1. Introduction
The various shapes of chitinozoan vesicles (e.g. discoidal, spherical, tubular, conical, etc.) are commonly represented in numerous unrelated fossil and extant unicellular organisms or reproductive cycles of metazoans. Thus, chitinozoan affinities based on shape have led to numerous radically different biological assignments (for a discussion, see Paris et al. Reference Paris, Grahn, Nestor and Lakova1999). The inferred chitinous composition of the chitinozoan vesicle wall was used by Eisenack (Reference Eisenack1931, Reference Eisenack1968), Collinson & Schwalb (Reference Collinson and Schwalb1955) and Jenkins (Reference Jenkins1970) to support particular inferred biological affinities. However, Voss-Foucart & Jeuniaux (Reference Voss-Foucart and Jeuniaux1972) and Jacob et al. (Reference Jacob, Paris, Monod, Miller, Tang, George and Bény2007) were unable to establish the presence of chitin in the organic vesicle wall. This presents two possibilities: (1) that the molecular structure of chitin is not preserved through geological time or (2) the wall of chitinozoan vesicles contained no chitin. The available data do not allow us to establish which is correct (Jacob et al. Reference Jacob, Paris, Monod, Miller, Tang, George and Bény2007).
Kozlowski (Reference Kozlowski1963) was the first to use the mode of chitinozoan aggradation in biological affinity arguments. Specimens of the genus Desmochitina Eisenack Reference Eisenack1931, which were contained within an organic-walled cocoon, were considered by Kozlowski to be similar to polychaete eggs enclosed in a similar structure. However, the various types of attachments to form chains or other types of vesicle aggregation cannot be evaluated for their biological affinity significance, since many invertebrates display a similar mode of egg laying.
Grahn (Reference Grahn1981) named the supposed marine metazoan parent organisms ‘chitinozoophorans’, and considered chitinozoans to be the reproductive bodies of a marine invertebrate. Paris (Reference Paris1981) discussed the possibility of small, pelagic or nectic, soft-bodied, wormlike (judging from the elongate coiled chains) animals as the parent organism, and based on the size of the chitinozoans, deduced that these would range from a few millimetres to a few centimetres in length. The distribution and biodiversification pattern of the chitinozoans may not exactly reflect those of the chitinozoophorans. A pelagic or necto-pelagic animal may use different strategies (see Paris & Nõlvak, Reference Paris and Nõlvak1999) in laying its eggs: (1) the eggs were freely spread in the water, or (2) they were attached to floating objects (e.g. seaweed) or the chitinozoophorans attached their eggs to any object that offered protection (e.g. Grahn, Reference Grahn1984b). It is likely that two modes of occurrence could be expected for such eggs in a fossil state: (1) evidence of eggs before laying, that is, the ‘intra-oviduct stage’ (e.g. frequently coiled chains persisted after the decay of the females; see Paris & Nõlvak, Reference Paris and Nõlvak1999; fig. 3), and (2) evidence of eggs after laying (cocoons, organized clusters, isolated vesicles). The chitinozoan vesicles were probably surrounded by a mucous or gelatinous layer (e.g. Paris & Nõlvak, Reference Paris and Nõlvak1999; fig. 4). This is corroborated by the occurrence of a chain of Lagenochitina esthonica Eisenack, Reference Eisenack1955, found in Tremadocian beds from England (Y. G., unpub. data), which was surrounded by framboidal pyrite where a mucous or gelatinous layer could be expected. Soft tissues, even of a gelatinous nature, are known to be frequently preserved through alteration of organic sulphur compounds to pyrite (Stanley & Sturmer, Reference Stanley and Sturmer1983). An important condition for such preservation is a quick burial, preferably in organic-rich sediments (Brett & Baird, Reference Brett and Baird1986). These clusters should not be confused with secondary stacking (e.g. faecal pellets, stuck vesicles, etc.).
