Surgical site infections (SSIs) are the most common hospital-acquired infections among surgical patients and the leading cause of hospital readmission after surgery; they impose a major financial burden.Reference Le Manach, Collins and Bhandari1, Reference Zimlichman, Henderson and Tamir2 It is generally accepted that the contamination of the surgical wound mainly occurs at the time of surgical procedure in the operating room (OR), eventually leading to SSI. Main routes of microbial entry into an open, clean, surgical wound include from the patient’s skin, from the surgical staff, by airborne microbes, or by contaminated surgical instruments.Reference Tammelin, Hambraeus and Ståhle3
The literature suggests an impact of surgical team behavior on the air microbial contamination and the SSI risk.Reference Birgand, Saliou and Lucet4 Door openings have been demonstrated to adversely affect air exchange, air quality, and positive pressure in the OR, affecting the air microbial contamination in the OR.Reference Mears, Blanding and Belkoff5 Current guidelines do not include specific recommendations regarding the best OR staff behavior (except for clothing rules and hand hygiene) to decrease the exogenous risk of SSI.Reference Allegranzi, Zayed and Bischoff6, Reference Berríos-Torres, Umscheid and Bratzler7 New technologies using motion-capture systems present an opportunity to objectively and continuously assess the global OR staff dynamics and behavior during surgical intervention in OR.Reference Lucet, Laouenan and Chelius8
In this study, we aimed to objectively describe and assess staff behaviors in the OR and their variability by recording staff movements using a motion tracking system and door-openings detection system. We also assessed correlations between movements of the OR personnel and the SSI risk, as approximated by surrogates of the exogenous infectious risk, in a panel of ORs from 2 clean surgical specialties.
Methods
Population and location of the study
This observational multicenter study was conducted at 10 facilities (5 university hospitals and 5 private hospitals) in France in a convenience sample of 13 ORs (6 in cardiac surgery and 7 in orthopedic surgery).Reference Birgand, Azevedo and Toupet9 Procedures requiring full median sternotomy and total hip replacement and knee replacement were included. The population observed comprised OR personnel and any other person likely to enter the OR during a surgical procedure. At the preoperative stage, patients were informed orally by surgeons, anesthetists, or infection control specialists of the ongoing study, and an information letter was systematically given. The ethics committee approved this study and granted a waiver of informed consent patients. Consent forms were obtained from the OR members.
System of motion capture
A technology of motion capture based on a video-tracking system was adapted for the objective, continued, and prolonged detection and characterization of movements in the OR. A network of 8 video cameras (VICON-Bonita, Vicon, Los Angeles, CA)Reference Isableu, Hansen, Rezzoug, Gorce and Pagano10 was fixed upright to the wall using suction-cup supports. Markers placed on the surgical caps/hoods of each person entering the OR were located in 3 dimensions (3D) using the Vicon Tracker software using spatial triangulation.Reference Birgand, Azevedo and Toupet11 The 68 LEDs situated on each camera produced an infrared light reflected by hemispherical markers and acquired by the optic sensors. The detection of the same marker by different cameras allows its 3D positioning. The motion capture was performed by continuous tracking of reflective markers placed on the surgical caps/hoods of each person entering the OR. Different markers distinguished different professional categories: surgeons, anesthesiologists (doctors, nurse and extracorporeal circulation personal), OR nurses, and others (including visitors). A study coordinator holding a marker stayed in the OR during the procedures, moving only for the sampling and to provide technical assistance.
Two autonomous wireless inertial sensors (HiKoB FOX, HiKoB, Villeurbanne, France) were fixed on each door of the OR and synchronized with the motion-tracking system. Door openings were determined offline based on data collected by the inertial sensors.
