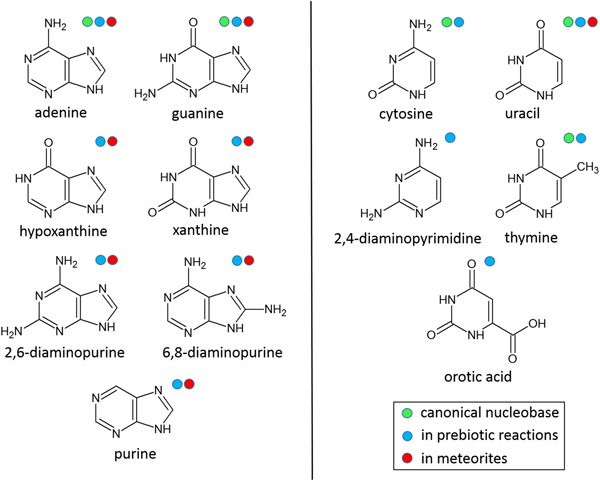
Introduction
Carbonaceous chondrites are meteorites that contain abundant indigenous organic compounds, including many of biological significance such as amino acids and nucleobases (Sephton, Reference Sephton2002; Burton et al., Reference Burton, Stern, Elsila, Glavin and Dworkin2012). Meteorites are continuously delivered to the Earth today (Halliday et al., Reference Halliday, Blackwell and Griffin1989) and were likely delivered at a much higher rate early in its history (Schoenberg et al., Reference Schoenberg, Kamber, Collerson and Moorbath2002; Gomes et al., Reference Gomes, Levison, Tsiganis and Morbidelli2005). Thus, these materials are significant in that they represent major initial inputs of organic materials to early solar system planetary surfaces and may have contributed to the origins of life on Earth (Oró, Reference Oró1961; Chyba and Sagan, Reference Chyba and Sagan1992). One generally accepted scheme for the synthesis of these meteoritic organics is that they are derived from the energetic processing of simpler compounds that had condensed on dust grains. Eventually, these grains were incorporated into larger parent bodies such as asteroids. Pre-terrestrial aqueous alteration, which is attributed to radiogenic heating and melting of ice on the parent asteroid shortly after accretion, likely influenced the organics found in meteorites as well (Browning et al., Reference Browning, McSween and Zolensky1996; Wilson et al., Reference Wilson, Keil, Browning, Krot and Bourcier1999).
In modern analyses of meteorites, the distribution and abundance of indigenous organic compounds may not necessarily reflect those originally present billions of years ago when carbonaceous chondrites were delivered to the prebiotic Earth. One potential reason for this is the prolonged radiolysis of organic compounds while in the dry state (after the aqueous alteration phase) since long-lived radionuclides likely contributed significant doses of high-energy radiation within meteorite parent bodies over their entire lifetimes (Urey, Reference Urey1955, Reference Urey1956). The early solar system contained a variety of radionuclides, which are well-documented in meteorites (Lee et al., Reference Lee, Papanastassiou and Wasserburg1976, Reference Lee, Shu, Shang, Glassgold and Rehm1998; McKeegan et al., Reference McKeegan, Chaussidon and Robert2000; Huss et al., Reference Huss, MacPherson, Wasserburg, Russell and Srinivasan2001). Though 26Al is relatively short-lived (t 1/2 ~ 7.2 × 105 y), it may have been especially abundant in early solar system materials (Diehl et al., Reference Diehl, Dupraz, Bennett, Bloemen, Hermsen, Knodlseder, Lichti, Morris, Ryan, Schonfelder, Steinle, Strong, Swanenburg, Varendorff and Winkler1995; Macpherson et al., Reference Macpherson, Davis and Zinner1995; Russell et al., Reference Russell, Srinivasan, Huss, Wasserburg and MacPherson1996). 40K, 235U, 238U and 232Th have long half-lives and undergo complex decay resulting in the emission of high-energy particles and γ-rays. These γ-rays are of sufficient energy to interact with matter via Compton scattering, effectively transferring photon energy to electrons and creating positive ion radicals, which then could lead to degradation or possibly complexification of organic compounds. Cosmic rays are also prevalent; however, they are not deeply penetrating and the absorbed energy would affect only the surface layers (on the range of meters) in asteroids (Draganic et al., Reference Draganic, Draganic and Vujosevic1984).
