Introduction
Evidence that birth outcomes predict a range of later outcomes related to health and human capital has heightened interest in the maternal factors that shape the gestational environment.Reference Barker 1 , Reference Gluckman, Hanson, Cooper and Thornburg 2 Although much of this work has focused on the importance of nutrient supply to the fetus, more recent work has highlighted the influence of the maternal immune system on fetal growth and development.Reference Guven, Coskun and Ertas 3 , Reference Tjoa, van Vugt and Go 4 Acute infections, including malaria and sexually transmitted diseases, have long-been recognized as important risk factors for giving birth to a smaller baby, operating through both fetal growth restriction and shortened gestational duration.Reference Akum, Kuoh and Minang 5 – Reference R and Moshe 7 Research has also shown that endogenous immune processes, notably chronic inflammation, can have a negative influence on birth outcomes.Reference Guven, Coskun and Ertas 3 , Reference Tjoa, van Vugt and Go 4 , Reference Bullen, Jones and Holzman 8 , Reference Pitiphat, Gillman and Joshipura 9 Normal pregnancy is associated with a modest and sustained increase in inflammation, reflected in elevated C-reactive protein (CRP), and by marked changes in immune priorities that suppress maternal rejection of the fetus by decreasing the ratio of T helper 1 to T helper 2 cells.Reference Hwang, Kwon, Kim, Park and Kim 10 – Reference McDade, Borja, Largado, Adair and Kuzawa 14 However, abnormally elevated CRP levels during pregnancy are associated with preeclampsia, preterm birth and impaired fetal growth.Reference Pitiphat, Gillman and Joshipura 9 , Reference Redman, Sacks and Sargeant 15
Despite the predominant focus on clinical outcomes in prior research, a small but growing number of studies demonstrate an association between maternal CRP levels and offspring outcomes across the full range of maternal inflammatory status.Reference Tjoa, van Vugt and Go 4 , Reference Han, Ha, Park, Kim and Lee 16 , Reference Lowe, Metzger and Lowe 17 Among mothers who experience clinically normal pregnancy, CRP has been found to be negatively associated with birth weight for gestational age, explaining up to 25.9% of the variance in birth weight.Reference Tjoa, van Vugt and Go 4 , Reference Sen, Rifas-Shiman and Shivappa 18 Women with increased pregnancy CRP have also been shown to give birth to smaller and shorter babies with reduced adiposity and moderately decreased head circumference.Reference Lowe, Metzger and Lowe 17
Although the pathways linking maternal inflammation with suboptimal fetal growth are not fully understood, elevated maternal CRP can indicate cellular damage and endothelial dysfunction characterized by dysregulated vasoconstriction and vasodilation.Reference Gershov, Kim, Brot and Elkon 19 , Reference Lam, Lim and Karumanchi 20 The resulting poor vascular function may contribute to restricted fetal growth by decreasing nutrient flow across the maternal–fetal interface.Reference Ernst, de Jonge and Hofman 21 In addition, there are feedbacks between maternal and placental immune regulation that can lead to dysregulated inflammation.Reference Ali, Bokhari and Zaki 22 For example, poor endometrial decidualization and/or placental perfusion can lead to placental hypoxia, ischemia and tissue death.Reference Lam, Lim and Karumanchi 20 , Reference Redman 23 , Reference Ertas, Kahyaoglu and Yilmaz 24 These conditions may lead to release of inflammatory stimuli by the placenta into maternal circulation which in turn stimulates placental production of proinflammatory cytokines, thus further inhibiting maternal–fetal nutrient delivery.Reference Ernst, de Jonge and Hofman 21 , Reference Ali, Bokhari and Zaki 22 , Reference Malek, Bersinger and Di Santo 25 Through these pathways, elevated maternal CRP can serve as a marker of restricted maternal–fetal nutrient exchange. Furthermore, there is evidence that inflammatory regulation in adulthood is related to prenatal and early postnatal conditions, such that being born small predicts elevated CRP in adulthood.Reference Sattar, McConnachie and O’Reilly 26 – Reference Skilton, Viikari and Juonala 30 To the extent that both relationships are causal, they suggest that inflammation may serve as an intergenerational pathway linking adverse health across generations.Reference McDade 28 , Reference Tzoulaki, Jarvelin and Hartikainen 31
In this study, we aim to clarify the role of maternal inflammation as an influence on offspring birth outcomes. To that end, we report maternal CRP measured during pregnancy, which we relate to offspring weight, length, head circumference and adiposity measured soon after birth. Data come from a well-characterized cohort in the Philippines, in which the original cohort are now of reproductive age and having offspring.