The motion-tracking system remained in place for 1 week in the same OR to allow people to acclimate to its presence and to take into account potential behavioral modifications due to the Hawthorne effect. Data acquisition started at skin incision and continued until wound closure. Door-opening sensors remained in some ORs for 1 additional week after the removal of the video cameras. The OR staff were not informed of the persistence of doors sensors. Thus, the comparison of the frequency of door openings during and after removal of the motion-tracking system allowed us to estimate the impact of the Hawthorne effect.
Surrogates of the infectious risk
Microbiological air counts were measured using an impactor air sampler (Air-test Omega, LCB, La Salle, France) at a flow rate of 100 L per minute for 5 minutes (500 L) on trypticase soy agar (BioMerieux, Marcy-l’Étoile, France), which was then incubated for 4 days at 30°C. Air counts were expressed as colony-forming units (CFU) per cubic meter. Samples were taken at the time of skin incision, 15 minutes after bone was cut (sternum or femur) and at wound closure.
Particle count was performed using a photodetection device (HandiLaz Mini, Boulder, CO) continuously from incision to wound closure.Reference Dharan and Pittet12 The particle analyzer sampled for 1 minute every 5 minutes from the patient entry into through exit from the OR at a rate of 28.3 L per minute (1.0 ft3/min). Particles were classified by diameter into 3 sizes: 0.3 µm, 0.5 µm, and 5 µm. Both particle and microbiological air counts were performed near the patient’s head.
A sample from the operated wound was performed before closure and prior to antiseptic aspersion. We used the sampling method described previouslyReference Hambraeus, Hoborn and Whyte13 using sterile pads of polyamide-polyester-viscose placed on subcutaneous tissue for 1 minute. Microorganisms were extracted by vortexing the pads in phosphate buffer (PBS) with Tween 80 at 2% and lecithin at 0.3% (Hyphen BioMed, Neuville sur Oise, France). For each pad, an aliquot of 0.5 mL phosphate buffer was cultured on blood agar after 48 hours of aerobic and anaerobic incubation, and colonies were counted without further identification.
Data collection
The following information was collected: (1) the surgical procedure, including the surgical specialty, procedure and technique used, incision time, preselected procedure periods described above and closure time; (2) surgical environment characteristics, including type of air filtration, either laminar airflow or turbulent, air changes per hour, positive pressure and the class of air cleanliness for airborne particulate level (ISO 14644). The architecture of the OR was also collected, including size and volume.
Statistical analysis
The results of particle counts were log10 transformed. Numbers of colony-forming units cultured from wounds in aerobic and anaerobic media were added up and computed to obtain the number of colony-forming units per square centimeter of wounds. Results of the wound culture were categorized into 3 classes: (1) negative culture, (2) 1–10 CFU/100 cm2, and (3) >10 CFU/100 cm2. Microbiologic air counts were also categorized into the following 3 different classes: negative, 1–10 CFU/m3, and >10 CFU/m3. These stratifications were performed using the 25th and the 75th percentile distributions. Continuous variables were compared using Mann–Whitney U and proportion using χ2 tests, as appropriate.
To determine potential risk factors for an increase of particles and air microbial counts, univariate linear mixed models for longitudinal data with a random intercept for each intervention and each OR and a random slope for time were used. The unstructured covariance matrix were used for the random-effects model. The Satterthwaite method was used to compute the denominator degrees of freedom for the tests of fixed effects models. Behaviors observed (ie, numbers of door openings, number of persons, or the total movements by persons) during the 5 minutes before the particle count (corresponding to the period between 2 particle counts) were considered to estimate associations. This period was pragmatically selected to consider the quasi-instantaneousReference Weiser, Shemesh, Chen, Bronson and Moucha26 impact of door openings on the positive pressure and airflow in the OR and to leave enough time to obtain explicative events (eg, door openings).
