1. Introduction
The hemipteran superfamily Cercopoidea Leach, Reference Leach and Brewster1815, also known as froghoppers or spittlebugs, comprises more than 2600 described recent species (Hamilton, Reference Hamilton2001; Dietrich, Reference Dietrich2002; Bartlett et al. Reference Bartlett, Deitz, Dmitriev, Sanborn, Soulier-Perkins and Wallace2018). In addition to their extant diversity, three extinct families (i.e. Cercopionidae, Procercopidae and Sinoalidae) from the Mesozoic have been attributed to Cercopoidea. The extinct family Procercopidae Handlirsch, Reference Handlirsch1906, the earliest lineage of Cercopoidea, is widely accepted as a stem group of the superfamily (Evans, Reference Evans1956; Shcherbakov & Popov, Reference Shcherbakov, Popov, Rasnitsyn and Quicke2002). Procercopidae, originally established to accommodate Procercopis Handlirsch, Reference Handlirsch1906 from the Lower Jurassic of Germany, can be traced back from the Early Jurassic to the Early Cretaceous of Eurasia and Australia (Handlirsch, Reference Handlirsch1906–8; Hamilton, Reference Hamilton1992). To date, nine valid genera of Procercopidae have been reported (Wang et al. Reference Wang, Szwedo and Zhang2012; Fu et al. Reference Fu, Huang and Engel2018), which include Procercopis Handlirsch, Reference Handlirsch1906–8, Procercopina Martynov, Reference Martynov1937, Sinocercopis Hong, Reference Hong1982, Anthoscytina Hong, Reference Hong1983, Cretocercopis Ren, Reference Ren, Ren, Lu, Guo and Ji1995, Liassocercopsis Ansorge, Reference Ansorge1996, Jurocercopis Wang & Zhang, Reference Wang and Zhang2009, Stellularis D Chen et al. Reference Chen, Yao and Ren2015 and Titanocercopis J Chen et al. Reference Chen, Zhang, Wang, Zheng and Wang2015a.
Cretaceous procercopids with three genera and seven species are confined to Siberia and northern China. They include Anthoscytina daica Shcherbakov, 1988 from the Upper Jurassic to Lower Cretaceous Glushkovo Formation of Chita, Siberia, Russia; Cretocercopis yii Ren, Reference Ren, Ren, Lu, Guo and Ji1995, A. trinervus Ren, Reference Ren, Ren, Lu, Guo and Ji1995 and A. pustulosus Ren, Reference Ren, Ren, Lu, Guo and Ji1995 from the Lower Cretaceous Lushangfen Formation of Beijing; Stellularis aphthosa (=Anthoscytina aphthosa) Ren et al. Reference Ren, Yin and Dou1998, S. macula (=A. macula) Hu et al. Reference Hu, Yao and Ren2014 and S. longirostris J Chen et al. Reference Chen, Wang, Zhang, Wang and Zheng2015b, from the Lower Cretaceous Yixian Formation at Huangbanjigou, Beipiao City, Liaoning Province.
Here we report two new genera and species belonging to Procercopidae from mid-Cretaceous Burmese amber, representing the first record of the family from this deposit.
2. Geological setting
Two well-preserved adult specimens (XFY10103 and XFY10104) are derived from amber deposits in the Hukawng Valley of Kachin Province, c. 100 km southwest of the village of Tanai, in northern Myanmar (Yin et al. Reference Yin, Cai and Huang2018: Fig. 1a) Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002) and Ross et al. (Reference Ross, Mellish, York, Crighton and Penney2010) provided an overview of the amber deposit and its geological settings. The age of Burmese amber had been controversial for a long time, even considered as young as Miocene (Grimaldi et al. Reference Grimaldi, Zhang, Fraser and Rasnistyn2005). The redeposition age of Burmese amber based on the U–Pb dating of zircon crystals was considered to be the earliest Cenomanian (98.79 ± 0.62 Ma; Shi et al. Reference Shi, Grimaldi, Harlow, Wang, Wang, Yang, Lei, Li and Li2012). In addition, available data suggest that the age of the Burmese amber was generally assigned to be around the Albian–Cenomanian boundary (Cruickshank & Ko, Reference Cruickshank and Ko2003; Ross et al. Reference Ross, Mellish, York, Crighton and Penney2010; Rasnitsyn et al. Reference Rasnitsyn, Bashkuev, Kopylov, Lukashevich and Ponomarenko2016; Mao et al. Reference Mao, Liang, Su, Li, Rao, Zhang, Xia, Fu, Cai and Huang2018; Smith & Ross, Reference Smith and Ross2018).