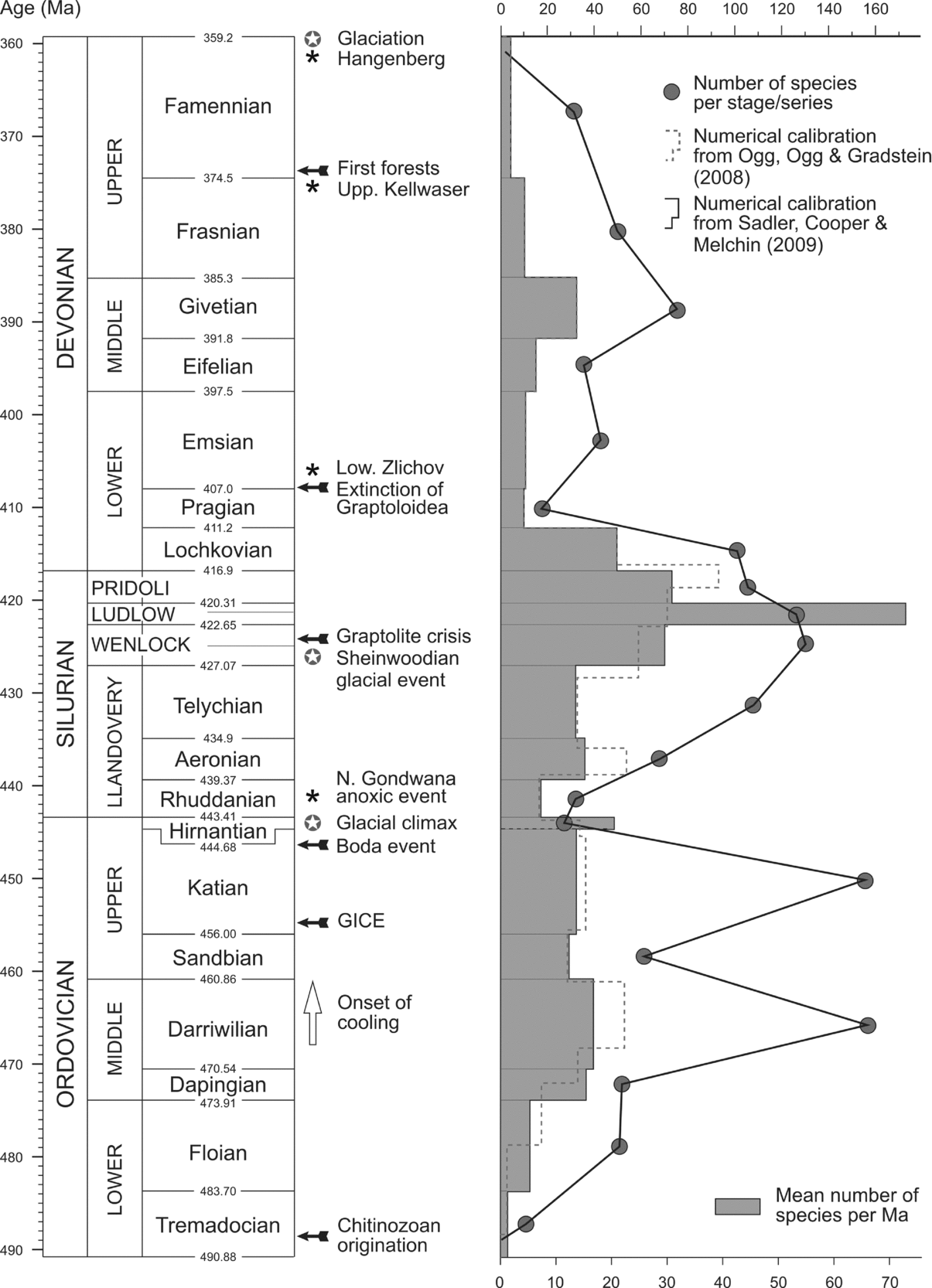
Chitinozoans evolved rapidly during Ordovician times. From their first occurrence in the early Tremadocian to the biodiversity crisis in the latest Ordovician, nearly 80 % of morphological innovations took place (Paris & Nõlvak, Reference Paris and Nõlvak1999; Paris et al. Reference Paris, Grahn, Nestor and Lakova1999). Chitinozoans had already reached their maximum Ordovician diversity by the late Darriwilian (Fig. 1). Until their extinction in latest Devonian times, the chitinozoan group survived several biodiversity crises: in the early Late Ordovician (Paris et al. Reference Paris, Achab, Asselin, Chen, Grahn, Nõlvak, Obut, Sennikov, Vecoli, Verniers, Wang, Winchester-Seeto, Webby, Paris, Droser and Percival2004), late Hirnantian, late Wenlock, earliest Emsian, and in the latest Frasnian (Kellwasser event). The general trend through time is shown in Figure 1.

Figure 1. Global evaluation of the chitinozoan biodiversification from the origin of the group in the early Tremadocian to its extinction in the latest Devonian. The solid circles indicate the number of species per stage (Ordovician and Devonian) or per series (Silurian). The graph represents the mean diversity of the chitinozoans per million years for the stages or series (durations based on Sadler, Cooper & Melchin, Reference Sadler, Cooper and Melchin2009). An alternative graph (dashed line) is based on the time calibration by Ogg, Ogg & Gradstein (Reference Ogg, Ogg and Gradstein2008). The most significant events are indicated along the time scale. Open arrow: Darriwilian cooling (Trotter et al. Reference Trotter, Williams, Barns, Lécuyer and Nicoll2008; Ainsaar et al. Reference Ainsaar, Kaljo, Martma, Meidla, Männik, Nõlvak and Tinn2010); black arrow: biological and oceanological events (Jaeger, Reference Jaeger1978, Reference Jaeger1991; Meyer-Berthaud, Scheckler & Wendt, Reference Meyer-Berthaud, Scheckler and Wendt1999; House, Reference House2002; Joachimski et al. Reference Joachimski, Pancost, Freeman, Ostertag-Henning and Buggisch2002; Kaljo et al. Reference Kaljo, Hints, Männik and Nõlvak2008; Servais et al. Reference Servais, Lehnert, Li, Mullins, Munnecke, Nützel and Vecoli2008; Bergström et al. Reference Bergström, Chen, Gutiérrez-Marco and Dronov2009a; Hints et al. Reference Hints, Delabroye, Nõlvak, Servais, Uutela and Wallin2010); black star: main anoxic events (Chlupáč & Kukal, Reference Chlupáč and Kukal1988; House, Reference House2002); circled star: main glacial events (Streel et al. Reference Streel, Caputo, Loboziak and Melo2000; Lehnert et al. Reference Lehnert, Männik, Joachimski, Calner and Fryda2010). The values of the biodiversity of the chitinozoan at species level are from the database ‘CHITINOVOSP’ of F. Paris.
The occurrence of chitinozoans in all types of sedimentary rocks (except for reefs and coarse, well-sorted sandstones), including black shales and cherts devoid of any bioturbation or evidence of benthic fauna, suggests that the chitinozoophorans were, most likely, part of the zooplankton (Vandenbroucke et al. Reference Vandenbroucke, Armstrong, Williams, Paris, Sabbe, Zalasiewicz and Nõlvak2010). It is probable that chitinozoophorans grazed on phytoplankton. This pelagic niche appeared in the Cambrian (Servais et al. Reference Servais, Lehnert, Li, Mullins, Munnecke, Nützel and Vecoli2008), but was only exploited from the Early Ordovician onward. It was also occupied by graptolites, which appeared a little earlier in the fossil record than chitinozoophorans (Cooper, Reference Cooper1999). However, the specific diversities of these two groups are inverted with relation to climatic belts: intertropical zones were dominated by highly diversified graptolite faunas and higher latitudes by chitinozoophorans. This is demonstrated for the early Sandbian by Vandenbroucke et al. (Reference Vandenbroucke, Armstrong, Williams, Paris, Sabbe, Zalasiewicz and Nõlvak2010). These authors also concluded that graptolites and chitinozoophorans did not share exactly the same ecosystem.
