Clostridium difficile is a Gram (+) spore forming bacteria, inhabiting the intestinal tract of several animal species, e.g. pigs, calves, dogs, horses, cats and mice (Arroyo et al. Reference Arroyo, Kruth, Willey, Staempfli, Low and Weese2005; Songer & Anderson, Reference Songer and Anderson2006; Avberse et al. Reference Avberse, Janezic, Patee, Rupnik, Zidaric, Logar, Vengust, Zemljic, Pirs and Ocepek2009). In piglets, C. difficile has been associated with neonatal enteritis (Hopman et al. Reference Hopman, Keessen, Harmanus, Sanders, van Leengoed, Kuijper and Lipman2011). In humans, C. difficile is an important cause of nosocomial diarrhoea, mainly antibiotic associated diarrhoea.
The microorganism is carried asymptomatically in about 50% of neonates, 20% of hospitalised patients and 2% of healthy adults (Matsuki et al. Reference Matsuki, OzakI, Shozu, Inoue, Shimizu, Yamaguchi, Karasawa, Yamagishi and Nakamura2005; Gursoy et al. Reference Gursoy, Guven, Arikan, Yurci, Torun, Baskol, Ozbakir and Yucesoy2007). In hospitalised individuals, proton pump inhibitors or antibiotics such as clindamycin, cephalosporins, fluoroquinolones and ampicillin can cause imbalance of the normal intestinal microbiota thus leading to overgrowth of intestinal C. difficile, or colonisation by environmental microorganisms that are normally present in health care centres (Schroeder, Reference Schroeder2005; Sunenshine & McDonald, Reference Sunenshine and McDonald2006). C. difficile is responsible for 90–100% of cases of pseudomembranous colitis (PMC), 60–75% of antibiotic-associated colitis and 30–60% of antibiotic-associated diarrhoea (AAD) (Limaye et al. Reference Limaye, Turgeon, Cookson and Fritsche2000).
The main virulence factors of this microorganism are two large protein toxins: TcdA (308 kDa) and TcdB (260 kDa). These toxins act as glycosyltransferases on small GTPases that are involved in actin polymerisation and cytoskeleton assembly (Jank et al. Reference Jank, Giesemann and Aktories2007). In the hamster model, TcdB but not TcdA, is an essential virulence factor in C. difficile infection (Lyras et al. Reference Lyras, O'Connor, Howarth, Sambol, Carter, Phumoonna, Poon, Adams, Vedantam, Johnson, Gerding and Rood2009). Some C. difficile strains produce a third toxin named binary toxin (CDT) (Popoff et al. Reference Popoff, Rubin, Gill and Boquet1988) an AB type toxin constituted by two components: CdtA (48 kDa) and CdtB (75 kDa). CdtB (receptor-binding component) form a heptamer onto cellular surface, allowing for the internalisation and enzymatic activity (ADP-ribosyl transferase) of CdtA (Barth et al. Reference Barth, Aktories, Popoff and Stiles2004). These events trigger cytoskeleton disorganisation, cell death and nutrient release into the extracellular milieu (Schwan et al. Reference Schwan, Stecher, Tzivelekidis, van Ham, Rohde, Hardt, Wehland and Aktories2009). Although relevance of CDT during pathogenic process of C. difficile infection has not been so far confirmed, this factor seems to be associated with higher severity in the infections (McDonald et al. Reference McDonald, Killgore, Thompson, Owens, Kazakova, Sambol, Johnson and Gerding2005).
Recommended therapy for C. difficile-associated diarrhoeas involves the use of antibiotics such as metronidazole for mild-moderate illness and high doses of vancomycin for severe illness (Cohen et al. Reference Cohen, Gerding, Johnson, Kelly, Loo, McDonald, Pepin and Wilcox2010). Several studies suggest that probiotics could constitute an alternative approach for the prophylaxis and/or treatment of C. difficile associated diarrhoea (CDAD). In this context, there are reports showing the correction of microbiota imbalances by administration of probiotics (Wullt et al. Reference Wullt, Hagslatt and Odenholt2003; Plummer et al. Reference Plummer, Weaver, Harris, Dee and Hunter2004; Segarra-Newnham, Reference Segarra-Newnham2007) or prebiotics (Lewis et al. Reference Lewis, Burmeister and Brazier2005).