There has been a long history of research to understand how organic compounds can be synthesized and survive in harsh cosmic environments through laboratory experiments using radiolytic and photolytic processing (Bernstein et al., Reference Bernstein, Sandford, Allamandola, Chang and Scharberg1995; Hudson and Moore, Reference Hudson and Moore2001). Multiple investigations have examined the effect of ionizing radiation on the survival and racemization of amino acids (Cataldo et al., Reference Cataldo, Ragni, Iglesias-Groth and Manchado2011b, Reference Cataldo, Angelini, Iglesias-Groth and Manchado2011a, Iglesias-Groth et al., Reference Iglesias-Groth, Cataldo, Ursini and Manchado2011) in an effort to explain the non-racemic mixtures of amino acids observed in meteorites (Cronin and Pizzarello, Reference Cronin and Pizzarello1997; Pizzarello and Cronin, Reference Pizzarello and Cronin2000; Glavin and Dworkin, Reference Glavin and Dworkin2009).
Here, various nitrogen heterocycles in the solid-state were exposed to γ-radiation from a 60Co source to investigate the stability of these compounds with respect to total radiation dose and dose rate. The nitrogen heterocycles studied (see Fig. 1) included the canonical nucleobases, which are universal to nucleic acids and closely related purines and pyrimidines that have been identified in cyanide reactions (Sanchez et al., Reference Sanchez, Ferris and Orgel1967; Ferris et al., Reference Ferris, Joshi, Edelson and Lawless1978; Ferris and Hagan, Reference Ferris and Hagan1984; Miyakawa et al., Reference Miyakawa, Cleaves and Miller2002) and/or extraterrestrial meteorites (Vandervelden and Schwartz, Reference Vandervelden and Schwartz1977; Stoks and Schwartz, Reference Stoks and Schwartz1979, Reference Stoks and Schwartz1981, Reference Stoks and