The αs1-casein locus (CSN1S1) is highly polymorphic in goats. According to their individual approximate contribution to the amount of protein in milk observed in previous studies, alleles for this locus have been grouped in 4 levels: strong alleles (A, B1, B2, B3, B4, C, H, L, and M), intermediate alleles (E and I), weak alleles (F, D, and G), and null alleles (O1, O2, and N) with no αs1-casein content (Grosclaude et al. Reference Grosclaude, Mahé, Brignon, Di Stasio and Jeunet1987; Martin et al. Reference Martin, Szymanowska, Zwierzchowski and Leroux2002; Ramunno et al. Reference Ramunno, Cosenza, Rando, Pauciullo, Illario, Gallo, Di Berardino and Masina2005; Sacchi et al. Reference Sacchi, Chessa, Budelli, Bolla, Ceriotti, Soglia, Rasero, Cauvin and Caroli2005; Sztankóová et al. Reference Sztankóová, Mátlová and Malá2007).
Previous studies have reported that the CSN1S1 can affect casein, protein, and fat levels, total solids, milk rheology, as well as cheese yield and quality (Pirisi et al. Reference Pirisi, Colin, Laurent, Scher and Parmentier1994; Clark & Sherbon, Reference Clark and Sherbon2000; Martin et al. Reference Martin, Szymanowska, Zwierzchowski and Leroux2002; Roncada et al. Reference Roncada, Gaviraghi, Liberatori, Canas, Bini and Greppi2002; Gómez-Ruiz et al. Reference Gómez-Ruiz, Miralles, Agüera and Amigo2004; Zeng et al. Reference Zeng, Soryal, Fekadu, Bah and Popham2007; Pagano et al. Reference Pagano, Pennisi, Valenti, Lanza, Di Trana, Di Gregorio, De Angelis and Avondo2010).
In addition to additive (individual allelic effects), dominance effects (specific effects for allele combinations), as well as interactions between CSN1S1 alleles and other genes of the studied populations (epistatic effects) are possible. Some evidence of several milk protein genes, acting as haplotypes on dairy traits has also been found (Hayes et al. Reference Hayes, Hagesaether, Ådnoy, Pellerud, Berg and Lien2006). Despite those possibilities, dominance effects, in this locus, had not been studied until recently in Norwegian goats (Dagnachew et al. Reference Dagnachew, Thaller, Lien and Åndøy2011) but there is no information published for Alpine and Saanen breeds.
The objective of this study was to evaluate both additive and dominance effects of CSN1S1 locus polymorphism on milk yield and milk composition traits in Alpine, Saanen, and Toggenburg goats.
Materials and Methods
Populations
Analysed data were obtained from 2003 to 2006 from Alpine, Saanen and Toggenburg in three herds located in Apaseo el Grande (at 1780 m above mean sea level), Guanajuato, Mexico. This area has a semi-dry temperate climate with an average temperature of 18°C, rain during the summer (approx. 70% of the annual rainfall of 605 mm, occurs from May to September) (CONAGUA, 2011). Goats were maintained in an intensive production system, fed with alfalfa hay and commercial concentrate, and were supplemented with vitamins and minerals. The milk produced in these herds is used for making cheese.
The official milk recording system used is the A4 of the International Committee for Animal Recording (ICAR, 2011). Fat, protein, lactose and total solids yields were obtained from milk yield and percentage measures for each monthly test day (based on twice daily milking). Fat, protein, lactose, and total solids contents were monthly obtained using an infrared milk analyser Bentley 150.
Lactation records with less than 3 test-day milk yield or milk components measurements information per goat (or less than 100 d in milk) were discarded. Records with less than 305 d in milk were projected to 305 d using methods described by Torres-Vázquez et al. (Reference Torres-Vázquez, Valencia-Posadas, Castillo-Juárez and Montaldo2009). The final data set consisted of 514 lactation 305-d records from 239 goats; 60 Alpine (130 lactations); 105 Saanen (210 lactations); and 74 Toggenburg (174 lactations). Analysed traits were milk (MILY), fat (FATY), protein (PROY), lactose (LACY), and total solids (SOLY) yields, fat (%FAT), protein (%PRO), lactose (%LAC), and total solids (%SOL) contents.
