Introduction
Living organisms are composed of a variety of complex organic compounds, with nucleotides and amino acids being the fundamental building blocks of the larger macromolecules employed in all life forms known on Earth: nucleic acids and proteins (Danchin and Sekowska, Reference Danchin and Sekowska2015). There are 20 different amino acids used to compose the proteins in organisms using the standard genetic code, with two additional amino acids being incorporated into proteins using alternative translation mechanisms (Danchin and Sekowska, Reference Danchin and Sekowska2015). These 20 (or 22) amino acids are termed proteinogenic amino acids, but there are hundreds of non-proteinogenic amino acids produced in Nature, as well as many different amino acids that can be synthesized in the laboratory and artificially inserted into proteins using solid phase synthesis and synthetic biology methods (Xie and Schultz, Reference Xie and Schultz2006; Neumann et al., Reference Neumann, Wang, Davis, Garcia-Alai and Chin2010).
Although extraterrestrial life may exist in many different forms, the conservative hypothesis in exploring other terrestrial planets and large satellites is that extended regions of liquid water, conditions that allow for the formation of complex organic molecules and an energy source or gradient must all be present for life, which is similar to life on Earth, to have evolved (Des Marais et al., Reference Des Marais, Nuth, Allamandola, Boss, Farmer, Hoehler, Jakosky, Meadows, Pohorille, Runnegar and Spormann2008; Hays et al., Reference Hays, Graham, Des Marais, Hausrath, Horgan, McCollom, Parenteau, Potter-McIntyre, Williams and Lynch2017). When exploring such potentially habitable environments, the identification of biosignatures in soil and water samples will be the first step in searching for recognizable signs of previous or current life (Hays et al., Reference Hays, Graham, Des Marais, Hausrath, Horgan, McCollom, Parenteau, Potter-McIntyre, Williams and Lynch2017). Although there are many possible biosignatures and the principles of biological evolution may not be universal, the identification of building blocks similar to our biosphere's molecular machinery would be an extremely strong indication of extraterrestrial life (Des Marais et al., Reference Des Marais, Nuth, Allamandola, Boss, Farmer, Hoehler, Jakosky, Meadows, Pohorille, Runnegar and Spormann2008; Burton et al., Reference Burton, Stern, Elsila, Glavin and Dworkin2012; Hays et al., Reference Hays, Graham, Des Marais, Hausrath, Horgan, McCollom, Parenteau, Potter-McIntyre, Williams and Lynch2017). Thus, the identification of DNA, RNA, proteins, or their precursors: nucleotides and amino acids, on potentially habitable planets or satellites would warrant more intense investigation of that site (Des Marais et al., Reference Des Marais, Nuth, Allamandola, Boss, Farmer, Hoehler, Jakosky, Meadows, Pohorille, Runnegar and Spormann2008; Hays et al., Reference Hays, Graham, Des Marais, Hausrath, Horgan, McCollom, Parenteau, Potter-McIntyre, Williams and Lynch2017). One proteinogenic amino acid (glycine) and several amino acid precursor molecules have already been discovered on comets and asteroids (Burton et al., Reference Burton, Stern, Elsila, Glavin and Dworkin2012; Elsila et al., Reference Elsila, Aponte, Blackmond, Burton, Dworkin and Glavin2016). Additionally, over 90 proteinogenic and non-proteinogenic amino acids have been found to exist in multiple different meteorites, with the number of non-proteinogenic amino acids outnumbering the number of proteinogenic amino acids on meteors (Alyssa and Ralph, Reference Alyssa and Ralph2014; Elsila et al., Reference Elsila, Aponte, Blackmond, Burton, Dworkin and Glavin2016).
Three likely locations in our Solar System which may satisfy, or have satisfied, the three criteria for a habitable body as discussed above are Mars, Enceladus and Europa (Chyba, Reference Chyba2000; Marion et al., Reference Marion, Fritsen, Eicken and Payne2003; Bibring et al., Reference Bibring, Langevin, Poulet, Gendrin, Gondet, Berthé, Soufflot, Drossart, Combes, Bellucci, Moroz, Mangold and Schmitt2004; McKay et al., Reference McKay, Porco, Altheide, Davis and Kral2008; Tosca et al., Reference Tosca, Knoll and McLennan2008; Barge and White, Reference Barge and White2017; Steel et al., Reference Steel, Davila and McKay2017). However, these locations have a much higher surface exposure to both ultraviolet (UV) and gamma irradiation than Earth (Des Marais et al., Reference Des Marais, Nuth, Allamandola, Boss, Farmer, Hoehler, Jakosky, Meadows, Pohorille, Runnegar and Spormann2008; Carlson et al., Reference Carlson, Calvin, Dalton, Hansen, Hudson, Johnson, McCord, Moore, Pappalardo, McKinnon, Khurana and Dotson2009; Schmidt et al., Reference Schmidt, Blankenship, Patterson and Schenk2011; Hays et al., Reference Hays, Graham, Des Marais, Hausrath, Horgan, McCollom, Parenteau, Potter-McIntyre, Williams and Lynch2017). Due to this higher energy irradiation, many postulate that if life were to have evolved it would have done so in subsurface habitats, as thick layers of liquid or solid water provide significant shielding against both of these radiation types (Boston et al., Reference Boston, Ivanov and McKay1992; Cleaves and Miller, Reference Cleaves and Miller1998; Levy et al., Reference Levy, Miller, Brinton and Bada2000; Weiss et al., Reference Weiss, Yung and Nealson2000; McKay et al., Reference McKay, Porco, Altheide, Davis and Kral2008; Parkinson et al., Reference Parkinson, Liang, Yung and Kirschivnk2008). However, the extent of this shielding is unknown and it is possible that any amino acids on these bodies would still be exposed to a much greater UV and gamma radiation dose than those found on Earth.