Significant variables at 0.1 were selected for the multivariate model. A backward selection was used on the multivariate model. Conditional studentized residuals were checked. A subanalysis was performed on interventions with video data to precisely evaluate the effect of number of persons and staff movements on increase of particles.Reference Littell, Milliken, Stroup, Wolfinger and Schabenberger14
The same method was applied to determine potential risk factors for an increase of air microbial count. Unlike the previous model, only 3 measures of air microbial count were done. Behaviors observed between the patient’s arrival and the first measure, the first and the second measure, and the second and the third measure were considered to estimate associations (Appendix Fig. A1). SAS version 9.3 statistical software (SAS Institute, Cary, NC) was used to perform all statistical analyses.
Results
General data
A total of 62 surgical procedures were observed from the May 14 through December 20, 2013. Three procedures were excluded due to incomplete data collection, for a total of 59 procedures (25 in cardiac and 34 in orthopedic surgery) included in the door-opening assessment. Data on intraoperative staff movements were comprehensively collected during 34 of the 59 procedures (Fig. 1).
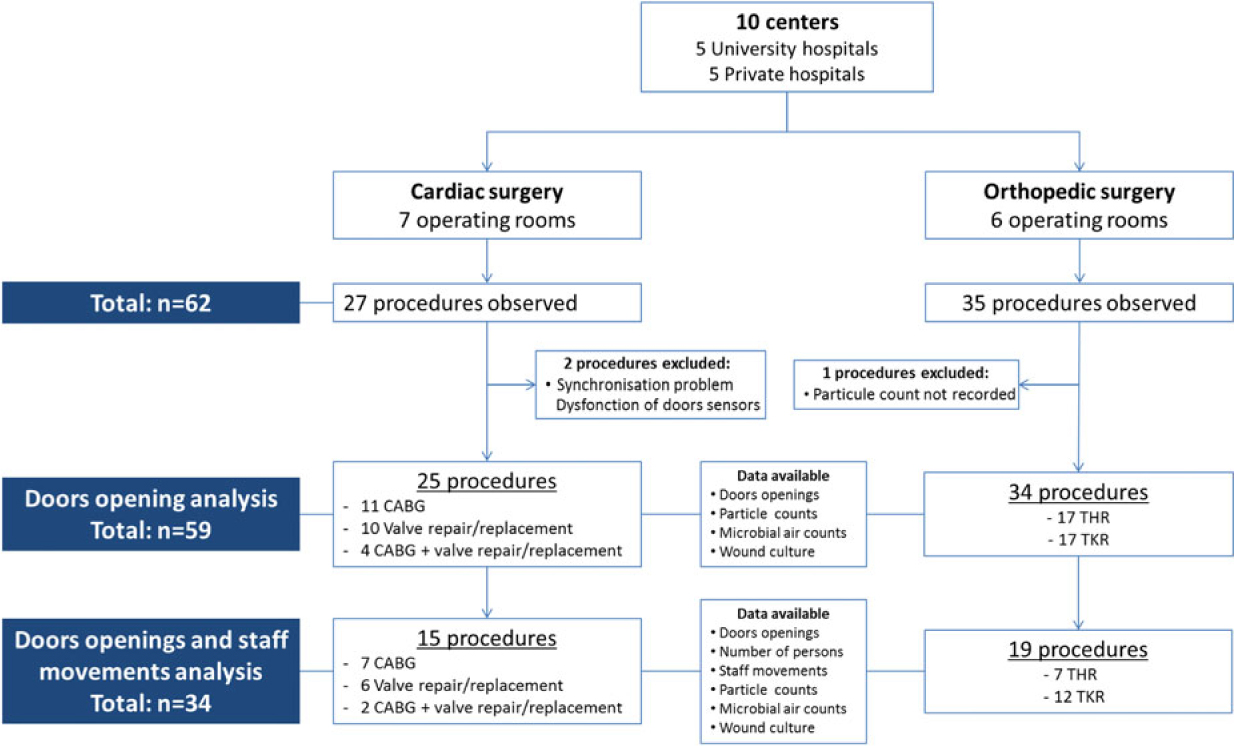
Fig. 1. Flow chart of procedures included in the analysis and data collected during orthopedic and cardiac surgeries.
