Ehrlichia were first associated with veterinary disease in Africa in 1925 by Cowdry, who identified Ehrlichia ruminantium in cattle. A decade later Donatien and Lestoquard described Ehrlichia canis in Algerian dogs (Refs Reference Donatien and Lestoquard1, Reference Cowdry2). Ehrlichioses continue to be important veterinary diseases, but are now also associated with newly identified human tick-borne zoonoses. At the end of the 20th century, a new tick-borne disease, human monocytotropic ehrlichiosis, emerged in humans and a novel aetiological agent – Ehrlichia chaffeensis – was identified (Ref. Reference Anderson3). Twelve years later, Ehrlichia ewingii, a recognised canine pathogen that infects granulocytes, was detected in four immunocompromised patients with symptoms of ehrlichiosis (Refs Reference Buller4, Reference Paddock5). Nearly a century after Cowdry's discovery, Ehrlichia are firmly established as zoonotic human pathogens of public health importance. Human monocytotropic ehrlichiosis is considered one of the most prevalent life-threatening tick-borne diseases in the USA, and ewingii ehrlichiosis is an important clinically indistinguishable disease in immunocompromised patients (Refs Reference Buller4, Reference Paddock5).
The order Rickettsiales contains two families of arthropod-transmitted obligately intracellular bacteria that cause human diseases, including spotted fever rickettsiosis, typhus, scrub typhus, anaplasmosis and ehrlichiosis. The genus Ehrlichia is a member of the family Anaplasmataceae, which also includes the genera Anaplasma, Wolbachia and Neorickettsia. In addition, the family Rickettsiaceae and respective genera Rickettsia and Orientia are also members of Rickettsiales. The Ehrlichia genus consists of six formally named members (E. canis, E. chaffeensis, E. muris, E. ruminantium, E. ewingii and E. ovis). E. chaffeensis and E. ewingii are recognised as human zoonotic pathogens that also cause significant disease in the animal hosts (Refs Reference Breitschwerdt, Hegarty and Hancock6, Reference Goldman7). E. canis, the aetiological agent of canine monocytic ehrlichiosis, is a globally distributed pathogen and has been recently associated with human infections (Refs Reference Perez8, Reference Keefe9). E. ruminantium is a veterinary pathogen that causes a severe acute infection known as heartwater in domestic ruminants localised primarily to sub-Saharan Africa (Ref. Reference Uilenberg10).
Ehrlichia have small genomes, yet have evolved elaborate and complex molecular strategies that enable adaptation to distinct hosts (invertebrate and vertebrate) and intracellular survival in innate immune effector cells. Using Ehrlichia as a model to understand the molecular interactions between pathogen and eukaryotic host cell provides an attractive and manageable system to advance our knowledge of the molecular pathobiology of intracellular microbes as well as the molecular biology of the eukaryotic cell. This review focuses on the molecular and cellular interactions of ehrlichiae and immunological responses that have clinical implications with regard to development of novel antimicrobial therapeutics or molecular countermeasures, and immunomodulatory approaches.
Physical characteristics and intracellular developmental biology
E. chaffeensis is confined to cytoplasmic membrane-bound vacuoles within monocytes/macrophages and dendritic cells (Ref. Reference Koh11), replicating to form microcolonies called morulae that contain one to >400 organisms (Ref. Reference Barnewall, Rikihisa and Lee12). Morphologically, individual ehrlichiae are coccoid and coccobacillary and exhibit two ultrastructural cell types: a larger reticulate cell and a smaller dense-cored cell (Fig. 1). Morphological comparisons to other intracellular bacteria such as Chlamydia have suggested that Ehrlichia reticulate cells and dense-cored cells represent analogous replicating and infectious forms of the organism. This hypothesis was confirmed by a recent study of ehrlichial developmental biology that demonstrated that the dense-cored cell form of E. chaffeensis binds to the host cell surface where it is rapidly (<1 h) internalised and completes the developmental cycle within 72 h (Ref. Reference Zhang16) (Fig. 2).
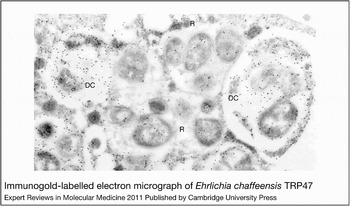
Figure 1. Immunogold-labelled electron micrograph of Ehrlichia chaffeensis TRP47. Within innate immune cells, ehrlichiae form membrane-bound microcolonies termed morulae. Individual ehrlichiae show two morphological forms: dense-cored cells (DC; 0.4–0.6 µm in diameter) and reticulate cell (R) forms (0.4–0.6 µm by 0.7–1.9 µm). Reticulate cells and dense-cored cells can be distinguished by the differential expression and secretion of two tandem-repeat-containing surface proteins – TRP47 and TRP120 – which are found only on dense-cored cells (Refs Reference Popov, Yu and Walker13, Reference Doyle14). The intramorular space in some morulae contains a fibrillar matrix of ehrlichial origin (Ref. Reference Popov15). Figure reproduced, with permission from American Society for Microbiology, from Ref. Reference Doyle14 (© 2006, American Society for Microbiology).
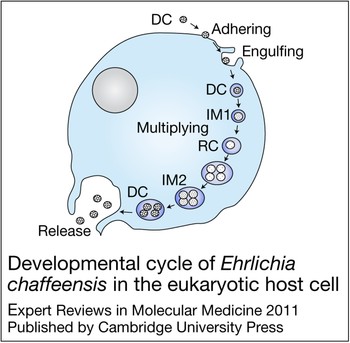
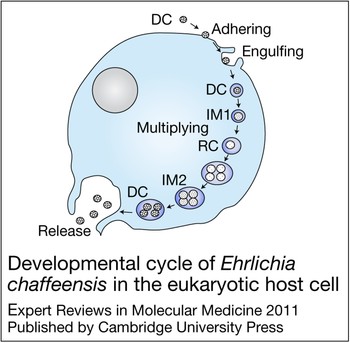
Figure 2. Developmental cycle of Ehrlichia chaffeensis in the eukaryotic host cell. Infectious dense-cored (DC) ehrlichiae attach and enter the host cell through receptor-mediated endocytosis, and within 1 h after entry transform into the intermediate (IM) 1, and then into the reticulate cell (RC). During the next 48 h RC replicates, doubling every 8 h, and then transforms into IM2 and matures to DC ehrlichiae within 72 h after initial cell contact (Ref. Reference Zhang16). DC ehrlichiae obtained 72 h post-infection showed much higher infectivity than E. chaffeensis RCs obtained 24 h post-infection. RC and DC ehrlichiae can be distinguished by two differentially expressed tandem repeat proteins – TRP47 and TRP120 – that are found only on DC ehrlichiae, and the major outer membrane protein p28-19, which is expressed only on the RC (Ref. Reference Zhang16). However, intermediate, presumably transitional, forms that co-express these proteins have also been described (Ref. Reference Zhang16). Although ehrlichiae and chlamydiae have similar morphological forms and developmental cycles, homologous genes for the histone H1 homologue proteins (Hc1 and Hc2) involved in condensation and decondensation of the chlamydial nucleoid are not present in the E. chaffeensis genome (Ref. Reference Zhang16). Figure adapted, with permission from John Wiley and Sons (http://www.interscience.wiley.com), from Ref. Reference Zhang16 (© The Authors).
