Lactobacillus delbrueckii is an important species for food fermentation. Lb. delbrueckii subsp. bulgaricus is essential for the production of yoghurt whereas Lb. delbrueckii subsp. lactis is mostly used in the manufacture of hard cheeses (Giraffa et al. Reference Giraffa, de Vecchi and Rossetti1998). Some authors have reported that Lb. delbrueckii is rarely found among the gut microbiota after ingestion due to its reduced capability to survive the restrictive conditions found during gastrointestinal digestion (Tannock, Reference Tannock, Fuller and Perdigón2003; Wall et al. Reference Wall, Fitzgerald, Hussey, Ryan, Murphy, Ross and Stanton2007). However, other researchers have provided evidence indicating that a proportion of these microorganisms can survive the gastrointestinal transit (GIT) (Marteau et al. Reference Marteau, Minekus, Havenaar and Huis In't Veld1997; Lick et al. Reference Lick, Drescher and Heller2001; Mater et al. Reference Mater, Bretigny, Firmesse, Flores, Mogenet, Bresson and Corthier2005; Elli et al. Reference Elli, Callegari, Ferrari, Bessi, Cattivelli, Soldi, Morelli, Goupil Feuillerat and Antoine2006). In the same way, we have recently found that a small fraction of Lb. delbrueckii subsp. lactis can survive simulated GIT when administered with milk (Burns et al. Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010).
Some strains of lactic acid bacteria (LAB) are able to produce exopolysaccharides (EPS). When produced in milk during fermentation by starter cultures, some of these polymers contribute to improve the sensory and structural properties of fermented products (Ruas-Madiedo et al. Reference Ruas-Madiedo, Salazar, de los Reyes-Gavilán and Ullrich2009b). EPS from LAB can generally be divided into homopolysaccharides (HoPS), which are polymers composed of one type of monosaccharide, and heteropolysaccharides (HePS), which are polymers of repeating units that are composed of two or more types of monosaccharides. A great diversity of HePS seems to exist among LAB regarding composition, structure and functionality (Mozzi et al. Reference Mozzi, Vaningelgem, Hébert, van der Meulen, Moreno, de Valdez and De Vuyst2006; Ruas-Madiedo et al. Reference Ruas-Madiedo, Salazar, de los Reyes-Gavilán, Moran, Brennan, Holst and von Itzstein2009c).
Some Gram-positive bacteria can develop an adaptive response in the presence of moderate stress conditions. In previous works we reported on the isolation, molecular and functional characterization of Lb. delbrueckii subsp. lactis bile-resistant derivatives (Burns et al. Reference Burns, Vinderola, Binetti, Quiberoni, de los Reyes-Gavilán and Reinheimer2008, Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010). The acquisition of stable resistance to bile could facilitate the arrival of these microorganisms to the intestine and the possibility to produce there, bacterial enzymes that can improve the digestion of nutrients, such as the β-galactosidase, or that can liberate bioactive peptides from caseins (Gilliland, Reference Gilliland, Marth and Steel1998; Hebert et al. Reference Hebert, Mamone, Picariello, Raya, Savoy, Ferranti and Addeo2008). However, it has been shown that the acquisition of bile tolerance can also modify other physiological and functional properties of the bile-adapted strain (Noriega et al. Reference Noriega, Gueimonde, Sánchez, Margolles and de los Reyes-Gavilán2004; Guglielmotti et al. Reference Guglielmotti, Briggiler Marcó, Vinderola, de los Reyes-Gavilán, Reinheimer and Quiberoni2007; Sánchez et al. Reference Sánchez, Ruíz, de los Reyes-Gavilán and Margolles2008), including the production of EPS (Ruas-Madiedo et al. Reference Ruas-Madiedo, Gueimonde, Arigoni, de los Reyes-Gavilán and Margolles2009a).
The strain Lb. delbrueckii subsp. lactis 193 and its bile resistant derivative Lb. delbrueckii subsp. lactis 193+ were described in a previous work (Burns et al. Reference Burns, Vinderola, Binetti, Quiberoni, de los Reyes-Gavilán and Reinheimer2008). Whereas the parental strain 193 showed no appreciable growth in the presence of 0·3% bile salts, the derivative 193+ displayed active growth in medium containing 0·5% bile salts (Burns et al. Reference Burns, Vinderola, Binetti, Quiberoni, de los Reyes-Gavilán and Reinheimer2008). The aim of the present study was to characterize the technological properties and the ability to survive simulated gastric and intestinal conditions of Lb. delbrueckii subsp. lactis 193 in milk, and to know whether the acquisition of a stable phenotype of resistance to bile could modify these properties.