Genotyping
The genomic DNA was extracted from the blood by Phenol-chloroform extraction. Discrimination procedure between CSN1S1 alleles E, F, N, A* (A, G, H, I, O1, O2) and B* (B1, B2, B3, B4, C, L) was developed by amplification of segments of the CSN1S1 gene by PCR and XmnI endonuclease restriction digestion according to Ramunno et al. (Reference Ramunno, Cosenza, Pappalardo, Pastore, Gallo, Di Gregorio and Masina2000) as in Torres-Vázquez et al. (Reference Torres-Vázquez, Vázquez, Montaldo, Ulloa-Arvizu, Valencia, Gayosso and Alonso2008).
Statistical procedures
Analyses were performed using linear mixed models and PROC MIXED of SAS® program (SAS version 9.0, SAS Institute Inc., Cary NC, USA). Final analyses considered two models; data analysed with model 1 included information from all available genotypes, while model 2 included only information from homozygote genotypes.
Model 1 included the fixed additive covariate effects of the alleles A*, B*, N and E coded representing the number of those alleles present in one genotype (2 for homozygotes; 1 for heterozygotes; and 0 for genotypes without that allele). The effect of the F allele was set to 0 (solution restriction) to avoid the singularity of the design matrix, which is associated with the covariates. Hence, the alleles additive effects are defined as deviations with respect to allele F. Besides additive effects, model 1 included fixed dominance effects defined by a group of covariates (1 to indicate the presence of the combination of two alleles in one observation; 0 to indicate its absence), then covariate values for all homozygote genotypes were 0.
Other fixed effects included in model 1 were breed-herd combination, season of kidding (1 from October to March; 2 from April to September), year of kidding, and lactation number (grouped as 1–2, 2–4, and 5 or more lactations). Based on differences observed in preliminary analyses, the interaction effect of additive allele by year of kidding was also included in this model.
Random effects included in model 1 were goat nested in breed-herd subclasses (to model repeated lactation information), and residual effects.
Equation for model 1 was:

where y ijklm is one observation of the response variables, b1, …, b4 are multiple regression coefficients associated to coded additive allelic effects A*, B*, N and E respectively, b5, …, b14 are multiple regression coefficients associated to coded dominance effects for combinations of alleles A*, B*, N and E respectively, and e ijklm is the random residual effect.
Model 2 was similar to model 1, but instead of additive and dominance effects, the genotype effect (considered as a categorical variable) was used. Tukey multiple range tests were used for genotype means comparison.
Equation for model 2 was:
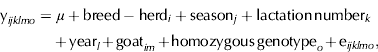
where y ijklmo is one observation of the response variables, genotype o is the effect of the homozygous genotype and e ijklmo is the random residual effect.
Results
Allelic and genotypic frequencies
In the Alpine breed, the frequencies of the alleles A*, B*, N, E, and F at the CSN1S1 locus were 0·150, 0·158, 0·175, 0·308 and 0·208, respectively. The same allelic frequencies for Saanen were 0·014, 0·105, 0·043, 0·629 and 0·210, respectively. And for Toggenburg they were 0·095, 0·216, 0·169, 0·182 and 0·338, respectively. Genotypes at CSN1S1 locus by breed are shown in Table 1.
Table 1. Genotypic frequencies of the CSN1S1 locus by breed

† A*=A, G, H, I, O1 and O2; B*=B1, B2, B3, B4, C and L
‡ Number of genotyped goats
§ EE=EE and EO1
In Alpine and Saanen breeds, alleles E (0·31 and 0·63, respectively) and F (0·21 in both breeds) were the most frequent. For Toggenburg breed, the most frequent alleles were F and B* (0·34 and 0·22. respectively). In general terms, the most frequent genotypes (Table 1) were EE (24·3%), EF (15·5%), B*E, and B*F (10·5%). The rest of the genotypic frequencies were lower than 10%. The frequency of the alleles A* and B* was low.
Additive allelic effects
Allele additive effects estimated using model 1 are presented in Table 2, while genotype least-square means obtained with model 2 are presented in Table 3. As allele F effect was set to 0 because its frequency was relatively high with homozygous FF goats present in all breeds (Table 1), and because it was also expected to be a weak allele. All additive effects are expressed as deviations from the mean effect of allele F.
