Introduction
Chemokines are a large group of cytokines with a short sequence in length. Because of their ability to induce chemotaxis in receptive cells, the name of these factors is derived from chemotactic cytokines. Chemokines have a mass of 8–10 KD approximately and have four cysteine residues in conserved locations, which are important to form their 3D shape. Based on these four-cysteine residues and space between two cysteines in N-terminus, chemokines are divided into four subgroups. These subgroups include C (only one N-terminal cysteine), CC (two adjacent cysteines), CXC (separated by one amino acid) and CX3C (separated by three amino acids) (Ref. Reference Griffith, Sokol and Luster1).
The expression of chemokines affects immune cell distribution in several tissues, organs and even the tumour microenvironment. Tumour cells and immune cells can express and secrete chemokines and their receptors in the tumour microenvironment. This process leads to the migration of various immune cells to the tumour microenvironment, thus regulating immune responses (Ref. Reference Marcuzzi2). The CXC chemokines have attracted much interest among chemokine subgroups because they are involved in the regulation of tumour development, invasion and metastasis. Based on the presence of ELR (glutamic acid-leucine-arginine) motif immediately before the first cysteine, CXC chemokines per se are divided into two groups, including non-ELR and ELR CXC chemokines (Ref. Reference Keeley, Mehrad and Strieter3).
CXCL16 and its receptor CXCR6
CXCL16, as a member of CXC chemokines, is a non-ELR and considered a fairly novel chemokine. CXCL16, having 254 amino acids, is the largest one compared with other CXC chemokines. CXCL16 is one of two chemokines that have been found in soluble (sCXCL16) and transmembrane (TM-CXCL16) forms (Ref. Reference Abel4). TM-CXCL16 has also been described as a scavenger receptor for oxidised low-density lipoprotein. Hence, it is also called SR-PSOX (scavenger receptor for phosphatidylserine and oxidised lipoprotein) (Ref. Reference Shimaoka5). CXCR6 (also named CD186) is the only known receptor for CXCL16. In addition to a receptor for CXCL16, CXCR6 was initially recognised as a co-receptor on CD4+ and CD8+ T cells for the human immunodeficiency virus (Ref. Reference Blaak6).
Genomic characterisation and tissue distribution
For the first time, Matloubian et al. described CXCL16 in human cells. They have located the human CXCL16 on chromosome 17p13. CXCL16 is the first discovered transmembrane CXC chemokine and has 70% similarity to mice CXCL16. In terms of organ distribution, northern blot analysis has shown that CXCL16 mRNA is expressed in lymphoid (including spleen, lymph nodes, Peyer's patches, thymus but not bone marrow) and non-lymphoid organs (Ref. Reference Matloubian7). The chemokine CXCL16 and its receptor CXCR6 have been shown to be expressed by a vast majority of immune cells. Although CXCL16 is mainly expressed by dendritic cells (DCs), macrophages, B-cells and monocytes, it is also constitutively expressed in bronchial epithelial cells, epidermal keratinocytes and renal podocytes (Ref. Reference Hald8).
It has been identified three forms of CXCL16 in human and mice up to now. The first one is the complete form of CXCL16, which is also called transmembrane CXCL16 or TM-CXCL16. Structurally, TM-CXCL16 comprised of 254 amino acids and has three domains, including a spacer or transmembrane domain (rich in serine, threonine and proline, which resembles mucin structure), a cytoplasmic domain (contains YXPV motif and able to be phosphorylated with tyrosine kinase) and a chemokine domain (resembles CC chemokines and contains six cysteines) (Ref. Reference Matloubian7). TM-CXCL16 is cleaved and is shed from cell surface by sheddase enzymes called ADAM10 and ADAM17 (a disintegrin and metalloproteinase domain-containing protein 10 and 17), producing secretory CXCL16 (sCXCL16) (Ref. Reference Abel9). The last form is CXCL16v (variant CXCL16), lacking the transmembrane and cytoplasmic domains produced by the mRNA alternative splicing process. CXCL16v form is uniquely expressed by tissue-resident DCs (Ref. Reference van der Voort10).
Signalling pathways
There are four proposed signalling pathways for CXCR6/CXCL16 complex in cancer until now (see Fig. 1). The ligation of CXCL16 to CXCR6 leads to intense phosphorylation of AKT. Activation of AKT initiates induction of p70S6K and 4E-BP1, as components of the mTOR signalling pathway, in prostate cancer. The activation of mTOR signalling pathway triggers the expression of CD44 and matrix metalloproteinases (MMPs), including MMP-2, 3 and 9. It has been shown that mTOR blocking by rapamycin inhibits CXCL16-dependent production of VEGF and IL-8 to induce angiogenesis, indicating activation of mTOR signalling pathway upon CXCR6 ligation (Ref. Reference Wang11).