Material and Methods
Bacterial strains and growth conditions
The strain Lb. delbrueckii subsp. lactis 193 and its bile resistant derivative 193+ (Burns et al. Reference Burns, Vinderola, Binetti, Quiberoni, de los Reyes-Gavilán and Reinheimer2008) were used in this study. Strains were grown in MRS (BioKar Diagnostics, Beauvais, France) at 37°C in a microaerophilic atmosphere containing 5% (v/v) CO2; bacterial stocks were kept at −80°C in MRS containing 200 ml glycerol per litre. As standard procedure, strains were cultured overnight from −80°C stocks and employed to inoculate (2% v/v) fresh MRS medium that was incubated for 24 h for its use in experiments performed in this work.
For specific purposes, strains were also grown in commercial pasteurized milk (Central Lechera Asturiana, Asturias, Spain) which was supplemented with 1% (w/v) Difco™ skimmed milk (Becton Dickinson, MD, USA) and pasteurized again at 90°C for 5 min. MRS cultures of each strain were washed twice with sterile PBS buffer pH 7·0 and were used to inoculate (2% v/v) 500 ml pasteurized milk which was incubated overnight (17± 1 h) in a water bath at 37°C. Following fermentation, a sample was collected in sterile conditions for bacterial counting, fermented milks were cooled-down afterwards to approximately 18°C with running tap water, and then they were stirred 20-times up and down with a spoon. After sample collection for several analyses, the stirred fermented milks were stored overnight at 4°C. Three replicated batches of fermented milks were performed for each strain.
Metabolic activity of strains in milk
For bacterial counts, serial dilutions of cultured milks were made in Ringer's solution (Merck, Darmstadt, Germany), deep-plated on agar-MRS and incubated at 37°C for 2 d. Counts were expressed as log cfu/g and the increase of the log units during milk fermentation was calculated. The pH of the fermented milks was directly measured with a MicropH 2001 pHmeter (Crison Instruments S.A., Barcelona, Spain).
Lactose consumption and organic acids production was determined by ion-exchange chromatography. A HPLC chromatographic system composed of an Alliance 2690 module injector, a Photodiode Array PDA 996 detector, a 410 refractive index (RI) detector and the Empower software (Waters, Milford, MA, USA) was used. The sample preparation and chromatographic conditions described by Salazar et al. (Reference Salazar, Prieto, Leal, Mayo, Bada-Gancedo, de los Reyes-Gavilán and Ruas-Madiedo2009) were used. Results were expressed in mm.
The volatile compounds in fermented milks were determined by means of a Head-Space (HS) GC-MS using a 6890N Agilent GC coupled with a HS automatic injector G1888 series and with a 5975B inert MS detector (Agilent Technologies Inc., Palo Alto, CA, USA). Data was collected and analyzed with the Enhanced ChemStation G1701 software (Agilent). Sample preparation and chromatographic conditions were those previously described by Salazar et al. (Reference Salazar, Prieto, Leal, Mayo, Bada-Gancedo, de los Reyes-Gavilán and Ruas-Madiedo2009). Results were expressed as μg/ml.
The apparent viscosity of stirred-fermented milks was measured using a Posthumus funnel (Hellinga et al. Reference Hellinga, Somesen and Koenraads1989). The funnel was filled with approximately 450 g stirred-fermented milk and the time (in seconds) taken to pass the mark inside the funnel was recorded. The measurements were carried out in a chamber refrigerated at 4°C.