Methods
Study population
Data come from the Cebu Longitudinal Health and Nutrition Survey, a longitudinal survey of 3080 singletons whose mothers were recruited during pregnancy between 1983 and 1984 in Metropolitan Cebu, Philippines.Reference Adair, Popkin and Akin 32 Of the 1447 original female cohort participants, 823 were interviewed in the 2009 survey (at ages 25–26). A special survey tracked new pregnancies among these women between 2009 and 2014. There were 383 who reported pregnancies (28% with 2–3 pregnancies) within the tracking period, yielding 507 pregnancy episodes. Women were visited in-home during pregnancy for anthropometric and questionnaire assessments, along with collection of a dried blood spot (DBS) – capillary whole blood collected on filter paper – for CRP measurement. A second visit was arranged right after delivery to obtain additional information from the mothers along with anthropometric measures of their newborns. This research was conducted under conditions of written informed consent, and with approval of the Institutional Review Boards of Northwestern University (Evanston, IL, USA), and the Office of Population Studies Foundation (Cebu, Philippines).
Sample inclusion criteria
Newborn anthropometric outcomes in these analyses included weight, length, head circumference and sum of five skinfold thicknesses (triceps, subscapular, suprailiac, bicep and calf), which were measured in-home by trained interviewers using standardized procedures.Reference Lohman, Roche and Martorell 33 Efforts were made to obtain these measurements as soon after birth as possible. The median and mean interval (in day) between birth and newborn anthropometry measurements were 3 and 4.5 days, respectively, with a range from 1 to 44 days. To minimize any impacts of the postnatal environment and postnatal growth on infant anthropometry, we limited analyses to infants who were measured within 2 weeks of birth, and adjusted for age at measurement in models (this excluded 17 individuals measured more than 2 weeks after birth). We further limited analyses to newborns born with gestational ages between 32 and 44 weeks, which excluded five very premature births and two implausibly late deliveries of around 46 weeks. Finally, we predicted newborn weight as a function of gestational age at birth and postnatal age at anthropometry measurement and excluded three individuals whose residuals were ⩾3 s.d. away from their predicted weights (e.g. individuals who were implausibly light for their gestational age at delivery and/or postnatal age at anthropometry measurement). After these exclusions, the final sample with all necessary biological and questionnaire data included 429 relatively healthy singletons born to 328 women. Regression models were clustered on mother to account for non-independence among siblings (see below).
Maternal covariates
We adjusted for mother’s age, parity and triceps skinfold thickness, at the time that the pregnancy interview and DBS collection were completed, and adult stature that had been collected during previous assessments. Because both CRP and birth outcomes are potentially impacted by the mother’s adiposity, we also adjusted for the mother’s pre-pregnancy body mass index (BMI). Maternal socio-economic status was measured using a pregnancy household assets scale reflecting whether the family had electricity, owned their home, owned an air conditioner, refrigerator, TV, vehicle and other appliances assessed, and a measure of household income that tallies all sources of income within the household (Adair et al. 2011). Because women were enrolled in the birth outcome sub-study after they were pregnant, we used height and weight measurements collected during prior surveys to estimate pre-pregnancy BMI. We used 2009 BMI when available, and then used 2007 and 2005 data as necessary. Under the assumption that women will tend to maintain a stable position within the population BMI distribution even as the population mean increases with age, we converted all BMIs to age-specific within-sample Z-scores before pooling into a single pre-pregnancy BMI variable. Supporting the validity of this approach, the correlation between Z-scores for BMI measured in 2005 and 2009 was very high (r=0.84).
CRP measurement
Samples were analysed for CRP in the Laboratory for Human Biology Research at Northwestern University using a modified high sensitivity enzyme immunoassay protocol previously developed for use with DBS.Reference McDade, Burhop and Dohnal 34 Prior validation of assay performance indicates that the DBS CRP method produces results that are comparable to gold standard plasma-based clinical methods, with a lower limit of detection of 0.03 mg/l.Reference McDade, Burhop and Dohnal 34 To minimize between-assay variation, all samples were analysed by the same technician using a single lot of capture antibody, detection antibody and calibration material. Between-assay CVs for low, mid and high control samples included with all runs were 11.8, 9.6, and 8.1%, respectively.
