Ketosis is a major metabolic disorder of dairy cows in early lactation (Li et al. Reference Li, Ding, Wang, Liu, Huang, Zhang, Guo, Wang, Li, Liu, Wu and Li2016a; Xu et al. Reference Xu, Xu, Chen, Yang, Xia, Yu, Zhu, Shen and Zhang2016). In dairy cows, ketosis occurs primarily in the first 3 weeks following calving, at which time up to 15%−60% of all dairy cows exhibit hyperketonemia (White, Reference White2015; Xu et al. Reference Xu, Xu, Chen, Yang, Xia, Yu, Zhu, Shen and Zhang2015). Ketosis is divided into type I and type II in dairy cows. The most common form of ketosis is type I ketosis, exhibiting hypoglycemia and hyperketonemia (Gordon, Reference Gordon2013). However, type II ketosis in dairy cows is common during early lactation (Gordon, Reference Gordon2013), and is characterized by hyperketonemia, hyperinsulinemia, hyperglycemia and significant hepatic fat accumulation (Holtenius & Holtenius, Reference Holtenius and Holtenius1996; Gordon, Reference Gordon2013). Insulin is a major endocrine regulator of hepatic lipid homeostasis (Chirieac et al. Reference Chirieac, Chirieac, Corsetti, Cianci, Sparks and Sparks2000; Saltiel & Kahn, Reference Saltiel and Kahn2001) whose concentration is markedly increased in type II ketotic cows (Hippen et al. Reference Hippen, She, Young, Beitz, Lindberg, Richardson and Tucker1999).
Human clinical studies showed that AMP-activated protein kinase alpha (AMPKα) plays a key role in regulating hepatic lipid metabolism in response to endocrine signals, and may be an important therapeutic target for metabolic syndrome that is a cluster of metabolic disorders, including insulin resistance, obesity, and type 2 diabetes (Li et al. Reference Li, Chen, Guan, Li, Lei, Liu, Yin, Liu and Wang2013; Deng et al. Reference Deng, Liu, Liu, Zhang, Yin, Shi, Wang, Yuan, Sun, Li, Yang, Guo, Zhang, Wang, Li and Li2015). AMPKα modulates hepatic lipid metabolism by regulating several lipid metabolism-related transcription factors such as peroxisome proliferator-activated receptor α (PPARα), sterol regulatory element-binding protein 1c (SREBP-1c), and carbohydrate responsive element-binding protein (ChREBP), all of which govern the expression of lipid metabolic enzymes (Chen et al. Reference Chen, Zhang, Li, Li, Sun, Yuan, Liu, Liu, Yin and Deng2013).
Cows with type II ketosis displayed high blood levels of insulin and massive hepatic lipid accumulation (Gordon, Reference Gordon2013). Accordingly, we hypothesized that hepatic fat accumulation might be associated with hyperinsulinemia in type II ketosis, mediated via the AMPKα signaling pathway.
Material and methods
Reagents
Insulin and HEPES were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Fetal bovine serum (FBS), collagenase IV, HepatoZYME medium and RPMI-1640 medium were obtained from Gibco (Grand Island, NY, USA). Dexamethasone acetate, vitamin C, ascorbic acid, and other chemicals were purchased from Baoman Biotechnology (Shanghai, China). AICAR (an AMPKa activator) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against SREBP-1c, ChREBP, PPARα, and β-Actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho-insulin receptor β, insulin receptor, AMPKα, p-AMPKα, ACC, p-ACC and histone were provided by Cell Signaling Technology (Danvers, MA, USA).
Isolation and primary culture of dairy cow hepatocytes
All of the animals used in this study were treated according to the International Guiding Principles for Biomedical Research Involving Animals. Hepatocytes were isolated from the liver of new born dairy calves as described previously (Song et al. Reference Song, Li, Gu, Fu, Peng, Zhao, Zhang, Li, Wang, Li and Liu2016; Du et al. Reference Du, Shi, Peng, Zhao, Zhang, Wang, Li, Liu and Li2017). Hepatocytes were isolated after collagenase perfusion of the caudate process and seeded into 6-well plates. After 4 h, the cells were incubated in basal culture medium with 10% FBS. Hepatocytes were cultured in an incubator which can keep a humidified atmosphere at 37 °C and 5% CO2. Cultures were re-fed every 24 h.