The fluctuation of the diversity of the chitinozoans through time, from the origination of the group in the early Tremadocian, to its final extinction in the latest Famennian, was evaluated using the ‘CHITINOVOSP’ database initiated by Paris & Bernard (Reference Paris, Bernard and Dorning1994) and updated by one of us (F. P.). All chitinozoan species described since Eisenack's first species description in 1931 are recorded in this database (1214 species). Besides the various taxonomic form fields, the database also includes palaeogeographic and stratigraphic information. The latter entries contain the total range of the recorded species at System, Series and Stage levels. The database has been periodically updated and the last international subdivisions adopted by IUGS are used (that is, the most recent Ordovician global stages).
The number of species per stage can be found by querying the database. These numbers should be regarded as approximate values, as the total range of each species is often a matter of estimation, related to the accuracy of the available stratigraphic information. In addition, ill-defined species included in the database add some further bias. Nevertheless, as the same treatment has been applied throughout the Palaeozoic record of chitinozoan species, the resulting general trends seem to reflect fairly well the actual biodiversification pattern of the group (Fig. 1), as supported by detailed sections providing a well-documented diversity trend for some short time intervals.
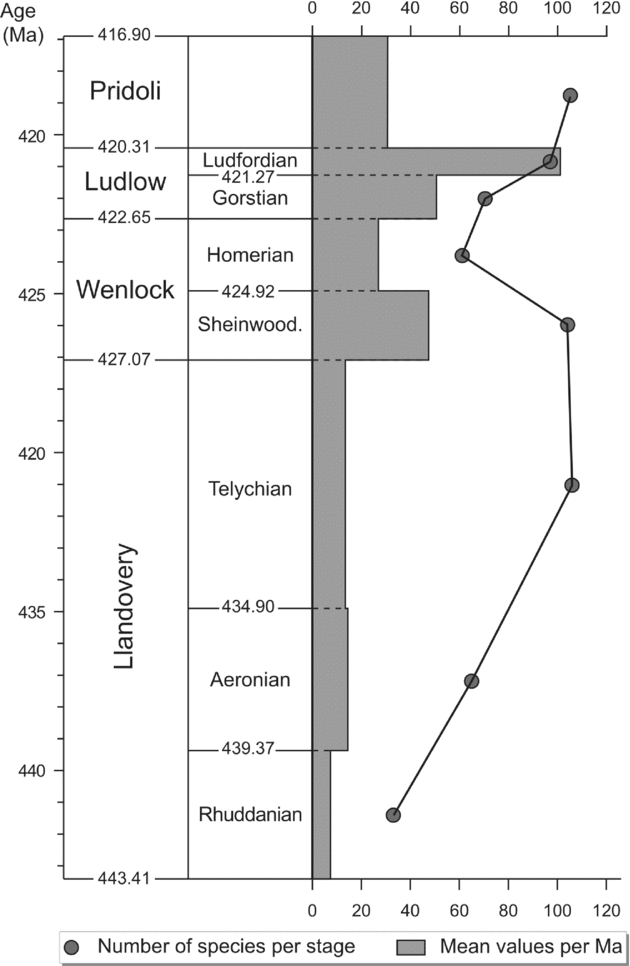
The durations of the Wenlock and Ludlow stages as calculated by Sadler, Cooper & Melchin (Reference Sadler, Cooper and Melchin2009) are much shorter than the duration of the Ordovician and Devonian stages. Consequently, in order to have a time slice roughly in the same range, the chitinozoan specific diversity is expressed at series level for Wenlock and Ludlow on Figure 1. However, a more detailed graph is provided for the Silurian (Fig. 2) with specific diversity also evaluated at stage level for the Wenlock and the Ludlow. This different time slicing points out a drop in diversity in the Homerian roughly contemporaneous with the late Wenlock graptolite crisis (see the lundgreni event in Section 5). The two graphs also illustrate the great influence of the time slicing on the diversity curves.
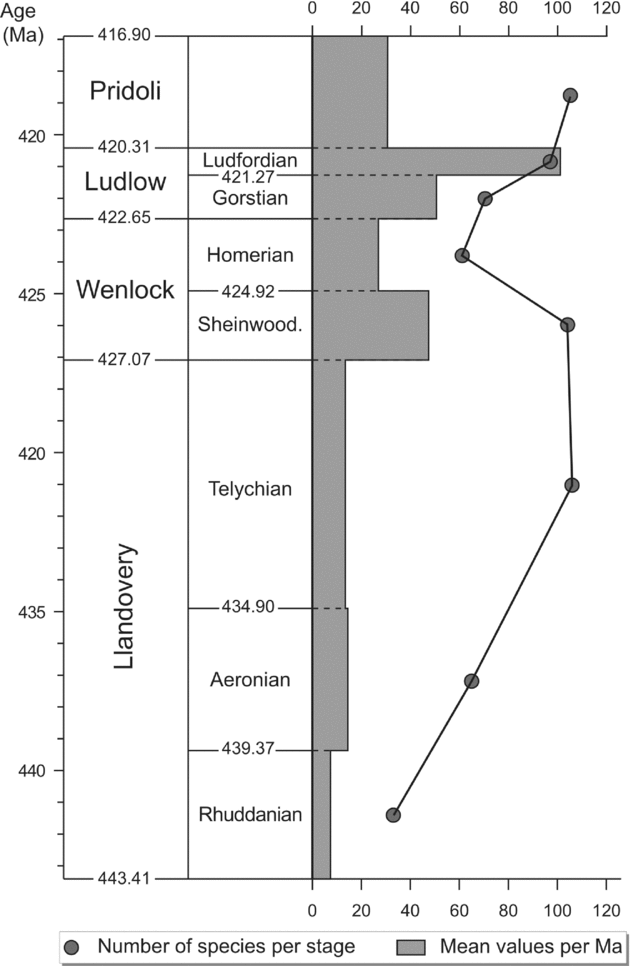
Figure 2. Global biodiversity of the Silurian chitinozoans species per stage (solid circles) and mean value of their specific diversity per million years for each stage. (Numerical calibration from Sadler, Cooper & Melchin, Reference Sadler, Cooper and Melchin2009.)
