It is estimated that millions of the world's children are growing up amid political violence, continuous war, tribal and racial terrorism, genocide, military attacks on civilian locations, or guerrilla warfare (Belsky, Reference Belsky2008; Masten & Narayan, Reference Masten and Narayan2012). Others, such as children of combat soldiers in the United States, experience the aftermath of war-related trauma through a parent (McFarlane, Reference McFarlane2009), while still others are exposed to a single act of terrorism, for instance, the September 11 attack (Chemtob, Nomura, & Abramovitz, Reference Chemtob, Nomura and Abramovitz2008) or the Oklahoma City bombing (Pfefferbaum et al., Reference Pfefferbaum, Nixon, Tucker, Tivis, Moore and Gurwitch1999). Although systematic research is often difficult in zones of war, several studies have described children's response to war and terrorism across the globe, including Lebanon (Bryce, Walker, Ghorayeb, & Kanj, Reference Bryce, Walker, Ghorayeb and Kanj1989), Gaza (Qouta, Punamaki, & El Sarraj, Reference Qouta, Punamaki and El Sarraj2003), Israel (Laor, Wolmer, & Cohen, Reference Laor, Wolmer and Cohen2001), Kosovo (Lopes Cardozo, Kaiser, Gotway, & Agani, Reference Lopes Cardozo, Kaiser, Gotway and Agani2003), Bosnia–Hercegovina (Smith, Perrin, Yule, & Rabe-Hesketh, Reference Smith, Perrin, Yule and Rabe-Hesketh2001), and Rwanda (Schaal, Jacob, Dusingizemungu, & Elbert, Reference Schaal, Jacob, Dusingizemungu and Elbert2010). Studies indicate that political violence compromises children's mental health, leading to anxiety, depression, posttraumatic symptoms, and conduct problems; that such problems tend to persist after the war has subsided; and that maternal factors, particularly the mother's well-being and support networks, function as resilience factors (Allwood, Bell-Dolan, & Husain, Reference Allwood, Bell-Dolan and Husain2002; Feldman & Vengrober, Reference Feldman and Vengrober2011). However, very little research has focused on infants and young children exposed to political violence, and we are aware of no study that tested hormones, observed parenting, and temperamental dispositions in war-exposed young children.
In this study, we recruited a large cohort of infants and young children aged 1.5 to 5 years living in a zone of continuous war. Our goal was to examine children's physiological stress response and assess maternal and child factors that may correlate with variability in young children's stress response in the context of war exposure. Two neuroendocrine markers of stress were tested, cortisol (CT) and salivary alpha amylase (sAA), which are considered as biomarkers of the two arms of the stress response: the hypothalamic–pituitary–adrenal (HPA) and the sympathetic–adrenal–medullary (SAM) systems (Hellhammer, Wüst, & Kudielka, Reference Hellhammer, Wüst and Kudielka2009; Nater & Rohleder, Reference Nater and Rohleder2009). Functioning of these systems is thought to be shaped by a range of proximate conditions (Juster et al., Reference Juster, Bizik, Picard, Arsenault-Lapierre, Sindi and Trepanier2011), and we examined the relations between children's stress response and several biological, parenting, and temperamental factors that have been linked with stress in young children. These include maternal CT and sAA, posttraumatic symptoms, and parenting style, and the child's reactivity and regulation. In addition to differences between exposed and nonexposed children, we were particularly interested in the differences between exposed children who developed posttraumatic stress disorder (PTSD) and those exposed to similar stressors who did not develop the disorder in an attempt to describe factors that may be associated with greater risk and resilience in the context of political violence.
Chronic Early Stress and Children's Stress Response
Chronic early stress initiates a cascade of physiological processes that alter the development of children's physiological stress systems (Gunnar & Quevedo, Reference Gunnar and Quevedo2007; Heim, Plotsky, & Nemeroff, Reference Heim, Plotsky and Nemeroff2004; Teicher et al., Reference Teicher, Andersen, Polcari, Anderson, Navalta and Kim2003). Exposure to a variety of early stressors, such as maltreatment, poverty, or institutional rearing, may interfere with the development of neurotransmitters and brain systems regulating arousal and emotions (Weinstock, Reference Weinstock2005), impair immune functioning and increase susceptibility to disease (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz and Edwards1998; Graham, Christian, & Kiecolt-Glaser, Reference Graham, Christian and Kiecolt-Glaser2006), increase the risk for behavior problems in childhood and mental illness in adulthood (Cicchetti & Toth, Reference Cicchetti and Toth1995; Fombonne, Wostear, Cooper, Harrington, & Rutter, Reference Fombonne, Wostear, Cooper, Harrington and Rutter2001), and disrupt the development of social reciprocity and empathy (Decety, Reference Decety2010). In light of research demonstrating links between early stress and children's stress physiology (Tarullo & Gunnar, Reference Tarullo and Gunnar2006), the first goal of the current study was to examine biomarkers of the stress response in young children exposed to continuous war. However, because the response of individual children to stressful contexts varies widely, our second goal was to assess maternal and child factors that may correlate with variability in children's stress response within the group of war-exposed young children. Such data may be useful for the development of more specific interventions for this group.