Perhaps the first quality an amino acid (or nucleotide) needs to have in order to exist in extraterrestrial living organisms is simply stability. In the case of Mars, for example, amino acids may need enhanced stability to UV and gamma radiation when under a largely carbon dioxide atmosphere. Alternatively, the subsurface oceans of Europa and Enceladus may exhibit high pressures and unique oceanic mineral compositions (Peterson et al., Reference Peterson, Holian and Garrison1969; Kvenvolden et al., Reference Kvenvolden, Lawless, Pering, Peterson, Flores, Ponnamperuma, Kaplan and Moore1970; Levy et al., Reference Levy, Miller, Brinton and Bada2000; Ehrenfreund et al., Reference Ehrenfreund, Bernstein, Dworkin, Sandford and Allamandola2001; Cataldo et al., Reference Cataldo, Ursini and Angelini2008, Reference Cataldo, Angelini, Iglesias-Groth and Manchado2011a, Reference Cataldo, Ragni, Iglesias-Groth and Manchado2011b, Reference Cataldo, Angelini, Hafez and Iglesias-Groth2013a, Reference Cataldo, Iglesias-Groth, Angelini and Hafez2013b; Iglesias-Groth et al., Reference Iglesias-Groth, Cataldo, Ursini and Manchado2011; Sarker et al., Reference Sarker, Takahashi, Kawamoto, Obayashi, Kaneko and Kobayashi2012 ). Therefore, in this work, an array of aqueous aromatic proteinogenic and non-proteinogenic amino acids were exposed to a selection of UV and gamma radiation exposures and the degree of degradation of the amino acids was then quantified using UPLC-MS (ultra-high performance liquid chromatography-mass spectrometry) (Swartz, Reference Swartz2005; Xu et al., Reference Xu, Fan, Rieser and El-Shourbagy2007; Waterval et al., Reference Waterval, Scheijen, Ortmans-Ploemen, Habets-van der Poel and Bierau2009; Want et al., Reference Want, Wilson, Gika, Theodoridis, Plumb, Shockcor, Holmes and Nicholson2010). Although there have been a few previous studies on the UV and gamma radiation stability of certain amino acids, to our knowledge there are neither any previous studies comparing the UV and gamma irradiation stability of the non-proteinogenic amino acids analysed in this study, nor any previous research directly comparing the stability of proteinogenic and non-proteinogenic amino acids with one another after exposure to identical conditions (Peterson et al., Reference Peterson, Holian and Garrison1969; Ehrenfreund and Charnley, Reference Ehrenfreund and Charnley2000; Ehrenfreund et al., Reference Ehrenfreund, Bernstein, Dworkin, Sandford and Allamandola2001; Kate et al., Reference Kate, Garry, Peeters, Quinn, Foing and Ehrenfreund2005; Ten Kate et al., Reference Ten Kate, Garry, Peeters, Foing and Ehrenfreund2006; Cataldo et al., Reference Cataldo, Ursini and Angelini2008, Reference Cataldo, Angelini, Iglesias-Groth and Manchado2011a, Reference Cataldo, Ragni, Iglesias-Groth and Manchado2011b; Reference Cataldo, Angelini, Hafez and Iglesias-Groth2013a, Reference Cataldo, Iglesias-Groth, Angelini and Hafez2013b; ten Kate, Reference ten Kate2010; Iglesias-Groth et al., Reference Iglesias-Groth, Cataldo, Ursini and Manchado2011; Sarker et al., Reference Sarker, Takahashi, Kawamoto, Obayashi, Kaneko and Kobayashi2012; Cherubini and Ursini, Reference Cherubini and Ursini2015).