Fig. 1. Microphotograph of holotype (XFY10103) of Paranthoscytina xiai gen. et sp. nov., from mid-Cretaceous Burmese amber. (a) Lateral–dorsal aspect; (b) lateral–ventral aspect; (c) dorsal aspect; (d) showing details of head structure with three ocelli (white arrows) and pronotum; (e) showing details of pygofer and genitalia; (f) showing details of legs. Scale bars = 2 mm in (a–c); 1 mm in (d); 0.5 mm in (e, f).
Burmese amber harbours probably the most diverse Mesozoic palaeobiota (Ross, Reference Ross2019). Among all the burmite bioinclusions, fossil insects are highly diverse. The number of species has risen exponentially over the past two decades (Ross, Reference Ross2019). However, Auchenorrhyncha from Burmese amber are comparatively rare, with most of them belonging to Fulgoromorpha (or planthoppers) (Ross, Reference Ross2019; Zhang et al. Reference Zhang, Ren and Yao2019). Recently, a few species of the infraorder Cicadomorpha (Cicadellidae, Sinoalidae and Tettigarctidae) have been reported from Burmese amber (Chen et al. Reference Chen, Szwedo, Wang, Zheng, Wang, Wang and Zhang2018, Reference Chen, Wang, Zheng, Jiang, Jiang, Zhang and Zhang2019; Poinar & Brown, Reference Poinar and Brown2017; Fu et al. Reference Fu, Cai and Huang2019; Wang et al. Reference Wang, Dietrich and Zhang2019).
3. Material and methods
Two well-preserved adult specimens (XFY10103 and XFY10104) are preserved in two clear yellowish amber pieces. The specimen XFY10104 also contains a thrip (Thysanoptera). Observations were made using an Olympus SZX7 microscope. Photographs were taken using a digital camera attached to a Zeiss Discovery V16 microscope; stacked using Helicon Focus 6 software; line drawings were drafted with CorelDraw X7 and Adobe Illustrator CC 2018 graphic software. The invert function in Photoshop CS6 software was used to invert colours of specimen images to show certain details more clearly. These specimens are deposited in the Lingpoge Amber Museum in Shanghai, China (Director: Fanguan Xia).
Wing venation terminology and cell nomenclature follow Bourgoin et al. (Reference Bourgoin, Wang, Asche, Hoch, Soulier-Perkins, Stroinski, Yap and Szwedo2015) and Nel et al. (Reference Nel, Prokop, Nel, Grandcolas, Huang, Roques, Guilbert, Dostál and Szwedo2012). The following standards were used for measurements: body length measured from the apex of the vertex to the apex of the abdomen; body width measured at the widest part of the abdomen; tegmen length measured from the base to the apex of the tegmen; tegmen width measured at the widest part of the tegmen from costal margin to posterior margin. All measurements are presented in millimetres. The nomenclatural acts established herein are registered under Zoo-Bank LSID urn:lsid:zoobank.org:pub:A75E71C6-E325-4B99-A85F-F88189DC11A4.
4. Systematic palaeontology
Order HEMIPTERA Linnaeus, Reference Linnaeus1758
Suborder CICADOMORPHA Evans, Reference Evans1946
Superfamily CERCOPOIDEA Leach, Reference Leach and Brewster1815
Family PROCERCOPIDAE Handlirsch, Reference Handlirsch1906–8
Genus Paranthoscytina Fu, Cai & Huang, gen. nov.
