During the peri-partum period, dairy cattle are more susceptible to several infection modalities as well as to metabolic diseases than at other times during the lactation cycle. Alterations in hormone profiles, metabolic demands, and the stress of parturition contribute to a reduced host defence. Shuster et al. (Reference Shuster, Lee and Kehrli1996) reported that peri-partum cows demonstrated an impaired ability to control the early growth of coliforms after an intramammary challenge when compared with mid-lactation cows. However, the authors observed that the rapid growth occurred before neutrophil recruitment into the mammary gland irrespective of lactation stage, which suggested deficiencies in aspects of immunity other than activation of the acute phase response (APR). Furthermore, early lactating cows activated the APR possibly to a greater extent as evidenced by a more rapid and elevated pro-inflammatory cytokine response and recruitment of neutrophils to the mammary gland. Using a cross-over design, Lehtolainen et al. (Reference Lehtolainen, Suominen, Kutila and Pyörälä2003) reported that cows in early lactation had a more robust APR than in late lactation in response to an intramammary lipopolysaccharide (LPS) challenge. The question remains as to whether the magnitude of the incurred pro-inflammatory response in early lactation is of benefit or detriment towards a progression to a pathological state. Therefore, strategies that limit the intensity or duration of the APR may benefit the health and ability of the cow to recover from a coliform infection in early lactation.
Increased intakes of the omega-3 (n-3) fatty acids (FA), eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6), in animal models other than ruminants alleviate inflammation (see Calder, Reference Calder2006). Furthermore, both enteral and parenteral supplementation of fish oil decreased the pathogenesis and increased survival in endotoxaemic and septicaemic models (Mascioli et al. Reference Mascioli, Dohoo and Duizer1989; Johnson et al. Reference Johnson, Griswold and Muakkassa1993). However, in addition to attenuating the APR, dietary fish oil impaired other aspects of the immune system including the production of reactive oxygen species (Rees et al. Reference Rees, Miles, Banerjee, Wells, Roynette, Wahle and Calder2006) and lymphocyte proliferation (Anderson & Fritsche, Reference Anderson and Fritsche2004) which might further compromise disease resistance during early lactation. There are no data in peri-partum dairy cattle on effects of supplemental fish oil on the APR to an intramammary challenge with LPS. Therefore, the objective was to determine the effects of supplementing fish oil during the peri-partum period on the APR following a LPS challenge.
Material and Methods
Experimental design, animals, and diets
Thirty multiparous Holstein cows were housed in individual pens and completely randomized to one of two treatment diets at 21 d before anticipated date of parturition. Treatments were supplemental lipid from either Energy Booster® (Milk Specialties Co., Dundee IL, USA; EB, n=15) or fish oil (Omega Proteins, Houston TX, USA; FO, n=15). Energy Booster was chosen as a control treatment because it is a highly saturated, rumen-inert lipid source. Treatment diets were fed from −21 d relative to expected date of parturition until 10 d post partum. Treatments were fed as a bolus prior to the morning feeding and were offered within 15 min of collecting the prior day's refusals. The dose of lipid during the pre-partum period was 250 g/d, whereas the amount of lipid supplemented post partum was adjusted to the level of intake, approximately 0·92% of the previous day's dry matter intake (DMI). The composition of the bolus was 150 g each of rolled barley grain, shredded beet pulp, and cane molasses plus 550 g of the respective total mixed ration plus the supplemental lipid source. After the entire bolus was consumed, typically within 15 min, each cow was offered ad libitum either a single pre-partum diet prior to parturition or a lactation diet post partum. Immediately after parturition cows were switched to the lactation diet and milked twice daily.
The amounts of feed offered and refused were measured daily. Samples of the total mixed ration were collected twice weekly and composited by month and frozen at −20°C until analysed at a commercial laboratory (Cumberland Valley Analytical Services, Maugansville MD, USA).