Aromatic amino acids were the focus of this study, with tyrosine, phenylalanine and tryptophan (Tyr, Phe, Trp) representing the proteinogenic aromatic amino acids. Twenty aromatic amino acids that were largely alternatively functionalized structural analogues of tyrosine, tryptophan, or phenylalanine were used as the non-proteinogenic amino acids. This study focused on aromatic amino acids due to their role in biological photoprotection, enzyme mechanisms and hydrophobic core formation in known proteins (Salih et al., Reference Salih, Larkum, Cox, Kühl and Hoegh-Guldberg2000; Makwana and Mahalakshmi, Reference Makwana and Mahalakshmi2015). The results showed that some, but not all, non-proteinogenic amino acids tested are as stable as proteinogenic amino acids when exposed to UV and gamma doses, while select (largely fluorinated) non-proteinogenic amino acids are more stable than their proteinogenic amino acid counterparts.
Materials and methods
Instrumentation and materials
An Acquity H Class UPLC-MS (Waters, Milford, MA) instrument, including PDA and QDa detectors (Waters, Milford, MA), Empower V3 software (Orlando, FL) and an autosampler with Waters screw top 12 32 mm2 vials with PTFE/Silicone septa (Milford, MA), was used for liquid chromatography and mass spectrometry analyses of all samples. A BEH C8 1.7 µm, 2.1 × 100 mm2 column (Water, Milford, MA) was used to separate amino acids from degradation products. Gamma irradiations were performed using a Shepherd 109-68R and a Gammacell 220 cobalt-60 gamma irradiator. The dose rates were measured as 10.6 and 0.104 krad min−1, using Fricke dosimetry, for the gamma radiolysis experiments (Fricke, Reference Fricke1934). A three wavelength UV-Lamp (254/302/365 nm) with 3 × 8 watt UV tubes from Thermo Scientific (Waltham, MA) was used for irradiation experiments.
All proteinogenic amino acids were from a Sigma-Aldrich L-amino acids analytical standard kit and non-proteinogenic amino acids were purchased from Sigma-Aldrich (St. Louis, MO), Santa Cruz Biotechnology (Dallas, TX), Enamine (Kiev, Ukraine), Bachem (Bubendorf, Switzerland), Iris Biotech (Marktredwitz, Germany), Abblis (Houston, TX), ACROS (Geel, Belgium), or MP Biomedical (Santa Ana, CA). Amino acids were suspended in Ultrapure filtered water that was purified using the Milli-Q (Billerica, MA) Advantage A10 water purification system and the ultrapure BioPak polisher. This ultrapure water was also used in solvent preparation. Optima LC/MS formic acid was purchased from Fisher Chemical (Hampton, NH) and Chromasolv MS grade acetonitrile was purchased from Fisher Scientific (Hampton, NH). Round bottom polypropylene cryogenic vials were purchased from Corning (Corning, NY).
Preparation and exposure of amino acid samples
A stock solution was initially prepared of all amino acid samples and aliquots from that initial stock solution was subsequently used for experiments in order to normalize the initial concentration. Amino acid stock solutions were 100 µM concentration, all amino acids fully dissolved in deionized, ultrapure water and solutions were stored in glass flasks in 4°C refrigeration. This stock was aliquoted into either cryogenic vials or UPLC glass autosampler vials in 1.8 ml aliquots for exposure to different temperatures, UV light, or gamma rays. However, the 24 h UV-B treatment of the 100 µM tryptophan analogues resulted in degradation such that the concentration of the remaining amino acids was below the detection limit of the single quadrupole MS. Therefore, the 24 h UV-B tryptophan analogue samples (Fig. 1) were from a 1 mM concentration stock solution instead of a 100 µM. Untreated samples, the controls, which the treated samples were compared with in order to determine the degree of amino acid degradation caused by the treatment, were pipetted into glass autosampler vials and run on the UPLC-MS. For UV and gamma irradiation experiments and high-temperature experiments, 1.8 ml of this initial stock of the amino acid was pipetted into borosilicate autosampler vials under ambient atmospheric conditions. During the UV and gamma irradiation experiments, the temperature in the laboratory ranged from 20°C to 25°C. However, the temperature within the vials during UV or gamma irradiation was not monitored. These capped vials were then immersed in an 80°C hot water bath for 24 h for the high temperature experiments and then analysed for degradation on the UPLC-MS. For the UV irradiation, the glass vials of the amino acids were placed under the UV lamp (4.5 cm from the light source) at either 254, 302, or 365 nm, which was enclosed within a box under a fume hood for 24 h and then analysed with UPLC-MS. Luminous emittance from the UV lamp at the three different wavelengths was measured using a Fisher Scientific Traceable dual Display light meter. The sampling time for the light meter was 0.4 s and the mercury lamp setting was employed, with the lamp reporting ± 4% accuracy and ± 1 lux resolution for the lux range that was determined. Illuminance readings using the light meter were taken at three different locations under the UV-lamp, with UV-A illumination yielding 350 ± 20 lux, UV-B illumination yielding 145 ± 21 lux and UV-C illumination yielding 104 ± 20 lux. For the gamma irradiations, all glass vials were placed within a large glass beaker (400 ml) and this beaker was placed inside the appropriate gamma source for the required time for 399 or 3.99 krad dosage.