Production of exopolysaccharides (EPS) in milk
The EPS fraction of fermented milks was isolated by mixing 40 g cultured milk with 10 ml of a 60% TCA solution and strongly stirred for 45 min at room temperature. Precipitated bacteria and proteins were removed by centrifugation (10 000 g, 4°C, 30 min) and the pH of supernatant was raised to 4·5±0·5. Finally, supernatants were intensively dialyzed for 3 d, with daily changes of ultrapure water, using dialysis tubes (Sigma Chemical Co., St. Luis, MO, USA) of molecular weight cut off 12–14 kDa and they were finally freeze-dried. To increase the purity level of the EPS fraction an additional procedure was applied. The initial EPS fraction was dissolved at 5 mg/ml in a buffer (50 mm-Tris-HCl, 100 mm-MgSO4·7H2O, pH 7·5) containing 2·5 μg/ml of DNAse type I (Sigma) and incubated at 37°C for 6 h. Afterwards, pronase E from Streptomyces griseus (Sigma) dissolved in 50 mm-Tris-HCl, 2% EDTA, pH 7·5 was added (50 μg/ml) and incubated at 37°C for 18 h. Following the enzymatic treatments, a 60% TCA solution was added to a final concentration of 120 g/l and the mixture was kept under mild stirring for 30 min at room temperature. Proteins and breakdown products were precipitated by centrifugation (10 000 g, 4°C, 30 min) and the supernatant was neutralised, dialysed and lyophilised as formerly described.
The protein content of the purified EPS fraction was measured using the commercial BCA protein assay kit (Pierce, IL. USA) following the manufacturer's instructions.
The EPS yield, molar mass distribution and radius of the molecule were determined by means of size exclusion chromatography (SEC). Samples were dissolved at 5 mg/ml in 100 mm-NaNO3 and separated in two columns placed in series: TSK-Gel G3000 PWXL + TSK-Gel G5000 PWXL protected with a TSK-Gel guard column (Supelco-Sigma). The mobile phase was 100 mm-NaNO3 and the separations took place at 40°C at a flow rate of 0·45 ml/min. The afore-mentioned HPLC apparatus was employed coupled with a third detector, the multi-angle laser light scattering (MALLS) Dawn Heleos II (Wyatt Europe GmbH, Dembach, Germany). The EPS yield was calculated from the data obtained with the RI detector by using the corresponding regression equations obtained from dextran standards and the PDA detector set at 280 nm was used to check the absence of protein in the EPS peak (Salazar et al. Reference Salazar, Prieto, Leal, Mayo, Bada-Gancedo, de los Reyes-Gavilán and Ruas-Madiedo2009). The weight average molar mass (Mw) and the weight average radius (Rw) of the EPS were directly measured with the MALLS detector.
The monosaccharide composition of the two EPS fractions was determined after hydrolysis with 3 m-trifluoroacetic acid (TFA), conversion in their corresponding alditol acetates and separation by gas-liquid chromatography (GLC) as described by Salazar et al. (Reference Salazar, Prieto, Leal, Mayo, Bada-Gancedo, de los Reyes-Gavilán and Ruas-Madiedo2009). The linkage types present in the EPS molecule were determined after methylation of the EPS according to the procedure described by Ciucanu & Kerek (Reference Ciucanu and Kerek1984). The permethylated polysaccharide was hydrolyzed with 3 m-TFA, and the monosaccharides released were reduced with NaBD4 and then acetylated to give corresponding partially methylated alditol acetates, which were analysed by GLC-MS under conditions previously described (Leal et al. Reference Leal, Jimenez-Barbero, Bernabé and Prieto2008). Analyses were carried out at “Centro de Investigaciones Biológicas” (CIB-CSIC, Madrid).