Type species Paranthoscytina xiai Fu, Cai & Huang, sp. nov., by original designation and monotypy.
Etymology. The generic name is prefixed para-, indicating that the new genus is similar to the genus of Procercopidae, Anthoscytina Hong, Reference Hong1983. Gender: feminine. The genus is
registered under LSID urn:lsid:zoobank.org:act:661347°c-A497-4F56-B4EB-884F82EA1FAB.
Diagnosis. The new genus is characterized by the following characters: head triangular; pronotum longer than wide, much longer than head, lateral margins bulge outward at mid-length; metatibia with single strong lateral spine; tegmen with large punctation from base to apex, postcostal cell extremely broad, costal margin distinctly convex, veins ScR+R, MP and CuA leaving basal cell at common point, MP branching basal of bifurcation of CuA, a veinlet segmenting median cell, connecting MP3+4 and CuA, cross-vein imp present; hind wing with cross-vein imp present.
Remarks. The new genus resembles Anthoscytina Hong, Reference Hong1983 by the metatibia with single lateral spine and several venation characters of tegmen such as radial area narrow, basal portion of ScP not reaching half of basal cell length, RP simple, MP varying from simple to three-branched, hind wing with MP two-branched. However, it differs from the latter by the following characters: (1) head triangular (head rounded apically in Anthoscytina); (2) pronotum expanded, much longer than head (pronotum often as long as head in Anthoscytina); (3) tegmen with postcostal cell wide, costal margin distinctly convex at one-fifth of tegmen length (postcostal cell relatively narrow, costal margin slightly arched at base in Anthoscytina); (4) vein ScR+R, MP and CuA leaving basal cell at common point (stem ScP+R+MP+CuA branching into ScP+R and MP+CuA leaving basal cell in Anthoscytina); (5) tegmen with MP branching basal of bifurcation of CuA (MP branching after or at the same level with bifurcation of CuA in Anthoscytina); (6) a veinlet segmenting median cell, connecting MP3+4 and CuA, and cross-vein imp present (the veinlet and imp absent in Anthoscytina); and (7) hind wing with cross-vein imp present (imp absent in Anthoscytina).
Paranthoscytina xiai Fu, Cai & Huang, gen. et sp. nov.
Etymology. The specific epithet is dedicated to Mr Fangyuan Xia, for his contribution to the study of this amber specimen. The species is registered under LSID urn:lsid:zoobank.org:act:60020852-919C-472F-B611-41C975D734FD.
Holotype. XFY10103, male, deposited in the Lingpoge Amber Museum in Shanghai, China.
Locality and Horizon. Burmese amber, from deposits near Tanai village in the Hukawng Valley of northern Myanmar. Late Albian.
Diagnosis. As for genus (see above) with some additional characters: body length c. 15 mm; tegmen with vein RA three-branched, RP single-branched; hind wing with MP two-branched.
Description. Body covered with punctures, length (including wings in repose) c. 15.2 mm (Fig. 1a, b, c); tegmen 11.6 mm long and 4.1 mm wide.
Head triangular (Figs 1d, 2a), c. 2.0 mm long and 2.5 mm wide from dorsal view; crown produced anteriorly; compound eye large, ovoid, located laterally; three ocelli on crown (Fig. 1d), with distance between lateral ocelli shorter than distance between ocellus and compound eye; median ocellus ovoid, lateral ocelli globular; postclypeus bulging, with distinct transverse grooves; rostrum obscure. Pronotum greatly expanded (Figs 1d, 2a), irregularly hexagonal, nearly 1.3 times as wide as head and 1.4 times as long as head; anterior margin straight; posterior margin slightly concave at middle length; lateral margins bulge outward at middle length. Mesonotum partly exposed, with mesoscutellum triangular, wider than long.