Models on allostatic load (McEwen & Stellar, Reference McEwen and Stellar1993) provided the theoretical framework for the study. Such models build on the concept of allostasis (the organism's adaptation to change) to describe the wear and tear caused by chronic stress that leads to persistent alterations in physiological systems (Juster et al., 2001). Across species, processes of allostatic load detail the ongoing trade-offs between the degree of stress and its persistence, the specific risks and resources embedded in the ecosocial niche, and differences related to the organism's dispositional response strategies (Korte, Koolhaas, Wingfield, & McEwen, Reference Korte, Koolhaas, Wingfield and McEwen2005). Thus, in studying children exposed to continuous war, allostatic load models would suggest to take into account specific environmental resources and temperamental dispositions. In the current study, we focused on three maternal factors as indices of the risks and resources embedded in the child's ecology: the mother's stress hormone, PTSD symptoms, and reciprocal parenting. In addition, two factors were tested as markers of the child's temperamental dispositions: emotional reactivity and regulation strategies. Such multivariate approach is consistent with Cicchetti and Rogosch (Reference Cicchetti and Rogosch2009), who suggest that parental factors, parent and child biology, and temperamental dispositions should each be considered in research on young children exposed to chronic stress.
CT, SAA, and Early Exposure to Continuous War
The stress response involves the coordinated functioning of two major anatomically distinct systems: the SAM, which initiates the fight-or-flight response by increasing blood flow, respiration, cardiovascular activity, and the release of catecholamines (Nater & Rohleder, Reference Nater and Rohleder2009), and the HPA system, which has a more gradual onset and is associated with physiological and behavioral withdrawal (Bauer, Quas, & Boyce, Reference Bauer, Quas and Boyce2002; Tarullo & Gunnar, Reference Tarullo and Gunnar2006). Whereas momentary stress induces immediate changes in each system, chronic stress exerts a lasting effect and may alter the balance between the functioning of the HPA and the SAM systems (Wolf, Nicholls, & Chen, Reference Wolf, Nicholls and Chen2008). It has been suggested that the assessment of basal levels, reactivity patterns, and functioning of both the HPA and the SAM systems may provide a fuller picture of the child's stress response under conditions of chronic stress (Del Giudice, Elllis, & Shirtcliff, Reference Del Giudice, Elllis and Shirtcliff2011; Korte et al., Reference Korte, Koolhaas, Wingfield and McEwen2005).
CT, the end product of activity of the HPA axis, is the most researched biomarker of the stress response; and its development in infancy is highly sensitive to the social context (Tarullo & Gunnar, Reference Tarullo and Gunnar2006). Higher baseline levels and greater CT reactivity were found among children exposed to early stress, including maltreatment (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001), maternal postpartum depression (Essex, Klein, Cho, & Kalin, Reference Essex, Klein, Cho and Kalin2002; Feldman et al., Reference Feldman, Granat, Pariente, Kanety, Kuint and Gilboa-Schechtman2009), or abuse (King, Mandansky, King, Fletcher, & Brewer, Reference King, Mandansky, King, Fletcher and Brewer2001). Studies have indicated that chronic stress impacts the body's response to trauma (De Bellis et al., Reference De Bellis, Baum, Birmaher, Keshavan, Eccard and Boring1999) and that ecological conditions may be related to the functioning of the HPA system following persistent exposure to traumatic conditions (Pervanidou, Reference Pervanidou2008).
Research on CT levels in childhood PTSD has shown inconclusive results. Several studies found that children with PTSD showed higher or comparable levels of CT to those of nonexposed controls (Carrion et al., Reference Carrion, Weems, Ray, Glaser, Hessl and Reiss2002; De Bellis et al., Reference De Bellis, Baum, Birmaher, Keshavan, Eccard and Boring1999). Others reported lower baseline CT and blunted CT response in cases of PTSD. For instance, Pfefer, Altemus, Heo, and Jiang (2007) found lower baseline and flatter diurnal curves in children exposed to the 9/11 attack who developed PTSD as compared to those who did not develop psychopathology. Similarly, lower CT has been described in adults with PTSD following childhood-onset trauma (De Bellis et al., Reference De Bellis, Baum, Birmaher, Keshavan, Eccard and Boring1999). Gunnar and Vazquez (Reference Gunnar and Vazquez2001) described a phenomenon termed hypocortisolism, the paradoxical underreactivity of the HPA system in children exposed to early trauma or severe adversity, and argued that this blunted response should be examined in research on chronic early adversity. Recently, Badanes, Watamura, and Hankin (Reference Badanes, Watamura and Hankin2011) found that young children growing in environments of high stress showed low baseline CT and little diurnal variability, particularly when combined with maternal depression, and this hypocortisolism predicted greater risk for internalizing disorders. Consistent with models on allostatic load, the authors concluded that hypocortisolism in young children is a marker of allostatic load and that the combination of low and flat CT with chronic contextual stress may increase the risk for psychopathology.