Gastrointestinal survival of strains
Resistance to the chemically simulated gastric and intestinal conditions
The survival of the strains in the GIT situation was studied by an in vitro model that chemically simulates the physiological conditions (Sánchez et al. Reference Sánchez, Fernández-García, Margolles, de los Reyes-Gavilán and Ruas-Madiedo2010). The following solutions were used: (i) simulated gastric juice containing 125 mm-NaCl, 7 mm-KCl, 45 mm-NaHCO3, and 0·3% (w/v) pepsin (Sigma) pH 2·0 adjusted with HCl, (ii) simulated duodenal juice containing 1% (w/v) bovine bile (Sigma) pH 8·0 adjusted with 10 mm-NaOH, and (iii) simulated ileal juice containing 0·3% (w/v) bovine bile, and 0·1% (w/v) pancreatin (Sigma) pH 8·0 adjusted with 10 mm-NaOH. To simulate the GIT conditions, cells from 24 h MRS-grown cultures of the parental and bile-resistant derivative strains were harvested by centrifugation (10 000 g, 15 min, 5°C), washed twice with a solution of 8·5 g NaC/l l and concentrated 10-fold. For each strain, 100 μl of the concentrated suspensions were centrifuged and resuspended either in 1 ml of simulated gastric juice or in 1 ml of simulated gastric juice containing 200 g skimmed milk/l, which increased the pH of the bacterial suspension to about 4·5. Bacterial suspensions were then incubated for 90 min at 37°C with a mild stirring (200 rpm). Afterwards, cells were harvested (10 000 g, 15 min), resuspended in the simulated duodenal juice and incubated for 10 min at 37°C in an anaerobic chamber (Mac 500, Down Whitley Scientific, West Yorkshire, UK) under 10% H2, 10% CO2, and 80% N2 atmosphere. After this step, cells were centrifuged again, resuspended in the simulated ileal juice and incubated for 90 min at 37°C in anaerobic conditions. Viable cell counts were obtained from the initial cultures and after the simulation of each condition tested and results were expressed as log cfu/ml.
Adhesion to the epithelial intestinal cell line HT-29-MTX
The adhesion capability of the strains was assessed with the epithelial intestinal cell line HT29-MTX that is able to constitutively produce mucin (Lesuffleur et al. Reference Lesuffleur, Barbat, Dussaulx and Zweibaum1990). The cell line was maintained in supplemented DMEM (Sigma) using standard procedures (Sanchez et al. Reference Sánchez, Fernández-García, Margolles, de los Reyes-Gavilán and Ruas-Madiedo2010). For experiments, 1×105 cells/ml were seeded in 24-well plates and incubated to confluence for 13± 1 day (about 1×107 cells/ml). The cell line was used between passes 26 and 28.
Bacterial suspensions were obtained from 24 h cultures of parental and bile-resistant derivative grown in MRS with different concentrations of bovine bile salts: the parental strain 193 was cultured in the presence of 0%, 0·1% and 0·3% of bile and the derivative 193+ with 0%, 0·3% and 0·5% of bile. Bacteria were harvested from cultures by centrifugation (10 000 g, 15 min, 5°C), washed twice with PBS buffer and resuspended in DMEM without antibiotics at a concentration of about 1×108 cfu/ml. Bacterial counts in MRS-agar were performed in order to determine the number of bacteria added. HT29-MTX monolayers were washed twice with Dulbecco's PBS buffer (Sigma-Aldrich) to remove the antibiotics and then bacterial suspensions were added in a ratio of epithelial cells: bacteria of 1:10. Plates were incubated for 1 h at 37°C, 5% CO2 in a Heracell® 240 incubator (Thermo Electron LDD GmbH, Langenselbold, Germany). After the incubation period, supernatant was removed and wells were softly washed three times with Dulbecco's PBS buffer to remove the non-attached bacteria. Finally, the monolayers were trypsinised and bacterial counts were carried out to determine the number of adhered bacteria. Results were expressed as the percentage of adhered bacteria with respect to the amount of bacteria added.
Statistical analyses
Data were statistically analyzed by means of one-way ANOVA tests using the SPSS 11.0 software for Windows (SPSS Inc., Chicago, IL). Tests were performed employing the strain as factor, with two categories: parental and derivative. For simulated gastric and intestinal transit, an additional ANOVA test was carried out using the presence and absence of milk as categories within each strain and gastric or intestinal condition tested.
Results and Discussion
Growth and metabolic activity of strains in milk
Main growth and metabolic activity parameters of Lb. delbrueckii subsp. lactis 193 and its bile resistant derivative Lb. delbrueckii subsp. lactis 193+ in milk are shown in Table 1. Lb. delbrueckii subsp. lactis 193 was able to grow and acidify milk efficiently at the expenses of lactose consumption, with accumulation of galactose. The apparent imbalance obtained between lactose consumed and galactose released and lactic acid formed, inferred from data presented in Table 1, may be attributed to limitations of the chromatographic separation of the different compounds and to the different sensitivity of detectors used for quantification (RI for sugars and PDA in the case of lactic acid). Production of acetaldehyde by the strain 193 was considerably higher than ethanol formation. Acetaldehyde is one of the main contributors to flavour in fermented milks and certain cheeses (Cogan, Reference Cogan1995; Qian & Reineccius, Reference Qian and Reineccius2003; Pinto et al. Reference Pinto, das Graças Clemente and Ronaldo de Abreu2009). In spite of the wide variation reported in literature on the profile of flavour compounds of different strains within the same species of LAB, values obtained by us were in the range of those previously reported for Lb. delbrueckii in milk which commonly produces more acetaldehyde than ethanol (Cogan, Reference Cogan1995; Beshkova et al. Reference Beshkova, Simova, Frengova and Simov1998; Shene & Bravo, Reference Shene and Bravo2007; Pinto et al. Reference Pinto, das Graças Clemente and Ronaldo de Abreu2009).