Before data analysis, DBS results were converted to plasma equivalent values using a conversion formula based on 69 matched DBS and plasma samples.Reference McDade 35 DBS samples were analysed using the same procedures, lot number of reagents and technician as applied to the study DBS samples. Plasma samples were analysed for high sensitivity CRP in a high throughput clinical laboratory on the Beckman Coulter Synchron DXC platform. The correlation between DBS and plasma values was high (Pearson’s R=0.98) and the resulting Deming regression conversion formula was as follows: plasma (mg/l)=1.64×DBS (mg/l).
Statistical analysis
All statistical analyses were conducted using Stata 13.0 (College Station, TX). We reported unadjusted means and standard deviations (or % for count variables) for the full sample and stratified on a median split of pregnancy CRP. We then ran a sequence of multivariate regression models (either linear or logistic) designed to assess relationships between maternal CRP and offspring gestational timing and weight, length, head circumference and sum of skinfolds measured soon after birth. All models were run with the cluster option in Stata, with clustering on mother’s ID, to account for the non-independence of siblings among women with multiple births in the analysis sample. CRP was right skewed and was therefore log-transformed before analysis. Because body fat can have both positive effects on birth outcomes (via nutrient supply) and negative effects on birth outcomes (via effects on proinflammatory cytokines) we assessed relationships between CRP and offspring outcomes before and after adjusting for maternal adiposity measures. Models predicting postnatal outcomes were adjusted for days after birth of anthropometry measurement, offspring gender, maternal parity and age during pregnancy visit and the mother’s adult height (coefficients not shown). Neither household assets nor income were close to significantly related to maternal CRP and adjusting for them did not substantially change model coefficients; we thus report each SES variable in the descriptive statistics for reference but omit them from models. To clarify the extent to which CRP influences offspring outcomes via effects on growth rate v. gestational duration, we evaluated models before and after adjustment for gestational age at delivery. Finally, to evaluate a possible role of preeclampsia, we assessed whether coefficients relating CRP to offspring outcomes were attenuated after further adjustment for the mother’s systolic blood pressure measured during the pregnancy interview.
Results
The women in this sample were an average of 27 years old during their pregnancy interviews (Table 1). When compared to women with below-median pregnancy CRP, women with above-median CRP had on average higher parity and thicker triceps skinfold thickness. There were no significant bivariate differences in any offspring trait between high/low maternal pregnancy CRP women.
Table 1 Characteristics of mothers during pregnancy and offspring after birth stratified on a median split of maternal pregnancy C-reactive protein (CRP)Footnote a
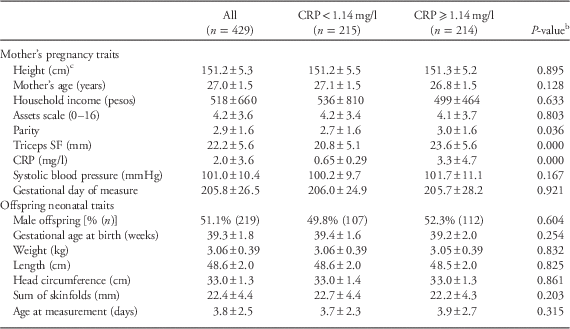
a Mean±s.d.
b From bivariate linear or logistic regression clustered on mother.
c Pre-pregnancy height (n=327 women).
SF, skinfold.
We next ran models linking maternal pregnancy CRP to offspring birth outcomes. In linear regression models predicting gestational age as a continuous variable, higher CRP during pregnancy was marginally associated with earlier delivery (P<0.081), but only after adjusting for adiposity measures (Table 2). In logistic models adjusting for maternal adiposity measures, CRP was not significantly related to preterm delivery (<37 weeks; P<0.109).
Table 2 Maternal pregnancy C-reactive protein (CRP) and adiposity as predictors of gestational age at delivery and preterm delivery

BMI, body mass index; SF, skinfold.
a From multiple regression models, coefficients β±s.e.
b From logistic regression models, odds ratio (95% confidence interval).
~P<0.1.