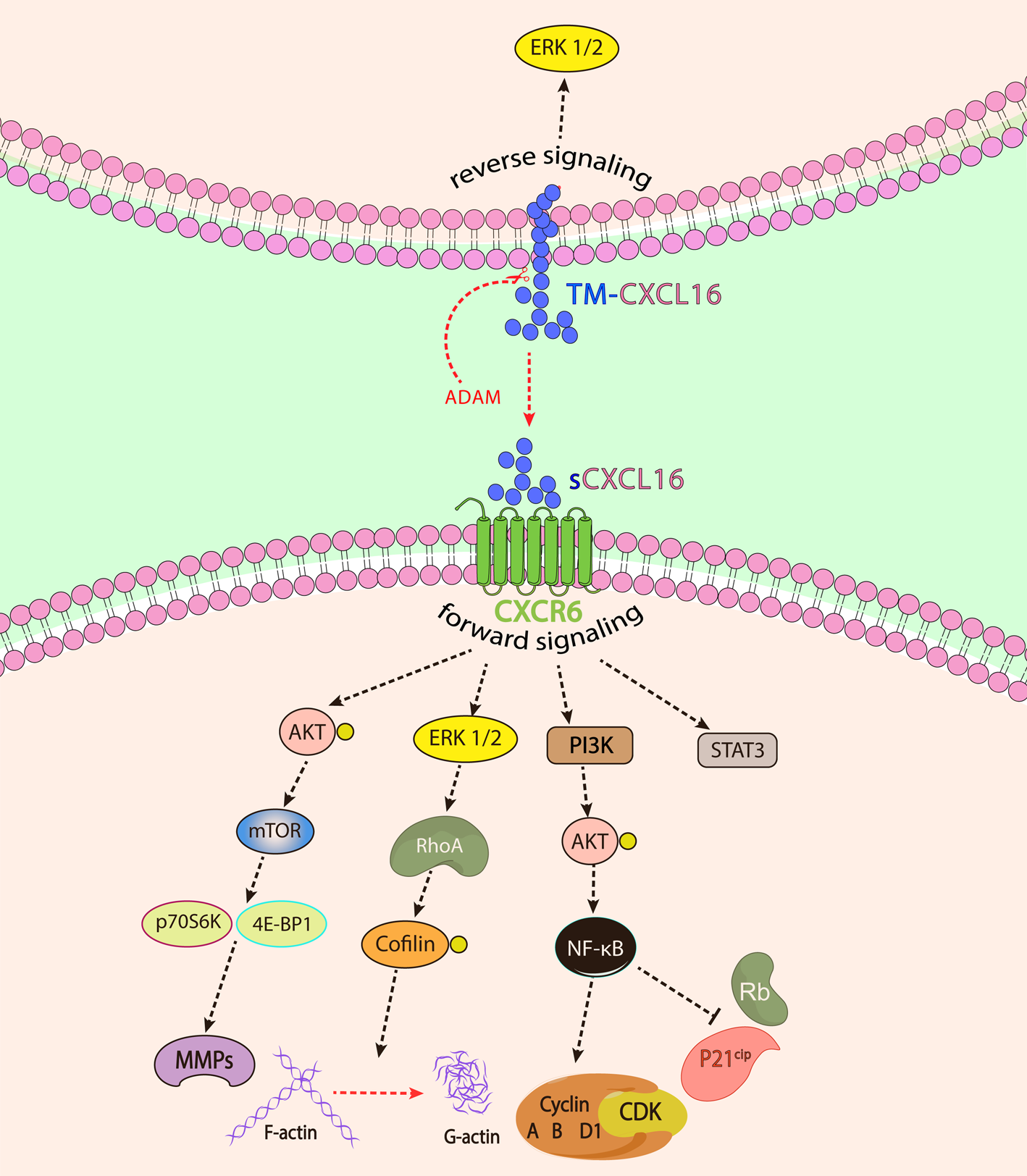
Fig. 1. The forward and reverse signalling pathways involved upon CXCL16 and CXCR6 interaction. In forward signalling, sCXCL16 or TM-CXCL16 binding to CXCR6 leads to signal transduction from CXCR6, whereas in reverse signalling, signal transduction is mediated by the intracellular domain of TM-CXCL16.
In the breast cancer model, it has been clarified that the interaction of CXCR6 and CXCL16 enhances the activity of the ERK1/2 pathway, which consecutively leads to small GTPase RhoA activation. RhoA activation results in actin cytoskeletal reorganisation and actomyosin-dependent contractility through indirect phosphorylation of cofilin and myosin light chain. This process eventually leads to the alteration of tight junction integrity and rearrangement of F-actin, which in turn mediates changes in the cytoskeletal system. Thereby, it has been concluded that high expression of CXCR6 in breast cancer cells has polarised cell morphology and enhanced cell mobility in these cells. Moreover, induction of high mobility and migratory properties in these cells has stimulated epithelial-mesenchymal transition (EMT) and metastasis (Ref. Reference Xiao12).
CXCL16 binding to CXCR6 also activates PI3K/AKT signalling to induce NF-κB translocation into the nucleus. The activation of NF-κB signalling also mediates the expression of IL-8, MMP2 and MMP9 to induce tumour progression (Ref. Reference Wang13). Moreover, the activation of NF-κB upregulates cyclin A, B and D1 and downregulates P21cip/Waf1 and retinoblastoma expression. Consequently, this process promotes cell cycle transition from G1 to S and induces cell proliferation in lung cancer cells (Ref. Reference Liang14). More recently, Ikeda et al. have introduced a new signalling pathway upon CXCL16 ligation on MKN45 cells. In this study, it has been demonstrated that CXCL16 induces STAT3 signalling (Ref. Reference Ikeda15).
In addition to forward signalling through CXCR6, Wang et al. have elucidated that CXCL16 mediates reverse signalling in transmembrane form. The interaction of recombinant CXCR6 with CXCL16 on cell surface results in ERK1/2 signalling from the intracellular domain in CXCL16-expressing glioma cells. The fast migration of glioma cells has been attributed to this reverse signalling pathway in vivo (Ref. Reference Adamski16). Furthermore, TM-CXCL16 has also been shown to act as a receptor for sCXCL16. Hattermann et al. have indicated that in RNAi-dependent CXCR6-silenced meningioma cells, the interaction of sCXCL16 with TM-CXCL16 leads to reverse signalling from the TM-CXCL16 intracellular domain. Following this signal transduction, intracellular ERK1/2 and AKT are activated and mediated proliferation and rescued cultured meningioma cells from apoptosis (Ref. Reference Hattermann17).