All calves received 6 l of frozen-thawed pooled colostrum within the first 24 h of life. Subsequently, all calves were fed 1·9 l of a 22·5% crude protein and 18% fat commercial milk replacer (Calva Products, Acampo CA, USA) twice daily. A commercial calf starter (Nutrena DairyWay, Cargill Inc., Minneapolis MN, USA) was offered ad libitum. The first blood sample taken from calves for ex-vivo immunological analyses was taken prior to colostrum feeding.
Fatty acid composition of plasma
On day 21 pre-partum and day 1 post partum, peripheral blood from a subsample of animals (n=5/lipid treatment) was collected, centrifuged, and plasma stored at −80°C until further analysis. Lipid was extracted from plasma with chloroform–methanol (2:1, v/v) and total phospholipids isolated by thin-layer chromatography using hexane–diethyl ether–acetic acid (90:30:1, v/v/v) as the elution phase. Fatty acid methyl esters were prepared by incubation with 2 m-KOH in methanol for 15 min at room temperature. The ester mixture was separated using a Hewlett Packard 5890 GC (Hewlett Packard, Avondale PA, USA). Unknown peak areas were compared with a known quantity of an external standard mixture containing all reported FA. All data are expressed as 100 g/kg of the total peaks recovered.
Ex-vivo immunological assays
Whole blood antimicrobial capacity against various microorganisms was determined in cows at 21 d pre-partum, and at 1 d and 21 d post partum, and in the calves at 2 h and 1 d and 21 d after birth. The general assay as described previously (Millet et al. Reference Millet, Bennett, Lee, Hau and Klasing2007) was optimized in Holstein cows and calves. The antimicrobial capacity was determined as the percent of the inoculum killed, which was calculated as [(1−(number of viable cfu after incubation/number of viable cfu inoculated)]. The sample CV was 15·8%. The ex-vivo capacity of peripheral blood mononuclear cell cultures to produce interferon-γ (IFN-γ) was determined only in cows on day 21 pre-partum and again at day 1 and day 21 post partum. The viability of calf peripheral blood mononuclear cells on day 1 after parturition was low (<50%); therefore cell cultures were discontinued. Thirty-ml of peripheral blood was collected, and peripheral blood mononuclear cells were isolated by density centrifugation using 1·083 g/l Percoll. After isolation, the peripheral blood mononuclear cells were re-suspended in RPMI 1640 and 10 g/l antibiotic–antimycotic solution (Gibco-Invitrogen, Carlsbad CA, USA). The number of viable cells was determined by trypan blue exclusion using a haemacytometer. Peripheral blood mononuclear cells were diluted to a working concentration of 106 peripheral blood mononuclear cells/ml in RPMI+50 g/l fetal calf serum. Two-hundred-μl of the working cell suspension were added in triplicate to a 96-well plate. Cell cultures to determine IFN-γ were stimulated with both 0 μg/ml and 5 μg/ml of phytohaemagglutin-P (PHA-P; Sigma-Aldrich Chemical Co., St. Louis MO, USA) for 72 h. Following incubation cell suspensions were centrifuged and the supernatant removed and stored at −80°C until assayed for IFN-γ by ELISA using a commercial kit (Endogen, Rockford IL, USA). All ELISA procedures followed the manufacturer's instructions. The intraplate and interplate CV was 3·5% and 5·4%, respectively.
Intramammary endotoxin challenge
On day 7 after parturition, one rear teat from each cow was cleaned with chlorohexidine diacetate and infused with 100 μg of purified LPS (Esch. coli 0111:B4, Sigma-Aldrich Chemical Co., St. Louis MO, USA) that was reconstituted in 5 ml of non-pyrogenic phosphate-buffered saline. The infused teat had low basal somatic cell counts. The dose of LPS was chosen because it was previously shown to cause acute effects on clinical and production performance of early lactating cows (Lehtolainen et al. Reference Lehtolainen, Suominen, Kutila and Pyörälä2003). Infusions occurred 30 min following the a.m. milking. DMI was recorded daily. Milk samples were collected immediately prior to and from the first six milkings following the LPS challenge from infused and un-infused quarters, with the latter consisting of a pooled milk sample from the three un-infused quarters. Milk weights were recorded. Milk samples were collected and analysed for somatic cell counts (Fossomatic 5000, Foss North America, Eden Prairie MN, USA) by a commercial lab (Silliker Labs, Modesto CA, USA). Additionally, skim milk was diluted 1/50 into Tris buffer (50 mm-Tris, 0·14 m-NaCl, 5 g/l BSA, and 0·5 g/l Tween 20) prior to storage at −20°C for determination of milk lactoferrin concentration, which was measured by a sandwich ELISA per the manufacturer's instructions (Bethyl Laboratories, Montgomery TX, USA). The intraplate and interplate CV was 1·2% and 6·3%, respectively.