The Ehrlichia genome: insight into host–pathogen interactions
The intracellular niche occupied by Ehrlichia has resulted in reductive evolutionary processes and a corresponding severe loss of genes associated with metabolic processes provided by the host cell. Hence the genome sizes (~1–1.5 Mb) of Ehrlichia are relatively small compared with those of extracellular bacteria. The genomes of three Ehrlichia species have been sequenced (Refs Reference Dunning Hotopp17, Reference Mavromatis18, Reference Collins19), and they show a high degree of genomic synteny, a low G+C content (~30%) and one of the smallest genome coding ratios, which is attributed to long noncoding regions and numerous long tandemly repeated sequences (Ref. Reference Frutos20). Long noncoding regions and low G+C content in other related Rickettsiales members are speculated to represent degraded genes in the final stages of elimination and excess GC-to-AT mutations (Refs Reference Andersson and Andersson21, Reference Andersson and Andersson22, Reference Andersson23). The large number of long tandemly repeated sequences that are a feature of Ehrlichia genomes have no phylogenetic relationships, suggesting that duplication occurred after diversification of the repeat-encoding DNA and divergence of the species (Ref. Reference Frutos24). The tandem repeats seem to be a result of locally occurring independent events, actively created and deleted through a mechanism compatible with DNA slippage (Ref. Reference Frutos24), and their generation might be a mechanism of adaptation to the host.
Features identified in Ehrlichia genomes associated with host–pathogen interactions include genes that encode tandem- and ankyrin-repeat-containing proteins, genes encoding actin polymerisation proteins, genes with poly(G-C) tracts encoding a family of proteins with short sequence repeats, and a multigene family encoding outer membrane proteins; in addition, there is an absence of genes for the biosynthesis of peptidoglycan and lipopolysaccharide (Ref. Reference Mavromatis18), the major pathogen-associated molecular patterns recognised by the innate immune system. Tandem repeats are associated with regulation of gene expression and phase variation, and Ehrlichia species show two types of tandem repeats: small (12 bp) and large (100–300 bp) period repeats (Ref. Reference Frutos24). Secretion of effector proteins into the host cell requires secretion systems, and such delivery mechanisms have been identified, including many of the known type IV secretion system (T4SS) components (Refs Reference Dunning Hotopp17, Reference Mavromatis18, Reference Collins19). There is no evidence of a type III secretion system (T3SS), although other intracellular bacteria such as Chlamydia have this system and some secreted ehrlichial tandem repeat proteins (TRPs) are predicted to have an N-terminal type III secretion transport signal. The Sec-dependent and Sec-independent protein export pathways for secretion of proteins across the inner membrane as well as a putative type I secretion system (T1SS) have also been identified (Refs Reference Dunning Hotopp17, Reference Mavromatis18, Reference Collins19). The Ehrlichia genomes also have genes that encode three response regulator two-component systems (composed of a sensor histidine kinase and a response regulator), which allow bacteria to sense signals and respond to changes in their environment through specific gene activation or repression (Ref. Reference Dunning Hotopp17).
Proteins associated with host–pathogen interactions
Tandem repeat proteins
The presence of long-period tandem repeats distributed in intergenic regions of Ehrlichia is well recognised and is associated with expansion and contraction of these regions. Interestingly, long-period tandem repeats are also found in a small subset of proteins, many of which are strongly immunoreactive, suggesting that they are exposed on the surface or secreted. TRPs in pathogenic bacteria have been associated with host–pathogen interactions such as adhesion and internalisation (Refs Reference Popov, Yu and Walker13, Reference Kumagai25), actin nucleation (Ref. Reference Jewett26) and immune evasion (Ref. Reference Gravekamp27). Many of these proteins in Ehrlichia have been molecularly characterised, and major continuous species-specific antibody epitopes have been mapped to the acidic serine-rich tandem repeats of E. chaffeensis TRP120, TRP47 and TRP32 (Refs Reference Doyle14, Reference Luo28, Reference Luo, Zhang and McBride29), and of the E. canis orthologues TRP140, TRP36 and TRP19, respectively (Refs Reference Doyle14, Reference Luo, Zhang and McBride29, Reference McBride30). Immunoelectron microscopy has shown that these TRPs are secreted by the ehrlichiae and are associated with the morular fibrillar matrix and the morula membrane (Refs Reference Barnewall, Rikihisa and Lee13, Reference Popov, Yu and Walker14, Reference Luo28). Other bacteria have effector TRPs that are secreted by the T3SS; however, the absence of an identifiable T3SS in Ehrlichia suggests that another mechanism such as the T1SS is involved. In preliminary work, we have explored the possibility of these TRPs as T4SS substrates, but they were not secreted in the well-characterised model of the VirB/VirD4-dependent T4SS of Agrobacterium tumefaciens (Ref. Reference Wakeel31).
The functional role of the tandem repeats in E. chaffeensis TRPs is not fully understood. However, homology between the tandem repeats and other functional protein domains and motifs of eukaryotic origin have been reported. The E. chaffeensis TRP47 contains seven 19-mer (ASVSEGDAVVNAVSQETPA) tandem repeats that dominate the C-terminal region of the protein, and approximately half of the TRP47 is represented by the tandem repeat domain (Ref. Reference Wakeel, Kuriakose and McBride32). The TRP47 tandem repeat region shows homology with eukaryotic proteins including the renin receptor (also known as ATP6AP2 and CAPER), the DNA polymerase III subunits gamma and tau-conserved domain, and ribonuclease E, suggesting similar functional characteristics (Ref. Reference Wakeel, Kuriakose and McBride32). TRP47 also has several N-terminal tyrosine and serine/threonine residues that are predicted sites of phosphorylation, and tyrosine phosphorylation has been detected on TRP47 with antiphosphotyrosine antibodies (Ref. Reference Wakeel, Zhang and McBride33). Other TRPs might be phosphorylated, such as TRP32, which has an unusually high frequency of tyrosine residues (20%) in the C-terminal tail (Ref. Reference Luo28). The tandem repeat domains of TRP120 and TRP32 do not have homology with other conserved protein domains; however, recent studies have reported that the tandem repeat region of TRP120 directly binds host cell DNA (Ref. Reference Zhu and McBride34).