Schwartz1982; Martins et al., Reference Martins, Botta, Fogel, Sephton, Glavin, Watson, Dworkin, Schwartz and Ehrenfreund2008; Callahan et al., Reference Callahan, Smith, Cleaves, Ruzicka, Stern, Glavin, House and Dworkin2011) and thought to be important for prebiotic chemistry and the origin of life on early Earth. Simulating hundreds of millions of years of radiation over the course of a few months raises reasonable questions about the realism of such simulations. As it is impossible to carry out such experiments on solar system timescales, we have designed our experiments as efficiently and practically as possible to investigate the radiolytic stability of nitrogen heterocycles and to better understand what the distribution and abundance of these compounds may have been in meteorites delivered to Earth approximately 4 billion years ago.
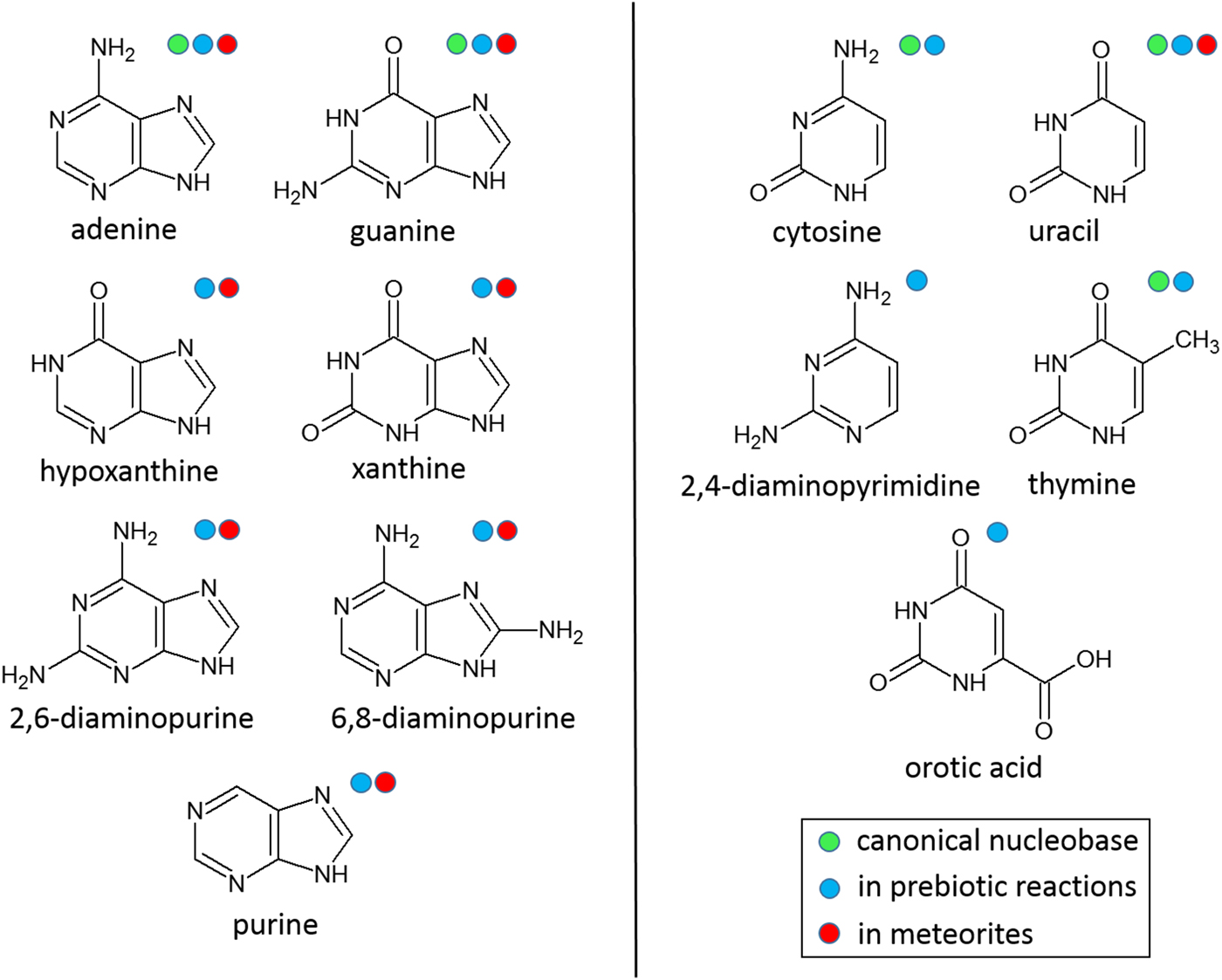
Fig. 1. Structures of nitrogen heterocycles that underwent γ-irradiation studies. These nitrogen heterocycles are considered important for prebiotic chemistry leading to the origin of life (i.e. they are identified in plausible prebiotic reactions and/or extraterrestrial meteorites) or are essential for biology.
Methods
Sample preparation of γ-irradiated samples and controls
All glassware used in these experiments was wrapped in aluminum foil and baked at 500 °C for 24 h under air to remove organic contaminants. Ultrapure water (18.2 MΩ·cm) produced by a water purification system was used for this study.
Individual solutions of nitrogen heterocycles were prepared by dissolving the reference standard with 0.1 M NH4OH to a concentration in the low millimolar range. 400 µl of each nitrogen heterocycle solution was added to individual glass ampoules and heated to dryness at 60 °C using an oven. Once dry, these ampoules were sealed under vacuum on a glass Schlenk line using a hand-held butane torch. For each nitrogen heterocycle, 11 ampoules were prepared: four non-irradiated controls and seven samples for radiolysis.