Clinical parameters, including heart rate, respiratory rate and rectal temperature were measured immediately prior to and at 1, 2, 3, 4, 5, 6, 8, 12, 24 and 72 h following the LPS challenge. Prior to and at 6 h and 24 h following the LPS challenge, peripheral blood was collected from a coccygeal vein to determine total white blood cell counts.
Statistical analyses
Of the 30 cows assigned to the experiment, data from 6 cows were not used for statistical analyses, 3 on each of the treatments, because the cow either calved within 14 d of initiating treatments or experienced a health disorder prior to the LPS infusion day. FA composition of plasma in both cows and calves was analysed by ANOVA using the general linear model procedure of SAS (SAS version 9.1, 2003) with treatment as the main effect. The change in the FA composition from baseline to parturition in the cows was calculated prior to analysis. Repeated measures data from the antimicrobial and cell culture cytokine assays were analysed by restricted maximum likelihood ANOVA using the Mixed procedure of SAS (SAS version 9.1, 2003). The full interaction model with treatment, day, and time as the main effects was fitted for the antimicrobial assay, whereas the full interaction model with treatment, day, and dose as the main effects was fitted for the cytokine cell cultures. For each model the random effect was either cow or calf nested within treatment. Data from each microorganism were analysed separately. Data from day 21 prior to expected parturition were used as a covariate. Repeated measures data following the LPS challenge were analysed by restricted maximum likelihood ANOVA using the Mixed procedure of SAS (SAS version 9.1, 2003). The model included the fixed effects of treatment, time, and the interaction of treatment and time; the random effect was cow nested within treatment. The SLICE option with a Tukey-Kramer adjustment was used to make multiple treatment comparisons on time for significant treatment and time interactions.
All repeated data were tested to determine the most appropriate covariance structures for the within-subject measurements and were chosen for each analysis based on the Schwarz's Bayesian Information Criterion. Least squares means (±sem) are reported throughout. Treatment difference of P⩽0·05 was considered significant and 0·05<P⩽0·10 was considered a tendency.
Results
Dry matter intake and plasma fatty acid composition
Cows were supplemented for 21·5±5·58 d and 22·5±5·35 d (mean±sd) during the pre-partum period for EB and FO, respectively. Ingredient and chemical compositions of the pre-partum and post-partum diets are presented in Table 1. The FA composition of the lipid supplements were as intended with the greater n-6:n-3 for EB compared with FO (Table 2). DMI and changes in body weight (BW) and body condition score during the peri-partum period were not different among treatments. Average DMI was 14·7 and 18·5 kg/d for pre-partum and post-partum periods, respectively. Furthermore, there were no differences between treatments with respect to milk yield or composition (data not shown). EPA and DHA were increased while there was a tendency for docosapentaenoic acid n-3 to increase in plasma with supplemental FO (Table 3). In EB cows, the plasma phospholipid concentrations of all n-6 FA decreased over the pre-partum period. Supplementing FO further numerically decreased the proportion of linoleic acid but the response was highly variable and not significant. Dihomo-γ-linolenic and adrenic (22:4n6) acids were decreased with supplemental FO.