We next considered CRP as a predictor of offspring weight, length, head circumference and sum of skinfolds measured soon after birth (Table 3). In each instance, negative relationships between CRP and each outcome were strengthened after adjusting for measures of maternal body fat. Relationships between CRP and each outcome were weakened slightly after further adjustment for gestational age. In fully adjusted models (Fig. 1), CRP had statistically significant inverse relationships with sum of skinfolds (P<0.006) and birth length and weight (both P<0.005), while the relationship with head circumference was only borderline significant (P<0.095). Although systolic blood pressure was borderline significantly inversely related to offspring skinfold thickness when added to the full model (P<0.055), adjusting for blood pressure left the coefficients relating CRP to all four outcomes unchanged (results not shown; coefficient relating blood pressure to offspring outcomes all P>0.5 for other three models).

Fig. 1 Offspring birth outcomes predicted by maternal C-reactive protein (CRP) during pregnancy. (a) Offspring birth weight, (b) offspring birth length, (c) offspring head circumference, and (d) offspring sum of skinfolds predicted by maternal CRP. Values represent residuals from models adjusted for gestational age at delivery, postnatal day of offspring anthropometry measurement, pregnancy order, offspring gender, maternal age, height pre-pregnancy body mass index and pregnancy triceps skinfolds (see models in Table 3).
Table 3 Multiple regression models predicting offspring birth outcomesFootnote a
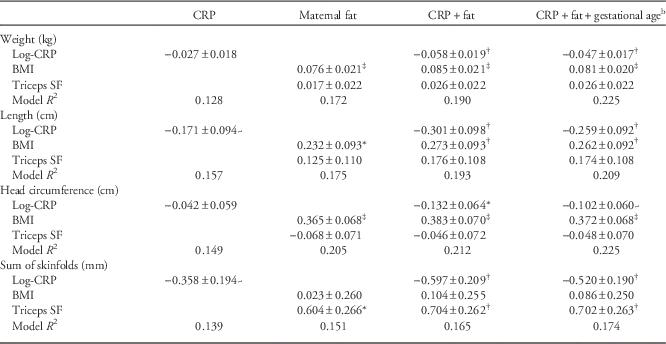
CRP, C-reactive protein; BMI, body mass index; SF, skinfold.
a All models also adjust for (not shown) days after birth of anthropometry measurement, offspring gender, maternal parity and age during pregnancy visit and mother’s adult height.
b Gestational age coefficient not shown.
~P<0.1; *P<0.05; † P<0.01; ‡ P<0.0001.
Discussion
In this sample of women from metropolitan Cebu, Philippines, a measure of inflammation, CRP, is inversely related to multiple offspring anthropometric outcomes measured soon after birth, including weight, length, adiposity, and to a lesser extent, head circumference. Although women with higher CRP had modestly truncated gestational ages at birth, the relationships with CRP were largely independent of gestational age, pointing to slower fetal growth as a likely effect. Furthermore, the inverse relationship between pregnancy CRP and offspring birth outcomes were linear across the full range of CRP, suggesting that any elevation in CRP predicts a proportionately attenuated offspring birth size. These findings point to maternal inflammation as an important influence on the gestational milieu that impacts fetal development, and by implication, long-term health in the next generation.
Our findings are consistent with the limited literature examining the effects of CRP variation in the normal range, in the absence of clinical pathology, as a predictor of offspring birth outcomes. In a subset of 1481 Hyperglycemia and Adverse Pregnancy Outcome study female participants, CRP levels in late pregnancy (24–32 weeks) were found to be negatively associated with offspring weight, sum of skinfolds and percent body fat at birth.Reference Lowe, Metzger and Lowe 17 Similarly, in a small study, women with normal pregnancies resulting in intrauterine growth restriction had higher CRP in early pregnancy (10–14 weeks) than those with expected fetal growth patterns.Reference Tjoa, van Vugt and Go 4 Furthermore, an inverse relationship between maternal CRP across all trimesters and birth weight Z-scores was found among Brazilian women.Reference de Oliveira, Franco-Sena, Farias, Rebelo and Kac 36 Together these findings demonstrate the negative association of moderately elevated maternal CRP on fetal growth among women experiencing normal pregnancy from diverse populations.