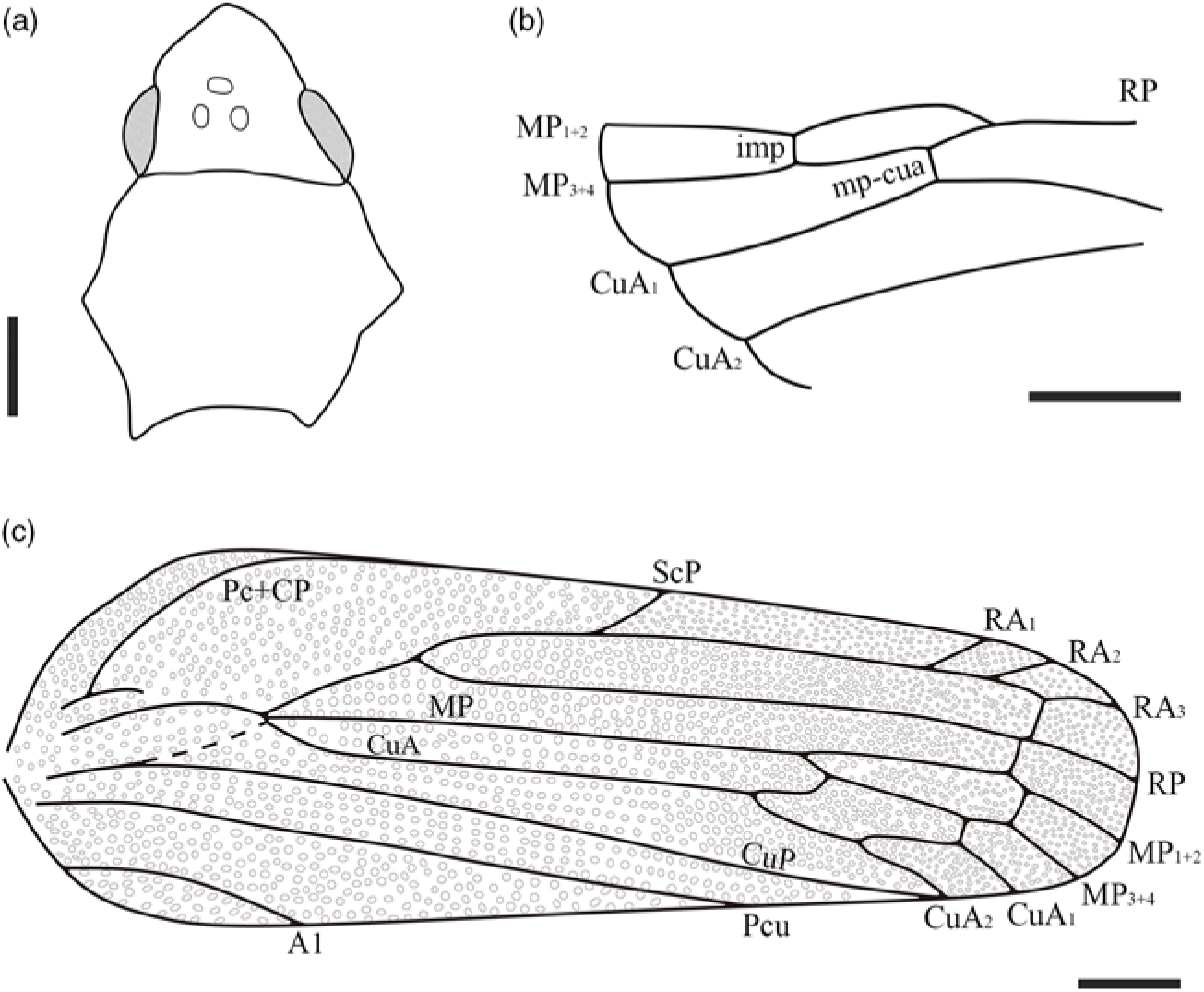
Fig. 2. Line drawings of Paranthoscytina xiai gen. et sp. nov. (a) Head and pronotum; (b) hind wing; (c) right tegmen. Scale bars = 1 mm in (a–c).