Table 1. Growth and metabolic activity parameters of Lactobacillus delbrueckii subsp. lactis 193 and its bile-resistant derivative strain 193+ in pasteurised milk incubated at 37°C for 18 h. ANOVA: *** P<0·001
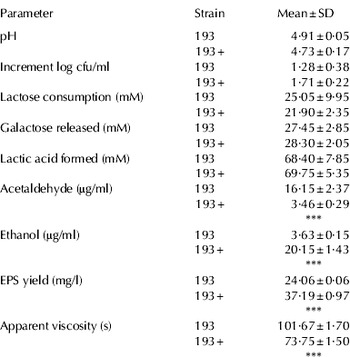
Adaptation to bile of sensitive strains has been proposed as a suitable way of overcoming the deleterious action of these compounds on beneficial strains intended to be used in human foods (Sarkar, Reference Sarkar2010). Although the species Lb. delbrueckii is not considered a probiotic, some health beneficial properties could derive from products containing LAB starters (Cenci et al. Reference Cenci, Rossi, Trotta and Caldini2002; Cogan et al. Reference Cogan, Beresford, Steele, Broadbent, Shah and Ustunol2007). Therefore, we compared growth and metabolic properties in milk of the bile-adapted strain Lb. delbrueckii subsp. lactis 193+ with respect to the bile-sensitive parental strain 193. No significant differences were found for pH values, lactose consumed, galactose released and lactic acid formed. However, formation of volatile compounds was markedly different between both strains. Notably, the production of ethanol by the bile-resistant derivative 193+ surpassed the production of acetaldehyde, levels of this last compound being considerably lower than those of the parental strain (P<0·001). Ethanol production by LAB occurs at the expense of enzymatic reduction of acetaldehyde (Axelsson, Reference Axelsson, Salminen, von Wright and Ouwehand2004); this reaction is usually unsuitable for yoghurt and dairy product production since flavour development may be negatively affected (Pinto et al. Reference Pinto, das Graças Clemente and Ronaldo de Abreu2009). In a similar way, Sánchez et al. (Reference Sánchez, Fernández-García, Margolles, de los Reyes-Gavilán and Ruas-Madiedo2010) found higher concentrations of ethanol and lower concentrations of acetaldehyde at the end of the cold storage period of fermented milks made with starter cultures and a bile-resistant derivative strain of Bifidobacterium animalis compared with products obtained with the parental strain. Relating to this, we have recently shown that adaptation to bile of the strain Lb. delbrueckii subsp. lactis 200 promoted a shift in the final products of glucose catabolism in MRS culture leading to an increase in the lactic/acetic acids ratio (Burns et al. Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010). Shifts in the catabolism of carbohydrates found in the present work, although not suitable for the development of sensory properties of dairy products, could however play an important physiological role in the bacterium. Thus, the reduction of pyruvate to lactic acid allows bacterial cells the regeneration of 2 mol NAD+ per mol of glucose consumed, whereas the conversion to ethanol provides 2 additional mol of regenerated NAD+. Therefore, it can be speculated that an increase of ethanol production may represent a mechanism of Lb. delbrueckii subsp. lactis 193+ to cope with oxidative stress imposed by bile salts, as has been previously suggested in other lactobacilli and bifidobacteria (Bron et al. Reference Bron, Marco, Hoffer, Van Mullekom, de Vos and Kleerebezem2004; Sánchez et al. Reference Sánchez, de los Reyes-Gavilán and Margolles2006; Lee et al. Reference Lee, Lee and Choi2008; Sánchez et al. Reference Sánchez, Ruíz, de los Reyes-Gavilán and Margolles2008).