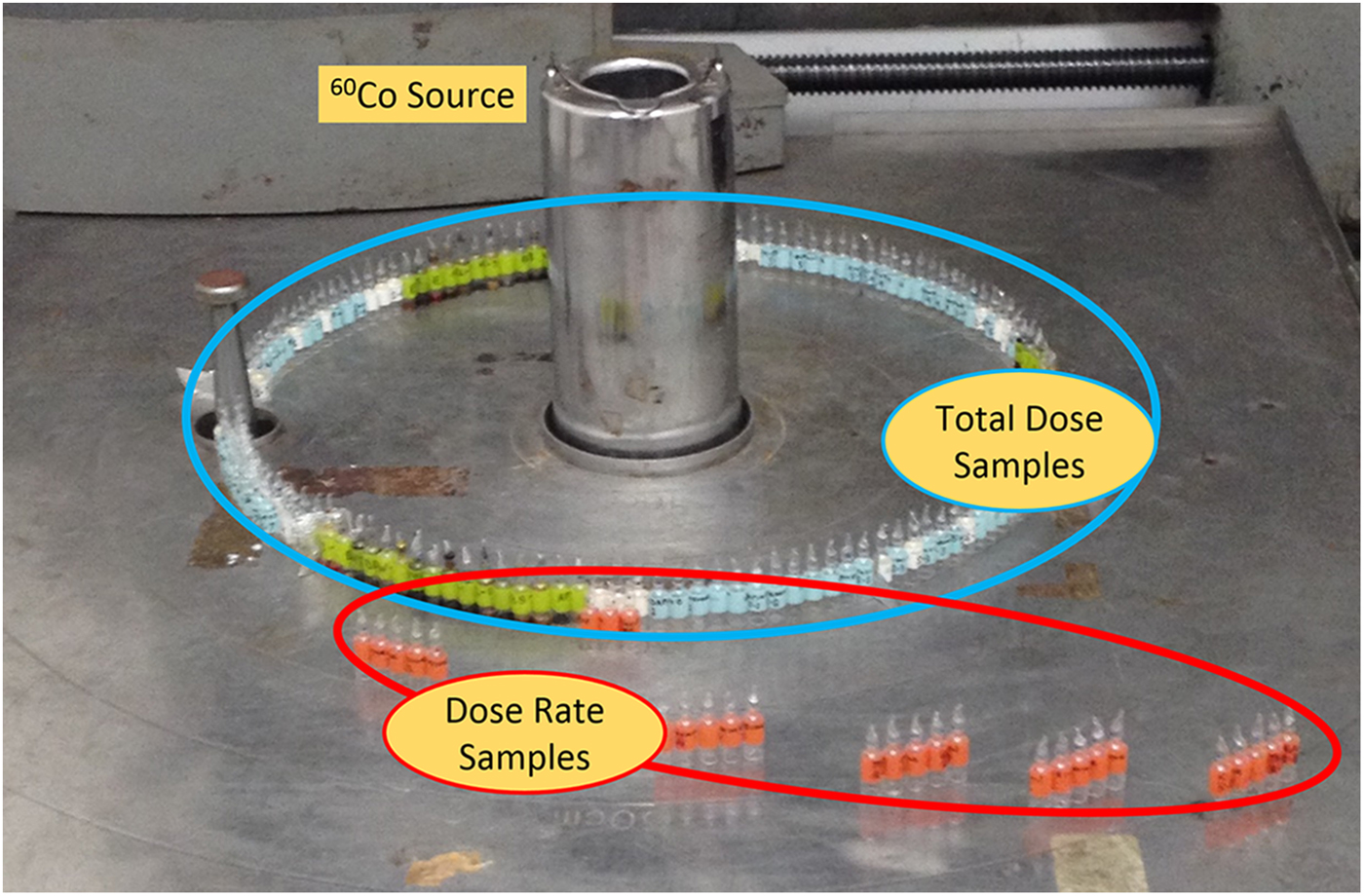
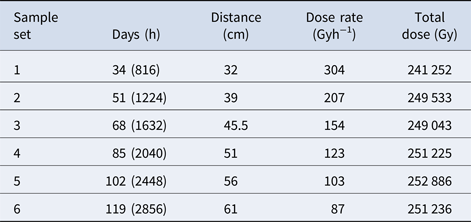
A 60Co source at the Research Laboratory for Nuclear Reactors at the Tokyo Institute of Technology was used to provide γ-radiation for this study. One set of ampoules for each heterocycle was placed at a distance of 30 cm from a 60Co source in order to receive a dose rate of ~350 Gy h−1. These ampoules were placed around the γ-radiation source (Fig. 2) and one ampoule from each set was removed from the source at ~17-day intervals in order to produce sets of samples with increasing doses of γ-radiation (see Table 1). The maximum total dose was ~0.992 MGy, which took 119 days to complete. Additionally, sets of canonical nucleobases were placed at varying distances from the radiation source (Fig. 2) in order to receive a total dose of ~250 kGy, but with different dose rates (see Table 2). A slower dose rate, corresponding to a sample placed further away from the 60Co source, means a longer time on the source in order for the samples to receive the same total dose of ~250 kGy. Irradiation experiments took place at room temperature. After irradiation of samples was complete, samples and non-irradiated controls were carefully packaged and shipped to Boise State University for work-up and sample analysis.
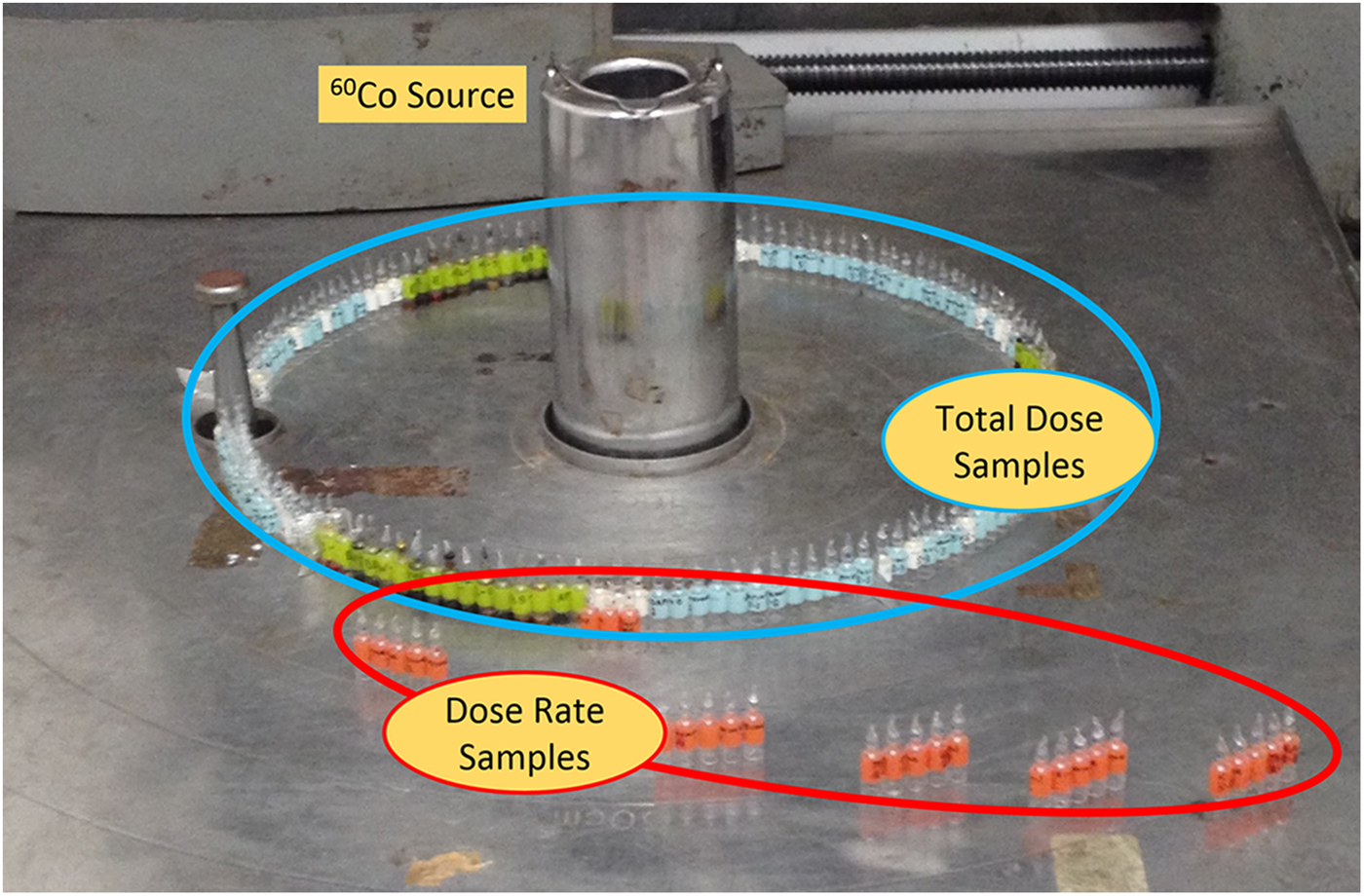
Fig. 2. Photograph of sample placement around the 60Co source. Samples of solid-state nitrogen heterocycles in vacuum-sealed glass ampoules are specifically placed for two separate studies: the effect of total dose (in blue circle) and dose rate (in red circle).