Although much less research investigated sAA, and data on its source, role, and effects are still emerging, sAA has been suggested as a biomarker of the stress response that indexes SAM activity and is associated with plasma norepinephrine levels in response to stress (Nater & Rohleder, Reference Nater and Rohleder2009). In contrast, Bosch, Veerman, de Geus, and Proctor (Reference Bosch, Veerman, de Geus and Proctor2011) argued that sAA is not a pure index of sympathetic activity and reflects both sympathetic and parasympathetic influences in ways that are still to be fully described. Notwithstanding this controversy, recent research examined sAA in relation to child health (Wolf et al., Reference Wolf, Nicholls and Chen2008), emotional reactivity (Spinrad et al., Reference Spinrad, Eisenberg, Granger, Eggum, Sallquist and Haugen2009), and attachment (Frigerio et al., Reference Frigerio, Ceppi, Rusconi, Giorda, Raggi and Fearon2009), and found links with stress-related processes. In general, no direct correlations emerged between CT and sAA (Granger, Kivlighan, el-Sheikh, Gordis, & Stroud, Reference Granger, Kivlighan, el-Sheikh, Gordis and Stroud2007), but associations were mediated by biological, genetic, or relational factors (Frigerio et al., Reference Frigerio, Ceppi, Rusconi, Giorda, Raggi and Fearon2009).
Several studies addressed the interaction of CT and sAA in predicting children's propensity for psychopathology. Higher CT was found to predict internalizing symptoms only among children high in sAA (El-Sheikh, Erath, Buckhalt, Granger, & Mize, Reference El-Sheikh, Erath, Buckhalt, Granger and Mize2008). Others found that the combination of low sAA and low CT predicted externalizing symptoms (Gordis, Granger, Susman, & Trickett, Reference Gordis, Granger, Susman and Trickett2006), suggesting that hypoactivity of the two systems may index greater risk. Among adolescents exposed to Hurricane Katrina, the combination of high CT and high sAA indicated greater resilience and was associated with higher self-esteem (Vigil, Geary, Granger, & Flinn, Reference Vigil, Geary, Granger and Flinn2010). Overall, research on stress biomarkers in children suggests that including CT and sAA in the context of developmental risk may provide a more comprehensive understanding of the child's stress response.
Maternal and Child Factors and Children's Stress Response
The most critical regulator of very young children's stress response is the mother (Hofer, Reference Hofer, Goldberg, Muir and Kerr1995). Three maternal factors have been associated with young children's stress response: maternal stress physiology, maternal behavior, and maternal well-being. Animal studies have demonstrated the lifelong effects of maternal antenatal stress hormones, particularly CT, on the fetus's developing HPA axis through the maternal–placental–fetal neuroendocrine axis (Lightman, Reference Lightman2008; Wadhwa, Reference Wadhwa2005; Weinstock, Reference Weinstock2005) and the programming of gluccorticoid gene expression in the amygdala, the hippocampus, and the hypothalamus (Fujioka, Fujioka, Ishida, Maekawa, & Nakamura, Reference Fujioka, Fujioka, Ishida, Maekawa and Nakamura2006; Welberg, Seckl, & Holmes, Reference Welberg, Seckl and Holmes2001). “Sensitive” maternal behavior (i.e., more licking and grooming) has been shown to exert epigenetic effects on glucocorticoid receptor gene expression in the pup's hippocampus (Weaver et al., Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma and Seckl2004), and maternal sensitivity has similarly been linked with more optimal HPA regulation in human infants (Haley & Stansbury, Reference Haley and Stansbury2003). In addition, several studies reported correlations between levels of maternal and child's CT (Feldman, Singer, & Zagoory, Reference Feldman, Singer and Zagoory2010; Ruttle, Serbin, Stack, Schwartzman, & Shirtcliff, Reference Ruttle, Serbin, Stack, Schwartzman and Shirtcliff2011; Sethre-Hofstad, Stansbury, & Rice, Reference Sethre-Hofstad, Stansbury and Rice2002) as well as maternal and child's sAA (Gordis, Margolin, Spies, Susman, & Granger, Reference Gordis, Margolin, Spies, Susman and Granger2010), indicating that maternal stress biomarkers may serve as important correlates of the child's stress response.
Mother–child reciprocity describes the synchronous component in the maternal style, and longitudinal studies demonstrated the effects of reciprocal parenting on children's stress modulation and social growth (Feldman, Reference Feldman2007). Mother–child reciprocity has been associated with lower baseline CT in mother and child, and with quicker return to baseline following stress (Feldman et al., Reference Feldman, Singer and Zagoory2010). Similarly, higher maternal responsiveness was related to lower infant baseline CT (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, Reference Gunnar, Brodersen, Nachmias, Buss and Rigatuso1996), whereas maternal psychopathology correlated with disruptions to the HPA response, expressed in child hypo- (Badanes et al., Reference Badanes, Watamura and Hankin2011) or hypercortisolism (Feldman et al., Reference Feldman, Granat, Pariente, Kanety, Kuint and Gilboa-Schechtman2009). Overall, these studies suggest that the mother's stress hormones, reciprocal behavior, and psychopathology may each be associated with the child's CT and sAA.
In addition to maternal factors, children's temperamental reactivity and negative emotionality have shown to increase the risk for externalizing and internalizing symptoms in childhood (Sanson, Oberklaid, Pedlow, & Prior, Reference Sanson, Oberklaid, Pedlow and Prior1991) and psychopathology in adolescence (Degnan, Almas, & Fox, Reference Degnan, Almas and Fox2010). In contrast, in addressing the effects of political violence on the development of psychopathology in children, Belsky (Reference Belsky2008) suggested that under conditions of continuous war, high physiological arousal and temperamental reactivity do not necessarily index higher risk but may be an adaptive response to the stressful context. Similarly, it has been suggested that under conditions of uncertainty, high physiological arousal may function to maintain vigilance and may be considered an appropriate response to stress (Belsky & Pluess, Reference Belsky and Pluess2009; Del Giudice et al., Reference Del Giudice, Elllis and Shirtcliff2011). It is thus possible that among war-exposed young children, high temperamental reactivity and high physiological arousal, as expressed in higher levels of CT and sAA, may not necessarily correlate with greater risk for psychopathology.