Insulin treatment
Insulin was dissolved in distilled water and the final concentration was adjusted to 10 µm. The hepatocytes were incubated with 100 nm insulin for 0, 0·5, 1, 3, 6, and 12 h, respectively. For the dose-response experiments, the cells were incubated for 1 h with different concentrations of insulin (0, 1, 10, or 100 nm) and AICAR (1 mm) (Ducommun et al. Reference Ducommun, Ford, Bultot, Deak, Bertrand, Kemp, Steinberg and Sakamoto2014) with three replications in each treatment. The insulin treatment concentrations in this study were chosen on the basis of the blood concentration of insulin in cows as previously described (Trenkle, Reference Trenkle1972).
AMPKα enzyme activity determination
The methods of Li et al. Reference Li, Chen, Guan, Li, Lei, Liu, Yin, Liu and Wang2013 were used. The hepatocytes were washed twice with ice-cold PBS and kept in lysis buffer for 30 min. The lysate was harvested by centrifuging at 12 000 g (4 °C) for 5 min. The enzyme activity of AMPKα in supernatant was determined by biochemical kit (Shanghai Bluegene Biotech Co., Ltd.).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from hepatocytes by Trizol (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China) according to the manufacturer's instructions. First strand cDNA was obtained by using 5 µg of total RNA using PrimeScript Reverse Transcriptase in a two-step method (TaKaRa Biotechnology Co., Ltd., Tokyo, Japan). The primers were designed by Primer Expression software (PE Applied Biosystems Inc., Foster City, CA, USA) and presented in online Supplementary File Table S1. The mRNA expression levels were determined by qRT-PCR using SYBR Kit in the 7500 Real-Time PCR System (Applied Biosystems, USA).
Western blot
The hepatocytes were treated for the indicated time, then the media were aspirated and the cells were washed twice with ice-cold PBS. The intracellular proteins were extracted using extraction kit (Sangon Biotech Co., Ltd., Shanghai, China). The samples were heated with SDS protein buffer at 95° for 5 min, and centrifuged, the supernatant was electrophoresed on SDS-PAGE gel (10%), after running the gel was transferred to polyvinylidene fluoride membranes (PVDF). The blots were blocked for 4 h at room temperature in 3–5% bovine serum albumin (BSA), and then incubated overnight at 4° with primary antibodies against PPARα (1 : 500, antigoat), SREBP-1c (1 : 500, antirabbit), ChREBP (1 : 500, antirabbit),β-actin (1 : 500, antigoat), phospho-insulin receptor β (1 : 1000, antigoat), insulin receptor (1 : 1000, antirabbit), AMPKα (1 : 1000, antirabbit), p-AMPKα (1 : 1000, antirabbit), ACC (1 : 1000, antirabbit), p-ACC (1 : 1000, antirabbit) and histone (1 : 1000, antirabbit). After incubation for 45 min with horseradish peroxidase-conjugated secondary antibodies antirabbit (1 : 5000), antimouse (1 : 5000) and antigoat (1 : 5000) at room temperature, washing four times in TBST. The blots were visualized using enhanced chemiluminescence and imaged by alpha ultrasensitive Fluorchem chemiluminescence imaging system (Protein Simple, USA).
TG content determination
Hepatocytes were harvested by cell scraper and washed twice with ice-cold PBS. After centrifugation at 800 g for 5 min at 4°, the cells were lysed by the SL-1000D ultrasonic cell disruption apparatus (Shunliu Instrument Company, Nanjing, China). Then, the lysate was centrifuged at 12 000 g for 5 min. The supernatant was stored at −80° for determining the TG content using automatic biochemical analyzer (Dirui Medical Equipment Co., Ltd., Changchun, China).
Abbreviations
ACC: Acetyl Co-A carboxylase, FA: Fatty acid synthetase, SCD-1: Stearoyl Co-A desaturase 1, ApoB: Apolipoprotein B, MTP: Microsomal triglyceride transfer protein, ApoE: Apolipoprotein E, ACO: Acyl Co-A oxidase, CPT1: Carnitine Palmitoyltransferase 1.
Statistical analysis
Data are expressed as the mean ± standard deviation (sd) and analyzed using SPSS (Statistical Package for the Social Sciences) 16·0 software (SPSS Incorporated, Chicago, IL, USA). Differences among groups were compared with one-way ANOVA. Compared to control group, a P value below 0·05 was considered significant.