CXCL16 expression in cancer
It has been shown that recurrent or chronic inflammatory conditions might predispose to various tumour types. Hence, lately, there has been growing attention to the relationship between chronic inflammation and tumour development (Ref. Reference Greten and Grivennikov18). Chemokines have been involved in tumour development processes, including transformation, cell proliferation, angiogenesis and lymphocyte recruitment to tumour sites (Ref. Reference Chow and Luster19). Given the role in recruiting leukocytes and inflammation, it has been demonstrated that CXCL16 influences the tumour microenvironment (Ref. Reference Hald8). The expression of CXCL16 and CXCR6 has been observed in numerous human cancers, including pancreatic cancer, thyroid carcinoma, breast cancer and-so-forth.
Several factors influence the expression of CXCL16 in cancer (Fig. 2). Notch receptor signalling is a highly conserved pathway involved in cell proliferation, differentiation and apoptosis. Dysregulation of the Notch signalling pathway has been reported in the vast majority of human cancers. It has been shown that the expression of Notch1 significantly upregulated in nasopharyngeal carcinoma (NPC) tissues and cell lines, and knockdown or downregulation of Notch1 prevents NPC growth, decreases cell migration and stimulates apoptosis. Since Notch1 knockdown inhibits CXCL16 expression in NPC, it has been concluded that CXCL16 might be a downstream target of Notch1 (Ref. Reference Guo20).

Fig. 2. The effect of various factors on the dysregulation of CXCL16 in cancer.
The product of the mutated KRAS gene is considered an oncogene. Mechanistically, inflammatory mediators such as IL-6 and CXCL16 induce the expression of KRAS in pancreatic ductal adenocarcinoma (PDAC) cells. KRAS initiates PI3K/AKT/NF-κB signalling and subsequently induces the expression of CXCL16. The produced CXCL16 per se induces PI3K/AKT/NF-κB and establishes CXCL16-PI3K/AKT/NF-κB loop. In fact, mutated KRAS is the initiator of this loop. Further examination has shown that somatostatin receptor subtype 2 (sst2) can block this loop. On the contrary, a loss-of-function mutation in sst2 has led to PDAC progression (Ref. Reference Chalabi-Dchar21).
Tumour necrosis factor-alpha (TNF-α) is a multifunctional cytokine that plays pivotal roles in cell apoptosis and cell survival. Yang et al. have proposed a positive feedback loop between CXCL16 and TNF-α. In diffuse large B-cell lymphoma (DLBCL), administration of exogenous TNF-α induced secretion of sCXCL16 substantially through induction of ADAM10 expression. In a positive feedback loop, sCXC16 secretion promoted TNF-α production via the NF-κB signalling pathway in DLBCL cells (Ref. Reference Yang22). In addition to TNF-α, it has also been shown that IFN-γ stimulated CXCL16 production from tumour cells in prostate cancer (Ref. Reference Darash-Yahana23).
The binding of the cleaved C5 component of complement (C5a) to its receptor (C5aR1) initiates signalling responses, contributing to cancer progression. The expression of C5aR1 has been verified in various immune and non-immune cells, such as tumour cells. C5a/C5aR1 interaction impedes anti-tumour responses through multiple mechanisms, including the infiltration of myeloid-derived suppressor cells (MDSCs) into the tumour microenvironment. Besides, Ajona et al. have indicated another mechanism for C5a immunomodulatory properties in the tumour. They have shown that C5a/C5aR1 signalling induces the expression of CXCL16 in lung cancer, which causes inflammation in the tumour microenvironment (Ref. Reference Ajona24).
Non-coding RNAs, including miRNAs and lncRNAs, are other regulators of gene expression. The expression of miR-451 in osteosarcoma has been decreased. This downregulation led to tumour development and progression. In this regard, Zhang et al. showed that the mechanism of miR-451 function in osteosarcoma relies on CXCL16. They have found that miR-451 has targeted 3′UTR of CXCL16 gene and decreased its expression. Downregulation of miR-451 in osteosarcoma resulted in the unrestrained expression of CXCL16, cell proliferation and tumour invasion (Ref. Reference Zhang25).
miR-221 is another regulator of CXCL16 expression. Staphylococcal nuclease domain-containing protein 1 (SND1) is a multidisciplinary protein that is upregulated in numerous cancers, including hepatocellular carcinoma (HCC) (Ref. Reference Jariwala26). SND1 is involved in cell signalling pathways (such as STAT, NF-κB and Wnt pathways), miRNA splicing process, gene silencing, cell viability and cell proliferation (Ref. Reference Chidambaranathan-Reghupaty27). Santhekadur et al. have shown that SND1 promoted NF-κB activation to induce the expression of miR-221 (Ref. Reference Santhekadur28). The elevation of miR-221 was attributed to the expression of CXCL16 in HCC cells. In terms of mechanism, it has been speculated that miR-221 has targeted and inhibited phosphatase and tensin homolog (PTEN) and tissue inhibitor of metalloproteinases-3 (TIMP3). Inhibition of PTEN activates AKT signalling, and TIMP3 inhibition leads to the activation of ADAM10 and ADAM17 for producing sCXCL16 (Ref. Reference Santhekadur29). In addition to miR-221 and miR-451, it has been shown that CXCL16 is the target gene of miR-873-5p. In papillary thyroid cancer (PTC), upregulation of miR-873-5p inhibited cell proliferation, migration and invasion, whereas these consequences were reversed upon treatment with CXCL16 (Ref. Reference Wang30).