The Current Study
In light of the above, the current study examined two sets of hypotheses: the first related to differences between war-exposed children and controls on physiological, maternal, and temperamental factors; the second assessing differences between war-exposed children who did or did not develop PTSD. Consistent with Cicchetti and Rogosch (Reference Cicchetti and Rogosch2009), multiple maternal and child biological and behavioral measures were collected. Children's stress response was indexed by two biomarkers: CT and sAA. Three maternal factors were examined as correlates of the child's stress response: maternal stress physiology indexed by the mother's CT and sAA, maternal reciprocity, and maternal PTSD symptoms. Three child temperamental factors were microcoded from a fear paradigm and similarly tested as correlates of the stress response: child negative emotionality and two emotion regulation strategies, comfort seeking and withdrawal.
With regard to the first set of hypotheses, we expected group differences on all of the aforementioned variables. War-exposed children were expected to show different levels of CT and sAA, more negative emotionality, and greater use of the two regulatory strategies. Mothers living in war zones were expected to report more posttraumatic symptoms, show lower reciprocity, and their stress physiology was expected to differ from that of controls. As to the second set of hypotheses, consistent with Badanes et al. (Reference Badanes, Watamura and Hankin2011), we hypothesized that children diagnosed with PTSD would exhibit hypocortisolism, expressed in low baseline CT and minimal reactivity, and would select withdrawal strategies to regulate fear as compared to exposed children without PTSD. Finally, among all children, we expected that children's baseline CT and baseline sAA would be associated with the various maternal and child factors. Maternal biomarkers were expected to correlate with the parallel biomarker in the child, maternal reciprocity was expected to be associated with lower baseline CT, and maternal PTSD and child negative emotionality were expected to correlate with higher baseline CT. Because research on sAA as a stress biomarker is still emerging, we examined the relations between these variables and sAA without directional hypotheses.
Method
Participants
Participants included 232 children from 232 families aged 1.5 to 5 years (M = 33.08 months, SD = 10.89) and their mothers (M = 31.27 years, SD = 5.55, range = 22.3–47.4). The sample included 47.6% males and 47.1% firstborns. Maternal and paternal education averaged 3.88 (SD = 1.44) and 3.51 (SD = 1.49), respectively, on a scale of 1 (elementary school) to 4 (college education). Among mothers, 31.6% worked full time, 42.6% worked part time, and 11% were single mothers. All children lived with their mothers since birth with no prolonged separation. In order to assess the unique effects of war-related trauma, children were not included in the study if there were official records of maltreatment or family violence, mothers reported physical or sexual abuse, or the child suffered a severe motor vehicle accident or other major trauma other than war exposure.
One hundred and forty-eight families comprised the war-exposed group. These included children living in the same neighborhoods in the town of Sderot, Israel, which is located 10 kilometers from the Gaza border (see Figure 1 for map). Citizens of Sderot were exposed repeatedly to rocket attacks from Gaza over a period of several years, had only 15 s to enter protected spaces after hearing the alert sirens, and were exposed to frequent mortar shelling to which no alert signals were provided. Testing was conducted during a period of repeated rockets and missiles attacks (January 2006–October 2008). During this period, rocket attacks on Sderot occurred unpredictably and continuously several times a month, and all children were seen at least 1 month after the period in which these attacks have begun. Visits were not conducted during the day of the attack or within the next 2–3 days. Figure 1 presents a map of Sderot, Israel, and shows the close proximity to Gaza, particularly to the town of Bet Hanun from which missiles were launched.

Figure 1. (Color online) A map depicting the Israeli town of Sderot in relation to the Gaza Strip. Citizens of Sderot, Israel, live within a 10-km range of missile attacks from the Gaza Strip and have 15 s from the sound of alarm to enter sheltered settings.
Eighty-four nonexposed children were recruited as controls from towns within the greater Tel-Aviv area that were equivalent to the town of Sderot in terms of population size, socioeconomic composition, and housing and employment opportunities. These areas were not exposed to missile attacks or acts of political violence during the study period, and controls were matched to the exposed group in age, gender, birth order (firstborn/later born), maternal and paternal age and education, and maternal employment and marital status. Prior to home visits, control families were screened by phone for major traumatic events in the child's life (e.g., motor vehicle accidents and physical or sexual abuse), and those reporting such trauma were excluded. The study was approved by the institutional review board, and all mothers signed an informed consent.