Results
Insulin decreases AMPKα phosphorylation in dairy cow hepatocytes
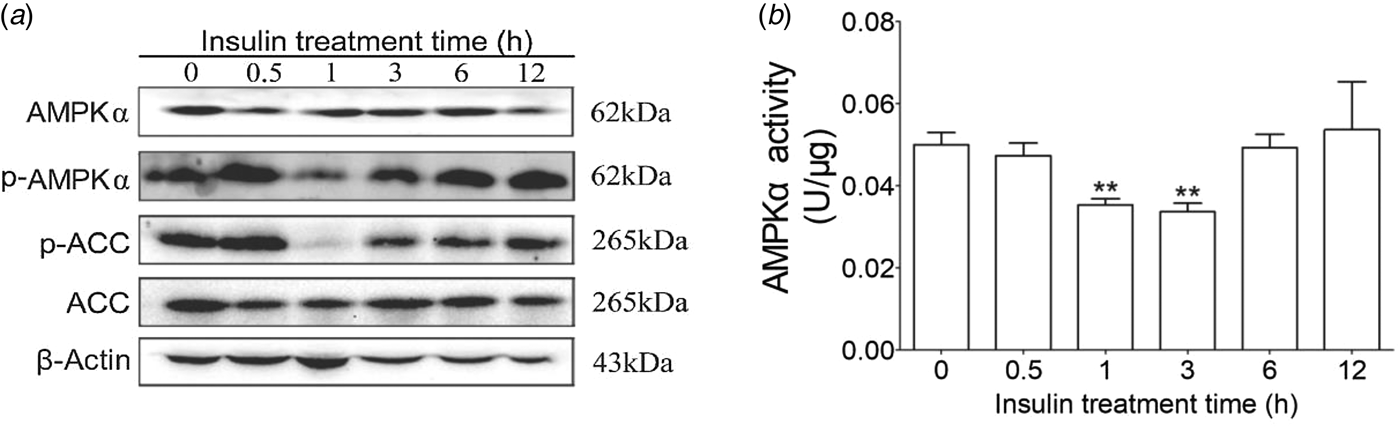
To determine the AMPKα signaling pathways induced by insulin in cow hepatocytes, the AMPKα phosphorylation levels and insulin receptor were detected. We observed that 100 nm insulin could significantly decrease AMPKα and its downstream molecule ACC phosphorylation at 1 h (Fig. 1). Therefore, the following studies were carried out based on 1 h of insulin treatment.
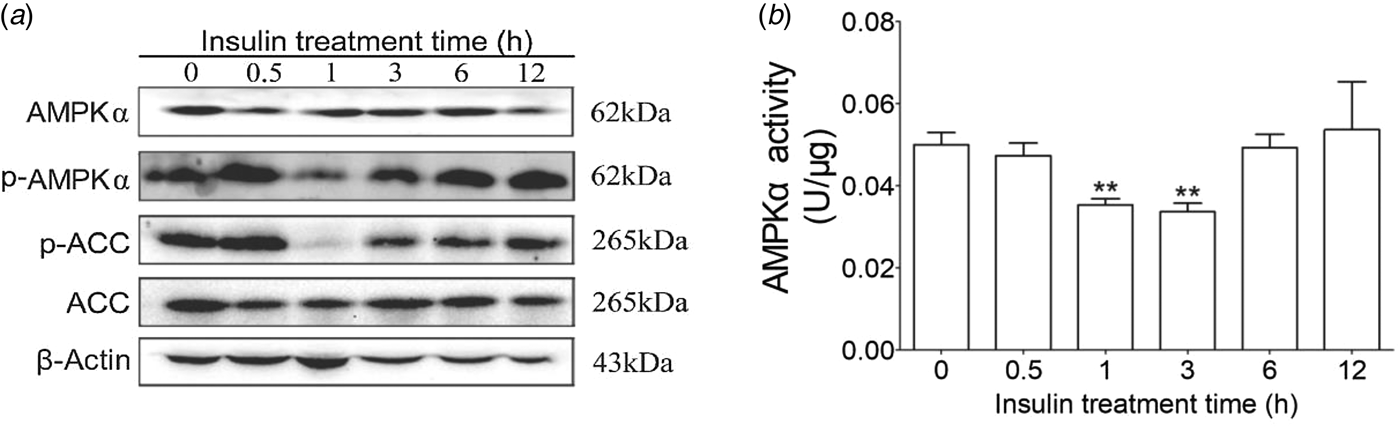
Fig. 1. Effects of insulin on AMPKα phosphorylation, activity and ACC phosphorylation. Hepatocytes were treated with 100 nm insulin for 0, 0·5, 1, 3, 6, and 12 h, respectively. Each treatment was replicated 3 times. (a) Western blotting results of p-AMPK, AMPK, p-ACC and ACC. (b) AMPKα enzyme activity. Actin was used as loading control. The data are presented as the mean ± sd. *P < 0·05, **P < 0·01 vs. the control group.