Ankryin repeat proteins
The ankyrin repeat (Ank) is a ubiquitous eukaryotic motif that can occur in combination with other types of domains and can cooperatively fold into structures that mediate molecular recognition by protein–protein interactions. Ehrlichia species are among only a few prokaryotes that are known to have Ank-containing proteins. The most extensively studied Ank protein in E. chaffeensis is a 200 kDa protein (Ank200) that has a central domain containing 19 Anks flanked by acidic (pI 4–5) C- and N-terminal domains with a predominance of glutamate and aspartate residues (Ref. Reference Luo35). In addition, like the TRPs, the E. canis and E. chaffeensis Ank200s have a high proportion of polar amino acids, including serine and threonine (Refs Reference Luo35, Reference Nethery36). Several species-specific antibody eptiopes have been mapped to acidic terminal domains of the Ank200s (Refs Reference Luo35, Reference Nethery36). Our preliminary data suggest that E. chaffeensis Ank200 is not secreted by the T4SS (Ref. Reference Wakeel31); however, Anaplasma phagocytophilum AnkA appears to be secreted by this mechanism (Ref. Reference Lin37). Although E. chaffeensis and A. phagocytophilum are closely related, they are different in many aspects; for example, they show tropism for different cell types, residence in different cytoplasmic compartments (Refs Reference Mott, Barnewall and Rikihisa38, Reference Webster39) and distinct immune evasion mechanisms (Refs Reference Barbet40, Reference Barbet41, Reference Wang42). In addition, an A. phagocytophilum VirD4, the T4SS substrate-coupling protein, shows a higher identity with A. tumefaciens VirD4 than with that of E. chaffeensis (Refs Reference Dunning Hotopp17, Reference Barbet41).
Major outer membrane proteins
A superfamily of immunoreactive outer membrane proteins that are members of the Pfam PF01617 group of proteins (http://pfam.sanger.ac.uk/) has been identified in the family Anaplasmataceae. E. chaffeensis has a paralogous family of 22 major outer membrane proteins (OMP-1/p28) encoded at a single locus upstream from the secA gene and downstream from a hypothetical transcriptional regulator gene (Ref. Reference Ohashi, Rikihisa and Unver44). They were originally proposed to be involved in antigenic variation of the organism. Although recombination of the major outer membrane proteins of closely related Anaplasma species occurs to create antigenic diversity, there is no evidence that recombination of the Ehrlichia OMP-1 family occurs. Differential expression of the OMP-1 genes in ticks and animal hosts has been reported, suggesting that they have a role in host adaptation (Refs Reference Unver45, Reference Seo46). Expression of only one OMP-1 gene (OMP-1B) has been reported in ticks and tick cell lines and appears to involve a temperature-sensitive regulation mechanism (Refs Reference Unver45, Reference Unver47). By contrast, all OMP-1 family members are expressed in mammalian hosts and cells, and antibodies against all OMP-1 proteins have been detected in experimentally infected dogs (Refs Reference Unver45, Reference Zhang48). Although the role of the outer membrane proteins in antigenic variation and immune evasion is still uncertain, other characteristics have been identified for the OMP-1 proteins, including transmembrane β-strand structural features and porin activity, suggesting that they might facilitate nutrient acquisition (Ref. Reference Kumagai, Huang and Rikihisa49).
Molecular and cellular biology of infection
Entry and characteristics of the ehrlichial vacuole
Infection of the host cell involves dense-cored ehrlichiae that express TRP120 on the surface. TRP120 has an important role in the binding and entry process (Refs Reference Popov, Yu and Walker13, Reference de la Fuente25), and a potential role has also been demonstrated for TRP47 in attachment to tick cells using the E. ruminantium orthologue (Erum1110) (Ref. Reference de la Fuente50). The stability of TRP120 and invasion of E. chaffeensis are regulated by the bacterial second messenger cyclic di-GMP and activity of ehrlichial surface serine protease HtrA (Ref. Reference Kumagai25). Binding to the host cell occurs through receptors such as E- and L-selectin and other glycosylphosphatidylinositol (GPI)-anchored proteins located in caveolae (Refs Reference Zhang, McBride and Yu51, Reference Lin and Rikihisa52), triggering receptor-mediated endocytosis that involves signalling events including transglutamination, tyrosine phosphorylation, phospholipase Cγ2 activation, production of inositol (Reference Donatien and Lestoquard1,Reference Buller4,Reference Paddock5)-trisphosphate and increases in intracellular calcium (Ref. Reference Lin, Zhu and Rikihisa53). Removal of surface-exposed GPI-anchored proteins by phosphatidylinositol-specific phospholipase C prevents the development of early inclusions (Ref. Reference Lin and Rikihisa52).
The vacuoles in which the organism enters contain caveolin-1, GM1 ganglioside and phospholipase Cγ2. Later (>3 h) after infection, vacuoles that contain replicating ehrlichiae show characteristics of early endosomes such as the presence of the small GTPase Rab5A (RAB5A), early endosomal antigen 1 (EEA1) and vacuolar (H+) ATPase, and they accumulate transferrin and transferrin receptor (Ref. Reference Barnewall, Rikihisa and Lee12). Replicative vacuoles of closely related A. phagocytophilum do not express these markers (Ref. Reference Mott, Barnewall and Rikihisa38). Other molecules found in Ehrlichia inclusions are vesicle-associated membrane protein 2, major histocompatibility class II and β2-microglobulin (Refs Reference Barnewall, Rikihisa and Lee12, Reference Mott, Barnewall and Rikihisa38). Ehrlichia vacuoles appear to be maintained in a caveolar trafficking system that interacts with recycling endosomal pathways (Ref. Reference Lin and Rikihisa52).
Modulation of host cell gene expression
E. chaffeensis appears to actively modulate host cell gene transcription and function through several mechanisms, including interactions with host chromatin and modulation of host signalling. One mechanism that has been identified involves the inhibition of host mitogen-activated protein (MAP) kinases by E. chaffeensis, leading to the downregulation of transcription factors and transcription of target genes related to host defence (Ref. Reference Lin and Rikihisa54). The discovery of DNA-binding proteins (TRP120 and Ank200) of Ehrlichia provides another mechanism by which host cell gene transcription can be modulated (Refs Reference Zhu and McBride34, Reference Zhu55).