Some discrepancies are noted when calibrating chronostratigraphic subdivisions with the most recent numerical scales. The mean chitinozoan diversity per million years shows higher values for the Darriwilian, the Aeronian and the Pridoli, when using the numerical calibration of Ogg, Ogg & Gradstein (Reference Ogg, Ogg and Gradstein2008), with regard to those proposed by Sadler, Cooper & Melchin (Reference Sadler, Cooper and Melchin2009). However, these different calibrations do not introduce significant bias, as the general trends of the resulting graphs are similar (Fig. 1). As demonstrated by a more detailed evalution of the diversity of Ordovician chitinozoans (Paris et al. Reference Paris, Achab, Asselin, Chen, Grahn, Nõlvak, Obut, Sennikov, Vecoli, Verniers, Wang, Winchester-Seeto, Webby, Paris, Droser and Percival2004), one of the critical points when elaborating such curves is the unbalanced quality of the available data; for instance, some time slices and some areas have been more extensively investigated than others (e.g. the diversity curve tends to mirror the number of available samples; see Paris et al. Reference Paris, Achab, Asselin, Chen, Grahn, Nõlvak, Obut, Sennikov, Vecoli, Verniers, Wang, Winchester-Seeto, Webby, Paris, Droser and Percival2004, fig. 28.2–3) and this must be kept in mind when discussing diversity fluctuations.
2. The emergence of the chitinozoan group
The first chitinozoans appear during a transgression event with shaly facies above the Cambrian sandstones and after the negative TOCE (Top of Cambrian Excursion) δ13C curve (Zhu, Badcock & Peng, Reference Zhu, Badcock and Peng2006). The chitinozoophorans colonized the niche as pelagic zooplankton together with dendroid graptolites (e.g. Dendrograptids, Anisograptids) and then with the first graptoloids. No undisputable chitinozoans have been recorded before the Ordovician, and the microfossils reported as chitinozoans from the Neoproterozoic Chuar Group by Bloeser et al. (Reference Bloeser, Scopf, Hordystir and Breed1977) are most probably testate amoebas (Porter & Knoll, Reference Porter and Knoll2000; Porter, Meisterfeld & Knoll, Reference Porter, Meisterfeld and Knoll2003). Simple, smooth, quite large chitinozoan forms (Lagenochitina, Conochitina) appear in the early Tremadocian. They are known from the middle part of the Fezouata Formation in Morocco (Elaouad-Debbaj, Reference Elaouad-Debbaj1988), where the first known chitinozoans (Lagenochitina destombesi) occur below the Adelograptus tenellus graptolite Zone, and above early Tremadocian taxa (Destombes, Holland & Willefert, Reference Destombes, Holland, Willefert and Holland1985; Paris, Reference Paris1990). Early Tremadocian chitinozoans have also been reported from the Yangtze area in south China (Chen, Paris & Zhang, Reference Chen, Paris and Zhang2008). During the late Tremadocian, chitinozoans spread to areas outside north Gondwana, and the morphological diversification now also includes species with smaller vesicles (Desmochitina, Euconochitina), together with large specimens from the Lagenochitina esthonica group. Early late Tremadocian (Adelograptus tenellus graptolite Zone) chitinozoans have been reported from the upper El Gassi Formation in Algeria (Poumot, Reference Poumot1964, Reference Poumot1968; Combaz, Reference Combaz1967; Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi and Ghienne2010) and from the New Fields Farm borehole (908.15 m), 5 km west of Southam, Warwickshire, England (Y. G., unpub. data). De la Puente & Rubinstein (Reference De la Puente and Rubinstein2009) described Lagenochitina from the Aorograptus victoriae graptolite Zone (Saladillo Formation), and chitinozoans from the lower Parsha Formation, Argentina. Chen, Paris & Zhang (Reference Chen, Paris and Zhang2008) reported Lagenochitina destombesi from the late Tremadocian in the Yichang area (Fenxiang Formation), Hubei Province, China. The same species has been recovered from the Varangu regional stage of Estonia (Nõlvak, Reference Nõlvak1999). During the latest Tremadocian, chitinozoophorans expanded to all the paleocontinents, for example, the upper Cienguillas and lower Obispo formations, east Codillera, Bolivia (Heuse, Grahn & Erdtmann, Reference Heuse, Grahn and Erdtmann1999), and the Montagne Noire/Aquitaine Basin, southwest France (Paris, Reference Paris1984). Outside Gondwana they are known from a number of places such as the Björkåsholmen Formation in Skåne, south Sweden (Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Grahn & Nõlvak, Reference Grahn and Nõlvak2010), Oslo Region, south Norway (Grahn & Nõlvak, Reference Grahn and Nõlvak2007a), and Isle of Rügen, NE Germany (Samuelsson, Reference Samuelsson, Kraft and Fatka1999); Leetse Formation, Estonia (Grahn, Reference Grahn1984a; Hints & Nõlvak, Reference Hints and Nõlvak2006); Cow Head, Ledge Section, Newfoundland, Canada (Williams et al. Reference Williams, Nowlan, Barnes and Batten1999); and Altai, Siberia (Sennikov et al. Reference Sennikov, Yolkin, Petrunina, Gladkikh, Obut, Izokh, Kipriyanova, Sennikov and Kanygin2008).