Hypoxia, radiation and gut microbiome are other CXCL16 regulators. As is well-known, a decreased oxygen level, called hypoxia, is a distinctive characteristic of most tumours. It has been shown that CXCL16 expression is upregulated in the hypoxic condition in the tumour microenvironment. Mechanistically, upregulation of transcription factor hypoxia-inducible factor-α (HIFA gene) might be involved in the overexpression of CXCL16 in triple-negative breast cancer (TNBC) (Ref. Reference Chaturvedi31). In addition, it has been shown that irradiation of breast cancer cells upregulates CXCL16 production in these cells dose- and time-dependently (Ref. Reference Yoon32). Furthermore, studies have revealed that irradiation of cells also influenced gene expression. In this regard, two independent studies showed that irradiation of breast cancer cells increased the expression of CXCL16 gene in these cells, resulting in the recruitment of CXCR6+ NK and T cells into tumour microenvironment (Refs Reference Yoon32, Reference Matsumura33).
It has also been shown that the gut microbiome, through regulating the bile acid ratio, plays a vital role in CXCL16 mRNA expression. The gut microbiome mediates the conversion of primary bile acid (such as β-muricholic acid (β-MCA)) into secondary bile acid (such as ω-muricholic acid (ω-MCA)). The elevation of ω-MCA downregulated CXCL16 expression, and β-MCA increased the expression of CXCL16 in liver sinusoidal endothelial cells (Ref. Reference Ma34).
The effect of CXCL16 on tumour development and metastasis
Chemokines and chemokine receptors have exerted a conflicting role in tumour development (Fig. 3). They mediate tumour progression directly via impacting survival, transformation, proliferation and metastasis of tumour cells or indirectly via enhancing leukocyte infiltration or angiogenesis (Ref. Reference Adamski35).

Fig. 3. Conflicting role of CXCL16 in cancer. The red area depicts the tumour-supportive role of CXCL16 in cancer, while the yellow area shows how CXCL16 can have a tumour-suppressive role.
As mentioned before, CXCL16 is a potent cell chemotactic factor. In a study by Jung et al., it has been cleared that CXCL16 recruited CXCR-expressing mesenchymal stem cells (MSCs) into the prostate cancer microenvironment. The signalling of CXCR6 in recruited MSCs leads to the conversion of MSCs into cancer-associated fibroblasts (CAFs), which these cells release stromal-derived factor-1 (also known as CXCL12) in tumour nest. The interaction of CXCL12 with its receptor (CXCR4) on tumour cells induces EMT and metastasis (Ref. Reference Jung36). Foamy cells are other CXCL16-dependent recruited cells into the tumour microenvironment. These cells are a type of tumour-promoting M2 macrophages that generally are considered lipid-laden macrophages. In papillary renal cell carcinoma (pRCC), the presence of these cells is the hallmark of tumour aggressiveness, which almost neither chem- nor radiotherapy have any therapeutic effect following surgery. It has been defined that CXCL16 accompanied by IL-8 and adipokine chemerin are involved in the formation of foamy macrophages (Ref. Reference Krawczyk37).
Receptor tyrosine kinase-like orphan receptor-1 and -2 (Ror1/Ror2) are considered a heterodimer receptor for Wnt5a that mediate non-canonical Wnt signalling during tissue repair, developmental morphogenesis, and tumour progression. Wnt5a and Ror2 are strongly overexpressed in cancer cells, and their consecutive activation leads to tumour progression and poor prognosis in PDAC and ovarian cancer. Secretion of CXCL16 from MSCs and its ligation on CXCR6-expressing MKN45 cells (cells derived from gastric cancer) has resulted in upregulation of Ror1 through activation of STAT3. This process indicates the effect of MSC-released CXCL16 to induce Wnt signalling and subsequently tumour formation and development (Ref. Reference Ikeda15). More interestingly, the Wnt5/Ror2 signalling augments CXCL16 expression, indicating the reciprocal feedback loop between CXCL16 and Wnt5/Ror2 signalling (Ref. Reference Takiguchi38). The consequence of this loop is the promotion of tumour cell proliferation induced by MSCs.
MMPs are extracellular matrix digesting enzymes and contribute to tumour cell invasion, migration and metastasis to the other tissue. It has been shown that treatment of ovarian cancer cell lines (SKOV-3 and OVCAR-3) with CXCL16 induces the expression of MMP2, 3, 9, 13 in cancer cells (Ref. Reference Mir39). Also, CXCL16 ligation on LNCap prostate cells promotes the expression of MMP2 and MMP9, resulting in prostate cancer cell metastasis to the bone (Ref. Reference Hu40). Therefore, the expression of CXCL16/CXCR6 and ADAM10 endows metastatic competencies to ovarian and prostate cancer cells (Refs Reference Mir39, Reference Hu40). Another study discussing the role of CXCL16 in ovarian cancer migration has shown that M2 macrophages induce CXCL16 production, and subsequently, its ligation to CXCR6 initiates PI3K/AKT pathway and induces cell migration and metastasis (Ref. Reference Hong41).
MDSCs and CAFs are the other important tumour-supportive and immune-suppressive cells in the tumour microenvironment. In TNBC, myeloid cells are recruited into the tumour nest in response to CXCL16. The recruited MDSCs express S100A9 and CD163, which in turn these markers stimulate fibroblast conversion into CAFs. Activated CAFs per se produce more CXCL16 in the tumour microenvironment and recruit more immature myeloid cells. These processes facilitate tumour stroma conversion into a much more active phenotype, which activates the tumour nest to become a high aggressive microenvironment (Ref. Reference Allaoui42). In HCC, CAF-secreted CXCL16 triggers TGF-β receptor (TGFβR) signalling and activates phosphorylation of downstream proteins, including TGFβR, Smad2 and Smad3, to mediate metastasis and EMT. However, the molecular mechanism of this process remains unclear (Ref. Reference Liu43).