Table 1. Total dose and irradiation time for N-heterocycles
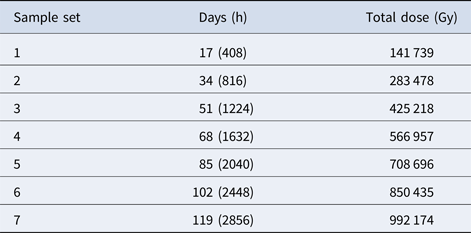
Table 2. Dose rate data
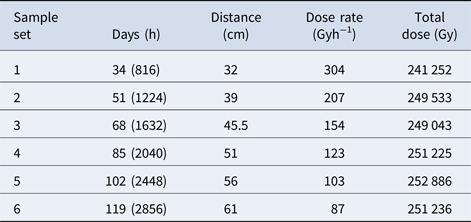
To prepare samples for high-performance liquid chromatography (HPLC) analysis, sealed ampoules were cracked open using disposable ampoule openers (a popping sound was observed upon opening ampoules, which indicated that the vacuum seal had not been compromised). 400 µl 0.1 M NH4OH was placed inside each ampoule and mixed using a vortex mixer on low setting for 30 s. The ampoules were then allowed to stand for 20 min in order to dissolve the individual nitrogen heterocycle before transferring to an HPLC vial. An additional 400 µl 0.1 M NH4OH was added to the ampoule and the process was repeated: vortex mix, let stand for 20 min, rinse the sides and then solution transfer. A final volume of 200 µl 0.1 M NH4OH was added and the procedure was performed one last time to ensure quantitative transfer. These steps provided a 1 ml sample. Typically, a 100-fold dilution for nitrogen heterocycle sample solutions was performed before HPLC analysis for accurate quantitation using a standard concentration curve.
Guanine does not readily dissolve in 0.1 M NH4OH; therefore, guanine samples were dissolved in 1 M NH4OH according to a slightly modified procedure from that used for the above samples. Two 400 µl and one 200 µl aliquots of 1 M NH4OH were used as outlined above. After this, nine successive 1 ml volumes of 1 M NH4OH were used to quantitatively rinse the ampoules for sample transfer into a 25 ml glass vial. From this guanine in 1 M NH4OH solution (10 ml), a 1:10 dilution using ultrapure water was performed, which resulted in the same 100-fold dilution as described earlier. Sample solutions were analysed immediately by HPLC.