We have demonstrated that growth and adhesion of the pathogen onto Caco-2 cells are significantly reduced by extracellular factors present in spent culture supernatants of bifidobacteria (Trejo et al. Reference Trejo, Minnaard, Pérez and De Antoni2006). In addition, co-culture of C. difficile with selected strains of bifidobacteria leads to a dramatic reduction of the biological activity of supernatants due to clostridial toxins (Trejo et al. Reference Trejo, Pérez and De Antoni2010). These findings are very interesting because bifidobacteria colonise the same intestinal region as C. difficile and they are included in the formulation of many dairy products thus allowing for the prevention/treatment of the infection by nutritional intervention.
Taking into account above-mentioned results we assessed the effect of administration of bifidobacteria on the course of an experimental infection with C. difficile in a hamster model.
Materials and methods
Preparation of bacterial suspensions
C. difficile strain 117 is a clinical isolate obtained from the Servicio de Bacteriología, Hospital Muñiz, Buenos Aires, Argentina. This strain was characterised in our laboratory as belonging to TcdA+/TcdB+ toxinotype (Trejo et al. Reference Trejo, Pérez and De Antoni2010). Clostridia were inoculated in BHI (Biokar, Diagnostics-Zac de Ther) supplemented with 0·05% w/v cysteine (Laboratorios ANEDRA, Argentina; BHI-Cys) and incubated anaerobically (AnaeroPak, Mitshubishi Gas Chemical Co, Inc) at 37 °C for 20 h. Fifty ml of bacterial culture were centrifuged at 12 000 g for 10 min. Bacteria were washed twice with sterile phosphate saline buffer (PBS: 0·144 g KH2PO4/l, 9 g NaCl/l, 0·795 g Na2HPO4/l, pH 7,5) and suspended in 4 ml sterile PBS. Bacterial concentration was evaluated in a haemocytometer and adjusted to 109 bacteria/ml in PBS.
Six bifidobacterial strains (Table 1) were selected according to their ability to antagonise growth, adhesion to enterocytes in culture and biological activity of spent culture supernatants of C. difficile (Trejo et al. Reference Trejo, Minnaard, Pérez and De Antoni2006; Trejo et al. Reference Trejo, Pérez and De Antoni2010). Bifidobacterium strains were grown in MRS broth (DIFCO, Becton Dickinson and Company Sparks, MD 21252, USA) supplemented with 0·05% w/v cysteine (MRS-cys) at 37 °C for 24 h in anaerobic conditions (AnaeroPak, Mitshubishi Gas Chemical Co, Inc). Bacterial suspensions were obtained from 1 l of Bifidobacterium culture. Afterwards, cultures were centrifuged at 12 000 g for 10 min, washed twice with sterile PBS and bacteria were suspended in sterile PBS and stored at −80 °C until use. Counts were performed by plating serial dilutions of the cultures on MRS-cys/agar 1·5% (w/v). Plates were incubated at 37° C for 72 h in anaerobic conditions (AnaeroPak, Mitshubishi Gas Chemical Co, Inc).
Table 1. Bifidobacterium sp. strains

Strain CIDCA 531 was isolated from a fermented milk product. Remaining Bifidobacterium strains were isolated from infant faeces (age between 6 d and 4 months), breastfed (Gomez Zavaglia et al. Reference Gomez Zavaglia, Kociubinski, Pérez and De Antoni1998; Pérez et al. Reference Pérez, Minnaard, Disalvo and De Antoni1998)
Infection protocol and probiotic administration
Specific pathogen free female Golden Syrian hamsters of 45–60 days old (100–150 g) were used (Instituto de Biología y Medicina Experimental, CONICET, Argentina). Animals were housed in polypropylene cages covered with polyester filters (4 or 5 animals per cage). Food, water, bedding, cages, wire lids and filter covers were sterilised (15 min, 121 °C) before use. Food and water were administered ad libitum throughout.