The transcription levels of a relatively small percentage (5%) of host cell genes are altered significantly within the first 24 h post-infection (Ref. Reference Zhang56). This transcriptional profile has provided new information on host cell processes targeted by Ehrlichia and has revealed key themes in pathobiology and disease pathogenesis. Specific cellular processes that appear to be modulated are apoptosis, regulation of the cell cycle and differentiation, signal transduction, and the expression of proinflammatory cytokines, biosynthetic and metabolic proteins, and membrane trafficking proteins. Host genes modulated during E. chaffeensis infection are distinct from those observed in infections by other intracellular bacteria, illustrating the complexity and diversity of intracellular pathogen–host interactions and survival strategies (Ref. Reference Zhang56).
Survival in mononuclear phagocytes requires the ability to evade innate and adaptive immune responses. E. chaffeensis represses the transcription of cytokines involved in the early innate immune response and cell-mediated immune response to intracellular microbes, including host cell cytokines that modulate innate and adaptive immunity to intracellular bacteria. Early in infection, genes for the proinflammatory cytokines interleukin (IL)-1β (IL1B), IL-8 and tumour necrosis factor β (TNF-β; also known as LTA) are upregulated, and others such as TNF-α (TNF) are not induced (Ref. Reference Zhang56). By contrast, most cytokines and receptors are downregulated, including IL-15, IL-18 and various chemokine receptors. These cytokines have fundamental roles in stimulating natural killer (NK) cells and T helper 1 (Th1) cells to produce interferon γ (IFN-γ; IFNG), which then activates macrophages to kill phagocytosed bacteria. IL-12 and IL-15 also activate NK cells and cytotoxic T lymphocytes to kill cells infected with intracellular bacteria. Thus, active modulation of genes associated with the immune response appears to be essential to the survival of E. chaffeensis.
Modulation of genes associated with inhibition of apoptosis and cell cycle regulators is observed early during E. chaffeensis infection, and is probably necessary for delaying host cell death, as discussed further below. E. chaffeensis infection upregulates transcription of apoptosis inhibitors such as IER3, BIRC3 and BCL2, but inhibits apoptosis inducers such as BIK and BNIP3L during the early stage of infection, thus impairing host cell apoptosis and maintaining a prolonged growth opportunity for ehrlichiae (Ref. Reference Zhang56).
E. chaffeensis lives in an early endosome and inhibits the maturation of the endosome to evade destruction by lysosomal enzymes (Ref. Reference Mott, Barnewall and Rikihisa38). In an effort to modulate this process, E. chaffeensis inhibits the transcription of genes involved in membrane trafficking and lysosomal fusion: the production of Rab5, synaptosome-associated protein 23 (SNAP23) and syntaxin 16 (STX16) is repressed, most dramatically during the first hour of infection. Interestingly, Rab5 is associated with E. chaffeensis inclusions. Depletion of Rab5 inhibits the fusion of the phagosome containing Listeria monocytogenes with lysosomes (Ref. Reference Alvarez-Dominguez57).
Acquisition of iron and cholesterol
Ehrlichia survival depends on an available supply of intracellular iron (Ref. Reference Barnewall, Ohashi and Rikihisa58), and this is also demonstrated by the fact that the antiehrlichial activity of IFN-γ is mediated by limiting the available cytoplasmic iron (Ref. Reference Barnewall and Rikihisa59). Interestingly, cytoplasmic vacuoles containing replicating ehrlichiae accumulate transferrin receptor, and transferrin receptor mRNA is upregulated during ehrlichial infection (Ref. Reference Barnewall, Ohashi and Rikihisa58), which might serve to counteract the reduction of surface transferrin receptor by IFN-γ. How Ehrlichia acquires iron from the host is not fully understood, but a conserved iron-acquisition mechanism has been identified in Ehrlichia involving the ATP-binding cassette transporter family, a mechanism shared by a diversity of bacterial species (Ref. Reference Doyle60). However, unlike most bacteria in which the genes encoding iron-acquisition proteins are found in an operon, Ehrlichia iron-acquisition genes are not part of a functional operon (Ref. Reference Doyle60). The iron-binding protein (Fbp) of Ehrlichia is conserved among other known iron-binding proteins in bacteria, and similarly it binds Fe(III). Notably, Ehrlichia Fbp has been observed outside the bacterium within the morular space containing dense-cored cells, suggesting that iron is obtained from intracellular pools derived from transferrin and shuttled by Fbp to the bacterium (Ref. Reference Doyle60).
Although Ehrlichia lack structural membrane components such as peptidoglycan and lipopolysaccharide, they appear to use cholesterol as a structural substitute (Ref. Reference Lin and Rikihisa61). Ultrastructural changes are observed in Ehrlichia in the presence of cholesterol-extraction reagents. Cholesterol is incorporated as a component of the outer membrane, and Ehrlichia have an unidentified, but specific, direct uptake mechanism (Ref. Reference Lin and Rikihisa61). The structural integrity of the organism is dependent on cholesterol, and it is essential for maintaining an infectious state (Ref. Reference Lin and Rikihisa61).
Evading innate host defences
As indicated above, Ehrlichia have evolved strategies that allow them to survive in phagocytes and evade innate host defence mechanisms. Ehrlichia manipulate innate immune defence mechanisms, including production of reactive oxygen species (ROS), apoptosis, lysosomal fusion and IFN-γ responsiveness. A major host defence mechanism is production of ROS that have strong antimicrobial effects. E. chaffeensis is highly sensitive to O2− killing (Ref. Reference Lin and Rikihisa62) and lacks genes involved in the detoxification of ROS (Ref. Reference Dunning Hotopp17). However, E. chaffeensis actively blocks O2− generation in monocytes stimulated with phorbol myristate acetate and causes degradation of NADPH oxidase subunit p22phox in monocytes, but not neutrophils (Ref. Reference Lin and Rikihisa62). Degradation of p22phox appears to involve nonproteasomal proteolysis and heat-labile factors or proteins from E. chaffeensis. Degradation can be detected in isolated monocyte membrane fractions, which suggests that intracellular signalling events are not required (Ref. Reference Lin and Rikihisa62).