The architecture of the 13 participating ORs was characterized by a median surface of 42 (interquartile range [IQR], 36–47) m², including a median of 2 doors (IQR, 1–5). The air ventilation system was turbulent in 8 of 13 ORs (6 of 7 in cardiac surgery and 2 of 6 in orthopedic surgery). The median baseline air renewal was 53 (IQR, 45–64) changes per hour, with a median positive pressure of 19 Pa (IQR, 12–33).
In cardiac surgery, only the first procedure of the day in the OR was included. In orthopedic surgery, 19 procedures were in first position, 11 were in second position, and 4 were in third position during the same day. In orthopedic surgery, the median duration from patient entry to exit and from incision to closure was 2.5 hours (IQR, 2–3.1) and 1.3 hours (IQR, 1–1.8), respectively. In cardiac surgery, the same duration measures were 5.1 hours (IQR, 4.7–6.2) and 3.5 hours (IQR, 3–4.3), respectively (Table 1).
Table 1. Descriptive Analysis on Door Openings From Cutaneous Incision to Closure During Orthopedic and Cardiac Surgery

Note. SD, standard deviation; IQR, interquartile range; OR, operating room; CABG, Coronary artery bypass grafting; TKR, total knee replacement; THR, total hip replacement.
Surrogates of the infectious risk
The median log10 of 0.3 µm, log10 of 0.5 µm, and log10 of 5 µm of the 1,747 particle counts performed measured during the 59 procedures are displayed in supplementary Table S1 and Fig. 2A. The counts of log10 of 0.3 µm particles varied according to ORs and procedures. The log10 of 0.3 µm varied according to ORs and procedures, with a mean in ORs with laminar airflow of 6.8 (standard deviation [SD], 1) and 6.8 (SD, 0.9) during orthopedic procedures. These values were consistently below those observed in ORs with turbulent ventilation systems (mean, 7.2; SD, 0.9) and during cardiac surgery (mean, 7.3; SD, 0.9) (P < .01) (Appendix Fig. A2 online).

Fig. 2. Boxplots describing the variability of (A) log10 0.3 µm particle counts (n = 59 procedures), (B) the frequency of door openings per hour (n = 59 procedures), and (C) cumulated movements by the team per hour (n = 34 procedures), according to the surgical specialty, the operating rooms and the type of ventilation system.
The median air microbial count at 3 moments in all 59 procedures was 3 CFU/m3 (IQR, 0–8). Among the 177 air samples, 50 (28%) were sterile, 90 (51%) carried 1–10 CFU/m3, and 37 (21%) >10 CFU/m3. For this last category, the median CFU value in air sampling was 21 CFU/m3 (IQR, 14–29, range 11–47), and 33 of 37 were in cardiac surgery and 35 of 37 were in OR with a turbulent ventilation system.
Among the 59 cultures of wound samples, 33 (56% of patients) were sterile, 18 (30%) had 1–10 CFU/100 cm², and 8 (14%) had >10 CFU/100 cm². Wounds in orthopedic surgery were significantly less contaminated at closure than in cardiac surgery: 24 versus 9 sterile cultures; 9 versus 11 cultures with 1–10 CFU/100 cm², and 0 versus 6 cultures with >10 CFU/100 cm² (P = .002).
Door openings
Among the 59 procedures observed, the median frequency of 19.4 openings per hour (IQR, 13.9–25.5), with large variation across ORs (Table 1 and Fig. 2B). Doors of aseptic preparation rooms were the most frequently opened, and door openings were mainly generated by the anesthetics team and persons not directly involved in the procedure (ie, assistant nurse or visitors).