High performance liquid chromatography
Irradiated samples, non-irradiated controls and reference standard solutions were analyzed using a Thermo Scientific Accela HPLC/UHPLC coupled to an Accela photodiode array detector (PDA). Nitrogen heterocycle separation was achieved by injecting 10 µl sample solution onto a Phenomenex Synergi 4 µ Fusion reverse phase column (2 mm × 150 mm; 80 Å pore size). The flow rate was 200 µl min−1 and the column temperature was maintained at 30 °C. Mobile phase A was composed of 20 mM ammonium acetate buffer at pH 4.5 and mobile phase B was acetonitrile. The ammonium acetate buffer was prepared via NH4OH titration of 20 mM acetic acid solution to pH 4.5. The following HPLC gradient was used: 0–10 min 0–45% B, 10–12 min 45–100% B, 12–17 min 100% B, 17–19 min 100–0% B and 19–29 min 0% B (equilibration of column). The PDA detector recorded the UV spectrum from 200–400 nm, although 260 nm was typically used for nitrogen heterocycle analysis. All samples were analysed in triplicate.
To prepare reference standard solutions, nitrogen heterocycles were dissolved in 0.1 M NH4OH to produce ~3 mM stock solutions. Serial dilutions were then performed to prepare 1, 10, 50 and 100 µM working solutions. Four-point calibration curves for each nitrogen heterocycle were measured and determined to be linear (R 2 > 0.99) in this concentration range.
Recovery of irradiated nitrogen heterocycles was calculated in two ways: (1) HPLC peak area of the irradiated nitrogen heterocycle compared with HPLC peak area of the non-irradiated control and (2) mole ratio of the irradiated nitrogen heterocycle to the non-irradiated control using the corresponding calibration curve. These two methods gave nearly identical results. Per cent recoveries shown in Tables 3–5 are based on the first method using the equation below:
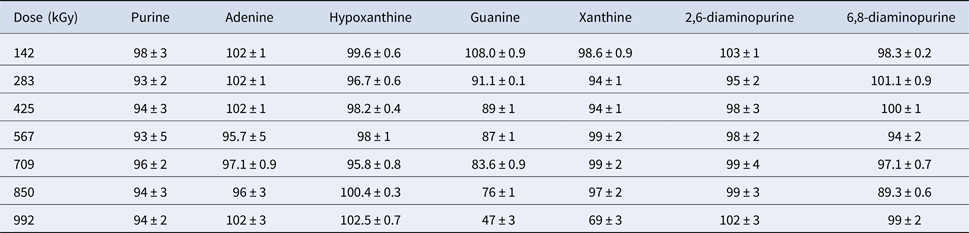
Table 3. Percent recovery of γ-irradiated purines

Table 4. Percent recovery of γ-irradiated pyrimidines

NS indicates no sample (ampoule was broken during shipping).
Table 5. Percent recovery of γ-irradiated nucleobases using different dose rates. Total dose was ~250 kGy
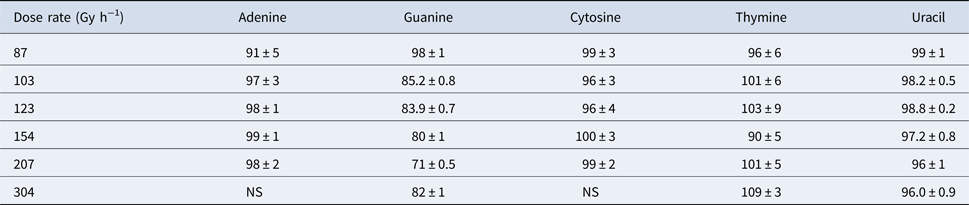
NS indicates no sample at this dose rate.
Results and discussion
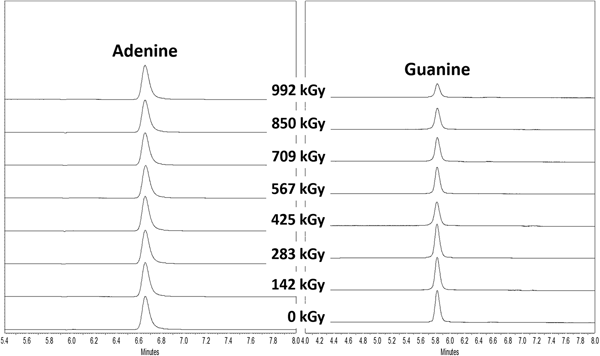
For all irradiated samples, only one peak was detected in the UV chromatogram, which corresponded to the nitrogen heterocycle of interest. Furthermore, no degradation products were detected in UV chromatograms using the full spectral range from 200 to 400 nm. This may be due to non-volatile degradation products lacking a chromophore in this UV detection range or that, more likely, degradation products were volatile and lost during the sample work-up. As an example, UV chromatograms of irradiated adenine and guanine along with their controls (non-irradiated adenine and guanine) are shown in Fig. 3.