Apoptosis is an innate cellular defence mechanism against microbes that is modulated by many bacterial pathogens, and there is new information that indicates that Ehrlichia also modulate host cell death. In nature, Ehrlichia survive by persistently infecting vertebrate hosts, so delaying or preventing apoptosis could be a means of enhancing survival by preventing host cell death and subsequent immune recognition. For most intracellular pathogens, induction of apoptosis leads to pathogen killing and clearance of infection, and this is thought to be beneficial to the host and enhance the immune response to the infection. In the case of Ehrlichia, there appear to be several mechanisms involved in apoptosis modulation by Ehrlichia. As mentioned above, E. chaffeensis upregulates the transcription of genes related to antiapoptotic activity and the cell cycle, including cyclin-dependent kinase expression, in THP-1 cells (Ref. Reference Zhang56). E. ewingii delays apoptosis in neutrophils by stabilisation of host cell mitochondria (Ref. Reference Xiong63).
Phagolysosomes represent another important innate host defence mechanism against pathogens. Ehrlichia are able to inhibit fusion of the phagosome containing ehrlichiae with lysosomes and thus prevent their destruction by this defence mechanism (Ref. Reference Barnewall, Rikihisa and Lee12). Although very little is known about how Ehrlichia avoid this host defence mechanism, recent studies have demonstrated that functioning two-component systems have an important role in preventing lysosomal fusion (Ref. Reference Kumagai64). Two-component systems consist of a sensor with a histidine kinase that detects environmental signals, and a response regulator that has DNA-binding activity and regulates gene transcription (Ref. Reference Parkinson and Kofoid65). E. chaffeensis has three two-component systems: PleC–PleD, NtrY–NtrX and CckA–CtrA (Ref. Reference Cheng66). Cells treated with closantel and incubated with E. chaffeensis have increased colocalisation between E. chaffeensis and lysosomal glycoprotein LAMP-1, and E. chaffeensis infection is completely inhibited by pretreatment with closantel (Ref. Reference Cheng66).
The macrophage-activating cytokine IFN-γ has an important role in innate and adaptive immune responses against intracellular pathogens. The activation of macrophages by IFN-γ induces several antimicrobial effector mechanisms, including regulation of iron homeostasis (Ref. Reference Collins67), that are necessary for the production of antimicrobial effectors, including ROS and nitrogen radicals (Ref. Reference Collins68). The ability to acquire iron is important for the survival of intracellular pathogens such as Ehrlichia, Salmonella, Listeria and Mycobacterium (Refs Reference Collins68, Reference Schaible and Kaufmann69). With respect to E. chaffeensis, macrophages that are stimulated with IFN-γ before infection or early in infection readily kill E. chaffeensis (Ref. Reference Barnewall and Rikihisa59). However, E. chaffeensis has developed strategies to circumvent the actions of IFN-γ. E. chaffeensis upregulates transferrin receptor expression to counteract the downregulation by the action of IFN-γ (Ref. Reference Barnewall, Ohashi and Rikihisa58). The inhibitory effects of IFN-γ on E. chaffeensis infection can be reversed by the addition of iron-saturated transferrin, and E. chaffeensis infection is inhibited by the intracellular iron chelator deferoxamine (Ref. Reference Barnewall and Rikihisa59), demonstrating the critical role of iron for E. chaffeensis infection and the role that IFN-γ has in regulating its availability. After E. chaffensis infection is established, IFN-γ is no longer effective, possibly because of the ability of E. chaffeensis to upregulate host iron-acquisition mechanisms. The ability of E. chaffeensis to block the antimicrobial mechanisms of IFN-γ is mediated at least in part by inhibition of the JAK–STAT IFN-γ signal transduction pathway (Ref. Reference Lee and Rikihisa70). Binding of a protein component of E. chaffeensis to the host cell blocks tyrosine phosphorylation of JAK1 and STAT1 normally induced by IFN-γ (Ref. Reference Lee and Rikihisa70). JAK–STAT inhibition has been linked to the upregulation of protein kinase A by E. chaffeensis (Ref. Reference Lee and Rikihisa70). Others have also suggested that avoiding IFN-γ activity might be influenced by transcriptional regulation of JAK–STAT expression mediated by the nuclear effector Ank200 (Ref. Reference Zhu55).
E. chaffeensis also appears to modulate the host innate immune response by influencing other cell signalling pathways. Antimicrobial activities of macrophages become progressively less responsive during E. chaffeensis infection in association with downregulation of pattern recognition receptors (PRR), including CD14, TLR2 and TLR4, and activity of the associated PRR transcription factor PU.1 (SPI1) (Ref. Reference Lin and Rikihisa54). Activation of PU.1 has been linked to a p38 mitogen-activated protein kinase (MAPK)-dependent pathway, and a p38-specific MAPK inhibitor shows similar effects on PU.1 and PRR activity and expression (Ref. Reference Wang, Lai and Yang-Yen71). Ehrlichia also appear to target host tyrosine kinases and phosphatases (FYN and PTPN2; described in more detail below), but the downstream processes affected by these interactions are not known (Ref. Reference Wakeel, Kuriakose and McBride32).
E. chaffeensis effectors and molecular host interactions
Significant progress has been made in the identification of host cell processes that are modulated by Ehrlichia in order to survive in phagocytes. However, the effector proteins involved in manipulating these host cell processes have been largely undetermined. Recent genome sequencing efforts and new biotechnology approaches to investigate molecular protein–protein interactions have focused attention on two small groups of Ehrlichia-encoded proteins: those containing tandem or ankryin repeats. These proteins appear to be effectors involved in novel, complex and multidimensional molecular strategies to reprogramme host cell defence mechanisms (Ref. Reference Wakeel72). Two proteins, TRP47 and Ank200, have been the focus of recent studies demonstrating interactions with host cell DNA and molecular interactions with host proteins associated with distinct host cell processes.
E. chaffeensis TRP47
Many of the small family of TRPs of Ehrlichia are strongly recognised by the host immune response, including TRP47 (Refs Reference Doyle14, Reference Luo28, Reference Luo, Zhang and McBride29). TRP47 is differentially expressed on dense-cored ehrlichiae and is found extracellularly, indicating that it is secreted (Fig. 1). A recent study to examine molecular interactions between TRP47 and the host identified several interactions with specific host cell targets, including polycomb group ring finger 5 (PCGF5), Src protein tyrosine kinase FYN, protein tyrosine phosphatase nonreceptor type 2 (PTPN2), adenylate cyclase-associated protein 1 (CAP1) and immunoglobulin lambda-like polypeptide 1 (IGLL1), with distinct cellular functions associated with signalling, transcriptional regulation, vesicle trafficking, and cellular proliferation and differentiation (Ref. Reference Wakeel, Kuriakose and McBride32) (Fig. 3). Notably, none of these host targets had been identified by previous studies related to the cell biology of Ehrlichia infection. The interactions between TRP47 and the host cell illustrate the complexity and diversity of pathogen–host interactions that occur and will require additional research to fully comprehend. Although the relevance of these ehrlichiae–host molecular interactions in the context of ehrlichial pathobiology remains to be determined, the host targets identified suggest that TRP47 is a multifunctional effector that has an important role in establishing bacterial infection and promoting intracellular survival (Fig. 4).

