Introduction
There has been increasing interest in life-course and cross-generation transmission of risk in relation to adult chronic diseases since Barker et al. first renewed attention on the issue.Reference Barker, Osmond and Winter 1 – Reference Hales and Barker 5 Much work in this area has focused on associations between infant birth weight (IBW) and subsequent adult health status and mortality,Reference Belbasis, Savvidou and Kanu 2 birth weight being seen both as the completed outcome of a pregnancy, but also as a general proxy for early life experience linked to longer-term outcomes in adulthood. Two analyses for instance from the large Nurses’ Health Study in the United StatesReference Rich-Edwards, Colditz and Stampfer 3 , Reference Rich-Edwards, Kleinman and Michels 4 contrast the influence of birth weight on long-term health outcomes such as diabetes and coronary heart disease. Birth weight was strongly inversely associated with type 2 diabetes in adulthood, after adjustment for adult body mass index (BMI). Higher BMI in adulthood was also a strong risk factor for coronary heart disease among women who were small at birth. Other studies have shown a positive association between birth weight and later life BMI.Reference Parsons, Power and Manor 6 , Reference Curhan, Willett and Rimm 7 A recent umbrella review of systematic reviews and meta-analyses on IBW and long-term health outcomes,Reference Belbasis, Savvidou and Kanu 2 cautions on the consistency of the evidence base in relation to IBW in itself.
The Lifeways cross-generational cohort study was designed a priori to examine associations across generations and it affords the opportunity of relating birth weight in cohort members to measures of health status across the life course.Reference O’Mahony, Fallon and Hannon 8 – Reference Murrin, Shrivastava and Kelleher 12 It is one of the very few studies with three generations followed prospectively. In previous published analyses we have shown that BMI in children at three age points is consistently associated with BMI in the adult maternal line, whereas height shows associations in both lineages.Reference Kelly, Murrin and Viljoen 10 We have also shown that family dietary patterns aggregate most strongly in the maternal lineReference Shrivastava, Murrin and Sweeney 11 and that maternal macronutrient intake in pregnancy is associated with children’s BMI aged 5.Reference Murrin, Shrivastava and Kelleher 12 Our objectives in this current analysis were to identify whether patterns of association existed for birth weights across generations, employing index child’s measured IBW and data on reported birth weights (RBW) of all adults in this cohort, linked to adults’ current health and social status. We further sought to identify whether index child’s IBW, as a proxy for intrauterine growth and development, was associated with adult cohort members’ characteristics, including in particular with adult BMI, as a measure of potential morbidity in those adults.
Methods
The Lifeways cross-generation cohort study was established in 2001 and its methodology has been described previously.Reference O’Mahony, Fallon and Hannon 8 , Reference Kelleher, Viljoen and Khalil 9 In brief, expectant mothers were recruited in two maternity hospitals in the East and West of Ireland during first hospital booking visit and at baseline completed a questionnaire. They were asked to include in the study their proband infant, the father if available and at least one of four potential grandparents. There are up to six adult lineages in the study, mothers, fathers, maternal grandmother (MGM), maternal grandfather (MGF), paternal grandmother, paternal grandfather (PGF) and a seventh lineage of index children from birth onwards. Families have been followed up since then, when index children were subsequently born, and were aged 3, aged 5 and aged 9. IBW and gestational age were recorded from hospital records for index children.
At baseline in a self-completed health status questionnaire in 2001–2003, adults RBW as well as social and lifestyle indicators such as whether they smoked or not, their level of education and their accommodation status. BMI in all adults was initially self-reported or measured at baseline. For mothers, BMI was again measured at a 5-year follow-up of the child, and for fathers it was self-reported. In 2011, a number of the East-based grandparents’ BMI was measured at a 10-year follow-up cardiovascular examination. For the purpose of this analysis, where more than one reported BMI for adults was available, it was decided to take the most recent BMI reported or measured, with priority given to measured variables, as previously this has shown to be a more accurate record.Reference Shiely, Perry and Lutomski 13
The purpose of the first part of the analysis was to examine whether RBW in adults was associated with their adult lifestyle risk factors or measures of social status, using a categorical analysis. RBW was divided into three categories (low being <3 kg, normal3 kg to ⩽4 kg and high as >4.0 kg). We took 3 kg as the cut-off for the lower birth weight category as that has been associated in a systematic review with subsequent development of type 2 diabetes.Reference Whincup, Kaye and Owen 14 We included measures of social position likely to relate to birth weight from the baseline adult questionnaire (e.g. education and accommodation status) and lifestyle (e.g. smoking and BMI) in the analysis, as well as sex. BMI was categorized into low/normal (<25 kg/m2), overweight (25–⩽30 kg/m2) and obese (>30 kg/m2). Smoking status was based on two categories: ‘Have you ever smoked?’ answering ‘yes or no’. The education and accommodation variables were divided, respectively, into three groups; ‘no schooling/completed primary school’, ‘completed secondary school’ and ‘completed third-level education’; those adults who lived in ‘detached houses’, were compared with those who lived in ‘semi-detached/mid-terrace’ housing and those who lived in ‘apartments/flats/other’. χ2 Tests were carried out as appropriate.