The primary step in cancer cell migration, invasion and metastasis is cytoskeletal rearrangement. Integrins, such as α vβ 3 and Ezrin, coordinate this process by regulating cytoskeletal polymerisation–depolymerisation and cellular adhesion. Chemokine receptor signalling has been shown to be involved in the regulation of cell migration and invasion. In prostate cancer, it has been established that aggressive tumour cells strongly express CXCR6, which is correlated to higher tumour invasiveness. The interaction of CXCR6 with CXCL16 induces FAK/PI3K/PKC signalling pathway and modifies cellular adhesion and motility. Further examination has shown that CXCR6 signalling phosphorylates Ezrin and induces α vβ 3 integrin clustering. The phosphorylation of Ezrin forms pseudopod, and integrin clustering mediates tumour cell adhesion to ECM as well as MMP1 and MMP13 upregulation. Thereby, CXCL16/CXCR6 complex signalling leads to prostate cancer cell migration and invasion into other tissues (Ref. Reference Singh44).
The role of CXCL16 in resistance to treatment in cancer
One of the fundamental complications in cancer is the presence of cells with the capability of self-renewing and sustained neoplastic growth. These cells, which are considered cancer stem cells, are highly proliferative and drug-resistant. It has been shown that melanoma-derived cancer stem cells express high levels of CXCR6 and ABCG2 (a transporter for drug efflux, mediating drug resistance in tumours), which correlates with asymmetric self-renewal phenotype in these cells (Ref. Reference Taghizadeh45).
Several signalling pathways are involved in developing drug-resistance, including PI3K/Akt/mTOR, NF-κB, Src family kinases, PTEN and ERK1/2. The mediators involved in these pathways facilitate the expression and induction of several pro- and anti-apoptotic proteins (Ref. Reference Kapur46). Kapur et al. have delineated that docetaxel treatment increases the expression and activation of CXCL16 and CXCR6 in prostate cancer cells. Moreover, docetaxel activates CXCR6/NF-κB signalling pathway. The activation of this pathway leads to the induction of CXCL16 cleavage by ADAM10, which consequently increases sCXCL16 production. The ligation of CXCL16 to CXCR6 following docetaxel administration phosphorylates NF-κB, GSK-3β and MAPK (ERK1/2) and subsequently promotes pro-survival signals. Since the administration of anti-CXCR6 antibody blocks docetaxel resistance, it is concluded that resistance to docetaxel in prostate cancer relies on CXCL16/CXCR6 signalling pathway (Ref. Reference Kapur46).
CXCL16 and tumour angiogenesis
An alteration in cytokine secretion pattern is a hallmark of many malignancies, including prostate cancer. The development of new blood vessels (angiogenesis) within the tumour nest is crucial for its development, progression and metastasis. It is worth mentioning the CXCL16 tumour-supporting role is partly because of its ability to mediate angiogenesis. CXCL16 is also an effective pro-angiogenic factor other than being a tumour cell growth factor and metastasis mediator. Interestingly, CXCR6 signalling is able to control the expression of pro-angiogenic factors such as interleukin IL-8 or VEGF, which participate in regulating tumour angiogenesis (Ref. Reference Wang11).
MDSCs are a group of immune-suppressive and tumour-supportive cells. These cells release a set of mediators that mediate tumour progression, metastasis and angiogenesis. It has been shown that supernatants from MDSC cultured media induce migration of human umbilical vein endothelial cells, form a capillary-like tube in these cells and eventually promote angiogenesis. Detailed information has indicated that the presence of CXCL16 and CCL2 in MDSC conditioned medium is responsible for angiogenesis so that neutralisation of these mediators suppresses angiogenesis (Ref. Reference Han47).
It has also been shown that CXCL16 induces the migration of monocytes to the tumour microenvironment. In the PTC microenvironment, monocyte polarisation to M2 macrophages is mediated by CXCL16. M2 macrophages are in a direct relationship with tumour angiogenesis. In fact, CXCL16 significantly upregulates M2 macrophage-related genes, including IL10, CD163 and CD206. Recruitment and accumulation of M2 macrophages in tumour nest have led to an aggressive phenotype of the thyroid tumour, including lymph node metastasis, extrathyroidal extension, tall-cell variant and higher TNM staging (Ref. Reference Cho48). Therefore, upregulation of pro-angiogenic factors, such as VEGFA, in CXCL16-induced M2 macrophages mediates angiogenesis (Ref. Reference Kim49).
The effect of CXCL16 on tumour regression
The analysis of CXCL16 expression in two breast cancer cell lines, MCF-7 and MDA-MB213, revealed a protective role of CXCL16 in breast tumorigenesis and displayed valuable pieces of evidence to better understanding the multifarious function of CXCR6/CXCL16 in cancers. The evaluation of CXCL16 in MCF-7 cells, a less aggressive breast tumour cells expressing both oestrogen and progesterone receptors, showed a high level of CXCL16 expression in these cells. In contrast, measurement of this chemokine in MDA-MB231 cells, a highly aggressive cell and triple-negative in terms of oestrogen, progesterone receptor and Her2, presented a lower level of CXCL16. This negative correlation between CXCL16 expression and tumour aggressiveness revealed that CXCL16 performs a tumour-suppressive role against the invasiveness and migration of breast cancer cells. Further evaluations have clarified that higher expression of CXCL16 in MDA-MB231 associates with upregulation of caspase-3 in this cell, which mediates apoptosis and growth inhibition (Ref. Reference Fang50). In addition to apoptosis induction, it is speculated that TM-CXCL16 on tumour cells binds to CXCR6-expressing stroma and prevents the detachment of individual tumour cells from the tumour mass similar to E-cadherin function, and eventually constrains cell invasion and migration (Ref. Reference Fang50).