Fig. 1. Stability of select aqueous tryptophan analogous amino acids (from Figs. 2 and 3) to UV-A (365 nm), UV-B (302 nm) and UV-C (254 nm) irradiation when under ambient atmospheric conditions. The darker triplet of bands (3) is the proteinogenic amino acid (Trp). All others (3-1 to 3-8) are non-proteinogenic amino acids whose structures are shown in Figs. 2 and 3. The y-axis shows the % of the amino acid remaining after exposure to the different UV wavelengths.
The cold temperature samples were pipetted into cryogenic vials (1.8 ml), in triplicate and immersed in liquid nitrogen for 24 h, thawed and pipetted into glass autosampler vials. Cold temperature controls for the amino acids were also produced in which the amino acid was pipetted into cryogenic vials, stored at 4°C for 24 h and then pipetted into glass auto-sampler vials.
UPLC-MS analysis of amino acid samples
The following solvents were used in the UPLC separation on a C8 column: Solvent B: Acetonitrile with 0.1% (v/v) formic acid and Solvent C: Water with 0.1% (v/v) formic acid. The amino acids were separated from reaction products using the following chromatographic conditions: solvents and columns were held at 30°C and the solvent flow rate was 0.4 ml min−1. The solvent gradient began at 5% Solvent B and 95% Solvent C from initial to 8 min and changed to 40% Solvent B and 60% Solvent C from 8.0 to 8.5 min, followed by 2.5 min of 40% Solvent B and 60% Solvent C flow. Solvent composition was then changed to 5% Solvent B and 95% Solvent C at 11 min for 1 min. The PDA detector was sampled at 20 points per second, recording absorbance at λ = 254 nm with a 4.8 nm resolution. The QDa detector cone voltage was set at 15 V, using a positive scan and 0.8 kV capillary voltage, sampling at 10 points per second. The mass spectrometer was a single quadrupole, 30–300 Da mass range, 0.2 Da accuracy and 0.7 Da mass resolution. All samples were performed in triplicate.
Tyrosine, phenylalanine and tryptophan were the proteinogenic amino acids tested in ultra-cold or hot temperatures and the initial round of UV-B exposure. Aza-tryptophan, L5-hydroxy-tryptophan, 5-methyl-tryptophan, 5-fluoro-tryptophan, 5-cyano-tryptophan, p-CN-phenylalanine, fluoro-phenylglycine (Fig. 2 = p-F-Phe), p-benzoyl-phenylalanine, p-azido-phenylalanine, H-4-(7-hydroxy-4-coumarinyl), 3 -(2-oxo-1,2,-dihydro-4-quinolinyl) alanine (oxo-ala in Fig. 2) and 4-boronic acid-phenyalanine, were the 12 non-proteinogenic amino acids tested for temperature degradation and the first round of UV-B exposure (Fig. 2). Based on the results from the first round of UV-B degradation, eight additional non-proteinogenic amino acids (Fig. 3: m-F-Phe, 4-Cl-Phe, p-Br-Phe, m-F-Tyr, 3-Cl-Tyr, 4-F-Trp, 5-Cl-Trp, 5-Br-Trp) were tested during the second round of UV irradiation with three wavelengths of UV light (254, 302, or 365 nm) and two doses of gamma-ray irradiation (399 and 3.99 krad).

Fig. 2. Structures of amino acids tested against 24 h + 80°C exposure and 24 h UV-B exposure. Select amino acids that exhibited UV-B stability were also tested against UV-A, UV-C and gamma irradiation exposure.

Fig. 3. Structures of additional amino acids tested against UV-B, UV-A, UV-C and gamma irradiation exposure
Data analysis of UPLC-MS results
The proteinogenic amino acids from the analytical standard kit, tyrosine, tryptophan and phenyalanine exhibited ideal chromatograms, while the non-proteinogenic amino acids varied in this respect, likely due to the reduced purity of these samples. The main peak in the non-proteinogenic amino acids was confirmed to be the amino acid via mass spectrum and all non-proteinogenic amino acids purchased were stated to be above 90% purity. The LC peak corresponding to the amino acid was identified and confirmed by extracting the mass spectrum of that particular peak and comparing it with the known molecular weight of the amino acid and the mass spectrum of the untreated, control amino acid (see Fig. 4 and Supplementary Information). The peak area of the intact amino acid following condition exposure was then compared with the peak area of the initial amino acid that had no treatment and all measurements were done in triplicate. The differences in integrated LC peak areas of the control and treated samples were used to calculate the percentage of amino acid degradation reported and the identity of LC peaks was confirmed with mass spectrum analysis.