Tegmen (Fig. 2c) distinctly exceeds the tip of abdomen, length/width ratio c. 2.8, broadest at 0.17 of tegmen length, with large punctures from base to apex, densely covered with piliferous granules; costal margin thickened, convex at about basal 0.17 tegmen length; postcostal cell wide; basal cell nearly 0.22 times as long as tegmen length; basal portion of ScP slightly shifted from stem R+MP+CuA, not reaching half of basal cell length; Pc+CP partly visible; stem ScP+R branching into ScP+RA and RP reaching 0.36 of tegmen length; ScP+RA slightly longer than ScP+R, branching into ScP and RA slightly after mid-point of wing, distinctly basal of MP branching; RA with three terminals, connecting to RP by cross-vein ir; RP single-branched, straight, connected to MP1+2 by cross-vein rp-mp, slightly basal of ir; MP straight, with two branches, branching into MP1+2 and MP3+4 distinctly basal of bifurcation of CuA, reaching 0.70 of tegmen length; cross-vein imp present, nearly at same level as ir; CuA curved anteriorly, branching into CuA1 and CuA2 at same level as Pcu termination, reaching 0.76 of tegmen length; CuA emitting a veinlet at 0.70 of tegmen length, nearly straight at base and arched upward, connecting MP3+4, segmenting median cell; cross-vein mp-cua straight and short, connecting MP3+4 and CuA1; CuP almost straight, terminating just basal of CuA2 termination; Pcu almost straight, ending slightly after mid-point of wing, proximally well separated from A1; A1 arcuate. Hind wing partly visible (Fig. 2b); cross-vein imp present; MP with two branches, connecting to CuA1 by cross-vein mp-cua; CuA with two branches.
Leg covered with dense setae (Fig. 1f); profemur robust; protibia much more slender than profemur, c. 1.7 mm long, nearly twice as long as protarsus; metacoxae enlarged; metatrochanter cylindrical in shape; mesocoxa without a triangular meron, mesofemur c. 1.0 mm long and 0.41 mm wide; mesotibia nearly as long as protibia; mesotarsus slightly longer than protarsus, pretarsal claw sharp apically, arolium present; metafemur almost 1.3 times as long as mesofemur; metatibia almost 1.2 times as long as mesotibia, with a lateral spine (Fig. 1f), distinctly widened apically, armed with two rows of tiny teeth; tarsi trimerous, metatarsus almost 1.3 times as long as mesotarsus, widened apically, basal and second tarsomeres armed with two rows of apical teeth, teeth of apical row with subapical setae.
Abdomen tapered, segments II to VIII becoming successively narrower; genital valve and genital plates unclear; genital styles elongate, distinctly exceeding apex of pygofer; anal style exposed, relatively long (Fig. 1e).
Genus Burmocercopis Fu, Cai & Huang gen. nov.
Type species. Burmocercopis lingpogensis Fu, Cai & Huang, sp. nov., by original designation and monotypy.
Etymology. The name is a combination of the specimen’s collection locality Burma (former name of Myanmar), and ‘Cercopis’, the type genus of the superfamily Cercopoidea. Gender: masculine. The genus is registered under LSID urn:lsid:zoobank.org:act:E15AE53B-D24C-48D5-B6BE-F508BD249503.
Diagnosis. The new genus is characterized by the following characters: head relatively long, strongly produced anteriorly; antennal flagellum with at least six elongate flagellomeres; pronotum with posterior margin strongly convex at middle portion, arcuately W-shaped; mesonotum greatly concealed; metatibia without lateral spine; tip of tegmen truncated; tegmen covered with large punctate from base to apex, basal cell lance-shaped, with a short common stalk of MP+CuA closing it apically, base of CuA zigzagged, hypocostal carinae present, MP branching distinctly basal of bifurcation of CuA.