3. Chitinozoan maximum diversity in the late Darriwilian
The chitinozoophorans quickly expanded during Early and Middle Ordovician times (Fig. 1), and reached their maximum Ordovician diversity (only to be exceeded in the early Wenlock and Pridoli) in the late Darriwilian (Paris & Nõlvak, Reference Paris and Nõlvak1999; Paris et al. Reference Paris, Grahn, Nestor and Lakova1999, Reference Paris, Achab, Asselin, Chen, Grahn, Nõlvak, Obut, Sennikov, Vecoli, Verniers, Wang, Winchester-Seeto, Webby, Paris, Droser and Percival2004; Hints et al. Reference Hints, Delabroye, Nõlvak, Servais, Uutela and Wallin2010) after about 15 Ma. The genetic potential was probably high with a ‘plasticity’ of the genome of the chitinozoophorans favouring new combinations (Paris et al. Reference Paris, Achab, Asselin, Chen, Grahn, Nõlvak, Obut, Sennikov, Vecoli, Verniers, Wang, Winchester-Seeto, Webby, Paris, Droser and Percival2004). The sea-levels were rising, but in the late Darriwilian a short lived regression (Dabard, Loi & Paris, Reference Dabard, Loi and Paris2007) occurred with the onset of a cooler climate (Trotter et al. Reference Trotter, Williams, Barns, Lécuyer and Nicoll2008; Ainsaar et al. Reference Ainsaar, Kaljo, Martma, Meidla, Männik, Nõlvak and Tinn2010). The regression and the climate change affected chitinozoophorans, and chitinozoan diversity decreased until a recovery in the Katian (Fig. 1).
4. The Hirnantian/Rhuddanian biodiversity crisis
The first major biodiversity crisis for chitinozoophorans on a global basis coincides with the Guttenberg δ13C excursion (GICE) in the early Late Ordovician (Paris & Nõlvak, Reference Paris and Nõlvak1999; Paris et al. Reference Paris, Achab, Asselin, Chen, Grahn, Nõlvak, Obut, Sennikov, Vecoli, Verniers, Wang, Winchester-Seeto, Webby, Paris, Droser and Percival2004; Achab & Paris, Reference Achab and Paris2007; Bergström et al. Reference Bergström, Chen, Gutiérrez-Marco and Dronov2009a, Reference Bergström, Chen, Schmitz, Young, Rong and Saltzmanb). The decline in chitinozoan species diversity (Fig. 1) is in general connected with decreases in sea-level, most likely caused by the development of restricted intra-continental ice sheets (Hamoumi, Reference Hamoumi1999; Ainsaar, Meidla & Martna, Reference Ainsaar, Meidla and Martna2004; Bourahrouh, Paris & Elaouad-Debbaj, Reference Bourahrouh, Paris and Elaouad-Debbaj2004; Loi et al. Reference Loi, Ghienne, Dabard, Paris, Botquelen, Christ, Elaouad-Debbaj, Gorini, Vidal and Videt2010), or by increased tectonic activity. Subsequently, a change in sedimentation led to a positive change in δ13C, extinction, and a microfaunal crisis. Glaciation pulses leading to the Hirnantian glaciation (Bergström, Saltzman & Schmitz, Reference Bergström, Saltzman and Schmitz2006; Kaljo et al. Reference Kaljo, Hints, Männik and Nõlvak2008) started in the late mid-Katian (Bourahrouh, Paris & Elaouad-Debbaj, Reference Bourahrouh, Paris and Elaouad-Debbaj2004; Loi et al. Reference Loi, Ghienne, Dabard, Paris, Botquelen, Christ, Elaouad-Debbaj, Gorini, Vidal and Videt2010). During the deglaciation of the Hirnantian ice sheet, most Ordovician genera and species became extinct. A few Ordovician genera (e.g. Acanthochitina, Armoricochitina) disappeared during the deglaciation of the Hirnantian ice sheet, when about 33 % of the chitinozoan genera became extinct during the Late Ordovician. Almost all the species that originated in the Ordovician became extinct during the last part of the Hirnantian. The first chitinozoans with Silurian affinity (Spinachitina oulebsiri) occurred in the latest Hirnantian (upper Normalograptus persculptus Zone). Continuous sedimentation across the Ordovician/Silurian boundary is rare, but known from Skåne, south Sweden (Grahn, Reference Grahn1978, Reference Grahn1998; Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Grahn & Nõlvak, Reference Grahn and Nõlvak2007b), possibly Anticosti Island, Canada (Achab, Reference Achab and Lespérance1981; Soufiane & Achab, Reference Soufiane and Achab2000; Bergström, Saltzman & Schmitz, Reference Bergström, Saltzman and Schmitz2006; Achab, Asselin & Desrochers, Reference Achab, Asselin, Desrochers, Kröger and Servais2008; Melchin, Reference Melchin2008) and Dob's Linn, Scotland (Verniers & Vandenbroucke, Reference Verniers and Vandenbroucke2006). In the former area, a barren zone occurs within the Normalograptus persculptus Zone, and before the appearance of Silurian chitinozoan lineages (e.g. Belonechitina postrobusta). At Dob's Linn the fossil record is not continuous. In Bohemia and southwestern France (A. Bourahrouh, unpub. Ph.D thesis, Univ. de Rennes, 2002), and Algeria (Paris, Bourahrouh & Le Hérissé, Reference Paris, Bourahrouh and le Hérissé2000; F. Paris, unpub. data), characteristic Ordovician species (e.g. Desmochitina minor, Armoricochitina nigerica, Calpichitina lenticularis, Tanuchitina elongata) thrived in open marine shelf environments after the end of the glaciation. They become extinct at the same level as in Skåne (that is, the uppermost Normalograptus persculptus Zone), but after the first occurrence of Silurian related taxa, such as Spinachitina oulebsiri-fragilis (Vandenbroucke et al. Reference Vandenbroucke, Gabbott, Paris, Aldridge and Theron2009b). The Hirnantian glaciation was therefore not directly responsible for the dramatic extinction of organic-walled microfossils. However, it certainly accelerated the extinction of lineages that had already been weakened since the Katian, and favoured development of taxa better adapted to the habitats available high in the water column above the anoxic sea-bottom environments that persisted in some northern Gondwana areas for 10–15 Ma (Paris, Bourahrouh & Le Hérissé, Reference Paris, Bourahrouh and le Hérissé2000; Le Hérissé et al. Reference Le Hérissé, Bourahrouh, Vecoli and Paris2003). Chitinozoans are abundant and highly diversified (Fig. 1) and recorded with other pelagic or epipelagic organisms such as graptolites, orthocones and leiospheres in the Silurian black shale. The poisoned anoxic sea-bottom was not suitable for any metazoan life (as indicated by lack of bioturbation, no benthic fossils, and no degradation of the organic matter). In western Gondwana the chitinozoophorans thrived during the early Silurian (Llandovery) deglaciations when the intracratonic basins had sea-way connections with the Rheic Ocean and subsequently shared the same fauna and phytoplankton (Grahn & Caputo, Reference Grahn and Caputo1992; Grahn, Reference Grahn2005; Villeneuve et al. Reference Villeneuve, Diallo, Keleba, Kourouma, Paris and Racheboeuf1989; S. De la Puente, unpub. Ph.D. thesis, Univ. Nacional de Córdoba, 2009).