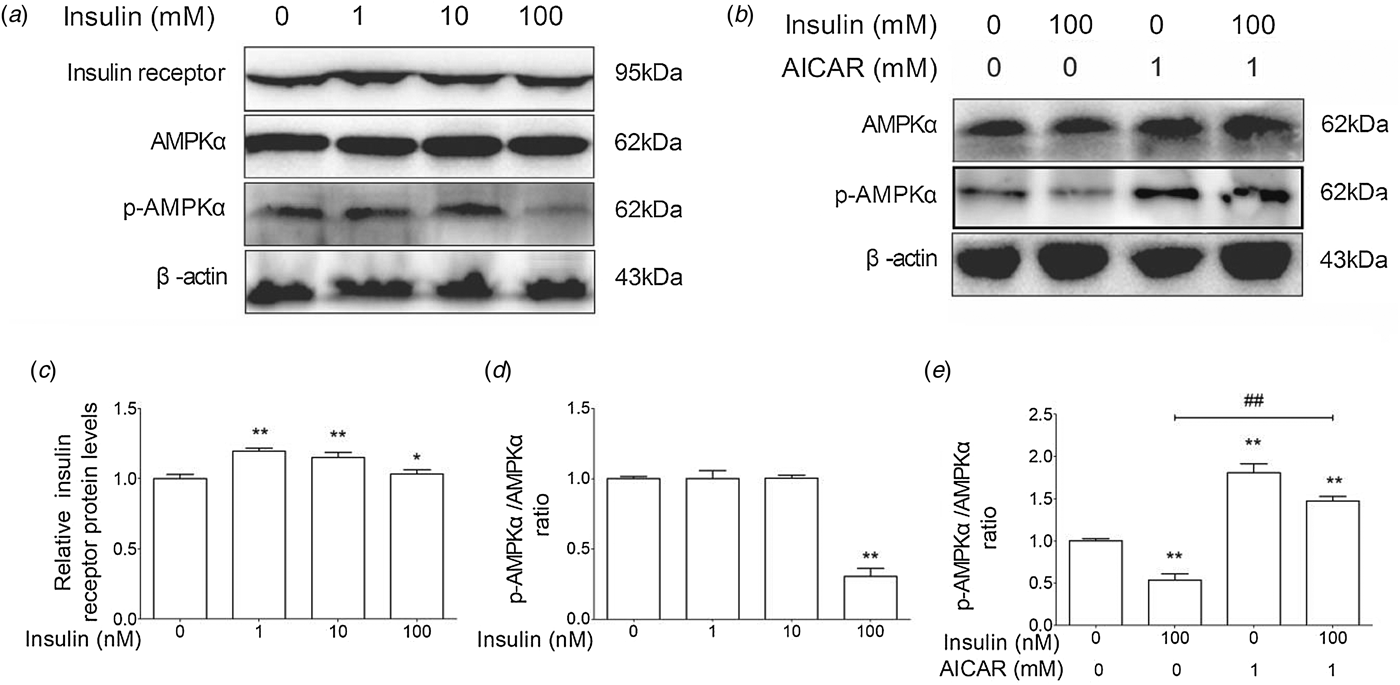
As shown in Fig. 2a, d, the AMPKα phosphorylation level was significantly lower in 100 nm insulin-treated group than in the control group. AICAR is an activator of AMPKα. AICAR treatment could markedly increase the phosphorylation of AMPKα and partially reverse the inhibitory effect of insulin on AMPKα in cow hepatocytes (Fig. 2b, e). Furthermore, insulin treatment could increase the protein levels of insulin receptor (Fig. 2c). These results suggest that insulin increases insulin receptor level and decreases the AMPKα phosphorylation level in cow hepatocytes.