Lb. delbrueckii subsp. lactis 193 and 193+ provided fermented milk with a smooth and creamy consistency, a desirable property for application in low-fat cheese making and manufacture of fermented milks (Ruas-Madiedo et al. Reference Ruas-Madiedo, Salazar, de los Reyes-Gavilán, Moran, Brennan, Holst and von Itzstein2009c). The high apparent viscosity in milk fermented by the strains under study strongly suggested that they were able to synthesize EPS. The viscosity-intensifying capability in milk of the strains 193 and 193+ was higher than that of traditional viscosifying yoghurt starter cultures containing Lb. delbrueckii subsp. bulgaricus and Streptococcus thermophilus strains (van Marle & Zoon, Reference van Marle and Zoon1995) and both viscosifying capability and EPS yield were comparable to that of HePS from other lactobacilli and lactococci from different origins (Ruas-Madiedo et al. Reference Ruas-Madiedo, Tuinier, Kanning and Zoon2002. 2005; Mozzi et al. Reference Mozzi, Vaningelgem, Hébert, van der Meulen, Moreno, de Valdez and De Vuyst2006; Salazar et al. Reference Salazar, Prieto, Leal, Mayo, Bada-Gancedo, de los Reyes-Gavilán and Ruas-Madiedo2009). Remarkably, the apparent viscosity of fermented milk made with the strain 193 was significantly higher, and EPS yield significantly lower (P<0·001), than that of fermented milks made with the bile resistant strain 193+; this suggests that the acquisition of bile resistance may have introduced some modifications in the composition/structure of the polymer that could have impaired its technological properties.
Physico-chemical characterisation of the EPS produced in milk
The synthesis in milk of viscosifying EPS is a property of great interest for improving the consistency of fermented milks and cheeses in a natural way. Firstly we characterized the EPS produced in milk by the strains under study (Table 2). The EPS fraction produced by Lb. delbrueckii subsp. lactis 193 presented a unique peak of high Mw (about 106 Da). This polymer was a HePS composed of glucose and galactose in a ratio 1:1. Mozzi et al. (Reference Mozzi, Vaningelgem, Hébert, van der Meulen, Moreno, de Valdez and De Vuyst2006) reported that most HePS produced by mesophilic and thermophilic lactobacilli from food origin displayed a unique EPS peak of Mw lower than 106 Da, mostly composed of galactose and glucose. In contrast, Salazar et al. (Reference Salazar, Prieto, Leal, Mayo, Bada-Gancedo, de los Reyes-Gavilán and Ruas-Madiedo2009) found that the presence of two peaks of high and low Mw is a common feature in EPS from bifidobacteria and lactobacilli of intestinal origin. No noticeable differences in Mw, Rw and monosaccharide composition were found between the EPS produced by the parental strain 193 and its bile resistant derivative 193+. Percentage of most linkage types found were similar in both strains, however notably the percentage of 1,2 Galf was higher in the bile resistant derivative 193+ than in the parental strain 193 (Table 3). Since this is the sole difference found by us between polymers of both strains, this suggests that the repeating unit of EPS 193+ may differ from that of EPS 193. Relating to this, we have recently reported variations in Mw of EPS fractions as well as in monosaccharide ratios between polymers synthesized by a bile-resistant Bifido. animalis strain and its parental sensitive strain (Ruas-Madiedo et al. Reference Ruas-Madiedo, Medrano, Salazar, de los Reyes-Gavilán, Pérez and Abraham2010). Whether these are changes linked to the acquisition of bile tolerance or they are just pleiotropic phenomena, is at present unknown. The physiological and ecological significance of these variations in the characteristics of EPS produced by bile resistant derivatives deserves further investigation.