Results
Stability of amino acids at varying temperature
As expected, no amino acids tested exhibited any statistically significant degradation due to 24 h immersion in liquid nitrogen (−196°C). Similarly, after exposure to 80°C temperature for 24 h, most of the amino acids in aqueous 100 µM solution did not undergo any statistically significant degradation (Supplementary Information Fig. 1). The following amino acids did degrade significantly (>4% degradation) after the 24 h 80°C exposure, listed in order of decreasing degradation: L-5-hydroxy-tryptophan by 12.6% (±0.9%), tryptophan by 8.7% (±0.2%) and oxo-alanine by 7.7% (±4.9%). Several of the tryptophan non-proteinogenic amino acid analogues showed slightly enhanced stability to high-temperature incubation when compared with tryptophan. 5-CN-Trp, Aza-Trp, 5-F-Trp and 5-methyl-Trp showed a slightly increased stability as compared with Trp after 80°C incubation, but no more than a 10% increase in any case.
Stability of amino acids following UV irradiation
Tyrosine, phenylalanine, tryptophan, aza-tryptophan, L5-hydroxy-tryptophan, 5-methyl-tryptophan, 5-fluoro-tryptophan, 5-cyano-tryptophan, p-CN-phenylalanine, fluoro-phenylglycine, p-benzoyl-phenylalanine, p-azido-phenylalanine, H-4-(7-hydroxy-4-coumarinyl), 3,(2-oxo-1,2,-dihydro-4-quinolinyl) alanine (oxo-ala in tables) and 4-boronic acid-phenyalanine were initially tested for degradation after 24 h UV-B exposure when under ambient atmospheric conditions (Supplementary Information Fig. 2). The –CN and -F group on Phe exhibited the most stability of the non-proteinogenic phenylalanine derivatives, while the azide and benzoyl functionalized Phe analogues resulted in nearly a complete transformation from the original amino acid with UV-B irradiation. Aza-Trp and the F- and hydroxy groups on the non-proteinogenic Trp analogues appeared to slightly enhance stability to UV-B irradiation, while the CN group reduced stability and a methyl group in the 5 position of Trp made no significant difference in UV-B stability.
Based on the results of the initial UV-B irradiation experiments, additional non-proteinogenic amino acids were tested with 24 h exposure to UV-B irradiation, as well as UV-A and UV-C irradiation under ambient atmospheric conditions. Different halogen substitutions on Phe, Tyr and Trp were selected due to the altered stability of fluoro-substituted non-proteinogenic amino acids in the first round of UV-B experiments and previous research showing an increased stability of engineered proteins following incorporation of fluorinated non-proteinogenic amino acids (Buer et al., Reference Buer, Meagher, Stuckey and Marsh2012; Buer and Marsh, Reference Buer and Marsh2012; Biava and Budisa, Reference Biava and Budisa2014; Marsh, Reference Marsh2014). As can be seen in Fig. 5, phenylalanine (1) did not undergo >10% degradation following exposure to UV-A, UV-B, or UV-C irradiation and tyrosine (2) did not significantly degrade with UV-A or UV-C exposure, but degraded by over 50% with UV-B irradiation. The non-proteinogenic amino acids p-CN-Phe (1-1), p-F-Phe (1-3) and m-F-Phe (1-6) all had similar stability to UV-A and UV-C irradiation as the proteinogenic Phe, but p-CN-Phe was less stable to UV-B irradiation. 3-amino-Tyr was similarly stable to all UV wavelengths as its proteinogenic tyrosine (2) counterpart. The coumarin amino acid and the oxo-ala amino acid (5,4), which are not simple analogues of proteinogenic amino acids, were not particularly stable at any UV wavelengths tested. Sample LC chromatograms and the mass spectra of UV-A, UV-B and UV-C results are provided in the Supplementary Information.

Fig. 5. Stability of select aqueous phenylalanine and tyrosine analogous amino acids (from Figs. 2 and 3) to UV-A (365 nm), UV-B (302 nm) and UV-C (254 nm) irradiation when under ambient atmospheric conditions. The darker triplet of bands (1) is the proteinogenic amino acid (Phe). Tyrosine (2) is also a proteinogenic amino acid. All others (1-1 to 5) are non-proteinogenic amino acids whose structures are shown in Figs. 2 and 3. The y-axis shows the % of the amino acid remaining after exposure to the different UV wavelengths.
As can be seen in Fig. 1, the tryptophan analogues were much less stable at all UV wavelengths, especially UV-B. 5-CN-Trp (3-1) and Aza-Trp (3-2) were more stable than the proteinogenic tryptophan (Trp) following UV-A and UV-C irradiation, but less stable to UV-B irradiation. 5-hydroxy-Trp, 4-F-Trp and 5-Br-Trp were all more stable than Trp at the UV-A wavelength, but less so at the UV-C wavelengths. Both 5-hydroxy-Trp and 5-methyl-Trp were slightly more stable than Trp at UV-B wavelengths, although 5-methyl-Trp had decreased stability at UV-A and UV-C wavelengths.