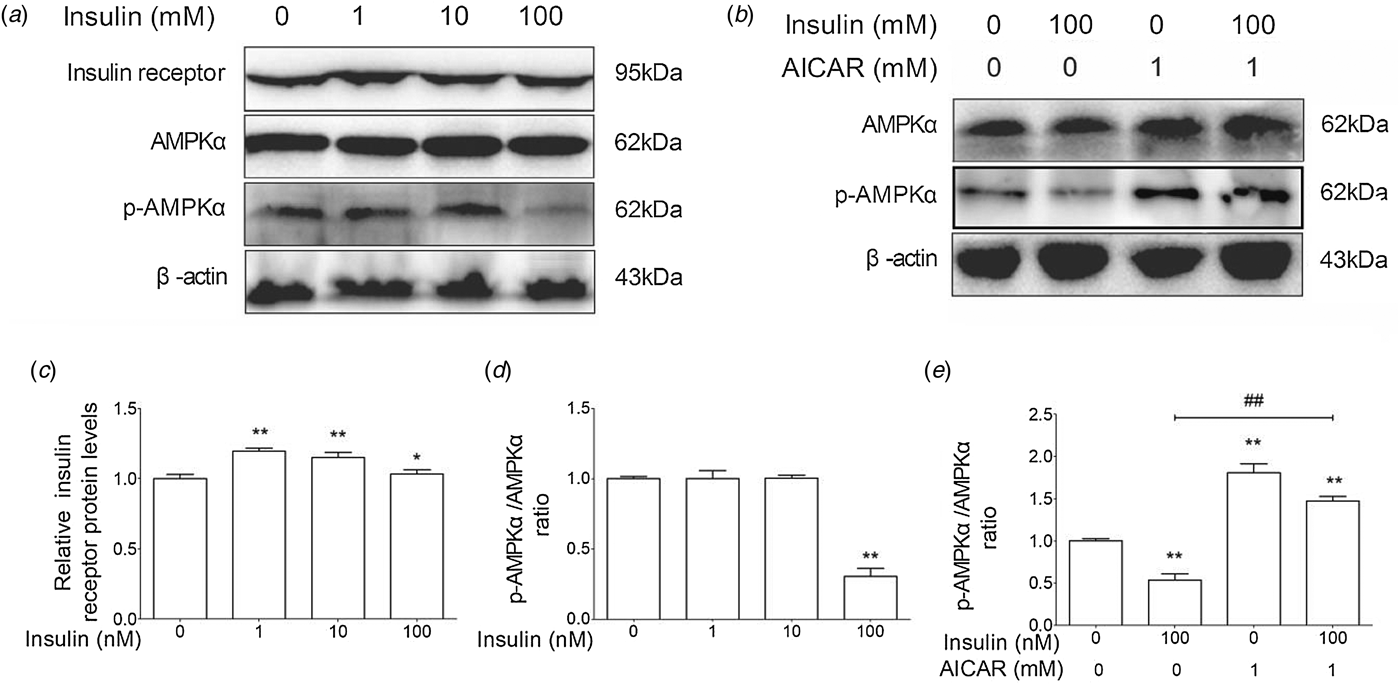
Fig. 2. Insulin suppressed the phosphorylation level of AMPKα in cow hepatocytes. Hepatocytes were treated with 0, 1, 10, or 100 nm insulin in the presence or absence of 1 mM AICAR, respectively. Each treatment was replicated 3 times. (a) The Western blot results of insulin receptor, p-insulin receptor, AMPKα, and p-AMPKα. (b) The Western blot results of AMPKα and phosphorylated AMPKα. β-Actin was used as control. (c) The protein level of insulin receptor. (d) and (e) The phosphorylation level of AMPKα. The data are presented as mean ± sd. *P < 0·05, **P < 0·01 vs. control group. #P < 0·05, ##P < 0·01 vs. 100 nm insulin-treatment group.