Table 2. Estimates of additive and dominance effects for milk yield and milk composition in goats of all the studied breeds

† Where MILY is milk yield; FATY is fat yield, PROY is protein yield, LACY is lactose yield; SOLY is total solids yield; %FAT is fat content; %PRO is protein content; %LAC is lactose content; and %SOL is total solids content
‡ b is the additive effect, estimated as a deviation from allele F; *P⩽0·05; **P⩽0·01; ***P⩽0·001
A*=A, G, H, I, O1 and O2; B*=B1, B2, B3, B4, C and L
Table 3. Least-squares means in homozygote genotypes† for milk yield and milk composition traits in the studied goats

† A*=A, G, H, I, O1 and O2; B*=B1, B2, B3, B4, C and L
‡ Where MILY is milk yield; FATY is fat yield, PROY is protein yield, LACY is lactose yield; SOLY is total solids yield; %FAT is fat content; %PRO is protein content; %LAC is lactose content; and %SOL is total solids content
§ EE=EE and EO1
¶ Genotypes without a common superscript letter are different (P<0·05)
Allele A* had a positive additive effect on %PRO (0·21%; P=0·002) and %SOL (0·66%; P=0·005), and a negative additive effect on %LAC (−0·25%; P=0·023). The allele E had a negative additive effect on %LAC (−0·08%; P=0·058). The additive affects for the other alleles were not significant (P>0·10).
Similar estimates from the model 1 were obtained with model 2 using only homozygous genotypes (Table 3). The additive effect of A* on %PRO, compared with F, was 0·23% (P<0·05) and −0·37% in the case of %LAC (P<0·05). Regarding %PRO, the additive effect of A* compared with E was 0·23% (P<0·05), and the difference for %SOL comparing A* and E was 0·65% (P>0·05). No statistically significant differences were observed between homozygote genotypes for other traits.
Dominance genotypic effects
Some traits were affected by significant dominance effects (Table 2). Favourable dominance interaction effects were found for several genotypes (P<0·05) for MILY (A*N and B*N), FATY (A*N and B*E), PROY (A*N and B*E), LACY (A*N) and SOLY (A*N). Also, unfavourable dominance effects were observed (P<0·05) for %PRO (A*B* and A*N) and %SOL (A*B*, A*N and A*F).
Within breed additive effects
In order to verify whether the effects found occur across all breeds, within-breed analyses using model 1, were done. Results for Saanen and Toggenburg revealed that additive and dominance effects might be confounded due to the information structure characterized by the absence of homozygote individuals for the allele A*. Within-breed analysis showed that only Alpine breed results agreed, in general, with those from the complete data analysis (Table 4). However, Alpine breed analysis allowed us to detect a significant additive negative effect of the allele A* on MILY (−81·4; P=0·046) and of the allele E on LACY (−4·2; P=0·039); additionally, allele N (null for αs1-casein production) was found to be significantly related to higher MILY (114·5; P=0·027) and PROY (3·64; P=0·016) (Table 4). These results for Alpine were very different with respect to the results from the general analysis (Table 1), in which N allele effect was not different from F allele for any trait, and therefore should be regarded with caution since they might be the product of some confounded effects. Dominance effects in Alpine breed agreed with complete data analysis. However, positive dominance effects were found (P<0·05) for MILY (A*E), FATY (B*N), PROY (A*E and NE), SOLY (A*E and B*N) and %SOL (NF); and a negative dominance effect was found (P<0·05) for MILY (NF).
Table 4. Estimates of additive and dominance effects for milk yield and milk composition traits† in Alpine goats

† MILY: milk yield; FATY: fat yield, PROY: protein yield, LACY: lactose yield; SOLY: total solids yield; %FAT: fat content; %PRO: protein content; %LAC: lactose content; and %SOL: total solids content
‡ b is the additive effect, estimated as a deviation from allele F; *P⩽0·05; **P⩽0·01; ***P⩽0·001
§ A*=A, G, H, I, O1 and O2; B*=B1, B2, B3, B4, C and L
Additive alleles by year interaction effects
Additive alleles by year interaction effects trends were found on several yield and composition traits (P<0·10). For milk yield, significant interaction additive by year effects were found for alleles A*, N, and E; and for composition traits a significant interaction of additive by year effects were found for alleles N and E. This suggests that environmental circumstances may modify additive allele effects.