The time line and general schedule of the experiments are depicted in Fig. 1. Animals were allocated to 3 experimental groups:
(a) IC (infected controls)
(b) IPT (infected probiotic treated)
(c) UC (uninfected controls)
Animals of the IPT group (Infected Probiotic-Treated) were administered daily with suspensions of bifidobacteria in drinking water at concentration of 2×108 CFU/ml. Probiotic administration started at day 0 and was continued until the end of the experiment. Fresh bacteria suspensions were given daily in order to administer high doses of viable microorganisms (around 1×109 CFU per animal per day). Infected controls (IC) and uninfected controls (UC) received drinking water throughout. The UC group was included in order to assess the effectiveness of the infection confinement measures.

Fig. 1. Time line of the study. Administration of bifidobacterial suspensions (1×109 per animal per day) begins at day 0 and continues until the end of the experiment. Clindamycin was administrated at a single dose (100 μl of a 30 mg/ml solution in PBS per animal).
On day 7 animals belonging to all experimental groups were intragastrically (i.g.) administered 100 μl clindamycin (Parafarm, Drogueria Saporiti, Argentina) solution (30 mg/ml in PBS) at dose 30 mg/kg/animal. In hamsters, clindamycin treatment results in microbiota imbalance predisposing animals to C. difficile infection (Chang et al. Reference Chang, Bartlett, Gorbach and Onderdonk1978). Four days later (day 11) animals of the IC and IPT groups received intragastrically C. difficile strain 117 (5×108 bacteria/animal). Uninfected controls (UC) received PBS instead of C. difficile. At day 4 post-infection (day 15), surviving animals were euthanatized by CO2 inhalation and cervical dislocation.
Two independent experiments were conducted: (1) Strain selection and kinetics: Six bifidobacterial strains. Groups IC (n=5); IPT (n=5) and UC (n=4) and (2) Strain CIDCA 5310: Groups IC and IPT (n=8 each).
All the procedures were performed according to international and local regulations related to animal welfare.
Infection markers
Animals were observed daily and mortality, morbidity and presence of diarrhoea were recorded. Criteria used to evaluated moribund animals were auto isolation, lethargy, skin erosions and stooped posture. Animals judged to be in a moribund state were euthanatized as described above.
Biological activity of caecal content
After sacrifice, 1 g caecal content was collected and homogenised with 1 ml PBS. The suspension was centrifuged at 12 000 g for 10 min and supernatants were filter sterilised (0·45 μm). Filtrates were stored at −80 °C until use.
Biological activity was assessed as previously described (Trejo et al. Reference Trejo, Pérez and De Antoni2010). Briefly, Vero cells, grown in 48 well plates for 48 h, were treated for 16 h (37 °C, 5% CO2 – 95% air atmosphere) with 2 fold serial dilutions of filtrates in DMEM. Detached cells were removed by washing with PBS. Remaining cells were fixed with 2% (v/v) formaldehyde and stained with crystal violet solution (0·13% w/v crystal violet; 5% v/v ethanol; 2% v/v formaldehyde in PBS).
Next, an extraction with 50% (v/v) ethanol was performed and OD540 was determined. Biological activity was expressed as the ratio of detached cells (rd), according to the following expression:
where:
ODs: optical density of sample.
OD0: optical density of well without cells (control of stain adsorption by the well).
ODc: optical density of untreated control cells
By using this equation, filtrate concentration leading to 50% of cell detachment was calculated (DD50). This value inversely correlates with the biological activity of the filtrates. To confirm that biological activity was associated with TcdB the assay was repeated in the presence of monoclonal antibody anti-TcdB (10 μl per 100 μl filtrate, Meridian Life Science, Inc., CA, USA). Biological activity was abrogated in the presence of anti-TcdB antibody.
Histological studies
Samples of caeca were removed, fixed by using 5% (v/v) paraformaldehyde and embedded in paraffin. Sections (5 μm) were hydrated and stained with haematoxylin/eosin.