Effect of insulin on PPARα, SREBP-1c and ChREBP in dairy cow hepatocytes
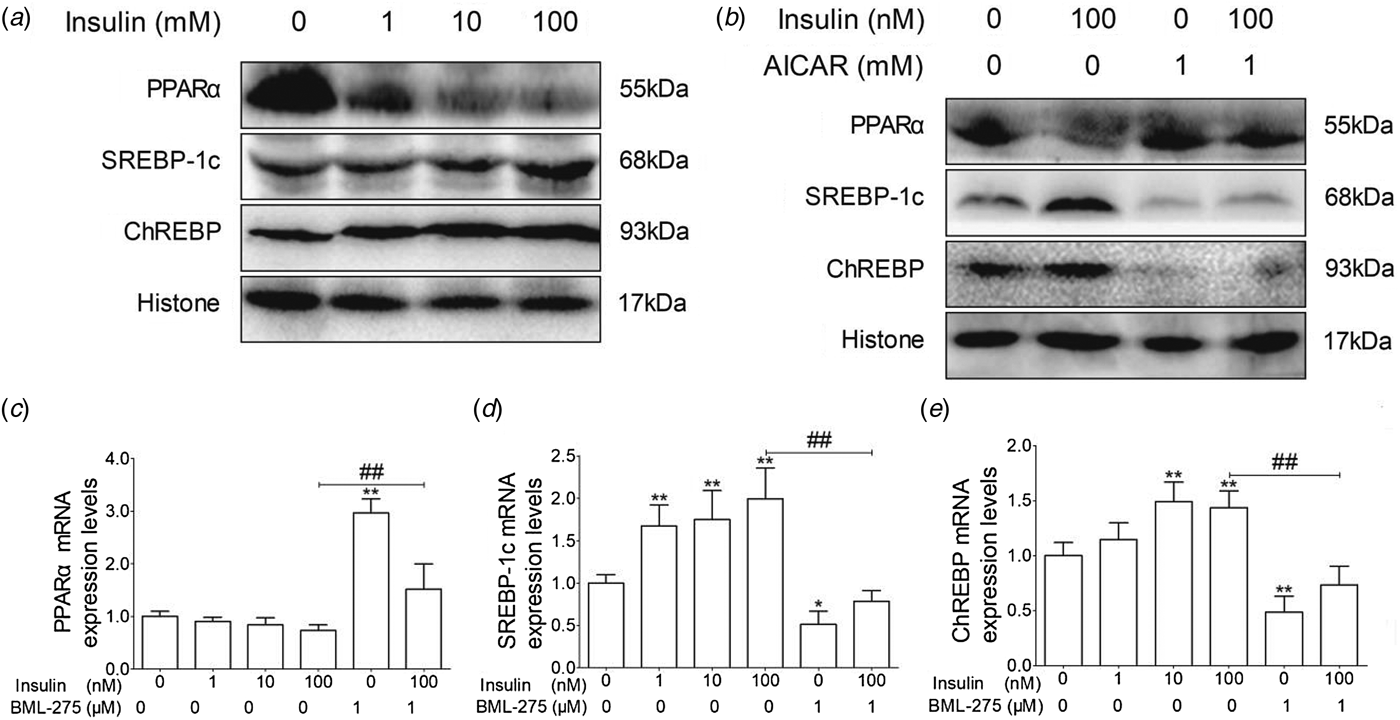
To evaluate whether insulin regulates lipid metabolism through the AMPKα signaling pathway, we examined the expression of PPARα, SREBP-1c, and ChREBP. The expression levels of PPARα protein and mRNA decreased in an insulin dose-dependent manner (Fig. 3a, c), while AICAR could partially reverse the inhibition of PPARα by insulin (Fig. 3b, c). Conversely, the expression levels of SREBP-1c and ChREBP were upregulated by insulin, but downregulated in AICAR and AICAR + insulin treatment groups (Fig. 3a, b, d, e). Taken together, these results indicate that insulin decreases the expression of PPARα and increases the expression of SREBP-1c and ChREBP in cow hepatocytes.

Fig. 3. The effect of insulin on the mRNA and protein expression levels of PPARα, SREBP-1c and ChREBP in cows hepatocytes. Hepatocytes were treated as described in Fig. 2. Each treatment was replicated 3 times. (a) and (b) The protein expression levels of PPARα, SREBP-1c and ChREBP. Histone was conducted as a protein loading control. (c) The mRNA expression level of PPARα; (d) The mRNA expression level of SREBP-1c; (e) The mRNA expression level of ChREBP. The data are presented as mean ± sd. *P < 0·05, **P < 0·01 vs. control group. #P < 0·05, ##P < 0·01 vs. 100 nm insulin-treatment group.
The effect of insulin on the expression of PPARα, SREBP-1c, and ChREBP target genes in cow hepatocytes
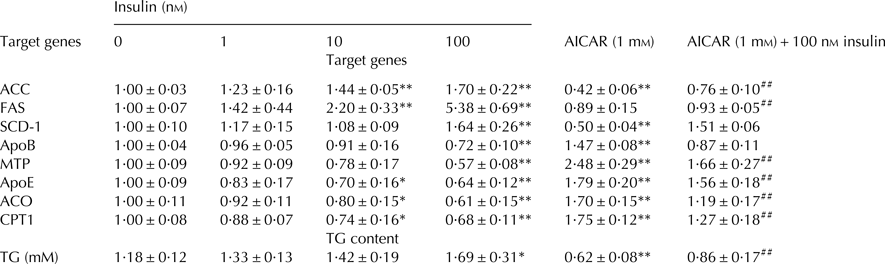
To further verify the role of insulin in hepatic lipid metabolism, we examined the downstream targets of PPARα, SREBP-1c, and ChREBP. ACC, FAS, and SCD-1 are target genes of SREBP-1c and ChREBP. The ACC, FAS and SCD-1 mRNA levels were significantly higher in 100 nm insulin-treated group than that in control group (Table 1). Conversely, the ACC and SCD-1 mRNA levels were significantly decreased when hepatocytes were treated with AMPK activator AICAR (Table 1).