Table 2. Physico-chemical characteristics of the EPS fractions isolated from Lactobacillus delbureckii subsp. lactis 193 and its bile-resistant derivative 193+

Table 3. Main sugar linkage types present in the EPS fractions isolated from Lactobacillus delbureckii subsp. lactis 193 and its bile-resistant derivative 193+. Galp: galactose residue in pyranose conformation, Galf: galactose residue in furanose conformation, Glcp: glucose residue in pyranose conformation

It has been indicated that linkage type into the backbone of repeating unit building HePS, as well as the presence of side chains or the branching degree, among others, are parameters contributing to the viscosity intensifying ability of these polymers in fermented milks (Ruas-Madiedo et al. Reference Ruas-Madiedo, Tuinier, Kanning and Zoon2002). Differences in percentage of linkage types could partly contribute to differences found in technological properties between the polymers produced by the sensitive and bile resistant derivative strains 193 and 193+. Other factors such as Mw, interactions between the EPS and the milk protein network (Hassan, Reference Hassan2008) or the acidification rate of the strains in milk (Lucey & Singh, Reference Lucey and Singh1998), among others, could also affect the viscosity of the fermented milk.
Survival of strains in simulated GIT conditions
Survival through the GIT may be a positive feature of microorganisms included in foods in order to reach the intestine alive. Therefore, the survival in simulated gastric, duodenal, and ileal juices, and the influence of milk on this process, was tested for both strains studied. Simulated gastric juice caused a decrease in the population of Lb. delbrueckii subsp. lactis 193 of ca. 5 log orders, although viable cells (about 5 log orders) were still observed at the end of the sequential passage through the different solutions (Fig. 1). Viability losses were in accordance with those previously reported for other strains of the same species (Vinderola & Reinheimer, Reference Vinderola and Reinheimer2003; Burns et al. Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010). The presence of skimmed milk counteracted the detrimental effect of simulated gastric juice on cell viability and improved the survival of the strain 193 in these conditions. However, no survival improvement was observed with skimmed milk for the subsequent steps (duodenal and ileal simulated juices, statistical analysis not shown). An increase of viable cell counts was found in ileal juice with respect to counts in duodenal juice either with and without skimmed milk which may be attributed to the transition from viable non-cultivable to viable cultivable state of cells (Lahtinen et al. Reference Lahtinen, Ouwehand, Reinikainen, Korpela, Sandholm and Salminen2006) probably favoured by lower concentrations of bile in simulated ileal juice compared with simulated duodenal juice. With respect to the strain 193+, exposure to simulated gastric and intestinal juices caused a viability loss of about 7 log units, and no improvement in survival was found with respect to the parental strain 193, which was coincident with results previously reported by us for other strains of the same species (Burns et al. Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010). The presence of skimmed milk improved the survival of the strain 193+ in simulated duodenal and ileal juices (significant differences at P<0·01; statistical analysis not shown) (Fig. 1). In spite of all this, considering jointly the two conditions tested in the present work (i.e. buffer and buffer + milk) the survival of Lb. delbrueckii subsp. lactis 193 and 193+ in simulated duodenal and ileal juices was similar, indicating that the acquisition of bile resistance did not actually provide an improvement of survival in simulated gastric and intestinal conditions. In contrast, Sánchez et al. (Reference Sánchez, Fernández-García, Margolles, de los Reyes-Gavilán and Ruas-Madiedo2010) reported an increase in the survival in simulated GIT conditions of a Bifido. animalis bile-resistant derivative with respect to its bile-sensitive parental strain. Additionally, Burns et al. (Reference Burns, Vinderola and Reinheimer2011) reported a higher survival of the bile-resistant derivative Lb. delbrueckii subsp. lactis 200+ in the intestinal fluid of mice compared with the parental bile-sensitive strain Lb. delbrueckii subsp. lactis 200. Different behaviour of bile-resistant derivatives could reflect physiological and molecular differences derived from their adaptation to this stress factor.

Fig. 1. Counts (log cfu/ml) of Lactobacillus delbrueckii subsp. lactis 193 (white bars) and its bile-resistant derivative strain Lb. delbrueckii subsp. lactis 193+ (grey bars) after chemical simulation of gastrointestinal conditions. GJ: simulated gastric juice, DJ: simulated duodenal juice, IJ: simulated ileal juice. ANOVA: * P<0·05, ** P<0·01, *** P<0·001.