Statistical analysis
Results were analysed by log-rank (Mantel-Cox) survival analysis, non-parametric test (Mann-Whitney) or Fisher's exact test (two-tailed) using GraphPad Prism version 5.00 for Windows, (GraphPad Software, San Diego California USA).
Results
Effect of C. difficile infection
Hamsters, administered with clindamycin and subsequently challenged with C. difficile strain 117 showed evident signs of infection at day 15 (4 d post infection). Indeed inflammation of caecum and colon, increased intestinal gas, viscous yellowish caecal content and tissue fragility were observed. These signs are evident in Fig. 2b that clearly contrasts with Fig. 2 A corresponding to an uninfected control. Above-mentioned findings were considered as markers of colitis. Diarrhoea was evidenced by wet tail and presence of faecal halo in the perianal region.

Fig. 2. Effect of C. difficile infection in hamster. (a) animal administered with B. bifidum CIDCA 5310 (2×108 CFU/animal) and infected with C. difficile (5×108 CFU/animal). (b) Infected control.
Probiotic strain selection
As shown in Table 2, infection of hamsters belonging to the IC group (infected controls) with strain 117 of C. difficile lead to diarrhoea in 4/5 animals, enterocolitis in 5/5 animals and 2/5 animals died before the final time point (4 d after infection). Interestingly, preventive administration of strain CIDCA 5310 significantly (P=0·02) reduced the ratio of animals with enterocolitis (1/5) as compared with the IC group group (5/5). An independent experiment conducted with strain CIDCA 5310 (not shown in Table 2) showed ratios of enterocolitis of 2/8 and 7/8 for the treatment and placebo group respectively. This represent a significant difference between the two groups (P=0·02).
Table 2. Ratios of animals with diarrhoea, enterocolitis or dead. Animals received clindamycin (30 mg/kg) at day 7 and they were infected with 5×108 CFU/animal C. difficile strain 117 at day 11. Results were recorded 4 d after infection. Control animals received antibiotic treatment but they were no infected nor probiotic-treated
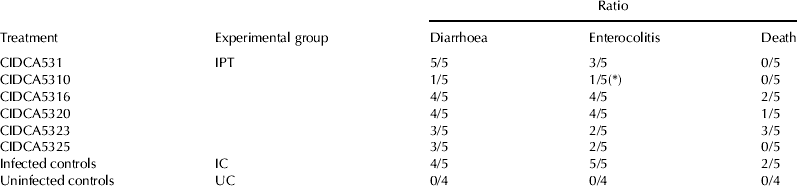
(*) Significantly difference from placebo group (Fisher's exact test, P<0·05)
Other strains under study, did not lead to a significant protective effect although strains CIDCA 5323 and CIDCA 5325 showed 2 out of 5 animals with enterocolitis thus leading to a trend (P=0·08) of protective effect. In the uninfected control group, neither enterocolitis or diarrhoea were observed and no deaths occurred. This indicates appropriate confinement of infection.
Effect of Bifido bifidum CIDCA 5310 on the kinetics of C. difficile associated diarrhoea
Kinetics of development of enterocolitis was significantly different when placebo and probiotic-treated groups were compared. As shown in Fig. 3, no mortality and only 1 hamster showing signs of diarrhoea were found in the IPT group at day 4 post-infection. In contrast, in the IC group diarrhoea and death were evident at days 1 and 2 post-infection respectively. At the end of experimentation period, 2/5 animals died and 5/5 showed signs of diarrhoea in IC group.

Fig. 3. Enterocolitis and death in hamsters infected with C. difficile strain 117 (5×108 CFU/animal). Infected Probiotic-Treated (IPT) animals were administered with 2×108 CFU/ml of B. bifidum CIDCA 5310 in drinking water starting at day 0 until end of experiment.