Figure 3. Colocalisation of PCGF5 and IGLL1 with Ehrlichia chaffeensis expressing TRP47 within infected THP-1 cells.E. chaffeensis-infected cells were dually labelled with anti-TRP47 (green) and PCGF5 or IGLL1 (red) and examined by confocal microscopy. PCGF5 and IgLL1 colocalise with E. chaffeensis TRP47-labelled morulae (yellow, right merged panels). Molecular interactions between PCGF5, IGLL1, FYN, CAP1 and PTPN2 were first reported in Ref. Reference Wakeel, Kuriakose and McBride32. Abbreviations: IGLL1, immunoglobulin lambda-like polypeptide 1; PCGF5, polycomb group ring finger 5; THP-1 cells, a human acute monocytic leukaemia cell line; TRP47, tandem repeat protein 47.
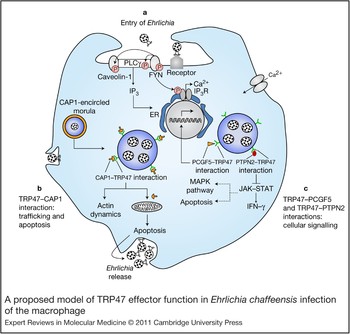
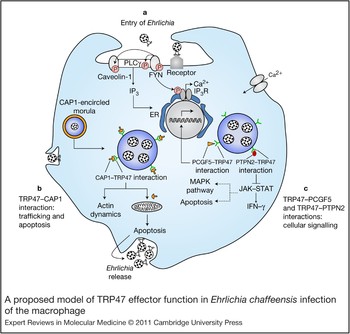
Figure 4. A proposed model of TRP47 effector function in Ehrlichia chaffeensis infection of the macrophage. The schematic diagram is based on defined pathogen effector molecular interactions (Ref. Reference Wakeel, Kuriakose and McBride32) and previous reports of cellular events during infection (Refs Reference Zhang16, Reference Lin and Rikihisa52, Reference Lin and Rikihisa54, Reference Zhang56, Reference Lee and Rikihisa74). (a) Entry of Ehrlichia. Binding of E. chaffeensis to its receptor on the surface of an innate immune cell such as a monocyte directly or indirectly activates FYN. Activated (phosphorylated) FYN tyrosine kinase phosphorylates and activates PLCγ, which hydrolyses membrane phospholipid phosphatidylinositol (3,4)-bisphosphate (not shown), resulting in increased levels of inositol (1,4,5)-trisphosphate (IP3) and thus release of Ca2+ from intracellular stores and Ca2+ influx. FYN might regulate the function of the IP3 receptor by phosphorylation and promoting release of Ca2+ from the endoplasmic reticulum. FYN also phosphorylates caveolin-1 involved in ehrlichial entry. (b) TRP47–CAP1 interactions. TRP47 is secreted by dense-cored Ehrlichia and associated with the morula surface, where it interacts with CAP1. This interaction might facilitate endocytosis and vesicle trafficking, because CAP1 promotes rapid actin dynamics in conjunction with ADF/cofilin and is required for cell morphology, migration and endocytosis; in addition, the TRP47–CAP1 interaction might promote apoptosis in the late stages of infection (which could have an important role in the release of dense-cored ehrlichiae), because mitochondrial shuttling of CAP1 promotes actin- and coflin-dependent apoptosis. (c) TRP47–PCGF5 and TRP47–PTPN2 interactions. TRP47–PCGF5 interaction modulates gene transcription associated with cell signalling and remodelling of the cytoskeleton, facilitating and supporting intracellular survival of E. chaffeensis. TRP47– PTPN2 interaction might modulate cytokine signalling events by exerting negative feedback on the JAK–STAT pathway by dephosphorylation of JAKs and STATs involved in intravacuolar maintenance and survival of E. chaffeensis. Dashed lines indicate processes the JAK–STAT pathway might affect indirectly. Abbreviations: CAP1, adenylate cyclase-associated protein 1; ER, endoplasmic reticulum; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; PCGF5, polycomb group ring finger 5; PLCγ: phospholipase Cγ; PTPN2, protein tyrosine phosphatase, non-receptor type 2; STAT, signal transducer and activator of transcription; TRP47, tandem repeat protein 47.
The strongest observed interaction between an Ehrlichia and host protein is between TRP47 and PCGF5 (Ref. Reference Wakeel, Kuriakose and McBride32) (Fig. 3). E. chaffeensis is known to modulate host cell gene transcription, and PCGF5 has been associated with DNA-dependent regulation of transcription, metal-ion binding and protein–protein interactions. PCGF5 is related to the polycomb group proteins (transcriptional repressors) Bmi-1 (PCGF4) and Mel-18 (PCGF2), which have important roles in the regulation of HOX gene expression, X-chromosome inactivation, tumourigenesis, self-renewal, maintenance of pluripotency of stem cells and stimulation of E3 ubiquitin ligase activity. Thus, it appears that TRP47-expressing dense-cored ehrlichiae might interact with PCGF5 in order to modulate host cell gene expression to favour survival (Fig. 4).
Another interesting interaction that has been characterised is between TRP47 and IGLL1, the surrogate light chain associated with the pre-B-cell receptor (Ref. Reference Herzog, Reth and Jumaa73) (Fig. 3). The pre-B-cell receptor is involved in transduction of signals for cellular proliferation, differentiation from the pro-B-cell to the pre-B-cell stage, allelic exclusion at the Ig heavy-chain gene locus and promotion of Ig light-chain gene rearrangements (Ref. Reference Herzog, Reth and Jumaa73). Thus, the significance of the interaction between TRP47 and IGLL1 might involve signalling and development, but suggests a novel role for IGLL1 in the macrophage and one that will require further study to understand.