The second part of the analysis aimed to look at the relationship between birth weights of all family members, including adults and index children. The index child’s IBW, taken from recorded hospital data was correlated separately to each individual adult RBW (e.g. infant v. mother, infant v. MGM). We employed Pearson’s correlations and also applied a Bonferroni’s correction to account for multiple testing.
The next part of the analysis examined adult BMI as an outcome variable relative to own RBW or IBW or both, in mixed models which also included adult respondents’ age, up to seven family lineage groups and adult respondents’ educational status. We treated adult BMI as an indicator of potential morbidity in adults and wished to see whether it related to own RBW or was associated with the index child’s IBW as a measure of cross-generation association. We also wished to assess whether the mother’s relationship with IBW contrasted with that of other adult cohort members. A number of multilevel mixed effects linear regression models were performed with family identification number, which is a common shared variable for all family members, as a random effect included in the models. The first model in all adults included IBW only, the second included IBW and also RBW for adults with available data and the third model was confined only to mothers, including their own RBW and IBW. The next model included just IBW and adults other than mothers. The final adjusted model looked at both IBW and RBW in adults, excluding mothers. Index child’s gestational age was adjusted for in all models.
As BMI was not normally distributed, different ways of transforming the data were examined and the inverse BMI seemed to fit the data best. However, as the results using inverse BMI were very similar to the non-transformed BMI, it was decided to show the non-transformed BMI results for ease of interpretation. Numbers of adults differ for different analyses due to missing values for covariates. Only adults with a singleton child were included in the analyses. All analyses were performed using the STATA statistical software package (version 13). All P-values were based on two-sided tests and considered statistically significant if <0.05.
We performed an analysis to identify whether the sub-sample of adults with RBW data differed significantly from other sub-samples randomly drawn from the larger data set. All samples were similar in respect of mean age, mean BMI, mean waist circumference and educational level. Those with RBW tended to have less reported morbidity, more likely to be General Medical Services means-tested medical card eligible and ever smokers, but the differences were small.
Results
The descriptive data for all adults, 1328 parents and 1218 grandparents, included in the analysis are reported in Table 1. The mean self-RBW of all adults (n=920 with data available) was 3.37 (s.d.=0.70) kg. The index child’s IBW (n=1075 with data available) was 3.51 (s.d.=0.58) kg and gestational age in weeks was 39.9 (s.d. 1.8), with data available for 959 children.
Table 1 Descriptive statistics of parents and grandparents in the Lifeways Cross-Generation Cohort Study (n=2546)

MGM, maternal grandmother; MGF, maternal grandfather; PGM, paternal grandmother; PGF, paternal grandfather; BMI, body mass index.
Table 2 shows patterns for RBW in adults split into three categories. Males reported significantly higher birth weights than females (P=0.006), a higher percentage of males are seen to be in the higher birth weight category, when compared with females. Table 2 also shows the relationship at uni-variate level between adult characteristics and self-RBW. RBW had no significant association with whether a person had ever smoked in their lifetime, as well as no significant relationship with BMI or accommodation. However, education showed a significant u-shaped association where adults who completed third-level education were relatively less likely to be in the low or high birth weight categories.
Table 2 Univariate analysis of adults’ reported birth weight v. adult risk factors in the Lifeways Cross-Generation Cohort Study (n=920)

a Numbers do not add up to total number because of missing values for covariates
Table 3 shows the correlation matrix for IBW and adults’ RBW. There was a significant association overall between maternal RBW and IBW and for both sexes of children. There was a borderline significant association between MGM’s RBW and that of her daughter. The PGF also showed some inverse associations, but with very small numbers. When Bonferroni’s corrections were applied, only the relationship between maternal RBW and IBW overall (P=0.0002) and for male IBW were significant (P=0.0084). No other correlations were significant. Figure 1 presents the scattergram for maternal RBW and IBW.