Fig. 3. UV chromatograms at 260 nm of γ-irradiated adenine and guanine at increasing doses along with their control samples (0 kGy).
In the solid state, most of the purines and pyrimidines in this study appear to be remarkably stable to γ-radiation up to doses of 1 MGy (see Tables 3 and 4). All of the canonical nucleobases, with the exception of guanine, were extremely stable to γ-radiation, which always showed >90% recoveries. Similar stability of nucleobases was also observed by Pilling et al. who used soft X-rays at 150 eV to irradiate solid-state adenine and uracil and concluded that only small reductions were measured by in situ Fourier transform infrared spectroscopy (Pilling et al., Reference Pilling, Andrade, do Nascimento, Marinho, Boechat-Roberty, de Coutinho, de Souza, de Castilho, Cavasso, Lago and de Brito2011). Past studies have also shown that nucleobases are much more resistant to ionizing radiation than amino acids, another class of organic compounds necessary for life as we know it (Pilling et al., Reference Pilling, Andrade, do Nascimento, Marinho, Boechat-Roberty, de Coutinho, de Souza, de Castilho, Cavasso, Lago and de Brito2011; Cataldo et al., Reference Cataldo, Angelini, Iglesias-Groth and Manchado2011a, Reference Cataldo, Ragni, Iglesias-Groth and Manchado2011b; Iglesias-Groth et al., Reference Iglesias-Groth, Cataldo, Ursini and Manchado2011; Cherubini et al., Reference Cherubini, Ursini, Cataldo, Iglesias-Groth and Crestoni2014).
Purine is very stable to γ-radiation. Adenine (6-aminopurine), 2,6-diaminopurine and 6,8-diaminopurine are also very stable to γ-radiation, which demonstrates that adding basic amine groups in different positions to purine does not reduce or enhance resistance to γ-irradiative decomposition. Hypoxanthine, a deamination product (replacement of an amino group with a carbonyl group) of adenine, was extremely stable to γ-radiation, which suggests that purines substituted at the 6-position are also relatively stable.
The recovery of guanine decreases as the radiation dose increases. Also, guanine displays the highest decomposition of all the compounds studied here, a reduction of 53% at ~1 MGy. Xanthine remained very stable to γ-radiation with the exception of the last data point (992 kGy), which showed a 31% decrease compared with its control. The exact reason for this decrease is unknown, although we may draw some insight by comparing xanthine to guanine. Xanthine is a deamination product of guanine and their structural similarity may explain their response to γ-radiation; guanine and xanthine were the two nitrogen heterocycles with the highest degree of decomposition. Methylation of xanthine does not alter its high radiolytic stability. In a previous study, theobromine (3,7-dimethylxanthine), theophylline (1,3-dimethylxanthine) and caffeine (1,3,7-trimethylxanthine) showed very little degradation to ionizing radiation (9.96 MeV electron beam) up to doses of 400 kGy (Marciniec et al., Reference Marciniec, Stawny, Olszewski, Kozak and Naskrent2013).
All five pyrimidines studied here were also very stable to γ-radiation and methyl, amine and carboxylic acid substitutions did little to alter this stability.
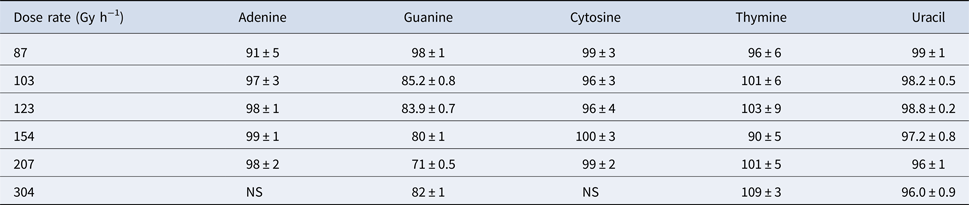
The second part of our study investigated the effect of dose rate on the degradation of the canonical nucleobases. Percent recoveries of γ-irradiated nucleobases were determined at different dose rates (between 87 and 304 Gy h−1) while the total dose remained constant (~250 kGy), which is shown in Table 5. For adenine, cytosine, thymine and uracil, the percent recoveries of γ-irradiated nucleobases were relatively high, ranging from 91 to 109%, which suggested that the radiolysis of these canonical nucleobases in the solid-state did not significantly depend on the dose rate. The lower recovery of adenine at a dose rate of ~87 Gy h−1 is calculated as an outlier at 95% confidence using a Dixon's Q-test; however, we cannot completely rule out that a lower dose rate does not have an effect on adenine.