During the 34 orthopedic procedures, the median frequency was 14.8 openings per hour (IQR, 12.2–21.2) from incision to skin closure. Doors stayed opened a cumulated duration of 4.2 minutes (IQR, 2.6–10.8), corresponding to 6% (IQR, 3.1%–10.4%) of the incision-to-closure period. During the 25 cardiac procedures, the median frequency of openings was 23.4 (IQR, 19.7–30) per hour from incision to closure. The cumulated duration of openings was 13.1 minutes (IQR, 10.7–21.3), corresponding to 7.3% of the operating time (IQR, 5.3%–10.6%).
The median frequency of door openings observed after the removal of the video-tracking system was 36.6 per hour (IQR, 33.3–42.6) from patient entry to exit versus 34.5 per hour (IQR, 23.6–48.8) in the presence of cameras in the OR (P = .50) (Appendix Table A2 online).
Number of persons and staff movements
Among the 34 procedures (19 orthopedic and 15 cardiac) with the recording of intraoperative staff movements, the median number of persons present from incision to skin closure was 10 (IQR, 8–11) (Table 1). The median cumulated time spent by individuals in the OR during a single procedure was 1.7 h (IQR, 1.1–2.4). Figure 2C displays the disparities of movements by specialty and OR. The cumulated movements by the entire team from incision to skin closure for 1 surgical procedure represented 12.1 km (IQR, 11.5–14). Each member of the team walked a median of 373 m (IQR, 324–461) from incision to skin closure in orthopedic surgery and 832 m (IQR, 629–877) in cardiac surgery.
Impact of behaviors on the surrogates of the exogenous infectious risk
The multivariate linear model performed on door openings collected during the 59 procedures revealed a significant positive link between the log10 0.3 µm particle counts and the number of door openings per period of 5 minutes (ß, 0.03; SD, 0.01; P = .01). In other words, 1 door opening during the 5 minutes preceding the particle sampling raised the log10 0.3 µm particles by 0.03.
The turbulent airflow was associated with an increased air microbial count (ß, 8.57; SD, 3.74; P = .04), as was the number of door openings per period (ß, 0.07; SD, 0.03; P = .03) (Table 2).
Table 2. Results of the Univariate and Multivariate Linear Mixed Models for the Particles Log10 0.3 µm (n = 1,747 Samples) and the Air Microbial Count (n = 177 Samples) During the 59 Included Interventions

Note. OR, operating room; SD, standard deviation; CABG, coronary artery bypass grafting; Ref., reference.
a The estimates represent the proportionality coefficient linking the explicative (eg, behaviors) and dependent variables (particle or air microbial count) during the prior time period considered (eg, 1 door openings during the 5 minutes preceding the particle sampling will raise the log10 0.3 µm particles by 0.03).
b These results were not confirmed in the multivariate analysis due to the nonvalidation of statistical assumptions.
The frequency of door openings and the mean of air bacterial counts from the incision to skin closure period were positively but not significantly correlated with the wound contamination before closure (r = 0.13; P = .32 and r = 0.15; P = .22, respectively).
The multivariate analysis performed on the 34 procedures with data on staff movements showed a significant association between the cumulated movements by the surgical team (ß, 0.003; SD, 0.0004; P < .01) and the log10 0.3 µm particle counts (Table 3, model 1). A subanalysis was performed to assess the relationship between the number of persons and their cumulated movements on the log10 0.3 µm particle counts. The inverse correlation found between both variables indicates the greater impact of staff movements on the log10 0.3 µm particle counts compared to the number of persons (Table 3, model 2).
Table 3. Results of the Univariate and Multivariate Linear Mixed Models to Evaluate the Effect of the Number of Persons and Staff Movements on the Particles Log10 0.3 µm During the 34 Interventions With Video Data and 1.072 Particle Counts

Note. SD, standard deviation; OR, operating room; CABG, coronary artery bypass grafting.
a Multivariate linear mixed models assessing variables associated with the particles log10 0.3 µm.
b Subanalysis performed to evaluate the combined effect of number of persons and staff movements on increase of log10 0.3 µm particles. The number of persons present in the OR appears negatively associated with particle counts after adjustment on cumulative movements. This model suggests that a high number of static persons in the OR will consistently generate fewer airborne particles and bacteria than a restricted number of persons with unregulated movements.
c These results were not confirmed in the multivariate analysis due to the nonvalidation of statistical assumptions.