Table 1. The effect of insulin on the mRNA expression levels of ACC, FAS, SCD-1, ApoB, MTP, ApoE, ACO, CPT1 and TG content in cow hepatocytes
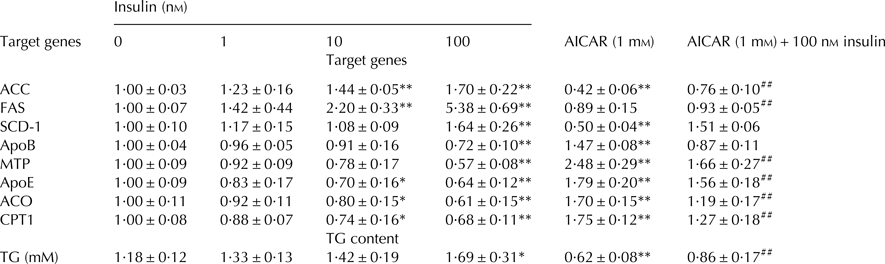
ACC, Acetyl Co-A carboxylase; FA, Fatty acid synthetase; SCD-1, Stearoyl Co-A desaturase 1; ApoB, Apolipoprotein B; MTP, Microsomal triglyceride transfer protein; ApoE, Apolipoprotein E; ACO, Acyl Co-A oxidase; CPT1, Carnitine Palmitoyltransferase 1.
The data are presented as mean ± sd. *P < 0·05, **P < 0·01 vs. control group. #P < 0·05, ##P < 0·01 vs. 100 nm insulin-treatment group.
ACO1 and CPT1 are PPARα target genes, and are the rate-limiting enzymes in fatty acid β-oxidation. As shown in Table 1, the mRNA expression levels of ACO and CPT1 were decreased in an insulin dose-dependent manner and were significantly lower in 10 and 100 nm insulin-treated groups than in control group, while AICAR treatment could reverse this inhibition.
Intrahepatic TG disappearance occurs mainly through either hydrolysis or secretion via VLDL in cows. Therefore, we have measured the expression of molecules that are involved in VLDL assembly. As shown in Table 1, the mRNA expression levels of APOB, MTP, and APOE were significantly decreased in 100 nm insulin-treated group, while AICAR treatment markedly reversed the inhibitory effect of insulin.
TG content
As shown in Table 1, compared with control group, the content of TG was increased by insulin in a dose-dependent manner and was markedly higher in the 100 nm insulin-treated group, while significantly lower in both AICAR and AICAR + insulin-treated groups.
Discussion
Dairy cows with type II ketosis display hyperglycemia, hyperinsulinemia and hyperlipidemia as well as hepatic TG accumulation (Li et al. Reference Li, Ding, Wang, Feng, Li, Wang, Liu and Li2016b), and insulin plays a crucial role in the hepatic lipid metabolism (Chirieac et al. Reference Chirieac, Chirieac, Corsetti, Cianci, Sparks and Sparks2000; Saltiel & Kahn, Reference Saltiel and Kahn2001). Therefore, we hypothesized that hepatic TG accumulation is associated with hyperinsulinemia. The data reported here support this hypothesis, since they indicate that high levels of insulin increase the intracellular TG content through inhibiting the AMPKα-PPARα/SREBP-1c/ChREBP pathway in cow hepatocytes. This might at least in part explain the hepatic TG accumulation that occurs in dairy cows with type II ketosis.