Stability of amino acids following gamma irradiation
The higher gamma dose at ambient atmospheric conditions, 399 krad, resulted in nearly complete degradation of most proteinogenic and non-proteinogenic amino acids tested (1, 2, 4, 5, 3-1, 3-5, 3-7, 3-8). 51 and 96% of the p-F-Phe (1-3) and p-boronic amino acids fully degraded with the high gamma irradiation, as compared with full degradation of the proteinogenic Phe. The 3-amino-Tyr was also more stable under high gamma irradiation then native tyrosine, with only 35% of 3-amino-Tyr (2-1) degrading as compared with 100% of tyrosine degrading following a 399 krad gamma dose. The 5-F-Trp, 5-hydroxy-Trp and 4-F-Trp all had slightly enhanced stability under high gamma doses as compared with the proteinogenic Trp, with 85, 91 and 95% of these non-proteinogenic amino acids being degraded after a 399 krad dose, as compared with 100% of native Trp degrading. The lower gamma dose at ambient atmospheric conditions was two orders of magnitude less than the high gamma dose, at 3.99 krad and at this dose, all tested amino acids maintained significant (>80% intact) stability (Fig. 6). Sample LC chromatograms and the mass spectra of all 3.99 krad gamma exposed amino acids are provided in the Supplementary Information.

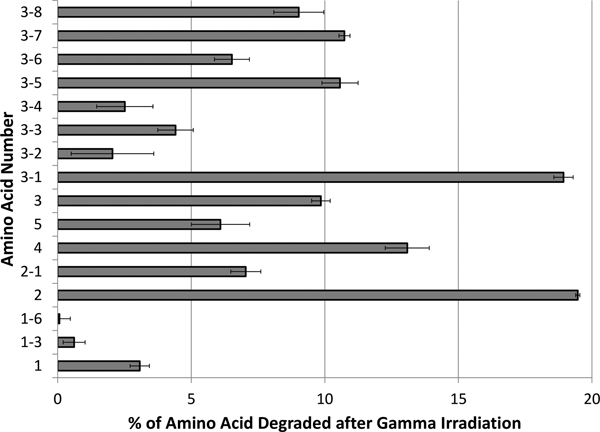
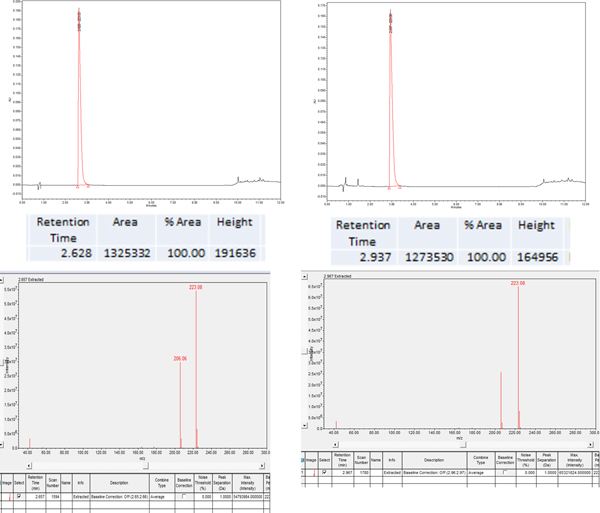
Fig. 6. Sample LC and MS chromatogram of 5-F-Trp (3-3) before (left) and after (right) exposure to 3.99 krad of gamma radiation. The values reported in Fig. 4 are the average of three trials. The LC absorbance at 254 nm was integrated and the mass spectrum extracted at the corresponding retention time in order to positively identify the non-degraded amino acid peak. 5-F-Trp had 95.6% (±0.66%) of the original amino acid remaining (so 4.4% degraded) following 3.99 krad of gamma radiation, as compared with proteinogenic tryptophan, which had 90.1% (±0.35%) of the original amino acid remaining (so 9.9% degraded) following 3.99 krad of gamma radiation.
Fluorine functionalization of Phe and Trp slightly increased the stability of these non-proteinogenic amino acids to gamma irradiation as compared with the proteinogenic Phe and Trp (Fig. 4). Likewise, amino-, and hydroxyl-functionalization of Tyr and Trp also slightly increased the stability of these non-proteinogenic amino acids to 3.99 krad gamma irradiation, as compared with proteinogenic Tyr and Trp (Fig. 4). However, the increased stability was not a large increase, ranging from 2 to 12%.
Discussion
This study focused on one class of amino acids, the aromatic amino acids, mainly phenylalanine (Phe), tryptophan (Trp) and tyrosine (Tyr) derivatives. The initial round of non-proteinogenic amino acids was chosen in order to represent a wide range of functional substitutions on the Phe, Tyr, or Trp scaffold, as well as test a couple of truly alternative, but still aromatic, non-proteinogenic amino acids (4 and 5).