Table 1. Ingredient and nutrient composition of the basal pre-partum and post-partum diets fed to multiparous Holstein cows during late pregnancy and early lactation

† Mix contained a minimum of 8·5% Ca, 4·5% P, 6·5% Mg, 1·0% K, 1·6% S, 500 mg of Cu/kg, 2000 mg Mn/kg, 17 mg Se/kg, 3250 mg Zn/kg, 44 mg I/kg, 400 000 i.u. of vitamin A/kg, 140 000 i.u. of vitamin D/kg, and 3150 i.u. vitamin E/kg
‡ Mix contained a minimum of 3·0% Ca, 1·0% P, 0·4% Mg, 1·0% K, 145 mg of Cu/kg, 615 mg Mn/kg, 3·7 mg Se/kg, 4·5 mg I/kg, 97 000 i.u. of vitamin A/kg, 22 000 i.u. of vitamin D/kg, and 1650 i.u. of vitamin E/kg
§ SoyChlor 16-7 (West Central Soy, Ralston, IA)
¶ Nutrient content based on monthly composites of TMR samples (n=7 for each TMR). Values for NFC and DCAD were estimated by the NRC (2001) model using mean composition data
Table 2. Fatty acid profileFootnote † of Energy Booster and fish oil
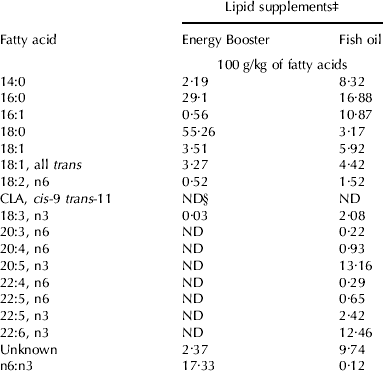
† Fatty acids profile of interest, reported proportion of total recovered peaks
‡ Energy Booster (Milk Specialties Co., Dundee IL) and Fish oil (Omega Proteins, Houston TX)
§ ND=non-detectable
Table 3. Change in the fatty acid composition of plasma phospholipids in the cow from 21 d before expected parturition to parturition in response to supplemental lipid source. Values are Least Squares Means±sem for n=12
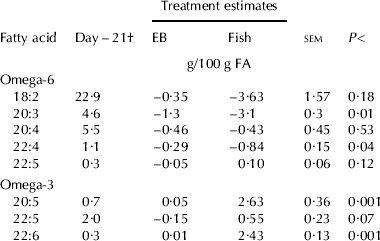
† Mean of pretreatment values
Ex-vivo immunological assays
Antimicrobial activity of whole blood was not affected by supplemental FO (Table 4). In addition, there were no interactions of treatment and day or treatment and time. There was a day effect (P<0·001) in the antimicrobial activity of whole blood against Esch. coli and Sal. typhimurium and it was apparent in both the cow and calf (Table 4). The day effect for the Cand. albicans only reached a tendency (P=0·08) in the cows and there was no day effect for the Cand. albicans in the calves. The production of IFN-γ was not different between treatments. Furthermore, no time effect was evident.
Table 4. Temporal anti-microbial capacity of whole blood in both the cow and her calf. Values are Least Squares Means±sem for n=12

† Assay run on days −21, 1, and 21 (cow); days 0 (2 h post partum; before feeding colostrum), 1, and 21 (calf) relative to parturition. day −21, in the cow analyses, was used as a covariate and represents the mean
‡ No significant interactions between the main effects were evident
§ Day relative to parturition
¶ Incubation time: 15 and 30 min for Escherichia coli and Salmonella typhimurium; 2 h and 4 h for Candida albicans
Intramammary lipopolysaccharide challenge
Intramammary infusion of 100 μg of purified LPS caused an acute and severe APR. Clinical signs of an APR were apparent within 2 h of the LPS challenge; however, the supplemental FO had no effect on the responses (Table 6). DMI of cows was suppressed (P<0·001) following the LPS challenge. Data from EB and FO were pooled, and DMI decreased by 16·8% and 12·5% on the day of infusion and 1 d post-infusion, respectively. DMI returned to the baseline by day 2. Intramammary LPS decreased (P<0·001) milk production in both un-infused and infused quarters. When EB and FO data were pooled, milk production in the LPS infused quarter decreased by 20·8, 38·1, 20·5 and 13·2% during the first four milkings, respectively, and returned to baseline yields by the fifth milking. More modest decreases (P<0·001) in milk yield were apparent in the un-infused quarters decreasing during the first two milkings by 13·1 and 8·3%, respectively, and returning to baseline yields by the third milking.