As mentioned before, there is a regulatory feedback loop between TNF-α and sCXCL16. Yang et al. have indicated that sCXCL16 reinforces cell proliferation inhibition and promotes TNF-α-dependent cell death and apoptosis in DLBCL cells. The activated apoptosis pathway induced by TNF-α is mediated by mitochondrial apoptotic pathway and Fas-associated death domain caspase signalling, which had been inhibited by anti-CXCR6 antibody (Ref. Reference Yang22). Correspondingly, CXCL16 reinforces the activation of TNFα-induced apoptosis in colorectal cancer (CRC). In CRC, it has been shown that CXCL16 upregulates interferon regulatory factor 8 (IRF8; a transcription factor that regulates Fas-mediated apoptosis) expression and sensitises tumour cells to TNF-α-induced apoptosis. On the other hand, CXCL16 increases the infiltration of M1 macrophages into the tumour microenvironment and subsequently induces the expression of TNF-α in these cells (Ref. Reference Kee51). Collectively, CXCL16 production in DLBCL and CRC mediates tumour inhibition through TNF-α-induced apoptosis.
Liver-resident natural killer T (NKT) cells are the high-expressing CXCR6 cells. These cells move towards the place with a high concentration of CXCL16. The recognition of tumour cells and antigens by NKT cells induces IFN-γ to stimulate cytotoxic T cells and fight against tumour cells. Therefore, the infiltration and accumulation of NKT via CXCL16/CXCR6 interaction are favourable for tumour regression (Ref. Reference Cullen52). Moreover, the infiltration of NKT cells in the murine model of CRC suppresses the metastasis of these cells to liver tissue (Ref. Reference Kee53). In another observation, alteration in the gut microbiome, through affecting CXCL16, influences the accumulation and infiltration of CXCR6+ NKT cells and subsequently suppresses liver tumour (Ref. Reference Ma34). In another study, the lower levels of mast cell proteases such as tryptase, carboxypeptidase A3 and chymase have decreased CXCL16 level in the tumour microenvironment, which in turn diminishes NKT cell responses against melanoma (Ref. Reference Grujic54). Investigation of Hojo et al. also has assumed that high expression of CXCL16 in CRC cell recruits and infiltrates CD8+ and CD4+ tumour-infiltrating lymphocytes (TILs) into the tumour microenvironment and represses tumour growth (Ref. Reference Hojo55).
CXCL16/CXCR6 expression as diagnostic and prognostic markers in cancers
Cancer prognosis is an evaluation of the prospective course and outcome of the tumour. The prognosis of cancer-suffering patients is often regarded as the chance of efficacious cancer treatment and patient recovery. Many factors can impact the prognosis of cancer. The location and type, stage and grade of cancer are the most important cancer prognosis factors. Also, genetic properties and the biological factors expressed in the cancer cells are the other factors that affect cancer prognosis. Hence, defining a non-invasive biomarker for cancer diagnosis and prognosis is essential. There are many prognostic biomarkers identified in cancer. In the meantime, several studies have indicated that high expression of CXCL16 in cancer could be applied as a prognostic biomarker. However, the impact of CXCR6 and CXCL16 expression on patients survival differs noticeably among different malignancies. All studies investigating the effect of CXCL16 in cancer and prognosis have been summarised in Table 1.
Table 1. Altered expression of CXCL16 in various cancers

There are studies indicating a negative correlation between high expression of CXCL16/CXCR6 and good prognosis in cancer. Since normal lung tissue expresses high endogenous CXCL16, it is suggested that dysregulation of CXCL16 is linked to cancer development (Ref. Reference Day82). Mir et al. have indicated that higher expression of CXCL16 supports lung cancer cell proliferation and metastatic process in NSCLC via enhancement of MMPs and ADAM10 expression. They also have shown that in two NSCLC subtypes, adenocarcinoma and squamous cell carcinoma, differential expression of CXCL16/CXCR6 could be correlated to prognostic differences (Ref. Reference Mir79).
To distinguish PTC, an invasive type of thyroid cancer, from benign adenoma and normal thyroid tissue, CXCL16 has been proposed as a reliable marker. Immunohistochemistry on normal tissue, benign adenoma and PTC has shown that CXCL16 is highly expressed in PTC. Also, higher mRNA transcription of CXCL16 in PTC correlated to poor prognosis (Ref. Reference Kim49). CXCL16 recruits M2 macrophages to the tumour microenvironment, which in turn exacerbates tumour outcome. Using CXCL16 in combination with the expression of AHNAK2 and THBS2 genes has also been proposed a prognostic biomarker panel for evaluating recurrence-free survival in PTC (Ref. Reference Kim49).
In a large multicentre cohort study with 535 prostate cancer-suffering patients, it has been shown that the expression of CXCL16 and CXCR6 independently could be considered as predictors for a worse clinical outcome. High expression of CXCR6 (hazard ratio 2.29; 95% confidence interval 1.10–4.82; P-value = 0.028) and CXCL16 (hazard ratio 2.52; 95% confidence interval 1.12–5.68; P-value = 0.026) has been established as independent predictors for clinical failure. Co-expression of CXCR6 and CXCL16 (hazard ratio 5.1; 95% confidence interval 1–15.9; P-value = 0.05) was highly correlated to negative prognostic factors, such as Gleason score ≥7, Gleason grade 4 + 3, positive surgical margins and vascular infiltration (Ref. Reference Richardsen70).
Minimally invasive colorectal resection (MICR) is a common method to remove the diseased portion of the digestive tract in CRC. Since colon resection increases the risk of tumour establishment and metastasis after surgery, it is necessary to determine a suitable biomarker for prolonged follow-up of these patients regarding inflammation and tumour development after surgery. It has been shown that CXCL16, as a pro-angiogenic factor, remains at a high level up to a month in the plasma. Therefore, elevated plasma levels of CXCL16 may encourage tumour angiogenesis in the first month after MICR and could be a useful marker for screening tumour recurrence after resection (Ref. Reference Shantha Kumara65). Also, Shibata et al. have identified that serum CXCL16 was a potential biomarker for the efficacy of therapy, including bevacizumab (i.e., anti-VEGF) in the treatment of advanced NSCLC. In this regard, pre- and post-chemotherapy assessment of serum CXCL16 levels showed that longer overall survival was observed in patients who had a significant decrease in serum CXCL16 levels (Ref. Reference Shibata83). Moreover, Farrow et al. reported that decreased expression of CXCL16 was associated with improved recurrence-free survival among the patients with resectable melanoma (Ref. Reference Farrow84).