As can be seen in Figs. 1 and 5, different amino acids had altered stability depending on the wavelength of UV light. In most cases, the least energetic UV-A radiation (365 nm) predictably caused the least amount of degradation in both the proteinogenic and non-proteinogenic amino acids. The absorbance spectrum from the UPLC-PDA of Tyr, Trp, Phe and all of the non-proteinogenic amino acids, except 4 and 5, show that these amino acids do not absorb at 365 nm radiation to any significant degree (see Supplementary Information for absorbance spectra of all amino acids). The greatest degree of UV degradation was seen with UV-B irradiation, instead of UV-C irradiation, even though UV-C rays are of higher energy than UV-B rays. Inspection of the absorbance spectra of Phe, Tyr and Trp, however, show a ‘valley’ in the absorbance spectrum of these amino acids in the 254 nm range (Supplementary Information; Beaven and Holiday, Reference Beaven, Holiday, Anson, Bailey and Edsall1952; Edelhoch, Reference Edelhoch1967). The UV-C lamp used in this study specified a wavelength of 254 nm. Although Tyr, Trp and Phe all absorb very strongly at shorter UV-C wavelengths (<220 nm) this low wavelength of UV-C radiation was not utilized in this study. The UV-B wavelength used was 302 nm, which is on the edge of absorbance of tryptophan, but outside the range of phenylalanine's absorption (Supplementary Information, Beaven and Holiday, Reference Beaven, Holiday, Anson, Bailey and Edsall1952). The results mimic this, with Phe barely degrading with this UV-B dose, Trp strongly degrading and Tyr being somewhere in the middle.
The non-proteinogenic phenylalanine analogues (Fig. 5) did not show a dramatic change in UV-stability as compared with native Phe. An exception to this is the p-CN-Phe (1-1), which had significantly decreased stability to UV-B irradiation as compared with native Phe. However, the Trp analogous non-proteinogenic amino acid stability varied significantly depending on UV wavelength exposure. Inspection of the absorbance spectrum of these amino acids obtained with the UPLC-PDA do not lend to a simple correlation of absorption spectra with stability (see Supplementary Information). For example, most substitutions at carbon 5 or 4 on tryptophan (3-3, 3-4, 3-5, 3-8) resulted in a red-shifted absorbance spectrum, as compared with native tryptophan. This slightly increased the amount of absorbed UV-B at 302 nm of these amino acids, which may correlate to decreased stability to the UV-B irradiation as compared with native tryptophan. While this was the case for 3-3 and 3-8, which had halogen functional groups at position 5, amino acids 3-4 and 3-5, which had a hydroxyl or methyl group at carbon position 5, had slightly increased stability to UV-B irradiation (Fig. 1). The changes in stability of the tryptophan analogues to UV-B irradiation correlated more closely as to whether an electron donating or an electron withdrawing group was added to tryptophan. For example, adding any electron withdrawing group to the tryptophan at carbon 5 or 4 (-CN, F, or Br) decreased the stability of that amino acid to UV-B degradation, whereas adding an electron donating group at carbon 5 (CH3 and OH) increased the stability of that amino acid to UV-B irradiation (Fig. 4).
Gamma rays have the smallest wavelength and the greatest energy of any wave in the electromagnetic spectrum. Gamma rays are ionizing radiation and as such have the ability to break molecular bonds in many compounds (such as DNA strands), as well as producing highly reactive radicals from the radiolysis of water molecules (Fricke, Reference Fricke1934; Henner et al., Reference Henner, Grunberg and Haseltine1982). There are many astronomical events that produce gamma rays and galactic cosmic rays and strong solar energetic particles can produce gamma rays and neutrons when impacting the surface of planets and moons (Dartnell et al., Reference Dartnell, Desorgher, Ward and Coates2007; Cooper et al., Reference Cooper, Sittler, Hartle and Sturner2008).
The gamma dosages in this study were chosen due to the Mars rover Curiosity radiation assessment detector data, which recorded approximately 20 mrad radiation exposure daily combined with 2–3 annual proton event spikes, each of which bombarded Mars with approximately a 2000 mrad dose (Hassler et al., Reference Hassler, Zeitlin, Wimmer-Schweingruber, Ehresmann, Rafkin, Eigenbrode, Brinza, Weigle, Böttcher and Böhm2013). This resulted in approximately 13 300 mrad of radiation per 365 days observed on the surface of Mars. Since microbial life on Earth is estimated to have taken at least 300 million year to evolve, 300 million (Earth) years on Mars would yield a radiation dose of 3.99 × 106 krad (Cavicchioli, Reference Cavicchioli2002; Schulze-Makuch et al., Reference Schulze-Makuch, Irwin and Guan2002; Griffin, Reference Griffin2013). Assuming atmospheric or surface shielding to block much of that, 399 krad was equal to 1/10 000 of the initial dose and 3.99 krad was equal to one millionth of the initial dose calculated and these values (399 and 3.99 krad) were the doses of gamma radiation that select amino acid solutions were exposed to using a cobalt-60 source.