Procedure
Children were visited at home for approximately 3.5 hr during the afternoon. Visits included hormone collection from mother and child, psychiatric diagnosis of child PTSD by interviewing mother when child was present, a series of mother–child interactions, a series of child emotion-eliciting paradigms, and a battery of self-report measures for mother. Following acquaintance, baseline saliva samples were collected from mother and child by mother placing a Salivette (Sarstedt, Rommelsdorft, Germany) in her own and in the child's mouth for 1 min (termed baseline). Following, mother and child were videotaped in several interactive episodes. To elicit the stress response, we used a detailed evocation of traumatic memories (see below), employed in research on CT reactivity in cases of PTSD (e.g., Smyth, Hockemeyer, & Tulloch, Reference Smyth, Hockemeyer and Tulloch2008), combined with a fear paradigm that involved the presentation of increasingly fearful novel objects adapted from the Lab-TAB (Goldsmith & Rothbart, Reference Goldsmith and Rothbart1996), consistent with research on CT reactivity in young children (Buss, Davidson, Kalin, & Goldsmith, Reference Buss, Davidson, Kalin and Goldsmith2004). A second salivary sample was collected from mother and child 20 min after the fear paradigm (termed following challenge), and a third set of samples from mother and child were collected 15 min thereafter (termed recovery). It is important to note that saliva collection was timed to the reactivity pattern of the HPA axis and not to that of the sAA. Our pilot study showed that it is not possible to draw more than three saliva samples from children at that age, and we selected to focus on the HPA axis as the central marker of the stress response. This should be noted as a study limitation with regard to the findings for sAA.
Psychiatric interview
Mothers in both the exposed and the control groups were interviewed when the child was present to diagnose child PTSD. Trained clinicians with a background in early childhood development and psychopathology visited families at home and diagnosed the child's PTSD using the Diagnostic Classification: Zero-to-Three (DC 0–3R; Zero to Three, 2005), which provides a validated tool for diagnosing psychiatric disorders in children 5 years and under (Chu & Lieberman, Reference Chu and Lieberman2010). Clinicians conducted an extensive interview with the mother while the child was present and elicited detailed information on the nature of the trauma, the mother and child's proximity to the traumatic event, the child's emotional reaction to specific traumatic events, specific symptoms in the four symptom clusters (reexperiencing, avoidance, hyperarousal, and fears and aggression), and the child's developmental regression or arrest. Clinicians were supervised by a senior clinical child psychologist and a child psychiatrist, and cases were conferred every few weeks. In addition, formal observation of young children's reaction to trauma evocation is suggested as central to the diagnosis of PTSD at this age (Chu & Lieberman, Reference Chu and Lieberman2010; Scheeringa, Zeanah, & Cohen, Reference Scheeringa, Zeanah and Cohen2011), and the videos of mother and child during the evocation of traumatic memories were coded for maternal and child emotional behavior. Following the clinical interview, mothers were asked to rate the child's symptoms on a list of 58 posttraumatic symptoms that were compiled after a year of pilot study in Israeli and Palestinian war-exposed locations. In the pilot study, extensive interviews were conducted with parents, caregivers, and therapists living in war zones on the typical symptoms children at that age exhibit following repeated exposure to war-related violence (Feldman, Vengrober, & Hallaq, Reference Feldman, Vengrober and Hallaq2007). For each symptom, mothers rated if she had not notice the symptom in her child and, if she did, whether the child exhibited the symptom infrequently (once a week or less) or frequently (daily or every 2–3 days). Consistent with the DC 0–3R guidelines, PTSD was diagnosed if the child was exposed to a firsthand war-related trauma that was followed by verbal and/or nonverbal expression of intense fear or horror and exhibited at least 1 reexperiencing, 1 avoidance, and 2 hyperarousal symptoms described as occurring “frequently.” According to the DC 0–3R criteria, both fears and aggression symptoms, and developmental regression are noted in detail during the diagnostic interview, but they are not included in the formal diagnosis of early childhood PTSD. PTSD was diagnosed in 56 of the exposed children (37.8%), and 92 children were defined as exposed-no-PTSD.
Control mothers were interviewed in a similar manner to the exposed group. Mothers were asked to describe events the child experienced as “traumatic,” stressful, or emotionally difficult during the past few months; and the interview and symptom list followed the same procedure as the exposed group. Mothers described mildly “traumatic” events in the child's life, such as loss of a grandparent, illness in the family, and moving to a new neighborhood.
Detailed evocation of traumatic memories
Child was present in the room and situated near the mother when the clinician elicited detailed information on the traumatic experience. The clinician asked about the nature of the trauma, including the specifics of the trauma exposure, the child and family members’ degree of exposure, and the child's emotional reaction to specific events, including the incidence of exposure; the child's proximity to the explosion; whether the child or family members were injured and how severely; the child's behavioral expression of fear, horror, and other emotions in response to each traumatic exposure; the specific symptoms the child expresses in the reexperiencing, avoidance, hyperactivity, and fears and aggression domains; and the nature of the developmental regression.
Mother–child free play
Mothers and children engaged in free play for 10 min. The experimenter placed a box of preselected toys in front of mother, and instructions were “Play with your child as you normally do.”
Fear regulation
In this procedure, adapted from the Lab-TAB (Goldsmith & Rothbart, Reference Goldsmith and Rothbart1996), the experimenter puts on four masks of increasing fearfulness while the child is looking: clown, pet animal, scary animal, and ghost. The experimenter puts on each mask, calls the child's name, and leaves it on for 10 s.
Maternal PTSD
Maternal PTSD symptoms were assessed with the Posttraumatic Diagnostic Scale (Foa, Reference Foa1995). This scale provides symptom scores in the three major symptom clusters (reexperiencing, avoidance, and hyperarousal) and a general PTSD score with good reliability and validity.