Fig. 1 Correlation between mother’s birth weight and index child’s infants birth weight in the Lifeways Cross-Generation Cohort Study (n=613).
Table 3 Correlations of family birth weights in the Lifeways Cross-Generation Cohort Study

IBW, infant birth weight; BW, birth weight; MGM, maternal grandmother; MGF, maternal grandfather; PGM, paternal grandmother; PGF, paternal grandfather.
a Correlation coefficient remained significant after Bonferroni’s correction was applied.
The results of the multilevel models for adults’ BMI are given in Table 4. This showed expected significant associations between both education status and lineage and BMI, but also that the IBW was significantly associated with adult BMI (P=0.0042). In the multi-level mixed effects linear regression model which then included adults’ RBW also (n=728), age was significantly associated with adult BMI (0.030), as well as IBW (0.004). The older a person was and the higher the IBW, the higher the adult BMI. However adults’ own RBW had no association with BMI. Mothers had significantly lower BMI relative to fathers (acting as the reference group).
Table 4 Results of multilevel mixed effects linear regression models on adults’ body mass index (BMI) in the Lifeways Cross-Generation Cohort Study

IBW, infant birth weight; RBW, reported birth weight; CI, confidence interval; MGM, maternal grandmother; MGF, maternal grandfather; PGM, paternal grandmother; PGF, paternal grandfather; ICC, intraclass correlation coefficient.
a Results were adjusted for other variables listed in the table.
In the next model, confined to maternal lineage only (n=483), again the IBW was associated with maternal BMI, with an effect of maternal education also, mothers with completed secondary education having relatively higher BMI than those with third-level education. When mothers were excluded from the overall model, a different pattern emerged. Lineage remained significantly associated with adult BMI, but IBW and education were no longer significantly associated with adult BMI. In the final model (Table 5), for adults other than mothers with available RBW, that variable was significantly associated with adult BMI, but IBW showed no association and there was an inverse association with accommodation and BMI, as a marker of social position.
Table 5 Results of multilevel mixed effects linear regression models on adults’ body mass index (BMI) (excluding mothers) in the Lifeways Cross-Generation Cohort Study
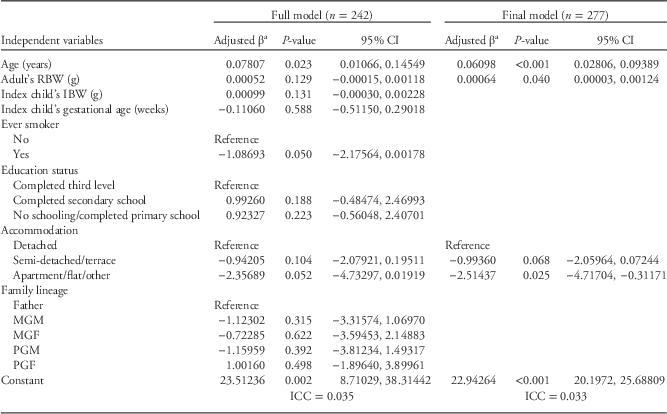
CI, confidence interval; RBW, reported birth weight; IBW, infant birth weight; MGM, maternal grandmother; MGF, maternal grandfather; PGM, paternal grandmother; PGF, paternal grandfather.
a Results were adjusted for other variables listed in the table.
Discussion
There are very few cohort studies with examination data across three generations and seven lineages and this analysis appears to be novel in the literature in that it includes birth weights of both adult and children family members simultaneously in the models. Our analysis suggests there are patterns of association seen for birth weight across generations, in keeping with other studies.
It is increasingly well established that infants at the normal extremes of birth weight are more likely to develop adult chronic disease.Reference Hales and Barker 5 , Reference Cnattingius, Villamor and Lagerros 15 , Reference Durmuş, Arends and Ay 16 A number of cohort studies have shown familial associations also for parents and their children, some with contrasting patterns in maternal and paternal lineages.Reference Durmuş, Arends and Ay 16 The 1958 birth cohort in the United Kingdom is a rich data set which was able to prospectively assess birth weight in relation to own BMI at five different time points in the lifecourse from 7 to 33 years old and also had reported anthropometric information on both parents of participating children.Reference Parsons, Power and Manor 6 This analysis showed a direct association between IBW and own BMI in early life, becoming more j-shaped by the age of 33. The authors point out the usefulness of examining birth weight relative to genetic potential, in this case BMI of both parents and adult children. They concluded that maternal weight largely explained the association between own birth weight and adult BMI in the offspring.
