference Burada, Hänggi, Marchesoni, Schmid and Talkner2009) and Bruna & Chapman (Reference Bruna and Chapman2013) for passive diffusing spheres.
Acknowledgements
Some of the initial work on this project was carried out with J. Gloe. The authors are grateful to S. Spagnolie for many discussions, and to several anonymous referees for constructive comments.
Declaration of interests
The authors report no conflict of interest.
Appendix A. Derivation of the MRT
In this appendix, we show how to from the reversal time formula (6.6) to the more explicit form (6.7) by eliminating the constant ![]() $\mathcal {C}$. First note that the numerator of (6.5) is
$\mathcal {C}$. First note that the numerator of (6.5) is
 \begin{equation} \int_{-{\rm \pi}}^{\rm \pi} \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta = \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta + \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta + {\rm \pi})}{\tilde{\mathcal{P}}(\vartheta + {\rm \pi})}\,{\mathrm{d}}\vartheta. \end{equation}
\begin{equation} \int_{-{\rm \pi}}^{\rm \pi} \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta = \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta + \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta + {\rm \pi})}{\tilde{\mathcal{P}}(\vartheta + {\rm \pi})}\,{\mathrm{d}}\vartheta. \end{equation}
We wish to relate ![]() $\tilde {\mathcal {P}}(\theta + {\rm \pi})$ to
$\tilde {\mathcal {P}}(\theta + {\rm \pi})$ to ![]() $\tilde {\mathcal {P}}(\theta ) = \tilde {c}_1\,w(\theta )\,\mathrm {e}^{\varPhi (\theta )}$. We have
$\tilde {\mathcal {P}}(\theta ) = \tilde {c}_1\,w(\theta )\,\mathrm {e}^{\varPhi (\theta )}$. We have ![]() $w(\theta + {\rm \pi}) = w(\theta )$. The change in
$w(\theta + {\rm \pi}) = w(\theta )$. The change in ![]() $\varPhi$ over a period is, from (5.4),
$\varPhi$ over a period is, from (5.4),
since ![]() $\mu (\theta )$ is
$\mu (\theta )$ is ![]() ${\rm \pi}$-periodic. Here,
${\rm \pi}$-periodic. Here, ![]() $\bar {\mu }$ is the period-averaged
$\bar {\mu }$ is the period-averaged ![]() $\mu (\theta )$ from (5.11);
$\mu (\theta )$ from (5.11); ![]() $\varPhi (\theta )$ is only
$\varPhi (\theta )$ is only ![]() ${\rm \pi}$-periodic when
${\rm \pi}$-periodic when ![]() $\bar {\mu }=0$, or equivalently
$\bar {\mu }=0$, or equivalently ![]() $c_2=0$. It follows from (A2) that
$c_2=0$. It follows from (A2) that ![]() $\varPhi$ must have the form
$\varPhi$ must have the form
where ![]() $\tilde {\Phi }(\theta )$ is
$\tilde {\Phi }(\theta )$ is ![]() ${\rm \pi}$-periodic. We conclude from
${\rm \pi}$-periodic. We conclude from ![]() $\tilde {\mathcal {P}}(\theta ) = \tilde {c}_1\,w(\theta )\,\mathrm {e}^{\varPhi (\theta )}$ that
$\tilde {\mathcal {P}}(\theta ) = \tilde {c}_1\,w(\theta )\,\mathrm {e}^{\varPhi (\theta )}$ that ![]() $\tilde {\mathcal {P}}(\theta + {\rm \pi}) = \mathrm {e}^{{\rm \pi} \bar {\mu }}\,\tilde {\mathcal {P}}(\theta )$. Similarly, for
$\tilde {\mathcal {P}}(\theta + {\rm \pi}) = \mathrm {e}^{{\rm \pi} \bar {\mu }}\,\tilde {\mathcal {P}}(\theta )$. Similarly, for ![]() $-{\rm \pi} \le \theta \le 0$,
$-{\rm \pi} \le \theta \le 0$,
 \begin{align} \tilde{G}(\theta + {\rm \pi}) &= \int_{-{\rm \pi}}^{\theta + {\rm \pi}} \tilde{\mathcal{P}}(\vartheta)\,{\mathrm{d}}\vartheta\nonumber\\ &= \int_{-{\rm \pi}}^0 \tilde{\mathcal{P}}(\vartheta)\,{\mathrm{d}}\vartheta + \int_0^{\theta + {\rm \pi}} \tilde{\mathcal{P}}(\vartheta)\,{\mathrm{d}}\vartheta \nonumber\\ &= \tilde{G}(0) + \mathrm{e}^{{\rm \pi}\bar{\mu}}\,\tilde{G}(\theta). \end{align}
\begin{align} \tilde{G}(\theta + {\rm \pi}) &= \int_{-{\rm \pi}}^{\theta + {\rm \pi}} \tilde{\mathcal{P}}(\vartheta)\,{\mathrm{d}}\vartheta\nonumber\\ &= \int_{-{\rm \pi}}^0 \tilde{\mathcal{P}}(\vartheta)\,{\mathrm{d}}\vartheta + \int_0^{\theta + {\rm \pi}} \tilde{\mathcal{P}}(\vartheta)\,{\mathrm{d}}\vartheta \nonumber\\ &= \tilde{G}(0) + \mathrm{e}^{{\rm \pi}\bar{\mu}}\,\tilde{G}(\theta). \end{align}Returning to (A1), we obtain
 \begin{align} \int_{-{\rm \pi}}^{\rm \pi}
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
&= \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
+ \int_{-{\rm \pi}}^0 \frac{\tilde{G}(0) +
\mathrm{e}^{{\rm \pi}\bar{\mu}}\,\tilde{G}(\theta)}
{\mathrm{e}^{{\rm \pi}\bar{\mu}}\,\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
\nonumber\\ &= 2 \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
+ \tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}.