Hormonal assays
CT
Salivettes were kept cooled until thawed before being centrifuged at 4°C at 1000 × g for 15 min. The samples were then stored at –20°C until assayed. CT levels were assayed using a commercial ELISA kit (Assay Design, MI). Measurements were performed in duplicate according to the kit's instructions. CT levels were calculated by using MatLab-7 according to the relevant standard curves. The intraassay and interassay coefficients are less than 10.5% and 13.4%, respectively. Missing values were estimated by the curve when one assessment was missing, and when two assessments were missing (n = 21, 9% for mothers and n = 24, 10% for children), the subject was taken out of the analysis.
sAA
The concentration of AA in saliva samples was determined by Salimetrics assay kit according to instructions. Most samples were diluted 1:100 prior to assaying. Samples exceed 400 U/ml were rerun at dilution of 1:500. Values too low to read at 1:100 dilution were rerun at dilution of 1:50.
The sAA concentration was calculated by the equation provided, taking into account appropriate dilution. Intraassay and interassay coefficient variances were 2.5 and 13.6, respectively.
Coding
Mother–child reciprocity
Interactions were coded using the Coding Interactive Behavior Manual, a global rating system for adult–child interactions with 45 codes aggregated into several composites. The system has shown adequate psychometric properties in multiple studies of healthy and high-risk populations at this age (Feldman & Eidelman, Reference Feldman and Eidelman2009; Feldman, Keren, Gross-Rozval, & Tyano, Reference Feldman, Keren, Gross-Rozval and Tyano2004; Feldman & Masalha, Reference Feldman and Masalha2010). Two coders, trained to 90% reliability and blind to psychiatric information, coded. Reliability κs on 40 interactions averaged 0.84 (range = 0.78–0.95). The dyadic reciprocity construct (α = 0.93) was used, which includes the following codes: mother and child engage in give and receive interaction, mother adapts to child signals, and interactions are fluent and rhythmic.
Fear regulation
The fear episode was microcoded in 0.01-s frames using a computerized system for four categories of behavior, each including a set of mutually exclusive codes: gaze, affect, vocalization, and regulatory behavior, consistent with previous research (Feldman et al., Reference Feldman, Granat, Pariente, Kanety, Kuint and Gilboa-Schechtman2009). Interrater reliability for 40 interactions averaged κ = 0.82 (range = 0.73–0.91). Three constructs were used as indices of temperamental emotional reactivity and regulation. Negative emotionality was the sum frequencies of the following codes: child expresses negative affect, child cries, and child emits fussy vocalizations. Comfort seeking was the sum frequencies of the child gazes to mother when seeing the scary object, child seeks maternal comfort, and proximity seeking. Withdrawal was the sum frequencies of gaze avert, child no talk, and child self-soothing behavior.
Results