Guanine is a different story. At a total dose of ~250 kGy, the recovery of guanine steadily declines from 98% to 71% as the dose rate increases from 87 to 207 Gy h−1; however, at the highest dose rate of 304 Gy h−1, the recovery of guanine is around 82%. Nonetheless, the effect of dose rate on guanine is readily apparent when compared with the other canonical nucleobases. It is also interesting to note that the canonical nucleobases (adenine, cytosine, thymine and uracil) that were most stable to the highest doses of γ-radiation seemed to be the least affected by different dose rates. For guanine, appreciable decomposition due to γ-radiation may indicate greater sensitivity to dose rate. Further investigation of more nitrogen heterocycles would be necessary to see if these trends are consistent. Finally, these results suggest that both total dose and dose rate should be examined with each particular compound for a full understanding of its radiation chemistry.
Comparison of results with estimated cosmic radiation doses
Draganic et al. have estimated that a cometary nucleus of 10 km radius would contain radionuclides that would emit ~14 MGy of radiation over 4.5 billion years (the age of the Solar System); the majority of this radiation (11 MGy) occurring in the first tens of millions of years due to 26Al decay (Draganic et al., Reference Draganic, Draganic and Vujosevic1984). Many other studies investigating the radiolysis of organic compounds have adopted this dose of 14 MGy for asteroids and comets in general (Cataldo et al., Reference Cataldo, Angelini, Iglesias-Groth and Manchado2011a, Reference Cataldo, Ragni, Iglesias-Groth and Manchado2011b; Iglesias-Groth et al., Reference Iglesias-Groth, Cataldo, Ursini and Manchado2011; Cherubini et al., Reference Cherubini, Ursini, Cataldo, Iglesias-Groth and Crestoni2014). Our total dose of ~1 MGy approximates hundreds of million years’ worth of γ-radiation emitted in meteorite parent bodies due to slow radionuclide decay. Linear extrapolation of our results suggests that there would be only small differences, less than an order of magnitude, between the purine nitrogen heterocycle abundances measured in carbonaceous chondrites today with those from the early solar system (with the exception of guanine). It is important to point out that our experiments involve the radiolysis of pure nitrogen heterocycles only and do not take into account other organics and mineral matrices that co-exist within asteroids. Therefore, we would consider any extrapolation to organic abundance as a first approximation only. Nonetheless, we conclude that nitrogen heterocycles would be very stable in dry parent bodies and persist for billions of years, hence their detection in carbonaceous chondrites today (Callahan et al., Reference Callahan, Smith, Cleaves, Ruzicka, Stern, Glavin, House and Dworkin2011; Smith et al., Reference Smith, Callahan, Gerakines, Dworkin and House2014).
Conclusions
Many purine and pyrimidine nitrogen heterocycles in the solid state are remarkably stable when γ-irradiated to ~1 MGy, which is the dose equivalent of γ-radiation released by radionuclide decay in asteroids in ~350 million years (assuming a total radiation dose of 14 MGy and simple linear decay only). The effect of dose rate on the survival of nitrogen heterocycles may be compound dependent; however, we observed that different dose rates influenced the recovery of guanine, which turned out to be the nitrogen heterocycle most prone to decomposition. From our radiolysis data, we suggest that the abundance of these purine nitrogen heterocycles, with the exception of guanine, as measured presently in CM2 carbonaceous chondrites is largely reflective of their abundance at the time aqueous alteration stopped in the parent bodies. The absence of other nitrogen heterocycles (derived from NH4CN chemistry) in carbonaceous chondrites may be due to degradation that occurred during the period of aqueous alteration. Finally, we suggest that guanine may have been more abundant in young parent bodies of carbonaceous chondrites (compared with guanine found in carbonaceous chondrites today) and that increased abundances may have made this nucleobase particularly favourable for prebiotic chemistry leading to a primitive genetic material.
Acknowledgements
This project was supported by a Seed Grant (6PRJ000890) from the ELSI Origins Network (EON), which is supported by a grant from the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation. This work was also partially supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas ‘Hadean Bioscience’, grant number: JP26106003. Phillip Hammer acknowledges support from a 2016–2017 NASA Earth and Space Science Fellowship (NNX16AP10H). We also thank Dr Karen Smith (Boise State University) for reading this manuscript and providing helpful comments.