5. The late Wenlock crisis (C. lundgreni event) and earliest Emsian (pre-basal Zlichov event) graptoloid extinction
At the end of the Wenlock, a regression (Johnson, Kaljo & Rong, Reference Johnson, Kaljo, Rong, Bassett, Lane and Edwards1991; Johnson & McKerrow, Reference Johnson and McKerrow1991; Kaljo & Märss, Reference Kaljo and Märss1991) severely affected the monograptids (C. lundgreni event) on a global basis (Koren & Urbanek, Reference Koren and Urbanek1994; Štorch, Reference Štorch1995; Kozlowska-Dawidziuk, Lenz & Štorch, Reference Kozlowska-Dawidziuk, Lenz and Štorch2001). Only Pristograptus dubius survived from the monograptid line. Although the chitinozoophorans shared part of the same niche as graptolites, they were less affected, but nevertheless the diversity decreased considerably (Figs 1, 2) in the late Wenlock–early Ludlow (Paris & Nõlvak, Reference Paris and Nõlvak1999; Paris et al. Reference Paris, Grahn, Nestor and Lakova1999). No glaciations or extraterrestrial (Jaeger, Reference Jaeger1991) events (as indicated by the lack of unusually high presence of iridium) are known from the end of the Wenlock that can explain the graptolite crisis on a global basis. Quinby-Hunt & Berry (Reference Quinby-Hunt and Berry1991) discussed a hydrochemical explanation. A high global temperature during the Silurian, and a low oxygen concentration in the atmosphere, probably led to an extensive oceanic anoxia (Quinby-Hunt & Berry, Reference Quinby-Hunt and Berry1991; Koren & Urbanek, Reference Koren and Urbanek1994). A possible scenario is, therefore, a change in reduction conditions in the oceans leading to anoxic waters at low depths, far from the bottom, and expanding into the graptolite habitat, which would lead to only a thin layer of pelagic waters suitable for life (Quinby-Hunt & Berry, Reference Quinby-Hunt and Berry1991; Koren & Urbanek, Reference Koren and Urbanek1994). The appearance of dolomites with interbedded graptolitic shales in the latest Wenlock corroborates the presence of anoxia in the oceans. Deep-sea dolomites occur only under an increased reducing potential of sediments. A global oceanic disturbance, as yet unidentified, which severely affected graptolites, should consequently be reflected in carbon isotope (δ13C) curves. These show depletion in some sections in the late Wenlock–early Ludlow (Corfield & Siveter, Reference Corfield and Siveter1992; Corfield et al. Reference Corfield, Siveter, Cartlidge and McKerrow1992; Kaljo, Kiipli & Martma, Reference Kaljo, Kiipli, Martma, Landing and Johnson1998). Chitinozoophorans were less affected (see Nestor, Reference Nestor2009) since they dominated in upper layers of the lower offshore to nearshore environments, while graptolites inhabited the pelagic (Vandenbroucke et al. Reference Vandenbroucke, Armstrong, Williams, Zalasiewicz and Sabbe2009a) or alternatively the deeper parts of the ocean (Cooper, Fortey & Lindholm, Reference Cooper, Fortey and Lindholm1991).
Graptoloid and chitinozoophoran diversity decreased dramatically during a regressive phase in the Pragian and earliest Emsian (Fig. 1), which resulted from the same oceanographic conditions as during the latest Wenlock (Jaeger, Reference Jaeger1991). In the Prague Basin the last graptoloids became extinct in the uppermost Dvorce-Prokop Limestone (Jaeger, Reference Jaeger1978), very close to base of the bursa chitinozoan biozone and to the former Pragian–Emsian transition (F. P., unpub. data). However, it must be stressed that this level is significantly younger than the controversial GSSP of the Emsian defined by the FAD of the Polygnathus kitabicus conodont index species (Yolkin et al. Reference Yolkin, Kim, Weddige, Talent and House2000). Only benthic dendroids survived the event (Chlupáč & Kukal, Reference Chlupáč and Kukal1988). During a transgressive phase in the early Emsian (basal Zlichov event), the chitinozoans were still abundant but fairly poorly diversified (e.g. Paris, Reference Paris1981). However, the disappearance of graptoloids had no major impact on the chitinozoan distribution, as new pelagic competitors occupied this more or less vacant pelagic niche after the disappearance of the graptoloids (e.g. ‘Thuringian ecotype’ ostracods; see Lethiers & Raymond, Reference Lethiers and Raymond1991).