All amino acids listed in Fig. 6 were tested with both high (399 krad) and low (3.99 krad) gamma-ray exposure. Gamma-ray exposure to water creates hydroxyl radicals, hydrated electrons and hydrogen atoms via radiolysis of water (Buxton et al., Reference Buxton, Greenstock, Helman and Ross1988). At the low (100 µM) aqueous concentrations of amino acids tested, it is assumed that these radicals are the species that are affecting and degrading the amino acids, and not the gamma rays directly (Buxton et al., Reference Buxton, Greenstock, Helman and Ross1988). Many of the amino acids tested completely degraded following the 399 krad gamma exposure. However, as mentioned in the Results section, p-F-Phe (1-3), p-boronic amino acid (1-5), 3-amino-Tyr (2-1), 5-F-Trp (3-3), 5-hydroxy-Trp (3-4) and 4-F-Trp (3-6) all had enhanced stability under high gamma doses as compared with the proteinogenic Phe, Tyr and Trp.
As expected, the lower gamma dosage did not completely destroy the original amino acids or create as many unresolvable side products that interfered with LC peak interpretation (Bonifačić et al., Reference Bonifačić, Štefanić, Hug, Armstrong and Asmus1998). No amino acids tested (Fig. 4) degraded by more than 20% following the low gamma dosage since this dose equates to approximately 24 µM of reactive radical species from the 3.99 krad of gamma irradiation. Similarly to what was seen following the high gamma irradiation dosage, fluorination of both Phe and Trp slightly increased the stability of these non-proteinogenic amino acids to gamma degradation, as compared with the proteinogenic Phe and Trp (Figs. 4 and 6). Likewise, amino-, and hydroxyl- functionalization of Tyr and Trp also slightly increased the stability of these non-proteinogenic amino acids to 3.99 krad gamma irradiation, as compared with proteinogenic Tyr and Trp (Fig. 4). However, the increased stability was not a large increase, ranging from 2 to 12%.
Interestingly, protein researchers and synthetic biologists have also found that substituting fluorinated non-proteinogenic amino acids for regular (non-fluorinated) amino acids often significantly increases the stability of the resulting engineered proteins due to the increased stability of the resulting hydrophobic core, suggesting fluorine may have a unique role in constructing super-stable proteins (Schröder and Meesters, Reference Schröder and Meesters2005; Buer and Marsh, Reference Buer and Marsh2012; Buer et al., Reference Buer, Meagher, Stuckey and Marsh2012). One of the reasons it is assumed that fluorine is not used in biological building blocks on Earth because the unusually strong C-F bond, as well as the stronger hydrogen bonding capability of the F-H functional group, creates molecules that have an energetic barrier that is too high for typical metabolic reactions that require rapid and dynamic bond breakages with minimal energy expenditure. However, extraterrestrial environments with high radiation exposures may favour biological macromolecules with the stronger bond formation and enhanced macromolecular stability that fluorinated monomers would impart to biological polymers.
Conclusions and future work
Many non-proteinogenic acids tested herein have equal, or greater, stability than their proteinogenic counterparts when exposed to varying wavelengths of UV radiation and gamma radiation. Increased stability of non-proteinogenic amino acids to UV and gamma irradiation was not dramatic (<12% increase), but it is notable that similar to thermal and denaturing stability studies in synthetic biology, fluorination of both tryptophan and phenylalanine increases their stability to gamma radiation degradation (Buer and Marsh, Reference Buer and Marsh2012; Buer et al., Reference Buer, Meagher, Stuckey and Marsh2012). These results suggest that certain non-proteinogenic amino acids may be more stable than the proteinogenic aromatic amino acids under high radiation conditions that may be found extraterrestrially, making them possible alternative building blocks for the evolution of extraterrestrial life. Future work will further characterize these amino acids under more intermediate gamma irradiation exposure, analyse the main transformation products, as well as testing the relative UV and gamma stability of non-aromatic amino acids.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1473550418000381
Acknowledgements
The work described herein was supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy, through award DE-FC02-04ER15533. We would like to thank Karen Dunbar and Matt Courtney at Ivy Tech Community College for their assistance in finding suitable community college students to participate in this research, and Professors Bruce Hrivnak and Mike Watters at Valparaiso University in their assistance in finding funding sources. The radiolysis experiments were conducted at the Radiation Laboratory, the University of Notre Dame, which is supported by the US Department of Energy (DOE #5220). This project was funded due to grants from the Indiana Space Grant Consortium-National Aeronautics Space Agency, a National Science Foundation Major Research Instrumentation Grant with which our University purchased the UPLC-MS instrument (NSF-MRI, Grant # 1531360) and the Creative Works Committee grants from Valparaiso University.
Conflict of interest
The authors have no competing interests.