Discussion
Our results showed that alleles E and F were the most frequent in Alpine (E: 0·308 and F: 0·208) and Saanen (E: 0·629 and F: 0·210). In these breeds, the observed frequencies agree with those reported in France (Saanen, E: 0·41 and F: 0·43; Alpine, E: 0·34 and F: 0·41), Italy (Saanen, E: 0·49 and F: 0·46; Alpine, E: 0·35 and F: 0·59), Mexico (Saanen, E: 0·42 and F: 0·37; Alpine, E: 0·24 and F: 0·28) and USA (Saanen, E: 0·71 and F: 0·30; Alpine, E: 0·36 and F: 0·46) (Grosclaude et al. Reference Grosclaude, Mahé, Brignon, Di Stasio and Jeunet1987; Martin & Leroux, Reference Martin and Leroux2000; Torres-Vázquez et al. Reference Torres-Vázquez, Vázquez, Montaldo, Ulloa-Arvizu, Valencia, Gayosso and Alonso2008; Maga et al. Reference Maga, Daftari, Kültz and Penedo2009). In general, alleles E and F were the most frequent. This may help to explain the low content of protein in goat milk in high-yielding breeds, which limits cheese yield.
In this study, some confounding effects are possible since A* allele may contain O1 and O2 null alleles which are related to low protein percentages. However, previous research (Martin & Leroux, Reference Martin and Leroux2000; Maga et al. Reference Maga, Daftari, Kültz and Penedo2009) indicates that frequencies of these alleles in Alpine, Saanen and Toggenburg breeds are low, most probably below 0·05. Soares et al. (Reference Soares, Rodrigues, Mognol, Ribeiro, Silva and Brancalhão2009) found O1 allele frequencies of 0·01 and 0·02 in Alpine and Saanen goats, respectively. Therefore, probably these null alleles have a small effect in the estimated additive or dominance effects involving allele group A* in our study. Most previous studies considered that main allelic groups for this locus, in these breeds, are A, B, E, F and O (Martin & Leroux, Reference Martin and Leroux2000), but with different allele aggregation, and more recently A, B, E, F and N (Maga et al. Reference Maga, Daftari, Kültz and Penedo2009). Low frequencies for several alleles make it impossible to obtain accurate estimates of their individual effects.
Results showed that %PRO, %LAC and %SOL were significantly influenced by allele A* when compared with F. Our findings regarding %PRO (additive effects varying from 0·21 to 0·26%) (Tables 2 & 4) agree with previous studies, which ranged from 0·9 to 3·2 g/l, corresponding approximately to a difference varying from 0·09 to 0·32% (Remeuf, Reference Remeuf1993; Analla et al. Reference Analla, Angulo-Heras and Serradilla2000; Chilliard et al. Reference Chilliard, Rouel and Leroux2006) and are essentially the same to those observed by Manfredi et al. (Reference Manfredi, Barbieri, Bouillon, Piacère, Mahe, Grosclaude and Ricordeau1993). In general terms, our results are very similar to most French studies for Alpine and Saanen breeds (Mahé et al. Reference Mahé, Manfredi, Ricordeau, Piacere and Grosclaude1993; Manfredi et al. Reference Manfredi, Barbieri, Bouillon, Piacère, Mahe, Grosclaude and Ricordeau1993; Remeuf, Reference Remeuf1993; Barbieri et al. Reference Barbieri, Manfredi, Elsen, Ricordeau, Bouillon, Grosclaude, Mahé and Bibé1995).
No significant effects of allele A* on PROY were found. Alleles N and E on this trait had similar effects to allele F, contrary to other studies where allele E effect was shown to be intermediate. However, superiority of allele A over alleles E and F for PROY had been observed also in some studies (Mahé et al. Reference Mahé, Manfredi, Ricordeau, Piacere and Grosclaude1993; Remeuf, Reference Remeuf1993). It is important to mention that the contrast of the effects of allele E and F on %PRO was not significant, while it was smaller for %LAC, similar to the case of allele A* (Table 2).
We found no evidence that allele B* effect was larger for %PRO (Tables 2 & 4), because allele B* behaved as a weak allele. This may be caused by the existence of several subtypes of alleles B (B1, B2, B3 and B4) whose effects on milk protein have been shown to be different in a Poitevine goat population (Ricordeau et al. Reference Ricordeau, Manfredi and Amigues2000). It is worth mentioning that inconsistencies regarding the allelic effects on milk traits have been already observed by other authors (Hayes et al. Reference Hayes, Hagesaether, Ådnoy, Pellerud, Berg and Lien2006).
Although there was not a significant effect of the allele A* on %FAT, the estimated effect was positive and similar to that found for %PRO. Also, the effect of the allele A* on %SOL was important with an increment ranging from 0·62 (P=0·031) to 0·66% (P=0·005) when compared with allele F (Tables 3 & 5). While the effect of the allele A* on %LAC was negative (−0·25; P=0·023), results suggest that there is a favourable effect of the allele A* on %FAT that could not be statistically detected due to its higher variability (Table 2).