The univariate analysis of log10 0.5 µm (ß, 0.003; SD, 0.0004; P < .001) and log10 5 µm particle counts (ß, 0.003; SD, 0.0005; P < .001) were significantly associated with the cumulated movements by the surgical team during the 5-minute period but not with the number of persons. The nonvalidation of statistical assumptions (residuals not normally distributed) did not allow an interpretation of the multivariate analysis.
Discussion
Door openings and staff movements were highly heterogeneous, varying ∼4-fold according to ORs and procedures in each specialty. Both had a significant impact on the air contamination by particles and microorganisms during procedures. The cumulated movements of the surgical team significantly affected the log10 0.3 µm, log10 0.5 µm, and log105 µm particle counts. This association was confirmed in the multivariate analysis for log10 0.3 µm particle counts. The results of the multivariate model for log10 0.5 µm and log10 5 µm particle counts were not interpretable due to the nonnormal distribution of the residuals.
The variability of behaviors observed, despite comparable procedures, may be explained either by the case mix, a lapse in the discipline of individuals or teams, or by the OR architecture and organization. In the present study, doors were mainly opened by nurses and visitors during orthopedic surgery. In cardiac surgery, anesthetists and external participants performed the most door openings. In the literature, most entries and exits occurring during procedures are explained by the frequent need for supplies or social activities. However, a substantial number of openings were unexplained, which suggests room for improvement.Reference Birgand, Saliou and Lucet4
The results confirm the findings of previous studies suggesting that door openings may affect the air sterility of the OR.Reference Andersson, Bergh, Karlsson, Eriksson and Nilsson15–Reference Mathijssen, Hannink and Sturm20 Door movements are known to alter the efficacy of ventilation systems by a disruption of the positive pressuresReference Mears, Blanding and Belkoff5 and the air flow.Reference Brohus, Balling and Jeppesen21 Our data suggest that controlling the movements of staff members inside the OR may be more efficient than restricting their number to prevent air particle contamination (Table 3, model 2). The number of airborne particles produced per person has been estimated at 10Reference Birgand, Saliou and Lucet4 per minute at rest and up to 3×10Reference Berríos-Torres, Umscheid and Bratzler7 during exertion.Reference Hambraeus22 Thus, a high number of static persons in the OR will consistently generate less airborne particles and bacteria than a restricted number of persons with unregulated movements.
The quantity of microorganisms cultured from the wound before closure was influenced by the cumulated movements by the team but not by the number of door openings. These results must be considered with caution. A large number of surgical wounds (89% in cardiac surgery) are contaminated at closure.Reference Bernard, Sadowski and Monin23, Reference Tammelin, Hambraeus and Ståhle24 The combination of endogenous and exogenous organisms can confound the relationship between the quantitative presence of organisms in the air and those colonizing the wound during surgery. In addition, the rather low number of wound samplings might not suffice for attaining a statistical association.
A recent meta-analysis concluded that laminar airflow may not be efficient in reducing the risk of SSIs in total hip and knee arthroplasties, or in abdominal surgery.Reference Bischoff, Kubilay, Allegranzi, Egger and Gastmeier25 After adjustment, our results showed a significant and independent increase air microbial contamination in ORs with a conventional airflow system in comparison to a laminar airflow system. Moreover, the airborne particle concentration was consistently lower at incision in ORs with laminar airflow versus conventional airflow and decreased faster during the procedures (Appendix Fig. A2 online). These findings support the current low-quality evidence on the advantage of laminar airflow to prevent SSI in clean surgery.