Rectal temperatures increased (P<0·001) rapidly and peaked 5 h after the challenge at approximately 3°C above baseline. LPS also caused rapid increases (P<0·001) in both heart and respiratory rates, and similarly to the febrile response peaked at 5 h and there was no effect of supplemental FO. The somatic cell count response in both un-infused and infused quarters was affected by time. There was also a treatment by time interaction (P<0·01) in the infused quarter; however, examination of the plots and sliced effects (not shown) revealed no significant differences at any of the times following the challenge. Relative to baseline, peripheral blood total white blood cell counts decreased at 6 h and increased at 24 h post-infusion (P<0·001). Milk lactoferrin increased in both the un-infused (P=0·06) and infused quarters (P<0·001) following LPS challenge. In the infused quarters, lactoferrin peaked at the fourth milking and had not returned to baseline concentrations by the sixth milking. Furthermore, in un-infused quarters, the lactoferrin response was less, and returned to baseline concentrations within the fifth milking.
Discussion
Daily DMI and the changes in both BW and body condition scores were unaffected by either supplemental lipid or source of the lipid supplement. The increased EPA, docosapentaenoic acid n-3 and DHA in plasma phospholipids of FO-supplemented cows reflected the intake of each FA, which was consistent with previous reports in ruminants (Ashes et al. Reference Ashes, Siebert, Gulati, Cuthbertson and Scott1992). Furthermore, the incorporations of the n-3 FA into plasma were consistent with moderate supplementation of (EPA+DHA) into plasma phospholipids in man (Yaqoob et al. Reference Yaqoob, Pala, Cortina-Borja, Newsholme and Calder2000). Supplementing FO decreased the n-6 FA, dihomo-γ-linolenic and adrenic acids, in plasma phospholipids. Supplementing either 4 g EPA+DHA/d or 2·1 g EPA/d for 4 weeks to human patients reduced the proportion of dihomo-γ-linolenic acid in serum phospholipids by approximately 50% (Laidlaw & Holub, Reference Laidlaw and Holub2003; Miles et al. Reference Miles, Banerjee and Calder2004). However, the lack of a decrease in plasma arachidonic and docosapentaenoic n-6 acids in cows supplemented with FO suggested that the relationship between preformed n-3 long-chain polyunsaturated FA in the diet and the proportions of each n-6 long-chain polyunsaturated FA in plasma was complex and not all n-6 long-chain polyunsaturated FA responded in a similar manner.
The antimicrobial assay provides an index of the blood's ability to protect against potential pathogens. Differences were evident in the antimicrobial capacity of blood against the various pathogens; however, fish oil did not affect the ability of cows or their calves to control the growth and kill each microorganism. In contrast to the moderate dose of FO used in the present study, a high dose of FO, 18% of the diet, impaired the resistance of mice to the intracellular bacterium Listeria monocytogenes (Fritsche et al. Reference Fritsche, Irons, Pompos, Janes, Zheng and Brown2005).
Synthesis of the cytokine IFN-γ is imperative for cell-mediated immunity. Production of IFN-γ was unaffected by day (Table 6) whereas others found an inhibition around parturition (Shafer-Weaver & Sordillo, Reference Shafer-Weaver and Sordillo1997; Lessard et al. Reference Lessard, Gagnon, Godson and Petit2004). Furthermore, supplemental fish oil had no influence on the production of IFN-γ (Table 5). A predominant number of studies in animal models other than ruminants revealed that supplemental FO suppressed cell-mediated immunity as measured by lymphocyte proliferation (Calder et al. Reference Calder, Yaqoob, Thies, Wallace and Miles2002; Anderson & Fritsche, Reference Anderson and Fritsche2004). Shapiro et al. (Reference Shapiro, Wu and Meydani1993) reported that prostaglandin (PG) E3 was equally or more potent as PGE2 at suppressing lymphocyte proliferation to mitogens in vitro. Furthermore, the addition of polyunsaturated FA to a Jurkat T cell line displaced cytoplasmic signalling proteins from the lipid rafts and subsequently reduced signal transduction (Stulnig et al. Reference Stulnig, Berger, Sigmund, Raederstorff, Stockinger and Waldhäusl1998). These data provide strong evidence for an inhibitory effect of EPA and DHA on cell-mediated immunity; however, Calder et al. (Reference Calder, Yaqoob, Thies, Wallace and Miles2002) reported that only high levels of FO suppressed lymphocyte responses. The present results were consistent in that moderate incorporation of EPA and DHA had no influence on mitogen-stimulated PBMC IFN-γ production (Yaqoob et al. Reference Yaqoob, Pala, Cortina-Borja, Newsholme and Calder2000).