6. Latest Frasnian anoxic crisis (Kellwasser event)
The latest Frasnian anoxic crisis (Kellwasser event) may be the consequence of a multiplicity of impacts (e.g. Alamo, Siljan, Flynn Creek). Moreover, these contributed to successive crises in the Frasnian (House, Reference House2002), and finally resulted in the latest Frasnian mass extinction (McGhee, Reference McGhee2001). Kellwasser sediments are characterized by a general decrease of detrital input, and an increasing burial of organic matter. There was a decrease in oceanic CO2 concentrations, that were very high during the Devonian, and an acceleration of terrestrial weathering (Elick, Driese & Mora, Reference Elick, Driese and Mora1998). The increasing bioproductivity and eutrophication of the epiric seas (Joachimski et al. Reference Joachimski, Pancost, Freeman, Ostertag-Henning and Buggisch2002; Filipiak, Reference Filipiak2002; Racki et al. Reference Racki, Racka, Matyja and Devleeschouwer2002) caused a decrease of oxygen levels and the development of anoxic sea-bottom conditions. Major tectonic movements (Racki, Reference Racki1998) in the late Frasnian are reflected in a higher hydrothermal volcanic influence (Pujol, Berner & Stüben, Reference Pujol, Berner and Stüben2006). A transgressive phase in the end of the Frasnian (Kellwasser event) occurred during a warm climate (Streel et al. Reference Streel, Caputo, Loboziak and Melo2000 and references therein). A regression in the beginning of the Famennian (Streel et al. Reference Streel, Caputo, Loboziak and Melo2000; House, Reference House2002) was caused by a cooler global climate (possibly a short-lived glaciation in the earliest Famennian). The exceptional high concentration of chitinozoans in the basal Famennian beds at La Serre, France, is probably not related to any physical mechanisms alone (Paris et al. Reference Paris, Girard, Feist and Winchester-Seeto1996). The Kellwasser event affected benthic fauna and probably also chitinozoan predators, and the chitinozoophorans could therefore expand in the cooler earliest Famennian environment. Despite the very high abundance of chitinozoans in the lowermost Famennian bed at La Serre, the assemblage is monospecific (Paris et al. Reference Paris, Girard, Feist and Winchester-Seeto1996). This drop of biodiversity was counterbalanced during the Famennian by a diversification of the group (Grahn & Melo, Reference Grahn and Melo2002) prior to the latest Famennian extinction. The peak in the 87Sr/86Sr curve (Burke et al. Reference Burke, Denison, Hetherington, Koepnick, Nelson and Otto1982; Veizer et al. Reference Veizer, Buhl, Diener, Ebeneth, Podlaha, Bruckschen, Jasper, Korte, Schaaf, Ala and Azmy1997) indicates an increase of silica in the oceans that might have been caused by the onset of the Eovariscan uplift and a mountain building-enhanced continental weathering (Averbuch et al. Reference Averbuch, Tribovillard, Devleeschouwer, Riquier, Mistiaen and van Vliet-Lanoe2005).
7. Extinction of the chitinozoophoran group
Despite numerous palynological investigations of early Carboniferous marine strata, no chitinozoans have been recorded in situ. However, Middle and Late Devonian chitinozoans are frequently found reworked into Tournaisian strata. Tasch & Hutter (Reference Tasch and Hutter1978) reported finding chitinozoans from the Carboniferous. However, these are reworked from the Devonian and we interpreted some of them (blistered structures) as cyanobacteria colonies. The last records of chitinozoans in situ are from Brazil (Grahn & Melo, Reference Grahn and Melo2002; Grahn, Loboziak & Melo, Reference Grahn, Loboziak and Melo2003) in the late Famennian prior to the latest Famennian glaciation (lower VH Zone = upper VCo Zone) and from the Retispora lepidophyta biozones in the Illizi Basin, Algeria (Abdesselam-Rouighi & Coquel, Reference Abdesselam-Rouighi and Coquel1997; Boumendjel et al. Reference Boumendjel, Loboziak, Paris, Steemans and Streel1988). In both areas, Fungochitina fenestrata is generally followed by a monospecific Fungochitina ultima assemblage (Paris et al. Reference Paris, Winchester-Seeto, Boumendjel and Grahn2000; Grahn & Melo, Reference Grahn and Melo2002; Grahn, Loboziak & Melo, Reference Grahn, Loboziak and Melo2003). The disappearance of the chitinozoans and therefore the extinction of the chitinozoophorans (Fig. 1) coincide with a regression and fall in sea-level (Hangenberg event) in connection with the glaciation in western Gondwana at the end of Famennian (lepidophyta biozones).
There are, however, several possible contributing factors to the extinction of the chitinozoophorans:
(1) The closing of oceans also disturbed the currents and thus the distribution of the food supply, as well as areas of upwelling. The assembly of Pangaea did not destroy the habitat of the chitinozoophorans as these planktic animals were in all Devonian oceans, including in the Panthalassa Ocean surrounding Pangaea. Moreover, suitable shallow marine environments were still available in the Early Carboniferous.
(2) The first forests developing in the early Famennian (Meyer-Berthaud, Scheckler & Wendt, 1999) drastically modified the terrigenous input in the ocean. The resulting chemical changes in the oceans and seas possibly affected the entire marine food chain, especially the phytoplankton.
(3) The development of these first significant forests led to an increase in the atmospheric oxygen level and possibly a decrease in the CO2 pressure. The influence of the latter on chitinozoan diversity may be better evaluated when well-documented δ13Corg curves are available and can be calibrated with chitinozoan biodiversity curves.
(4) The proliferation of more efficient predators in the pelagic niche, such as ‘Thuringian ecotype’ ostracods (see Lethiers & Raymond, 1991, fig. 6), generated a drastic increase in competition with the chitinozoophorans for the use of the food supply. Moreover, some components of this microfauna were potential chitinozoan consumers and thus affected the number of vesicles reaching the sea-bottom. The arrival of new competitors happened earlier with the development of the ostracods of ‘Thuringian ecotype’ during the Frasnian (Lethiers, Baudin & Casier, Reference Lethiers, Baudin and Casier1998), and even earlier with the entomozoidea ostracods in the Silurian. Because no dramatic consequences are noted for the abundance and diversity of the pre-Famennian chitinozoans, the role of these predators in the extinction of the chitinozoans should not be overestimated.