Our study has several strengths. This is the first multicenter study using motion-tracking systems to precisely and continuous assessment of the intraoperative staff behaviors, including critical movements inside ORs. The absence of a Hawthorne effect due to the presence of video camera, compared to a period with door-opening collection (hidden to staff) but without video cameras, suggests the reliability of our results. The cutaneous incision of a sterile site in cardiac and orthopedic surgeries increased the potential impact of environmental contamination on subsequent SSI. The high reproducibility of procedures and techniques improves the generalizability of these results. Finally, the statistical method allowed adjustment of the analysis with a random intercept for each intervention and each operating room and a random slope for time, preventing bias due to important confounding factors.
We acknowledge several limitations of our study. First, the end points were surrogates of the environmental infectious risk and not SSI. The SSI rate would have been an ideal but unreachable end point. Indeed, obtaining a benchmarked SSI rate in these surgical units would have required a long duration of surveillance, and many confounding factors should have been collected. Second, air samples were not strictly performed at the sterile site. This bias was minimized by positioning the counters near the patient’s head, under the laminar air flow when present, and at a height above sterile drapes separating the sterile site and the anesthesia area. The impact of door openings on the positive pressure and airflow in the OR is quasi-instantaneous.Reference Weiser, Shemesh, Chen, Bronson and Moucha26 The 5-minute period chosen to analyze the impact of behaviors on the air particle contamination appeared to be the best compromise between a period enough large to include events (eg, door openings) and their proximity to the counts. The longer periods used for the air microbial contamination may relate more to the long-term effect of intraoperative behaviors. Longitudinal modeling focused on the log10 0.3 µm particle and the air microbial counts. A previous study suggested that the 3 ranges of particle size were strongly correlated with airborne bacterial counts and likely represent a surrogate of overall air contamination during the surgical procedure.Reference Birgand, Toupet and Rukly27 The variability and large values obtained for 0.3 µm particles offered the possibility to satisfy the statistical assumptions and precisely model and to assess the relationship between the traffic flow and environmental contamination. Finally, 42% of surgical procedures were excluded from the analysis of staff movement due to noncomprehensive collection of staff positions by the motion-tracking system. Moreover, due to the typical duration of cardiac procedures and the amount of time required for study-specific setup, we only included the first scheduled cardiac procedure, which is potentially not representative of full-day behaviors.
This study highlights the importance of the intraoperative discipline of staff, suggesting that a restriction of staff movements and door openings may prevent airborne contamination and the associated SSI risk. The awareness of surgical staff in this field may improve behaviors and quality of care.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.35.
Acknowledgements
We thank the bacteriology laboratories that performed bacterial cultures and all the people who participated in the study. We thank Sebastien Bailly for his help in reviewing the statistical method. The following contributors provided and cared for study patients: Pierre Squara, Corinne De Diesbach, Alain Brusset, Marie-Françoise Vogel, François Gouin, Sophie Touchais, Jacqueline Lepennec, Gérard Babatasi, Emmanuel De Thomasson, Mathieu Debauchez, Christian Mazel, Pascal Bizot, Philippe Rosset, Patrick Nataf, Philippe Massin, Agnès Jue-Denis. The following contributors collected data: Gilles Antoniotti, Philippe Souchoix, Xavier Richomme, Marie-Noëlle Deschamps, Didier Lepelletier, Florence Legallou, Nathalie Ferronnière, Audrey Mouet, Xavier Lecoutour, Véronique Aguelon, Claire Lesteven, Carole Pornet, Jean Baptiste Stern, Jacques-Yves Nizou, Yves-Marie Vandamme, Maurice Tanguy, Marie-Laure Joly-Guillou, Nathalie Van Der Mée - Marquet, Aurélie Thomas-Hervieux. No preregistration exists for the reported studies reported in this article.
Financial support
This study was partly supported by the French Ministry of Health (grant no. PREQHOS 2011).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.