Table 5. Effects of supplemental fish oil during the peri-partum period on the ex-vivo production of interferon-γ by peripheral blood mononuclear cells when stimulated with phytohaemagglutin-P. Values are Least Squares Means±sem for n=12Footnote †,Footnote ‡

† Assay run on days −21, +1, and +21 relative to parturition. Day −21 was used as a covariate
‡ No significant interactions between the main effects were evident
§ Concentration of phytohaemagglutin-P in cell culture media
Infusing 100 μg of purified LPS into one mammary quarter activated an APR as evident by changes in both local and systemic signs of inflammation. Supplementing cows with FO during the peri-partum period had no significant effect on any physiological, clinical or production parameter during the APR (Table 6).
Table 6. Effects of supplemental fish oil on the acute phase response following an intramammary lipopolysaccharide challenge. Values are LSM±sem for n=12

† Statistics from analyses of change over baseline
‡ Linear somatic cell count=Log 2 (Somatic cell counts/100 000)
Limiting the pathogenesis of the APR during coliform mastitis was the objective of many studies (Burvenich & Peeters, Reference Burvenich and Peeters1982; Anderson et al. Reference Anderson, Kindahl, Smith, Davis and Gustafsson1986; Vangroenweghe et al. Reference Vangroenweghe, Duchateau, Boutet, Lekeux, Rainard, Paape and Burvenich2005). In those studies, pharmacological doses of non-steroidal anti-inflammatory drugs were given either before the challenge or at the onset of clinical signs of disease. Non-steroidal anti-inflammatory drugs had positive effects on limiting the clinical pathogenesis, but had limited or no effects on production performance. Similarly to non-steroidal anti-inflammatory drugs, supplementing FO altered the synthesis of eicosanoids in man (Goldman et al. Reference Goldman, Pickett and Goetzl1983) and ruminants (Baguma-Nibasheka et al. Reference Baguma-Nibasheka, Thomas Brenna and Nathanielsz1999; Mattos et al. Reference Mattos, Staples, Arteche, Wiltbank, Diaz, Jenkins and Thatcher2004). A high daily dose of intravenous emulsions of EPA and DHA (0·3 g EPA+DHA/kg BW) given to sheep completely abrogated the parturition-induced production of PGE2 compared with intravenous emulsions of soybean oil (Baguma-Nibasheka et al. Reference Baguma-Nibasheka, Thomas Brenna and Nathanielsz1999). More recently, supplementing a high daily dose of FO (~50–60 g absorbable EPA+DHA, assuming 65% ruminal escape from biohydrogenation and an intestinal absorption of 70%) moderately attenuated the post-partum elevation in plasma PGF-metabolite (Mattos et al. Reference Mattos, Staples, Arteche, Wiltbank, Diaz, Jenkins and Thatcher2004). The daily dose of absorbable EPA+DHA when expressed in terms of BW, assuming a 700-kg cow, was approximately 0·079 g EPA+DHA/kg BW. Although effective in reducing PG production, supplementing unprotected FO at that concentration had adverse effects on DMI. In the present study, assuming similar efficiencies of biohydrogenation and intestinal absorption as Mattos et al. (Reference Mattos, Staples, Arteche, Wiltbank, Diaz, Jenkins and Thatcher2004) the dose of absorbable EPA+DHA was approximately 0·042 and 0·033 g/kg BW daily pre-partum and post partum respectively, which was approximately half the dose administered in the study by Mattos et al. (Reference Mattos, Staples, Arteche, Wiltbank, Diaz, Jenkins and Thatcher2004). The productions of PG were not determined in the present study, but inferences based on the finding that there was no effect of supplemental FO on the clinical response to LPS suggests that the dose of FO either did not significantly alter the synthesis of PG or was not sufficient to elicit a biological response.