The results are presented in two sections. In the first, we examine differences among the three groups (controls, exposed children with PTSD, and exposed-no-PTSD) on (a) child and maternal CT and sAA (b) maternal factors, including maternal PTSD and mother–child reciprocity, and (c) child temperament, including negative emotionality and regulatory strategies. To control for variability in child age, child age in months was entered in all analyses as a covariate. The second section presents two hierarchical regressions predicting child CT and sAA from maternal and child factors for the entire sample.
Differences among exposed children with PTSD, exposed children without PTSD, and nonexposed controls
Child and maternal biomarkers
CT
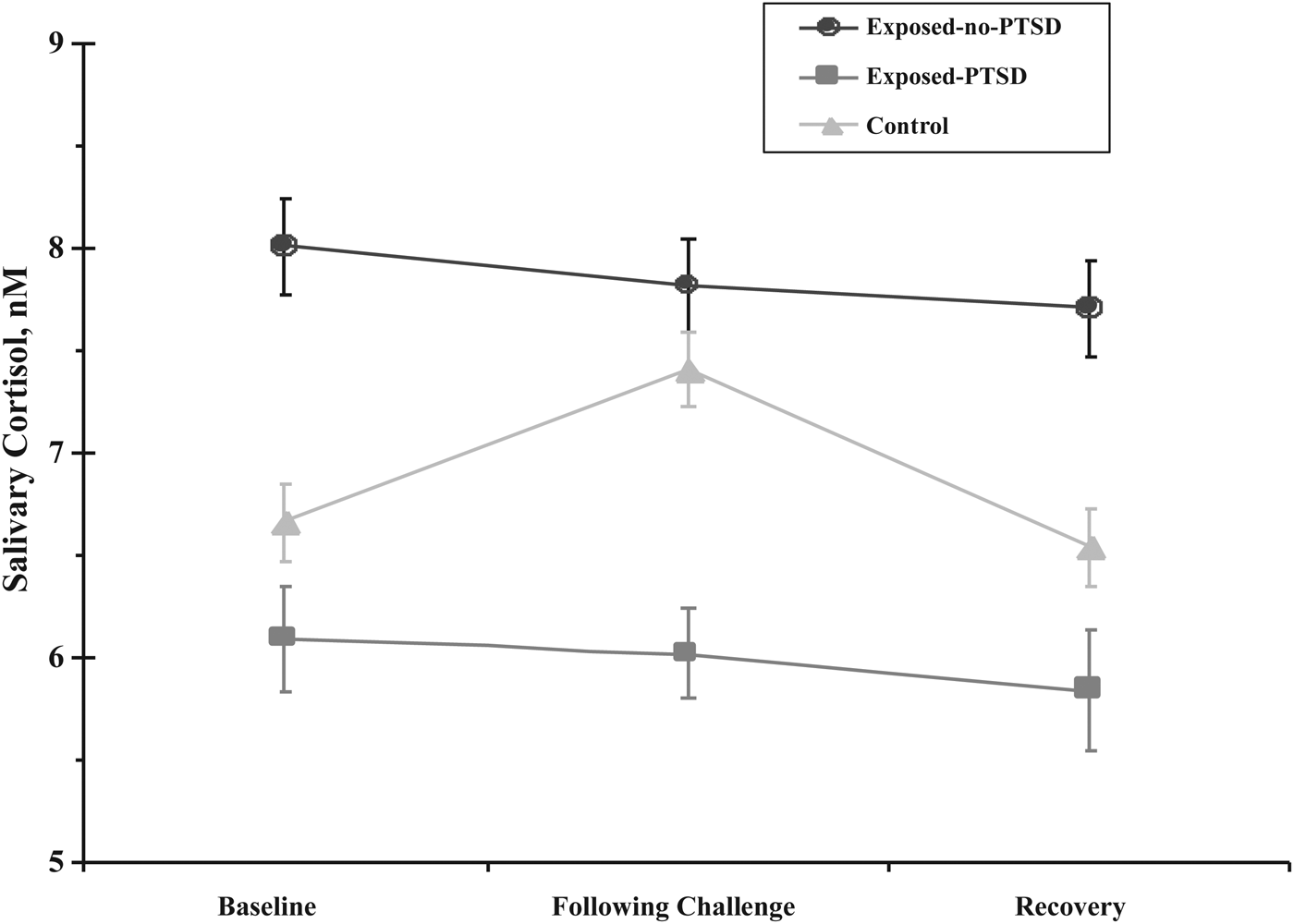
A repeated-measure multivariate analysis of covariance (MANCOVA) with group (controls, exposed children with PTSD, and exposed-no-PTSD) and child gender as the between-subject factors and child age as a covariate was computed for the three measurements of CT (baseline, following challenge, and recovery). The results revealed an overall effect for measurement, Wilks F (2, 201) = 7.18, p < .001, effect size (ES) = 0.07. This suggests that CT changed across assessments for all children. An overall between-subject effect was found for group, F (2, 201) = 16.58, p < .001, ES = 0.17. This finding demonstrates that CT levels were different among the three groups. Means are presented in Table 1 and show that group differences in CT were significant for all three assessments: baseline, following challenge, and recovery. Finally, an interaction of CT and group was found, Wilks F (4, 402) = 2.32, p = .042, ES = 0.03. This interaction indicates that the pattern of change across the three assessments differed as a function of group. Among the two exposed groups, CT did not change across assessments in more than 10%, thus showing little reactivity to stress. Among controls, however, CT showed the typical increase–decrease pattern (Figure 2). Stress reactivity scores were computed by subtracting the child's CT levels at baseline from the CT levels following challenge. The results indicated significantly greater stress reactivity among controls as compared to the exposed children, F (2, 197) = 6.37, p < .01, with no differences between the two exposed groups. Computing stress reactivity as regression coefficients (residuals resulting from regressing baseline CT on CT levels following challenge) showed similar results, with higher reactivity found for controls as compared to the exposed groups, F (2, 197) = 5.82, p < .01. It thus appears that although exposed children with PTSD showed consistently low CT and exposed children without PTSD showed consistently high CT compared to controls, in both groups there was little reactivity of the system in response to momentary stress or recovery of the system following induced stress.

Figure 2. Cortisol at baseline, following challenge, and recovery in war-exposed children diagnosed with posttraumatic stress disorder (PTSD), war-exposed children without PTSD, and controls.
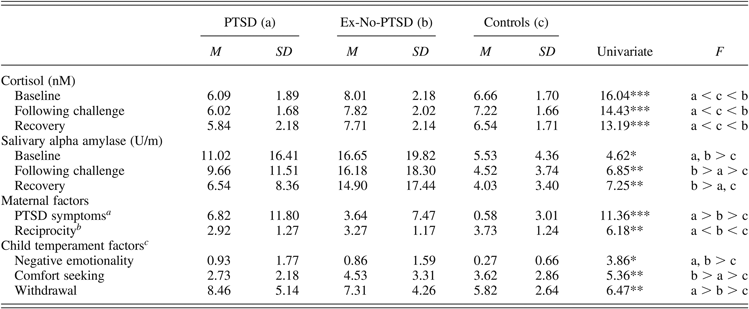
Table 1. Biomarkers, mothering, and temperament in war-exposed children with PTSD, war-exposed children without PTSD, and nonexposed controls

Note: PTSD, posttraumatic stress disorder; Ex-No-PTSD, war-exposed children without PTSD.
aAssessed by the Posttraumatic Diagnostic Scale.
bThe numbers represent the frequencies during the fear paradigm.
cThe numbers are coded on a scale of 1–5.
*p < .05. **p < .01. ***p < .001.