Histology
As shown in Fig. 4, caeca of animals belonging to the IC group show evidence of cellular infiltration with enlarged sub-mucosal region (Fig. 4 B1), typical volcanic eruption lesion (Fig. 4 B2) and oedema (Fig. 4 B3). In contrast, normal appearance was demonstrated in histological sections of CIDCA 5310-treated infected animals (Fig. 4 C1, C2 and C3). Histological characteristics of this group were similar to those of uninfected controls (Fig. 4 A1, A2 and A3). Intestinal epithelium breakdown produced during C. difficile pathology allows passage of polimorfonuclears cells from submucose though luminal area (Fig. 4b). Viscous aspect of intestinal content, shown in Fig. 2b, normally is associated with fibrin effusion that gives rise to the characteristic volcanic eruption lesion found in histological analysis (Waters et al. Reference Waters, Orr, Clark and Schaufele1998).

Fig. 4. Histology of caecum stained with Haematoxylin-Eosin. (A1, A2, A3): uninfected control (UC). (B1, B2, B3) infected control (IC). (C1, C2, C3) B. bifidum CIDCA 5310-treated infected animal (IPT) e: oedema, sm: sub-mucosa, lp: lamina propria, i: cellular infiltrate, L: lumen, ve: volcanic eruption.
Biological activity of caecal content
As depicted in Fig. 5, biological activity of filtrates of caecal content revealed significant differences between groups. Indeed, samples from the placebo group had lower DD50 (higher biological activity) than those belonging to the 5310-treated group (P=0·016). Non-biological activity associated to TcdB was evidenced in non-infected control group (data not shown).
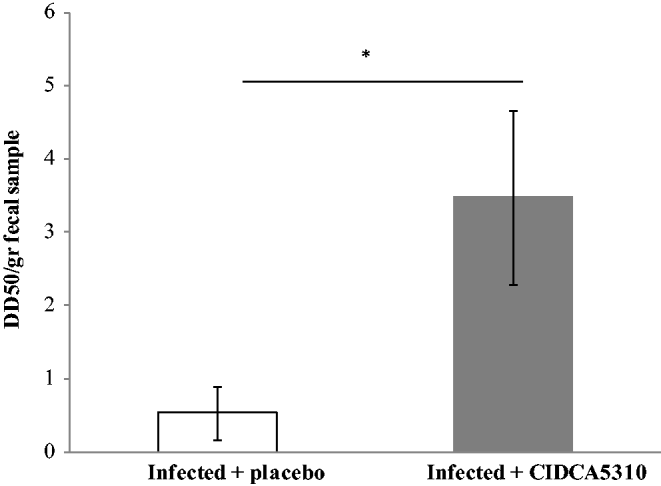
Fig. 5. Biological activity of faecal filtrates from hamsters belonging to the IPT or IC groups. Animals of the IPT group were administered with strain CIDCA 5310. Results are expressed as mean of DD50/gr of faecal sample ±sd. *Significant difference by Mann–Whitney test (P=0.0159).
Discussion
Treatment of C. difficile associated diarrhoea (CDAD), generally performed with metronidazole or vancomycin has proved to have an effectiveness of 95% (Kelly et al. Reference Kelly, Pothoulakis and LaMont1994). However, there is evidence of increasing failure of this conventional therapy (Aslam et al. Reference Aslam, Hamill and Musher2005) as well as relapse ratios ranging from 20 to 50% (Musher et al. Reference Musher, Aslam, Logan, Nallacheru, Bhaila, Borchert and Hamill2005; Pepin et al. Reference Pepin, Alary, Valiquette, Raiche, Ruel, Fulop, Godin and Bourassa2005).
Since CDAD is associated with disruption of the intestinal microbiota, strategies encompassing administration of probiotic microorganisms constitute a promising approach. These nutritional interventions have been addressed in human trials that revealed the suitability of probiotics to improve the course of this pathology (reviewed in Gougoulias et al. Reference Gougoulias, Tuohy and Gibson2007).
C. difficile can lead to severe infectious diarrhoea in pigs (Songer & Anderson, Reference Songer and Anderson2006) but the most suitable animal model to mimics human infection is the Syrian Golden hamster (Mesocricetus auratus) (Chang et al. Reference Chang, Bartlett, Gorbach and Onderdonk1978). It has been reported that administration of nontoxigenic strains of C. difficile 3 d before challenge with toxigenic strains prevented colonisation of hamster (Sambol et al. Reference Sambol, Merrigan, Tang, Johnson and Gerding2002). This protective effect has been correlated with decrease in colonisation by clostridia.