\end{align}
\begin{align} \int_{-{\rm \pi}}^{\rm \pi}
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
&= \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
+ \int_{-{\rm \pi}}^0 \frac{\tilde{G}(0) +
\mathrm{e}^{{\rm \pi}\bar{\mu}}\,\tilde{G}(\theta)}
{\mathrm{e}^{{\rm \pi}\bar{\mu}}\,\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
\nonumber\\ &= 2 \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
+ \tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}.
\end{align}
The denominator of ![]() $\mathcal {C}$ in (6.5) is
$\mathcal {C}$ in (6.5) is
 \begin{equation} \int_{-{\rm \pi}}^{\rm \pi} \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} = \int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} + \int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta + {\rm \pi})} = (1 + \mathrm{e}^{-{\rm \pi}\bar{\mu}}) \int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}. \end{equation}
\begin{equation} \int_{-{\rm \pi}}^{\rm \pi} \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} = \int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} + \int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta + {\rm \pi})} = (1 + \mathrm{e}^{-{\rm \pi}\bar{\mu}}) \int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}. \end{equation} \begin{equation} \mathcal{C}\int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} = \frac{1}{1 + \mathrm{e}^{-{\rm \pi}\bar{\mu}}} \left ( 2 \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta + \tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}} \int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} \right ). \end{equation}
\begin{equation} \mathcal{C}\int_{-{\rm \pi}}^0 \frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} = \frac{1}{1 + \mathrm{e}^{-{\rm \pi}\bar{\mu}}} \left ( 2 \int_{-{\rm \pi}}^0 \frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta + \tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}} \int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)} \right ). \end{equation}We use this in the reversal time (6.6):
 \begin{align} \tau_{{rev}} &=
\mathcal{C} \int_{-{\rm \pi}}^0
\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
- \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta \nonumber\\ &= \frac{1}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}} \left ( 2 \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
+ \tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
\right ) - \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta \nonumber\\ &= \frac{1}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}} \left
(\tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
+ (1 - \mathrm{e}^{-{\rm \pi}\bar{\mu}}) \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta \right )\nonumber\\ &=
\frac{\tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
+ \frac{1 - \mathrm{e}^{-{\rm \pi}\bar{\mu}}}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}} \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta,
\end{align}
\begin{align} \tau_{{rev}} &=
\mathcal{C} \int_{-{\rm \pi}}^0
\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
- \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta \nonumber\\ &= \frac{1}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}} \left ( 2 \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}\,{\mathrm{d}}\vartheta
+ \tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
\right ) - \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta \nonumber\\ &= \frac{1}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}} \left
(\tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
+ (1 - \mathrm{e}^{-{\rm \pi}\bar{\mu}}) \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta \right )\nonumber\\ &=
\frac{\tilde{G}(0)\,\mathrm{e}^{-{\rm \pi}\bar{\mu}}}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}}
\int_{-{\rm \pi}}^0\frac{\,{\mathrm{d}}\vartheta}{\tilde{\mathcal{P}}(\vartheta)}
+ \frac{1 - \mathrm{e}^{-{\rm \pi}\bar{\mu}}}{1 +
\mathrm{e}^{-{\rm \pi}\bar{\mu}}} \int_{-{\rm \pi}}^0
\frac{\tilde{G}(\vartheta)}{\tilde{\mathcal{P}}(\vartheta)}
\,{\mathrm{d}}\vartheta,
\end{align}
which is (6.7).