Although overexpression of CXCR6 and CXCL16 was observed in many cancers, and their increased expression has been related to a more aggressive phenotype and advanced tumour stage, some investigations have demonstrated that their increased expression is independently associated with good survival. For example, Hojo et al. have found that high levels of CXCL16 expression in CRC were a favourable prognostic factor, and it was associated with increased TIL levels (Ref. Reference Hojo55). Similarly, Xing et al. have shown that nuclear CXCL16 expression in gastric cancer is associated with improved survival and decreased cancer aggressiveness. This study has also conducted that patients with mixed-type carcinoma or with lymphatic invasion carcinoma showed lower CXCL16 concentrations than those with diffuse-type carcinoma or without lymphatic invasion. The serum concentration and expression of CXCL16 could specify the prognosis and aggressiveness of gastric carcinomas (Ref. Reference Xing63).
The study of Gutwein et al. showed that the low expression of CXCL16 and CXCR6 in renal cell carcinoma is linked to decreased overall survival and proposed that the evaluation of CXCL16 in RCC could be used as an independent prognostic marker to determine better patient survival (Ref. Reference Gutwein81). Moreover, Liu et al. have shown that the expression of CXCL16 was relatively lower in non-Hodgkin lymphoma (NHL; a lymphoma with bad prognosis) than in Hodgkin lymphoma specimens (HL; a lymphoma with a fairly good prognosis), reflecting the different microenvironments of the two types of lymphoma and indicating that CXCL16 is a good prognostic biomarker in this type of cancer. Further examinations on the effect of CXCL16 on the fate of these two types of lymphoma have shown that CXCL16 induces proliferation of CD4+ T cells to fight against HL cells rather than NHL cells (Ref. Reference Liu69).
Therapeutic approaches
CXCL16 is a transmembrane and secretory protein and hypothetically druggable so that neutralizing antibodies, aptamers and small inhibitors can be established to modulate its function. Owing to inadequate physiological data on the CXCL16 function, the potential adverse effects of CXCL16 antagonists are difficult to predict.
Escin, a natural mixture of triterpene saponins, is a Chinese herbal medicine and one of the major active compounds derived from Aesculus hippocastanum (horse chestnut) seed. It has been shown that Escin has anti-inflammatory, vascular protective, veinotonic and anti-cancer effects. In terms of the anti-cancer effect, Lee et al. have shown that Escin drastically decreased the secretory level of CXCL16 and migration of AGS cells (gastric adenocarcinoma cells). Further examination has shown that Escin inhibits FAK (a protein tyrosine kinase) and AKT phosphorylation in these cells and subsequently inhibits tumour growth (Ref. Reference Lee64).
Blocking the expression of CXCL16 using shCXCL16 in PTC cell lines significantly decreased cell viability. Inoculation of CXCL16-silenced tumour cells into mice, as ectopic tumour model, showed significantly delayed tumour growths from day 10 to day 30 compared with CXCL16 + tumour cells. The inhibitions of CXCL16 expression by targeting either the stromal cells or tumour cells could be a promising therapeutic strategy for an advanced and aggressive type of thyroid cancer (Ref. Reference Kim49). Also, since CXCL16 is one of the important factors in forming foamy macrophages in pRCC, the blockade of CXCL16 could be promising for alleviating tumour severity when surgery and common therapeutic approaches fail (Ref. Reference Krawczyk37).
Inhibitors of signal transduction proteins gained much attention among investigations during the last decade. As mentioned above, ERK/MEK plays a pivotal role in the signalling of CXCR6 and reverse signalling from TM-CXCL16. U0126, as a MEK inhibitor, has been used to treat NPC cell lines. The results showed that the administration of U0126 inhibits the expression of CXCL16, cell proliferation and NPC cell metastasis into the bone, dose- and time-dependently. This idea has provided a novel therapeutic strategy for metastatic NPC by targeting the CXCL16/CXCR6 signalling pathway (Ref. Reference Zhu68).
As discussed above, KRAS mediates the expression of CXCL16 in PDAC and, on the contrary, sst2 blocks the activation of CXCL16 transcription. Using sst2 activators has been suggested as a therapeutic approach in KRAS-mutated PDAC. Octreotide, an octapeptide that acts as a new synthetic pharmacological analogue for natural somatostatin, has reversed sustained activation of KRAS/PI3K/AKT/NF-kB and proposed a promising approach for future consideration (Ref. Reference Chalabi-Dchar21).
Using monoclonal antibodies against tumour growth factors has attracted much focus among researchers. Since CXCL16 has been described as a tumour growth factor in several cancers, application of anti-CXCL16 antibody has been tested. In this regard, Kim et al. have declared that anti-CXCL16 antibodies in PTC murine model significantly reduced tumour volume (Refs Reference Kim49, Reference Hu80).
Since the transmembrane and secretory type of CXCL16 shows different functions in various tumours, these two variants have paradoxical performance. It has been concluded that the inhibition of the converting enzymes, including ADAM10 and ADAM17, might be promising in cancer treatment. In this regard, Gooden et al. have shown that the administration of ADAM10-specific inhibitor (GI254023x) or ADAM10/ADAM17 inhibitor (TAPI-2) reduced CXCL16 membrane shedding and significantly decreased migration and metastasis of ovarian cancer cells (Ref. Reference Gooden74). Other drugs or agents that can be mentioned for inhibiting or downregulation of CXCL16 expression in tumours is Namitecan (ST1968) (Ref. Reference Cassinelli85).