Remarks. The new genus differs distinctly from other known genera in Procercopidae by: (1) head strongly produced anteriorly (head relatively rounded apically in other genera); (2) specialized pronotum with posterior margin strongly convex, W-shaped (posterior margin of pronotum nearly straight or slightly concave at mid-length in other genera); (3) tegmen with ScP+R separated, short common stalk of MP+CuA closing basal cell, base of CuA zigzagged (stem ScP+R+MP+CuA branching into ScP+R and MP+CuA leaving basal cell, base of CuA slightly arched in other genera); (4) tip of tegmen truncated (tegmen elongated in other genera).
Burmocercopis lingpogensis Fu, Cai & Huang, gen. et sp. nov.
Etymology. From ‘Lingpoge’, indicating the amber is deposited in the Lingpoge Amber Museum in
Shanghai, China. The species is registered under LSID urn:lsid:zoobank.org:act:5A57C093-8405-4DD3-ACBD-6FA96F365F6E.
Holotype. XFY10104, male, deposited in the Lingpoge Amber Museum in Shanghai, China.
Locality and Horizon. Burmese amber, from deposits near Tanai village in the Hukawng Valley of northern Myanmar. Late Albian.
Diagnosis. As for genus (see above) with some additional characters: body length c. 5.2 mm; tegmen with vein RA and RP single-branched; hind wing with RA, RP and MP single-branched.
Description. Body covered with punctures, length (including wings in repose) 6.8 mm (Fig. 3a, b); tegmen 4.9 mm long and 2.2 mm wide.

Fig. 3. Microphotograph of holotype (XFY10104) of Burmocercopis lingpogensis gen. et sp. nov., from mid-Cretaceous Burmese amber. (a) Dorsal aspect; (b) ventral aspect; (c) showing details of antenna; (d) showing details of forewing and hind wing; (e) showing details of punctate. Scale bars = 1 mm in (a, b, d); 0.2 mm in (e); 0.1 mm in (c).
Head subtriangular (Fig. 4b), 1.5 mm long, and 1.8 mm wide in dorsal view; crown distinctly produced anteriorly; compound eye large, ovoid; antenna partly visible (Fig. 3c); three ocelli on crown, ovoid (Fig. 4b); flagellum with at least six elongate flagellomeres, flagellomeres I–V becoming successively thinner; postclypeus bulging, with distinct transverse grooves, widest at middle; clypeus triangularly elongate; rostrum straight, extending to base of metacoxa, c. 1.3 mm long. Pronotum greatly expanded (Fig. 4b), nearly 1.5 times as wide as head, anterior margin nearly straight, posterior margin strongly convex at middle portion; mesonotum shortened.

Fig. 4. Line drawings of Burmocercopis lingpogensis gen. et sp. nov. (a) Left tegmen; (b) head and pronotum; (c) hind wing. Scale bars = 1 mm in (a–c).
Tegmen (Figs 3d, 4a) exceeds the tip of abdomen, length/width ratio c. 2.2, with large punctures from base to apex, densely covered with piliferous granules (Fig. 3e); costal margin with tiny spines; postcostal cell wide; basal cell narrow, somewhat lance-shaped, c. 1.3 mm long and 0.15 mm wide, with a short common portion of MP+CuA closing it apically; hypocostal carinae wide, sclerotized; Pc+CP extremely long, extending to termination of RA; ScP+R branching into ScP+RA and RP reaching 0.45 of tegmen length; ScP+R slightly longer than ScP+RA; ScP+RA branching into ScP and RA basal of MP branching, reaching 0.58 of tegmen length; RA simple, connecting to RP by cross-vein ir; RP single-branched, connecting to MP1+2 by cross-vein rp-mp; MP with two branches, branching into MP1+2 and MP3+4 distinctly basal of bifurcation of CuA, reaching 0.64 of tegmen length; cross-vein imp absent; CuA with two branches, branching into CuA1 and CuA2 reaching 0.71 of tegmen length, CuA1 arcuate, more than twice as long as CuA2; cross-vein mp-cua connecting MP3+4 and CuA1; CuP straight, terminating just basal of CuA2 termination; Pcu almost straight, ending slightly after mid-point of wing; A1 simple, arcuate. Hind wing with peripheral membrane wide (Figs 3d, 4c); RA simple, connecting to RP by cross-vein ir; MP simple, connecting to CuA1 by cross vein mp-cua; CuA with two branches.