Ketotic cows display hepatic lipid metabolism disorder, which might be associated with high level of insulin. In our present study, the phosphorylation level of insulin receptor was significantly increased but the phosphorylation level of AMPKα was markedly decreased in insulin-treated cow hepatocytes. These results demonstrate that insulin suppresses AMPKα activity in cow hepatocytes. AMPK is an energy sensor that modulates hepatic lipid metabolism, acting as a key ‘master switch’ by regulating target transcription factors involved in lipid metabolism, including PPARα, SREBP-1c and ChREBP. PPARα dominates lipid oxidation gene expression, such as CPT1 and ACO, involved in fatty acid β-oxidation in hepatocytes (Kim, et al. Reference Kim, Kiyosawa, Burgoon, Chang and Zacharewski2013). CPT1 plays a key role in lipid oxidation by transferring long-chain acyl-CoAs into the mitochondria for β-oxidation (Li et al. Reference Li, Chen, Guan, Li, Lei, Liu, Yin, Liu and Wang2013). ACO is the rate-limiting enzyme in the progress of peroxisomal β-oxidation to very long straight-chain fatty acids (Tugwood, et al. Reference Tugwood, Issemann, Anderson, Bundell, McPheat and Green1992). In the present study, our results indicate that insulin could significantly decrease PPARα expression through inactivation of AMPKα, and then decrease the mRNA levels of ACO and CPT1, thereby decreasing lipid oxidation in cow hepatocytes. Our data demonstrate that insulin inhibited hepatic lipid oxidation through AMPKα-PPARα pathway. This supports a previous study showing that fatty acid oxidation of normal or obese rats injected with AMPKα activator-AICAR was significantly increased (Merrill et al. Reference Merrill, Kurth, Hardie and Winder1997). SREBP-1c and ChREBP govern lipogenesis through the transcriptional regulation of lipogenic genes, including ACC1, FAS, and SCD-1 (Li et al. Reference Li, Huang, Gu, Du, Lei, Yuan, Sun, Wang, Li and Liu2015). The synthesis of malonyl-CoA is the first committed step of fatty acid synthesis, and the enzyme that catalyzes this reaction, ACC1, is the major regulatory site in fatty acids synthesis (Li et al. Reference Li, Chen, Guan, Li, Lei, Liu, Yin, Liu and Wang2013). FAS catalyzes fatty acid elongation step. FAS is a determinant of the maximal capacity of the liver to synthesize fatty acids by de novo lipogenesis (Postic et al. Reference Postic, Dentin, Denechaud and Girard2007). SCD-1 is a rate-limiting enzyme in monounsaturated fatty acids synthesis and catalyzes the synthesis of monounsaturated fatty acids (Li et al. Reference Li, Chen, Guan, Li, Lei, Liu, Yin, Liu and Wang2013). In this study, our data showed that insulin treatment of cow hepatocytes markedly increased the expression of SREBP-1c and ChREBP, and then upregulated the expression of target genes ACC, FAS and SCD-1. Furthermore, AMPKα activator AICAR reversed these effects on lipid synthesis. This is in agreement with previous evidence in other species (mice, human) showing that DNA-binding activity and mRNA of SREBP-1c and ChREBP is significantly increased by insulin treatment (Poupeau & Postic, Reference Poupeau and Postic2011).
The secretion of VLDL is the main way to export TG from liver to blood. However, dairy cows synthesize hepatic VLDL very slowly but synthesize TG at rates that are comparable to those in most species (Pullen et al. Reference Pullen, Liesman and Emery1990). APOB100, ApoE, and MTP are the main structural and regulatory proteins for the synthesis and assembly of VLDL (Mason, Reference Mason1998; Greenow et al. Reference Greenow, Pearce and Ramji2005). We therefore measured the effect of insulin on the expression of APOB100, ApoE, and MTP, and found that high levels of insulin were inhibitory. Downregulation of APOB100, ApoE, and MTP further decreased VLDL assembly and further induce intracellular TG accumulation.
Conclusions
These results indicate that high levels of insulin suppress the AMPKα pathway in cow hepatocytes. AMPKα inactivation decreases the expression of PPARα, thereby downregulating the expression of lipid oxidation genes. Furthermore, AMPKα inactivation increases the expression of SREBP-1c and ChREBP, thereby upregulating the expression of lipogenic genes. In addition, high levels of insulin inhibit intracellular VLDL assembly through AMPKα pathway. Consequently, insulin increases lipid synthesis and decreases lipid oxidation and VLDL assembly in cow hepatocytes, which induces TG accumulation. The current study presents a possible explanation regarding the pathological mechanism of hepatic fat accumulation induced by hyperinsulinemia in dairy cows with type II ketosis.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S002202991800016X
This work was supported by the National Natural Science Foundation of China (Beijing, China; grant Nos. 31572581, 31502136, 31472247, and 31772810), the natural science foundation of Jilin Province (Changchun, China; grant No. 20170101148JC), and the National Key Research and Development Program (Beijing, China; grant Nos. 2016YFD0501007 and 2016YFD0501206).
Conflicts of interest
The authors declare no conflict of interest.