The effect of the allele A* on PROY and FATY was close to zero and not significant (P>0·05); this could be related to the possible negative effect of the allele A* on MILY, which was detected in Alpine goats (Table 4). Other authors have previously detected negative effects of the allele A on MILY (Barbieri et al. Reference Barbieri, Manfredi, Elsen, Ricordeau, Bouillon, Grosclaude, Mahé and Bibé1995; Ricordeau et al. Reference Ricordeau, Manfredi and Amigues2000). This agrees with the negative genetic correlations between milk yields and milk contents estimated in dairy goat populations (Torres-Vázquez et al. Reference Torres-Vázquez, Valencia-Posadas, Castillo-Juárez and Montaldo2009). In our study, the genetic effects of the alleles at the CSN1S1 locus on the production of fat and protein by lactation were not significant, in agreement with Ricordeau et al. (Reference Ricordeau, Manfredi and Amigues2000).
Dagnachew et al. (Reference Dagnachew, Thaller, Lien and Åndøy2011) found significant dominance effects of single SNP within CSN1S1 and CSN3 on contents of protein, fat, lactose and milk yield in Norwegian goats. Those findings are in agreement with our results. Dominance effects may compromise the efficiency of breeding programmes aimed simply to increase the frequency of strong alleles and to reduce the frequencies of weak and null alleles. With dominance, the mean for the %PRO could depend not only on the allelic frequencies at the CSN1S1 locus but also on the number and proportion of heterozygous genotypes in the population.
The effect of the alleles can be different, not only because the different level of the allele disaggregation used in a particular study, but also because the genes of the different dairy proteins could act as a whole. Studies are needed to examine the protein haplotypes through several dairy gene proteins to identify their effects accurately. Hayes et al. (Reference Hayes, Hagesaether, Ådnoy, Pellerud, Berg and Lien2006) detected significant effects of haplotypes involving several casein loci (CSN2, CSN1S2, and CSN3), in addition to CSN1S1 locus.
Based on our results, we conclude that there are favourable effects of the allele A* on %FAT, %PRO, PROY and %SOL compared with the effects of the F allele, but the rest of the studied alleles behaved rather as weak alleles regarding these traits. Increasing the frequency of the allele A* may increase mainly strong allele frequencies and therefore lead to larger cheese yield per unit of processed milk (Zeng et al. Reference Zeng, Soryal, Fekadu, Bah and Popham2007), but an important increment of the quantity of total solids, fat and protein per lactation on the population is not expected. Owing to the negative effects of the allele A* on %LAC, increasing its frequency may have a detrimental effect in the yield of products that contain or require lactose.
Despite the fact that some of the allelic effects are statistically significant, they do not allow the prioritizing of Genetic Marker-Assisted Selection (GAS) over conventional genetic evaluation methods. So far, the milk production and milk composition traits can be increased in a profitable way based only on phenotypic and pedigree information (BLUP evaluations), because they affect all loci involved on these traits (Montaldo & Manfredi, Reference Montaldo and Manfredi2002). Simulations have shown that using this polymorphism in the context of breeding programmes for dairy goats may increase the rates of genetic gain for %PROT, over conventional progeny testing programmes based on phenotypic information only, but not for PROY (Sánchez et al. Reference Sánchez, Ilahi, Manfredi and Serradilla2005).
A simple alternative to exploit the additive effect of the allele A* is to detect young males with high predicted breeding value based on best linear unbiased predictors (BLUP) for an economic index, which have at least one copy of the allele A*, or that have no weak alleles. These young males could have priority in breeding programmes with AI and progeny tests. However, the implications of this procedure, including the consequences on all the economically important traits and in the genetic variation of the population must be considered.
The existence of dominance effects complicates the use of this polymorphism in the genetic improvement of dairy goats, because as different genotypes are favourable/unfavourable for different traits, optimizing mating to obtain the desired genotypes in the population may be very difficult. Moreover, in practical goat breeding, the need of mating planning yield the ideal gene and genotype frequencies for this locus, may easily interfere with the breeding programme mating plan for other traits depending on polygenic effects (Montaldo et al. Reference Montaldo, Torres-Hernández and Valencia-Posadas2010).