We also wished to assess whether adult BMI, as a known proxy as well as a risk factor for adult chronic disease, was associated with either own RBW or index child’s IBW in mixed models that also included age, lineage group and educational status. We examined a direct association between birth weights and BMI, as that association tends to be positive in other studies,Reference Parsons, Power and Manor 6 , Reference Curhan, Willett and Rimm 7 whilst being inverse for outcomes such as hypertension, diabetes and cardiovascular disease.Reference Rich-Edwards, Colditz and Stampfer 3 , Reference Rich-Edwards, Kleinman and Michels 4 , Reference Whincup, Kaye and Owen 14
Again, we found expected associations with gender and social position for RBW, suggesting the coherence of the data. Having in effect adjusted for these associations, we find initially that child’s IBW, but not adults’ RBW, is significantly associated with the outcome of interest. IBW was consistently associated with adults’ BMI, in the first three models. This analysis confirms other similar analyses which show that adults including parents and grandparents, do show an association with the birth weights of their offspring.Reference Durmuş, Arends and Ay 16 – Reference Naess, Stoltenberg and Hoff 18 This is not to say there is necessarily a causal or temporal relationship, but rather that factors which may influence intrauterine growth or development may also be influencing the development of adult chronic disease in family members.
Very few human studies to date have examined transmission patterns across more than two generations.Reference Manor and Koupil 17 – Reference McCarron, Davey Smith and Hattersley 21 The findings in these three generation study reports are conflicting. Some have only maternal lineage data and show an inverse association between grandchild’s birth weight and grandparental cardiovascular diseaseReference Smith, Wood and White 20 or diabetes.Reference McCarron, Davey Smith and Hattersley 21 Naess et al. in a large-scale study in Norway have shown inverse relationships between IBW and mortality in both grandparental lineages, suggesting that for cardiovascular outcomes, this association may be explained by the maternal characteristics, including smoking, but may reflect also transmitted intrauterine effects for diabetes.Reference Naess, Stoltenberg and Hoff 18 None of these studies have RBW or measures such as BMI from grandparents as they are linkage in design, rather than prospectively followed families.
Our analysis further shows that it is the relationship between mother and infant that appears to be primarily driving the associations found. The only significant correlations for birth weight itself were between mother and child and when the mother is excluded from the adult analyses and considered separately, the patterns of association differ, IBW being no longer significantly associated with the remaining adults’ BMI. Evidence is accumulating across the lifecourse for offspring outcomes related to pregnancy. Weight gain during pregnancy has been shown to influence neonatal fat mass.Reference Crozier, Inskip and Godfrey 22 A longer-term follow-up of the Aberdeen Children of the Nineteen Fifties study showed risk of cerebrovascular disease might be increased in adult offspring whose mothers gained weight during pregnancy but not for all-cause mortality or cardiovascular disease, though the offspring’s own characteristics, including smoking and BMI, were strongly associated with cardiovascular outcomes.Reference Bhattacharya, McNeill and Raja 23 Maternal obesity during pregnancy has been shown to be associated with cardiovascular events in adult offspring.Reference Reynolds, Allan and Raja 24 Wells has highlighted the significance of so-called maternal capital, whereby different components of the maternal phenotype developed over her own lifecourse can influence offspring development.Reference Wells 25
Notwithstanding that consistent association it may partially reflect shared genetic traits. A recent Mendelian randomization analysis of Avon Longitudinal Study of Parents and Children data, replicated in the Generation R cohort, shows consistent associations between maternal and offspring BMI, attenuated significantly when robust genetic markers are adjusted for in the analysis.Reference Richmond, Timpson and Felix 26 A further Mendelian randomization analysis suggests maternal BMI is probably causally associated with higher IBW, consistent with the association we show in this analysis.Reference Tyrrell, Richmond and Palmer 27
We have treated IBW as a co-variable rather than an outcome variable in this analysis. However there are several possible pathways to consider. In the maternal line, maternal BMI may causally predict higher IBW, that infant going on to higher childhood BMI.Reference Cnattingius, Villamor and Lagerros 15 The MGM may in turn be associated with both BMI of daughter and that of grandchild.Reference Kelleher, Viljoen and Khalil 9 , Reference Kelly, Murrin and Viljoen 10 It is possible this path is mediated through IBW. Another explanation for the findings here is a clustering for risk of elevated BMI in families, with age, education and lineage, all playing a role both in determining IBW and adult BMI. The 1958 birth cohort also showed an association between babies’ birth weight and parental birth weight and additionally an association between height of the MGM and social class of the MGF and birth weight, suggesting inter-generational influences in the maternal line.Reference Lunde, Melve and Gjessing 28 A further very large linkage study from Norway also showed associations between parental birth weights and those of their offspring with the maternal association being stronger and suggesting that maternal genetic factors explained 22% of the variation in offspring birth weight.Reference Emanuel, Filakti and Alberman 29