The ability to transiently colonize the gut surfaces is related to the ability of microorganisms to adhere to mucus or intestinal epithelial cells. Adhesion to the intestinal cell line HT29-MTX at different bile salt concentrations was assessed (Fig. 2). The strains 193 and 193+ displayed a lower adhesion capacity (about 3·5 and 2·5%, respectively) than other lactobacilli (Schillinger et al. Reference Schillinger, Guigas and Holzapfel2005; Burns et al. Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010). Adhesion of both microorganisms clearly rose in the presence of bile salts (statistical analysis not shown) although the derivative 193+ was significantly less adhesive (P<0·01) than its parental counterpart. Differences between parental and derivative strains may be due to changes in surface properties that have been shown to occur in other microorganisms such as some lactobacilli and bifidobacteria as a consequence of the acquisition of bile salt resistance (Gomez-Zavaglia et al. Reference Gómez-Zavaglia, Kociubinski, Perez, Disalvo and DeAntoni2002; Gueimonde et al. Reference Gueimonde, Noriega, Margolles, de los Reyes-Gavilán and Salminen2005; Ruiz et al. Reference Ruiz, Sánchez, Ruas-Madiedo, de los Reyes-Gavilán and Margolles2007). The behaviour of these two strains was different from that found for the bile sensitive/bile resistant pair Lb. delbrueckii subsp. lactis 200 and 200+ since, in that case, adhesion values clearly diminished in the presence of bile (Burns et al. Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010). These differences between both pairs of microorganisms could account for different surface molecules involved in cellular adhesion of 193/193+ and 200/200+.

Fig. 2. Adhesion (% counts of bacteria adhered with respect to bacteria added) to the epithelial intestinal cellular line HT29-MTX of Lactobacillus delbrueckii subsp. lactis 193 (white bars) and its bile-resistant derivative strain Lb. delbrueckii subsp. lactis 193+ (grey bars) in the presence of different concentrations of bile salts. ANOVA: * P<0·05, ** P<0·01.
Although it has traditionally been considered that yoghurt starters do not survive the GIT, some recent studies seem to indicate that certain strains do at a reduced extent (Lick et al. Reference Lick, Drescher and Heller2001; Mater et al. Reference Mater, Bretigny, Firmesse, Flores, Mogenet, Bresson and Corthier2005; Elli et al. Reference Elli, Callegari, Ferrari, Bessi, Cattivelli, Soldi, Morelli, Goupil Feuillerat and Antoine2006; Burns et al. Reference Burns, Sánchez, Vinderola, Ruas-Madiedo, Ruiz, Margolles, Reinheimer and de los Reyes-Gavilán2010). From our results, it could be assumed that both strains of Lb. delbrueckii subsp. lactis tested in the present work could survive simulated gastric and intestinal conditions in certain numbers when they are ingested within a food matrix. In contrast to that observed in some bifidobacteria and lactobacilli in which the acquisition of bile resistance improved some functional properties (Sánchez et al. Reference Sánchez, Ruíz, de los Reyes-Gavilán and Margolles2008, Reference Sánchez, Fernández-García, Margolles, de los Reyes-Gavilán and Ruas-Madiedo2010; Burns et al. Reference Burns, Vinderola and Reinheimer2011), in the case of Lb. delbrueckii subsp. lactis 193, the acquisition of resistance to bile does not seem to provide any improvement in the viability and adhesion in simulated gastric and intestinal conditions.
In short, Lb. delbrueckii subsp. lactis 193 presents suitable properties for the manufacture of fermented dairy products and produces a HePS that confers viscosity intensifying properties to milk. The acquisition of a stable bile-resistant phenotype does not improve any of the properties tested. Thus, the use of bile-resistant derivative strains should be carefully evaluated in each specific application considering the influence that the acquisition of a stable bile-resistant phenotype could have in the viability and technological properties of microorganisms.
This work was financed by FEDER funds (European Union) and the Spanish Plan Nacional de I+D through projects AGL2007-62736, AGL2009-09445, and AGL2010-16525. Patricia Burns was funded by a 6-month grant from the Agencia Española de Cooperación Internacional for a research stay at IPLA-CSIC. Groups from Spain and Argentina shared a joint collaboration project CSIC-CONICET (reference 2005AR0047). Ana María Hernández-Barranco from IPLA-CSIC and Alicia Prieto from CIB-CSIC are acknowledged for their excellent technical assistance in the GLC and GC-MS analyses. The cellular line HT29-MTX was kindly supplied by Dr. T. Lesuffleur (INSERM U843 Paris, France).