Treatment with Saccharomyces boulardii reduces mortality of hamsters infected with C. difficile (Toothaker & Elmer, Reference Toothaker and Elmer1984). Even though in vitro studies have shown that Sac. boulardii is able to inactivate C. difficile toxins by proteolytic cleavage (Castagliuolo et al. 1996; Buts, Reference Buts2008) this effect has not been demonstrated in vivo.
In the present study, we show for the first time the protective effect of a selected strain of Bifido bifidum (CIDCA 5310) in a hamster model of CDAD. Continuous administration of strain CIDCA 5310 in drinking water starting 11 d before infection with C. difficile, leads to a significant amelioration of symptoms and increased survival ratio in the probiotic-treated group. Protective effect was observed at least for 4 d post infection.
Even though six bifidobacterial strains were tested, only strain CIDCA 5310 was able to antagonise the effect of C. difficile. Interestingly, other strains (Trejo et al. Reference Trejo, Minnaard, Pérez and De Antoni2006) showed higher in vitro inhibitory potential than strain CIDCA 5310 (e.g. CIDCA 5320 ad CIDCA 5323) and also higher capability to antagonise adhesion to cultured human enterocytes (e.g. strains CIDCA 5316, 5320, 5323 and 5325). Although none of these characteristics were found in strain CIDCA 5310, co-culture of this strain with toxinogenic C. difficile dramatically reduces biological activity of spent culture supernatants as compared with pure clostridial cultures (Trejo et al. Reference Trejo, Pérez and De Antoni2010). Furthermore, it has been demonstrated that culture of C. difficile in the presence of strain CIDCA 5310 leads to lower concentrations of TcdA and TcdB in the spent culture supernatants. These findings have been ascribed to either decrease of toxin release or synthesis diminution with no inhibition of C. difficile growth (Trejo et al. Reference Trejo, Pérez and De Antoni2010). Interestingly, in the present study we detected lower biological activity in faecal samples of probiotic-treated animals as compared with the control group.
In agreement with the above mentioned findings animals administered with strain CIDCA 5310 showed better general condition, lower ratio of enterocolitis and lower mortality compared with placebo-treated group.
It is worth noting that the protective effect of strain CIDCA 5310 was found even in a model that includes antibiotic administration. Indeed, in the present study hamsters were administered with clindamycin at day 7 to facilitate infection by C. difficile. It has been demonstrated (Larson & Borriello, Reference Larson and Borriello1990) that this antibiotic remains in the caecal content at potentially inhibitory concentrations (4–6 μg/g) for up to 11 d. These concentrations are higher than minimal inhibitory concentrations (MIC) reported by Xiao et al. (Reference Xiao, Takahashi, Odamaki, Yaeshima and Iwatsuki2010) but daily administration of bifidobacteria from day 0 until the end of the study provides daily intake of living microorganisms.
The effect of strain CIDCA 5310 could be related to several factors that involve balance of the intestinal microbiota and immunomodulation. However, the ability of strain CIDCA 5310 to decrease in vivo, the biological activity of faecal contents seems to be a finding of particular relevance given the main role of secreted TcdA and TcdB in the course of the pathology. Noteworthy, the low proteolytic activity of bifidobacteria precludes degradation of toxins in the gut as a mechanism for explaining the protective effect.
Results reported in the present study emphasise the importance of strain selection for improving the likelihood of successful interventions. Even though the mechanisms have not been elucidated, results presented here encourage further research on the use of bifidobacteria-containing products in the prophylaxis/treatment of CDAD.
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC-PBA) and Universidad Nacional de La Plata (UNLP). Authors are indebted to R. Rollet from the Servicio de Bacteriología of the Hospital Muñiz (Buenos Aires, Argentine) for kindly provide strain 117 of C. difficile.