Altered eicosanoid metabolism is not the only mechanism by which EPA and DHA attenuate the pathogenesis of the APR. Supplemental FO reduced neutrophil chemotaxis to chemotactic ligands (Lee et al. Reference Lee, Hoover, Williams, Sperling, Ravalese, Spur, Robinson, Corey, Lewis and Austen1985; Schmidt et al. Reference Schmidt, Pedersen, Varming, Ernst, Jersild, Grunnet and Dyerberg1991). Dietary supplementation of healthy human subjects with 3·2 g EPA+2·2 g DHA daily for 6 weeks decreased maximal chemotactic response to leucotriene B4 by 70% (Lee et al. Reference Lee, Hoover, Williams, Sperling, Ravalese, Spur, Robinson, Corey, Lewis and Austen1985). Further supporting the inhibitory effect of FO was that 6 weeks after stopping the EPA+DHA supplementation the chemotactic response returned to baseline values. Schmidt et al. (Reference Schmidt, Pedersen, Varming, Ernst, Jersild, Grunnet and Dyerberg1991) revealed chemotaxis was reduced in a dose-dependent fashion, but the most dramatic decrease was at the low concentration, 1·3 g/d supplemental EPA+DHA. When expressed in terms of BW, the EPA+DHA daily dose in man was only 0·019 g/kg BW, which is approximately half of the estimated absorbed dose in the current study. The lack of an effect on somatic cell counts in infused quarters and leucopenia in peripheral blood indicated no effect of FO on chemotaxis or margination of neutrophils into mammary gland tissue.
Based on data from man and rodents, the estimated dose of absorbable EPA and DHA in the present study should have been sufficient to attenuate aspects of the APR in these dairy cattle (Schmidt et al. Reference Schmidt, Pedersen, Varming, Ernst, Jersild, Grunnet and Dyerberg1991; Michaeli et al. Reference Michaeli, Berger, Revelly, Tappy and Chioléro2007). The exact reason for the discrepancy among species is unknown and biologically intriguing. Ruminants might differ from man and rodents in terms of the sensitivity of lipid-mediated effects on biological processes because they evolved under lower quantities of absorbable polyunsaturated FA owing to biohydrogenation in the rumen. Supplementing a large enough dose of FO to alter eicosanoid production (Baguma-Nibasheka et al. Reference Baguma-Nibasheka, Thomas Brenna and Nathanielsz1999; Mattos et al. Reference Mattos, Staples, Arteche, Wiltbank, Diaz, Jenkins and Thatcher2004) and possibly the APR in ruminants would probably have detrimental effects on DMI (Mattos et al. Reference Mattos, Staples, Arteche, Wiltbank, Diaz, Jenkins and Thatcher2004). The dose of FO in the present study was chosen because it was expected not to adversely affect DMI. Future research on the effects of EPA and DHA on immune competence should use protected lipid sources to achieve absorbable doses near or above those achieved by Mattos et al. (Reference Mattos, Staples, Arteche, Wiltbank, Diaz, Jenkins and Thatcher2004). Supplementing FO during the peri-partum period at the doses used in the current study did not protect early lactating cows from the deleterious effects of an excessive APR.
The authors thank M Yelle, D Kim, J Heguy, S Amato and M Simmons for their help in data collection. SJ Taylor provided laboratory support. In addition, the authors appreciate the constructive comments of JE Santos and KC Klasing during manuscript preparation. Finally we acknowledge the product contributions of Silliker Laboratories (Modesto CA), Omega Proteins Inc. (Houston TX), Milk Specialties Co. (Dundee IL), and Pfizer Animal Health (St. Louis MO). The cost of fatty acid analyses was supported through the Dairy Milk Components Laboratory at UC Davis.