(5) The drop in acritarch diversity and subsequently of the phytoplankton productivity during the Late Devonian has to be stressed (Riegel, Reference Riegel2008). This might represent an important factor in the survival of chitinozoophorans: that is, insufficient food supply and more efficient new competitors, such as pelagic ostracods.
(6) The latest Famennian glaciation generated a drop in the sea-level with drastic changes in marine environments: much shallower seas, uplift and even erosion of land, as demonstrated by the common reworking of Middle and Late Devonian palynomorphs into the Carboniferous. In western Gondwana the onset of the latest Famennian glaciation changed open marine conditions to brackish environments as indicated by the appearance of Protosalvinia (Niklas, Phillips & Carozzi, Reference Niklas, Phillips and Carozzi1976; Loboziak et al. Reference Loboziak, Melo, Quadros and Streel1997) that occur somewhat later than the last chitinozoans (Grahn & Melo, Reference Grahn and Melo2002). This suggests that the chitinozoophorans were holomarine and could not adapt to brackish water conditions.
The chitinozoophorans became extinct for multiple and in some cases related reasons:
(1) They possibly no longer had the genetic potential to develop innovations favouring successful adaptations to rapid environmental changes (intrinsic factors). The monospecific assemblage in the latest Famennian supports this possibility.
(2) Their predators became more and more efficient (extrinsic factors). There are examples of selective predation from the late Llandovery in Saudi Arabia, documented by faecal pellets with cracked vesicles of a large species of Cyathochitina (F. P., unpub. data). Based on the size of the pellets, the predators were not very large and would have been part of the zooplankton (e.g. entomozoidea ostracods, including the Devonian ‘finger-print’ ostracods) or of the necto-pelagos (small polychaetes or arthropods such as crustaceans or crustacean larvae, but the poor preservation potential has left no body fossils recorded).
(3) Their usual niche was invaded by a more efficient group, such as pelagic ostracods (extrinsic factor), but this can be envisaged only if the competitor group had a dramatic increase in abundance in the Famennian (e.g. the ‘Thuringian ecotype’ ostracods; see Lethiers & Raymond, Reference Lethiers and Raymond1991, fig. 6). Indeed, other pelagic ostracods (pelagic entomozoidea and myodocope ostracods) are reported from the Wenlock onwards (see Siveter, Vannier & Palmer, Reference Siveter, Vannier and Palmer1991); V. J. Perrier, unpub. Ph.D. thesis, Univ. Claude Bernard, Lyon, 2007; Perrier, Vannier & Siveter, Reference Perrier, Vannier and Siveter2007) and they had no lethal effects on the chitinozoophorans.
(4) Their usual food supply disappeared or was not sufficient to share with more efficient feeding groups. This is supported by the contemporaneous decline in phytoplankton.
As a hypothesis, the chitinozoan record may promulgate a false idea of the situation if the chitinozoophorans had drastically changed their mode of life (e.g. become parasites) or their usual environment. For instance, the chitinozoophorans may have moved onto land, with an insect-type behaviour and a subsequent dramatic change in their eggs (see Paris, Reference Paris1981, p. 83). That is, there would no longer be any need to control osmotic pressure, but new membranes might have been necessary for the survival of the embryos. There are a number of similarities in ultrastructures between chitinozoans and modern insect eggs (Grahn & Afzelius, Reference Grahn and Afzelius1980; Paris, Reference Paris1981). Arthropods are known to have colonized land in the Silurian when the chitinozoophorans were thriving, for example, chelicerates in the early Llandovery (F. P., unpub. data) including myriapods (Morrissey & Braddy, Reference Morrissey and Braddy2004) and arachnids (Jeram, Selden & Edwards, Reference Jeram, Selden and Edwards1990) in the late Silurian. However, no significant diversification changes are noticed in the chitinozoan group at this time.
8. Concluding remarks
The chitinozoan group existed for about 130 Ma, from early Tremadocian to latest Famennian times. Chitinozoophorans (the chitinozoan animal) were pelagic zooplankton and shared part of this niche with graptolites and others. They were therefore less affected than other groups by the development of anoxic conditions in the deeper part of the water column (Rhuddanian black shales, Kellwasser event). Extinction of typical Ordovician taxa took place during the Hirnantian deglaciation, and while not directly responsible for the dramatic extinction of organic-walled microfossils, it certainly accelerated the extinction of lineages that had already been weakened since the Katian. This event also favoured the development of taxa better adapted to low oxygen levels in the anoxic oceanic environments prevalent during the Early Silurian (Rhuddanian). These Silurian lineages first appeared in the latest Hirnantian (upper Normalograptus persculptus Zone). Extinction of the chitinozoan group occurred after a combination of events that restricted the environments for the chitinozoophorans and favoured new competitors. This, combined with the fact that lineages had been weakened since the Frasnian and were monospecific in the latest Famennian, meant that they no longer had the genetic potential to develop innovations to adapt to successive environmental changes. The contemporaneous decline in phytoplankton indicates that the food supply disappeared or was insufficient for the chitinozoophorans. Together with the pressure of more predators, these factors contributed to their extinction.
Acknowledgements
Yngve Grahn thanks the Faculty of Geology at Universidade do Estado do Rio de Janeiro (UERJ), and Dr C. S. Valladares, head of the post-graduate progam at the Faculty of Geology for access to the facilities, and the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq, PQ 309751/2007-1) which made his work possible through grants. Oliver Chang Paris improved and updated the database (CHITINOVOSP) used by Florentin Paris for the evaluation of the chitinozoan biodiversity at species level. The authors are greatly indebted to Theresa Winchester-Seeto (Sydney, Australia) for scientific and linguistic improvements of the manuscript. We are indebded to Aicha Achab (Québec, Canada) for updated information on the range of the Late Ordovician chitinozoans in Laurentia. The reviewers, Thijs Vandenbroucke (Lille University, France) and Olle Hints (Tallinn Technical University, Estonia), are warmly acknowledged for helpful comments on the manuscript and for extensive discussions on the palaeooceanological distribution of the chitinozoans.