There are significant limitations to the Lifeways data, which may have influenced these findings. First, just over a third of adult respondents provided birth weight data and it is self-reported. There are differing views in the literature as to the reliability of such informationReference Tehranifar, Liao and Flom 30 and grandparents were less likely to report their birth weight than parents. There is potential recall bias therefore to consider. As against that, in the reported data there were significant male/female differences and associations both with social position and infants’ measured birth weights. It is plausible that many respondents to our adult questionnaire did not complete this question because they did not know what they weighed at birth, suggesting those who did respond had more accurate recall. For the RBW data for adults, the findings were coherent, in that males’ birth weights were higher than those of females, as would be expected. There is a weak u-shaped association with education status, which is again an expected finding, as we know IBW in other studies is strongly associated with measures of social position, with both low and high birth weight associated with social disadvantage.Reference Durmuş, Arends and Ay 16 , Reference Manor and Koupil 17
How reliable is RBW in adults? Tehranifar et al.Reference Tehranifar, Liao and Flom 30 found reasonable level of agreement between recorded and RBW in a small cohort of adult women, though with variation according to educational status. The Nurses’ Health Study is an example of a very large cohort study employing RBW, which showed clear associations with major health outcomes.Reference Rich-Edwards, Colditz and Stampfer 3 , Reference Rich-Edwards, Kleinman and Michels 4 That study also reported validation of RBW in a sample of women as recalled by women themselves and their mothers against state birth records.Reference Troy, Michels and Hunter 31 RBW and adult hypertension and obesity were assessed in a cohort of over 20,000 men in the Health Professionals Follow-up study.Reference Curhan, Willett and Rimm 7 A twin study relying on RBW showed an inverse association with adult blood pressureReference Poulter, Chang and MacGregor 32 and mean age of respondents in that study was 56 years old. In our study the grandparental generation were of a comparable age in that they averaged 60 years old and were free-living in the community, so were neither extremely elderly nor frail.
There are also important statistical power and sample size issues to acknowledge in the Lifeways cohort, as numbers were small and as yet major outcome events including mortality have been measured in the grandparents only.Reference Shrivastava, Murrin and O’Brien 33 , Reference Viljoen 34 This may have limited the expected associations seen in the literature for own birth weight and long-term outcomes.
This is not the first time we have shown lineage specific associations in this cohort however. In a previous analysis we showed a positive association between index child’s IBW and all-cause mortality in the PGF but an inverse association with stroke and diabetes in the MGM.Reference Shrivastava, Murrin and O’Brien 33 Subsequently, ViljoenReference Viljoen 34 expanded on that analysis, with larger numbers and further follow-up, showing the same pattern for the PGFs. We have also seen contrasting associations, in that BMI was more strongly associated with the maternal line in two separate analyses but the PGF is associated with his male grandson’s BMI at the age of 9.Reference Kelly, Murrin and Viljoen 10 It is also biologically coherent that the maternal birth weight is correlated in this analysis with that of children of both sexes. Again, we have found maternal associations with offspring for diet, BMI and social position previously, so this analysis adds to those findings.Reference Kelleher, Viljoen and Khalil 9 – Reference Shrivastava, Murrin and Sweeney 11
In conclusion this study shows consistent associations between maternal RBW and that of offspring IBW. It also shows that there are patterns of association between birth weight and BMI across three generations of the same families, suggesting persistent common drivers for both outcomes, meriting further investigation.
Acknowledgements
The authors acknowledge with appreciation the participation of the families in the study and the contribution of the Steering Group and team members for successive sweeps of this study.
Financial Support
The Lifeways cross-generation cohort study has been funded across all sweeps by the Health Research Board of Ireland. S.M. received a Health Research Board summer student bursary to contribute to this analysis.
Conflicts of Interest
None.
Ethical Standard
Ethical approval has been obtained for all stages of the study, including most recently by UCD Human Research Ethics Committee, LS-11-07.