The location of TRP47 on the surface of dense-cored ehrlichiae suggests that this protein might be involved in host cell attachment or entry. The association with FYN tyrosine kinase suggests that such a role is possible (Fig. 4). Tyrosine kinases are known to be involved in ehrlichial entry; however, the specific kinases involved have not been determined (Ref. Reference Lin, Zhu and Rikihisa53). In addition, phosphorylation of host or bacterial proteins has been implicated in signalling pathways, triggering the entry of many intracellular pathogens. Ehrlichia enter through caveolae, and FYN is known to specifically phosphorylate caveolin-1 and is required for coxsackievirus internalisation and infection by caveolin-associated vesicles of polarised epithelial cells (Ref. Reference Coyne and Bergelson75). Evidence of tyrosine phosphorylation of TRP47 has been reported, suggesting that there is a functional significance and that FYN might be responsible (Ref. Reference Wakeel, Zhang and McBride33).
TRP47 also interacts strongly with PTPN2, also known as T-cell PTP, which catalyses the dephosphorylation of phosphotyrosine peptides and regulates phosphotyrosine levels in signal transduction pathways. It is ubiquitously expressed with particularly high expression in haematopoietic tissues and appears to broadly influence haematopoietic cell development, but recent findings also demonstrate a role in several human illnesses, from autoimmune disease to cancer (Ref. Reference Doody, Bourdeau and Tremblay76). PTPN2 has several substrates, including CSF1R, EGFR, PDGFR, INSR, p52Shc (SHC1), JAK1, JAK3, STAT1, STAT3, STAT5A/B and STAT6 (Ref. Reference Stuible, Doody and Tremblay77). The JAK–STAT pathway is inhibited by monocytotropic E. chaffeensis (Ref. Reference Lee and Rikihisa74), supporting the possibility that TRP47 might be involved not only in the inhibition of IFN-γ-induced tyrosine phosphorylation of STAT1, JAK1 and JAK2 by interacting with PTPN2, but also in the regulation of cellular development (Fig. 4). Ehrlichia might favour PTPN2 upregulation because the loss of PTPN2 results in STAT5 hyperactivation, increased production of IFN-γ, TNF-α, IL-12 and inducible nitric oxide synthase, increased tyrosine phosphorylation, recruitment of a complex of GRB2–GAB2–SHP2 (PTPN11) to the CSF1R and enhanced activation of ERK (MAPK1/3), and might affect transcription factor PU.1 signalling. Thus, TRP47 might influence a large number of important signal transduction pathways by interacting with PTPN2 (Fig. 4).
The membrane-bound vacuoles in which Ehrlichia reside in the host cell cytoplasm interact with endosomal recycling pathways. A specific interaction between TRP47 and the multifunctional protein CAP1 has been defined that appears to occur at the morula membrane interface, where CAP1 localises with the dense-cored morulae adjacent to the morula boundaries (membrane) (Ref. Reference Wakeel, Kuriakose and McBride32). CAP1 is a highly conserved monomeric-actin-binding protein that contains binding domains for actin (C-terminal region), adenylate cyclase and cofilin (N-terminal region), and profilin (central region), and it has an active role in actin turnover (Ref. Reference Hubberstey and Mottillo78). Genetic studies in yeast have implicated CAP1 in vesicle trafficking and endocytosis. In mammalian cells, CAP1 is associated with the SH3-domain-dependent complex of actin-binding protein 1 and dynamin that is involved in receptor-mediated endocytosis (Ref. Reference Kessels79). Thus, in an effort to survive in the intracellular niche, Ehrlichia might manipulate cytoskeletal components of the mononuclear phagocyte such as actin by modulating CAP1 (Fig. 4). Interestingly, CAP1 has also been implicated in promoting apoptosis by functioning as an actin shuttle to mitochondria. Similar to cofilin, BAD and BAX, CAP1 rapidly translocates to mitochondria independently of caspase activation, where it promotes apoptosis (Ref. Reference Wang80). Associations between ehrlichial morulae and mitochondria have been consistently observed (Ref. Reference Popov15). Thus, the TRP47 and CAP1 interaction might be multifunctional by facilitating endocytosis and vesicle trafficking, and promoting apoptosis in the late stages of infection (Fig. 4).
E. chaffeensis Ank200 interaction with host Alu elements
There are four genes in the E. chaffeensis genome that encode proteins with Ank repeats. One of these proteins, Ank200, is a well-characterised major immunoreactive protein that has several species-specific antibody epitopes located primarily in acidic N- and C-terminal domains (Ref. Reference Luo35). The E. chaffeensis Ank200 lacks a signal peptide, but is predicted to be secreted by a leaderless secretion system (Secretome 2.0), and it has a T4SS motif (Ref. Reference Rikihisa and Lin81). Ank200 has recently been detected in host cell nuclei of E. chaffeensis-infected cells where it interacts with an adenine-rich motif in promoter and intronic Alu elements (Ref. Reference Zhu55). Alu elements are short, interspersed mobile DNA elements distributed in a nonrandom manner that comprise approximately 5–10% of the human genome and are thought to be involved in transcriptional regulation as carriers of cis-regulatory elements (Refs Reference Tomilin82, Reference Matera, Hellmann and Schmid83). Alu elements have known binding sites for transcription factors including all MEF2 family members, as well as HNF1.03, OC.2, BARX2 and PAX4 (Ref. Reference Polak and Domany84). The global analysis of binding sites of Ank200 demonstrates that this protein binds to several regions distributed on nearly every chromosome by direct DNA interaction or via other DNA-binding proteins.
The host cell processes targeted by Ank200 have been classified, and include genes associated with transcriptional regulation, apoptosis, ATPase activity and structural associations with the nucleus (Ref. Reference Zhu55) (Table 1). Interestingly, genes associated with host cell processes known to be altered during E. chaffeensis infection were found to be targets of Ank200. In addition, Ank200 appears to bind genes associated with transcription, and there is evidence that a large number of genes associated with transcription are modulated during E. chaffeensis infection (Ref. Reference Zhang56).
Table 1. Selected E. chaffeensis Ank200 target genes identified by ChIP-on-chipa

a Information in the table is based on Ref. Reference Zhu55.
Abbreviation: CHIP-on-chip, chromatin immunoprecipitation and DNA microarray analysis.