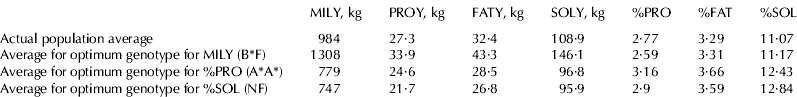
Genotypes for maximum MILY, %PRO and %SOL estimated from model 1 analyses are shown in Table 5. Means for all important milk traits are also displayed. The best genotype for MILY was BF*, the best for %PRO was A*A* and the best for %SOL was NF. Potential changes on the mean by substituting all population alleles by A*, are shown in Table 5. Changes for %PRO were 14% (from 2·77 to 3·16%), 11% for %FAT (from 3·29 to 3·66%), and 12% for %SOL (from 11·07 to 12·43%). As the change for MILY was −21% of the mean (from 984 to 779 kg), the results on PROY and SOLY for substituting all the alleles by A* were negative (−10 and −11%, respectively).
Table 5. Predicted averages for different genotypes† compared with actual population averages for yields of milk (MILY), fat (FATY), protein (PROY), and total solids (SOLY); and contents of protein (%PRO), fat (%FAT), and total solids (%SOL)‡

† A*=A, G, H, I, O1 and O2; B*=B1, B2, B3, B4, C and L
‡ Values for PROY, FATY and SOLY, were calculated from MILY and the appropriate content average
Consequences of maximizing the average for some traits by manipulating the genetic make up of the population for this locus is shown in Table 5. The higher values for PROY and SOLY were observed for genotype B*F, which gave the highest average for MILY. Therefore, even if the use of this polymorphism for breeding might allow for the increase of %PRO (genotype A*A*) and %SOL (genotype NF), that might be at the expense of total cheese production by lactation per goat. The genotype with the highest average for %SOL has also reduced PROY, and SOLY averages if compared with the current population averages (Table 5).
Whether increasing %PRO and %SOL is a priority on a particular population will depend on many factors, particularly the relative economic value of increasing one unit of %PROT compared with increasing one unit of MILY, particularly when there is a high economic value for increasing the cheese-making properties of milk (Montaldo & Manfredi, Reference Montaldo and Manfredi2002; Sánchez et al. Reference Sánchez, Ilahi, Manfredi and Serradilla2005). Moreover, because allele A* is in such small frequency in all studied breeds, increasing its frequency may be impossible without compromising the effective population size (Table 1), creating potential problems regarding conservation of genetic diversity and avoiding an increase of inbreeding rate.
Therefore, using this polymorphism in goat breeding may be only justified when breeding goals are clearly defined and when there is a breeding organization able to carry out the programme and transfer the benefits to farmers (Gama & Bressan, Reference Gama and Bressan2011). Significant interaction effects between additive allelic and environment level (year) effects in our study were also observed by Pagano et al. (Reference Pagano, Pennisi, Valenti, Lanza, Di Trana, Di Gregorio, De Angelis and Avondo2010). These interactions constitute a further complication in the use of this major gene in breeding programmes addressed to increasing productivity in dairy goat populations.
Low frequency of A* allele in these high milk yield populations may be the result of an unfavourable pleiotropic effect for a selected trait (i.e. milk yield) as found in Alpine breed in our study. Another possibility is a negative effect of the A* allele on some important fitness traits (i.e. natural selection effects) yet to be identified.
Further research on this locus with larger samples and more precise techniques for allele discrimination are needed to confirm the estimates of additive and dominance effects found in this study.
Conclusions
Significant additive effects for allele A* v. allele F were found on protein and total solids milk contents, by using a model that included additive and dominance effects. However, no significant effects of the allele B, compared with allele F, were observed.
The presence of significant dominance effects at the CSN1S1 locus, modifies the prediction of GAS results by using just a model of allelic replacement.
Potential benefits from increasing the frequency of the allele A* in the goat population to increase cheese yield, needs to be balanced with its potential disadvantages, such as a the deterioration of the mean or the reduction of genetic progress in other economically important traits, such as milk, protein and total solids yields, and a potential reduction of genetic variability of the population.
The presence of year by allele interaction effects may imply complications to accurately predicting the long-term effects of these alleles across production environments.
CONACyT Mexico supported the first author's Master in Science studies and funded partially this research through project CONACyT-SAGARPA 2003-152. H.H. Montaldo, R.A. Alonso, M. Valencia-Posadas and H. Castillo-Juárez are members of the National Research System of Mexico.