Protibia more slender than profemur, covered with dense setae, c. 1.1 mm long; protarsus 0.47 mm long, pretarsal claw large, sharp apically, arolium obscure; mesofemur robust, 0.68 mm long and 0.24 mm wide; mesotibia slightly longer and wider than protibia; metafemur nearly as long as mesofemur; metatibia without lateral spine, distinctly widened apically, armed with a circle of apical teeth, totally at least 12 in number; metatarsus covered with setae, c. 0.50 mm long; tarsi trimerous, first and second tarsomeres armed with some apical, long, stout teeth. Abdomen narrow, tapered.
5. Discussion
The two new genera here described can be tentatively placed in the extinct family Procercopidae based on the combination of the following characters: body and tegmen entirely punctate; metatibia without lateral spine or with immovable lateral spine(s) arranged in a single row, no more than three in number; vein MP of tegmen with one to three terminals, branching after mid-length of tegmen; and hind wing with peripheric membrane (Wang et al. Reference Wang, Szwedo and Zhang2012). However, two new genera share some unique characters previously unknown in Procercopidae, including head produced anteriorly, pronotum greatly extended, mesonotum concealed, tegmen covered with large punctures, and vein MP of tegmen branching basal of bifurcation of CuA.
Paranthoscytina gen. nov. is probably related to Anthoscytina Hong, Reference Hong1983, the largest genus of Procercopidae, reported from the Early Jurassic to the Early Cretaceous of northern Asia. However, some striking features of Paranthoscytina differentiate it from other procercopids, including tegmen with postcostal cell extremely broad, broadest near one-fifth of wing length; vein ScR+R, MP and CuA leaving basal cell at common point; a veinlet segmenting median cell; cross-vein imp present; and hind wing with cross-vein imp present. Burmocercopis gen. nov. bears the combination of characters also previously unknown in Procercopidae, including posterior margin of pronotum distinctly convex at mid-length, mesonotum greatly concealed; tip of tegmen truncated; ScP+R of tegmen separated, a short common stalk of MP+CuA closing basal cell, and base of CuA zigzagged.
Fossil record of Cercopoidea comprises all families except Machaerotidae from the Mesozoic to Miocene (Szwedo, Reference Szwedo2018). Procercopidae was distributed worldwide and flourished in the Jurassic, but the contemporaneous Sinoalidae Wang et al. Reference Wang, Szwedo and Zhang2012 confined to NE China from the Middle to Late Jurassic. Cretaceous procercopids are relatively rare, including only three genera and seven species from the middle to high latitudes areas of the Northern Hemisphere. Our discovery of two new genera of procercopids from mid-Cretaceous Burmese amber represents the first record of Procercopidae from the Mesozoic amber, which extends the duration for nearly 25 Ma from the Early Cretaceous to mid-Cretaceous. It also represents the first record of procercopids from southern Asia, adding a significant distribution to the family Procercopidae. Additionally, the newly described species also provides important data for our knowledge of the documented palaeodiversity and morphological disparity of Cretaceous procercopids, increasing our understanding of the origin and evolution of the peculiar hemipteran family.
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000, XDB18000000), the National Natural Science Foundation of China (41688103), and the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0706). We thank Mr Fangyuan Xia, Director of the Lingpoge Amber Museum, for his contribution to the study of the amber specimens.