Several Ank200 target genes have been linked to pathogenesis and immune evasion, including TNF-α, JAK2 and CD48. TNF-α expression is not induced early in infection (<48 h) (Refs Reference Lee and Rikihisa85, Reference Lee and Rikihisa86), but expression is upregulated approximately 30-fold by day 5 post-infection (Ref. Reference Zhu55). E. chaffeensis Ank200 might contribute to the induction of TNF-α by binding directly to the promoter and upregulating gene transcription. Studies have demonstrated that overproduction or high serum concentration of TNF-α on day 7 post-infection is closely associated with fatality in severe monocytotropic ehrlichiosis (Refs Reference Bitsaktsis and Winslow87, Reference Ismail88). One of the primary mechanisms by which E. chaffeensis survives in the host cell appears to be blocking macrophage responsiveness to IFN-γ (Ref. Reference Barnewall and Rikihisa59). Furthermore, JAK2 transcription appears to be silenced during E. chaffeensis infection, and JAK–STAT genes are also Ank200 targets, suggesting that E. chaffeensis uses several strategies, including directly modulating genes associated with the JAK–STAT pathway (Ref. Reference Zhang56).
Immunopathological mechanisms and disease
During E. chaffeensis infection, there is a relatively low bacterial burden in the blood and tissues in nonimmunocompromised patients. However, the clinical manifestations, which include fever, multiorgan failure and adult respiratory distress syndrome, suggest that the pathogenesis of ehrlichiosis might involve immunopathological responses that are described as a toxic-shock-like syndrome (Refs Reference Fichtenbaum, Peterson and Weil89, Reference Maeda90). Studies using a fatal murine model and a surrogate unnamed monocytic ehrlichia isolated from Ixodes ovatus ticks in Japan (IOE) suggest that such a mechanism is involved (Ref. Reference Sotomayor91). Mice inoculated with IOE develop histopathological lesions resembling those observed in human monocytotropic ehrlichiosis patients, and a similar disease course is observed in the IOE murine model. Lethal infections with IOE are accompanied by extremely high levels of serum TNF-α, a high frequency of splenic TNF-α-producing CD8+ T cells, decreased Ehrlichia-specific CD4+ T cell proliferation, low IL-12 levels in the spleen and a 40-fold decrease in the number of ehrlichial antigen-specific IFN-γ-producing CD4+ Th1 cells (Refs Reference Ismail88, Reference Ismail92). Furthermore, mice lacking TNF receptors I/II are resistant to IOE-induced liver injury (an apparent effect of reduced immunopathology), but show higher bacterial burdens (indicating reduced protective immunity) (Ref. Reference Ismail, Stevenson and Walker93). Others have also demonstrated immunopathological responses linked to CD8+ T cells (Ref. Reference Sotomayor87). Interestingly, fatal memory responses against homologous but not heterologous challenge are associated with decreased bacterial burden, enhanced inflammatory response in the liver, decreased T cell responses and defective maintenance of IFN-γ-producing T cells (Ref. Reference Thirumalapura94). CD1d-restricted NKT cells appear to be instrumental in the induction of immunopathological responses (Ref. Reference Stevenson95).
Clinical implications and applications underlying
Ehrlichiosis manifests as an undifferentiated febrile illness that is difficult to diagnose, and delayed treatment can lead to serious complications and poor prognosis. There are no vaccines for human rickettsial diseases, including ehrlichiosis, and therapeutic options are limited. The rapid growth in antibiotic resistance among microbes suggests that antibiotics could become ineffective for ehrlichial and rickettsial infections in the future. The lack of broader therapeutic options is complicated by the fact that there are no effective vaccines available to prevent disease in susceptible populations. Recent advances in our understanding of molecular pathogen–host interactions, virulence mechanisms, effector proteins, the cellular basis of infection and the immunopathological basis of clinical manifestations have provided new targets for therapies that block pathogen virulence mechanisms, host–pathogen interactions or eukaryotic cell processes, and have identified additional approaches involving immunomodulatory therapies that can be considered.
Antibodies that block interactions between effector proteins and host targets can be used therapeutically. For example, passive transfer of E. chaffeensis TRP120-epitope-specific antibodies reduces ehrlichial burden and demonstrates the potential of therapeutic antibodies against ehrlichial effector proteins (Ref. Reference Kuriakose96). These studies also suggest that vaccines containing defined antigens are feasible and will be developed in the future. Additionally, drugs that target specific pathogen virulence mechanisms such as secretion systems and signal transduction systems or inhibit acquisition of essential components for pathogen structural integrity and function might be viable alternative therapies that can be used for patient treatment. Defining molecular host–pathogen interactions and ultimately the three-dimensional structures of effector molecules will allow the development of small-molecule inhibitors that can disrupt the ability of the pathogen to manipulate host cell processes resulting in pathogen clearance by the immune system.
Managing patients who are experiencing manifestations and complications associated with severe disease requires understanding of the underlying immune dysregulation, to develop rational strategies to alleviate its effects. The overproduction of inflammatory cytokines or chemokines is responsible for serious clinical manifestations that can be managed by targeted immunotherapies that block cytokine production or neutralise cytokine effects by blocking a specific cytokine–receptor interaction and its immunopathological consequences. Such patient management strategies can be considered as the immune effectors are comprehensively defined.
Research in progress and outstanding research questions
The complexity of the pathogen–host relationship between Ehrlichia and the eukaryotic host cell is becoming better understood, and the mechanisms used by the organism to reprogramme cellular processes are becoming more defined. New pathogen–host interactions such as nuclear translocated proteins that bind host chromatin have been recently described, but much more research is needed to fully appreciate how these effector proteins modulate host cell transcription and whether they can be useful therapeutic targets. Other effector proteins and secretion mechanisms have been identified, but the mechanisms used by these effectors and how they modulate targeted host cell processes are areas of active research that will provide useful information for the development of therapeutics. Much research on the cellular processes that are affected during infection has been accomplished, and advancing knowledge regarding host cell processes that are targeted by the organism will be beneficial for designing therapeutic interventions for Ehrlichia as well as other rickettsial pathogens.
Development of immunomodulatory strategies for patient management will become more practical in the future, and research to understand the immunopathological mechanisms involved has taken large steps forward. Questions that need to be addressed are why some patients respond immunologically in a detrimental way and how we can accurately identify and manage these patients. More research needs to be devoted to the development of biomarkers for disease for diagnosis and development of rational strategies for patient management and therapeutic decisions.
Acknowledgements and funding
We thank all current and former laboratory members for discussions and scientific contributions towards understanding the molecular, cellular and immunobiological aspects of Ehrlichia infections. We also appreciate the helpful comments and suggestions provided by the referees to improve the review. J.W.M. and D.H.W. are supported by grants (AI0071145 and AI069270 to J.W.M.; AI 31431 to D.H.W.) from the National Institute of Allergy and Infectious Diseases (NIAID) and jointly by the Clayton Foundation for Research.