In the type of tumours in that CXCL16 has an anti-tumour function, the upregulation of this chemokine has clinical significance for cancer treatment. Radiotherapy is a widely-used and common treatment technique in cancer. In general, studies have confirmed that irradiation combined with chemotherapy plays a crucial role in tumour cell killing and is also known to be immune-supportive. Since Yoon et al. have shown that irradiation enhances the expression of CXCL16 and NK cell response against breast tumour cells, it is suggested that the optimal irradiation's dose and time might be promising in the treatment of those tumours that CXCL16 has tumour-suppressive activity (Ref. Reference Yoon32). In another study, ionizing radiation of TRAMP-C1, 67NR and 4T1 cells increased the expression of ADAM10 and ADAM17, which are responsible for the production of sCXCL16 and tumour inhibition (Ref. Reference Matsumura and Demaria61). Furthermore, Matsumura et al. have also shown that ionizing radiation therapy increases the activation of Th1 cells to induce cytotoxic T cells against breast tumour cells. This study also indicated that ionizing radiation elevates infiltration of CXCR6+ T cells into the tumour microenvironment (Ref. Reference Matsumura33). Overall, the investigations have emphasised the positive effect of irradiation on tumour cell inhibition mediated by the CXCR6/CXCL16 axis.
In some cancers such as RCC, it has been shown that the CXCL16 promoter is hypermethylated, and its re-expression inhibits tumour growth. Using methylation inhibitors or demethylating agents such as 5-azacytidine suppresses CXCL16 methylation and promotes tumour suppression (Ref. Reference Morris86). Also, since Hojo et al. have emphasised the role of CXCL16 in CRC regression and TILs infiltration into tumour nest to fight against tumour cells, it can be assumed that using recombinant CXCL16 to induce T cell infiltration into tumour microenvironment might be promising in combination with other therapeutic approaches (Ref. Reference Hojo55).
Dormancy in cancer refers to a state in which the tumour cells cease proliferation but survive in a quiescent state and wait for suitable environmental situations to begin dividing once more. Dormant cancer cells are generally resistant to drugs because they are not proliferating, while chemotherapy is ideal for inhibiting rapid dividing cells. Hence, dormancy endows drug-resistance to tumour cells. The exit of dormancy relies on multiple mechanisms. One of the mechanisms that inhibit cell dormancy and promote cell proliferation is the ERK signalling pathway (Ref. Reference Aguirre-Ghiso87). Since CXCL16 activates this signalling pathway, it is concluded that CXCL16 promotes cell proliferation. Inducing cell exit from dormancy by applying CXCL16 can be an approach in cancer therapy (Ref. Reference Adamski35).
Future directions and concluding remarks
In vitro studies, murine models and clinical observations have clarified that inflammation can cause tumorigenesis. Among vital mediators for inflammation, chemokines and their receptors play a pivotal role in this process. Various functions have been defined for chemokines in cancer biology, including direct effects on cancer cells (e.g., cell proliferation, transformation and survival) and indirect impacts (e.g., leukocyte infiltration, tumour protection and angiogenesis). Given the possible pro-tumorigenic role of chemokine in cancer, several studies have assumed that these cytokines, either produced by the cancer cells or cancer-associated leukocytes, irrespective of typical viewpoint, might contribute to tumour development through enhancement of leukocyte infiltration. However, some investigations believed that chemokines employ the immune system's anti-tumour arm, including NK, NKT, T cells, to fight against a tumour (Ref. Reference Chow and Luster19).
During the decade, CXCL16 and its unique receptor CXCR6 have drawn attention to itself in cancer studies. It has been demonstrated that CXCL16 in cancer plays a multifarious role. Mainly, the overexpression of CXCL16 ligand and CXCR6 receptor in cancer correlates to lower survival rates and poorer prognostic profile. However, it has also been made known that CXCR/CXCL16 signalling has tumour-suppressive functions, proposing that this pathway is highly context-dependent. It seems that the cell origin of CXCL16 plays a pivotal role in determining the fate of the tumour. For instance, in breast and liver cancers in which CAFs are the origin of CXCL16, it has been shown that the secretion of CXCL16 is not favourable for a good prognosis (Refs Reference Allaoui42, Reference Liu43).
Since CXCL16 exists either in secretory or transmembrane forms, and the latter could be cleaved into the secretory form, it is hard to discriminate which form is being investigated in cancer studies. Hence, this is one of the problems in the study of CXCL16 in cancers. Another problem is that the origin of CXCL16 in the tumour microenvironment is not defined. Moreover, the difference in tumour stage investigating CXCL16 is almost not been considered. Therefore, future studies should focus on these issues. The in vitro and in vivo conditions were quite different from each other so that the model of tumour studies is affected by various mechanisms, including leukocyte infiltrating and the stomal cells. Therefore, in vitro studies cannot be generalised to the other forms of studies. Moreover, the different roles of transmembrane and secretory forms of CXCL16 during tumour progression have indicated that a high serum level of sCXCL16 is a signal of poor prognosis while TM-CXCL16 appears to be a signal of good prognosis. The different expression patterns of sCXCL16 or TM-CXCL16 in tumours cleared that the different forms of CXCL16 might be responsible for their conflicting roles in different types of cancers.
By distinguishing the role of CXCL16 in the tumour, it is hoped that using CXCL16 would be promising in tumour therapeutic approaches and diagnosis.
Acknowledgements
None.
Financial support
None.
Conflict of interest
None.