In support of the findings of Lowe et al.,Reference Lowe, Metzger and Lowe 17 we find that higher levels of maternal CRP during pregnancy predict not only a reduction in birth weight and length, but also body fat, as measured by skinfold thicknesses. This finding suggests that maternal inflammation has effects on multiple, distinct domains of fetal growth. Body length reflects chondrocyte differentiation in the growth plates of growing bones, and is driven by nutrients and growth factors. Fat deposition, in contrast, is most rapid during the final trimester of gestation,Reference Widdowson and McCance 37 when the fat content of the fetus rises dramatically to the unusually high percentage body fat that characterizes human newborns.Reference Kuzawa 38 Fat deposition accounts for 50% of the energy costs of growth at 27 weeks of gestation, and this value rises to 90% by term.Reference Haggarty 39
We find similarly strong associations between pregnancy CRP and offspring body fat, body length and body weight. In contrast, the relationship with head circumference was only of borderline significance. Although differences in measurement error could contribute to differences in effect size across measures, it is notable that the measurement typified by the lowest technical error of measurement (TEM), head circumference,Reference de Onis, Onyango, Borghi, Garza and Yang 40 exhibited the weakest relationships with CRP, whereas that typically with the highest TEM, skinfolds, showed the strongest relationships with CRP. We interpret our findings as evidence that maternal inflammation reduces fetal fat deposition and skeletal growth. In contrast, the comparably modest effects on head circumference are consistent with brain sparing, which has been shown previously in the context of conditions that limit delivery of substrate or oxygen to the fetus, such as placental insufficiency.Reference Wladimiroff, Tonge, Stewart and Reuss 41 , Reference Baschat 42
Our prior analyses in Cebu showed that adult CRP is inversely related to that individual’s own weight at birth,Reference McDade, Rutherford, Adair and Kuzawa 43 a finding reported in a number of other populations.Reference Sattar, McConnachie and O’Reilly 26 , Reference Tzoulaki, Jarvelin and Hartikainen 31 Viewed alongside these studies, our present findings suggest that inflammation could serve as a pathway linking restricted fetal growth and elevated inflammatory status across generations. Specifically, factors that lead to chronically elevated inflammation and CRP during pregnancy predict giving birth to a modestly smaller baby, who in turn is predicted to have elevated CRP as a result of their small birth size. At Cebu, we have previously reported similar evidence for reciprocal effects between birth outcomes and adult phenotypes related to hypothalamic–pituitary–adrenal (HPA) axis function at Cebu,Reference Thayer, Feranil and Kuzawa 44 , Reference Lee, Fried, Thayer and Kuzawa 45 and alterations in glucose metabolism, especially with maternal obesity and gestational diabetes, can operate in a similar intergenerational fashion.Reference Benyshek 46 Future research will be needed to evaluate the collective impact of these interrelated metabolic, endocrine and immune pathways as pathways linking adverse health across generations.Reference Kuzawa and Sweet 47
Several limitations of this study warrant mention. Because our sample is a population-based survey (and not a hospital-based study), interviews and data collection occurred within participants’ homes. As a result, our anthropometric measures of offspring were not obtained immediately after birth, but on variable days after birth. Almost all of the data were collected within 4 days of pregnancy, and we adjusted for day of measurement, minimizing any impact of postnatal growth processes. In addition, we rely upon a single measure of CRP as an indication of maternal inflammatory status, where using the multiple of several measures would provide more reliable estimates. As such, our models, which successfully identify significant relationships with birth outcomes, almost certainly underestimate the true intergenerational impact of maternal inflammation.
In sum, we find that maternal inflammation during pregnancy, as measured by CRP, is inversely related to multiple neonatal measures of offspring anthropometry in this sample of women living in metropolitan Cebu, the Philippines. Relationships were present across the full range of CRP, pointing to negative effects of any incremental increase in maternal inflammation. Maternal CRP was negatively related to measures of skeletal growth and body fat deposition, while an attenuated relationship with head circumference was consistent with the principle of brain sparing. These findings point to endogenous maternal immune processes as an important contributor to the fetal gestational milieu, and highlight inflammation as a potential pathway linking maternal environments and health with development and health in the next generation.
Acknowledgments
The authors thank the researchers at the USC-Office of Population Studies Foundation, Inc., University of San Carlos, Cebu City, Philippines, for their role in the study design and data collection, and the study participants, who generously provided their time.
Financial Support
National Science Foundation (Grant no.: BCS-0746320 and BCS-1440564).
Conflicts of Interest
None.