A similar repeated-measure MANCOVA was computed for maternal CT in the three assessments. Mother's CT similarly showed a main effect for group, F (2, 203) = 3.84, p = .04, ES = 0.04. However, unlike the findings for children, differences were found only between the exposed and the control mothers and not between mothers of children with and without PTSD. Mothers in the two exposed groups displayed higher baseline CT (M = 7.92, SD = 2.01) than did mothers of controls (M = 7.11, SD = 1.63).
sAA
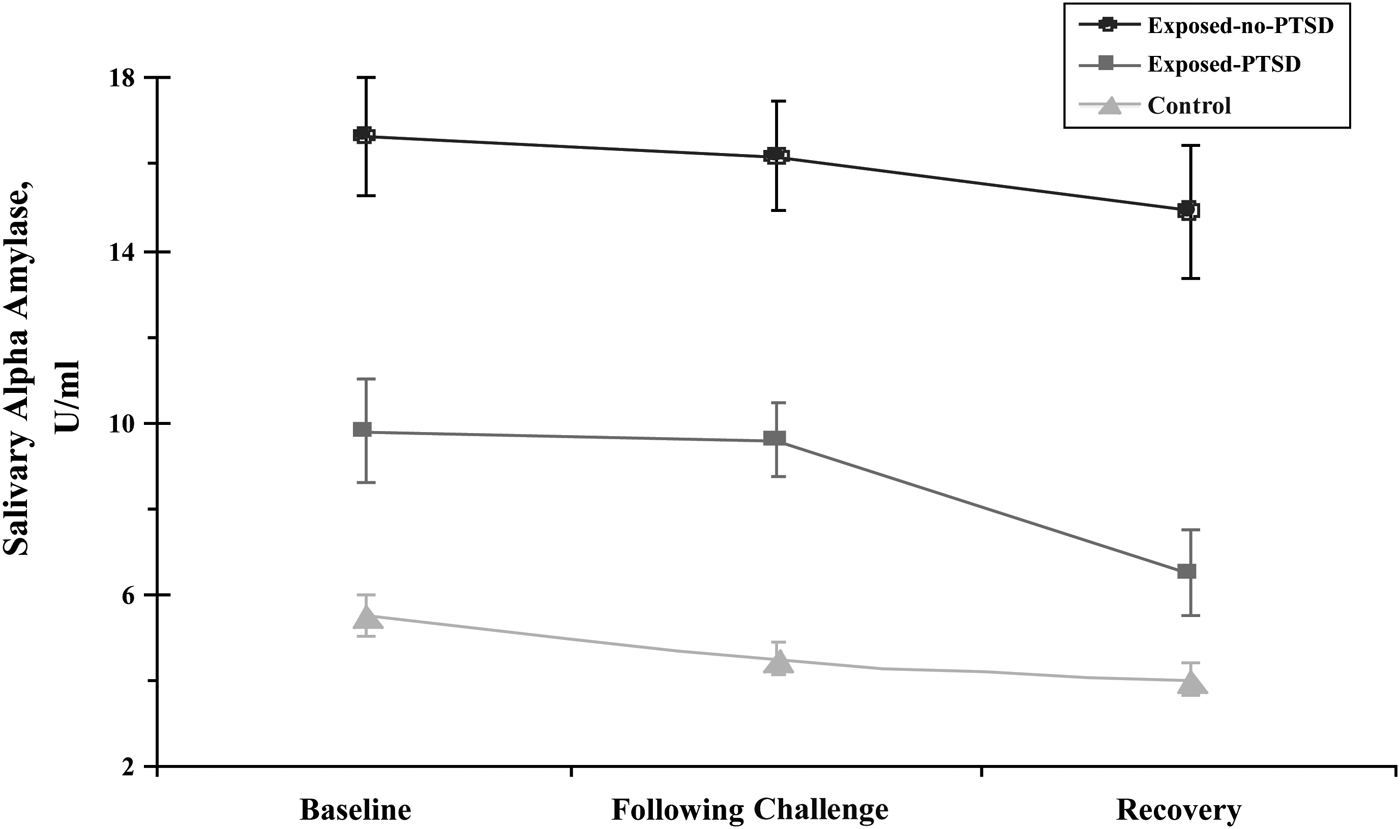
A repeated-measure MANCOVA with group (controls, exposed children with PTSD, and exposed-no-PTSD) and child gender as the between-subject factors and child age as the covariate showed no overall change across assessments, indicating that sAA did not change meaningfully across assessments. Possibly, because the reactivity patterns of sAA are quicker than those of CT, the timing of the collection did not allow for a clear reflection of sAA reactivity. A significant main effect was found for group, F (2, 201) = 4.33, p = .03, ES = 0.04. As seen in Table 1 and Figure 3, control children had the lowest sAA of all groups. Among exposed children, exposed-no-PTSD had significantly higher sAA in the following challenge and recovery assessments as compared to children with PTSD. No interaction effect was found.

Figure 3. Salivary alpha amylase at baseline, following challenge, and recovery in war-exposed children diagnosed with posttraumatic stress disorder (PTSD), war-exposed children without PTSD, and controls.
A similar MANCOVA assessing maternal sAA for the baseline, following challenge, and recovery assessments showed an overall main effect for group, F (2, 203) = 4.02, p = .03, ES = 0.04. Again, differences in maternal sAA were found only between the exposed and nonexposed mothers, and not between mothers of children with and without PTSD. Mothers in the two exposed groups had higher sAA at baseline (M = 17.36, SD = 14.22) than did controls (M = 5.89, SD = 4.41), F (1, 215) = 5.77, p = .004, at the following challenge assessment, exposed: M 16.55, SD = 15.45; controls: M = 6.16, SD = 5.41; F (1, 215) = 5.09, p = .008, and at recovery, exposed: M = 13.66, SD = 16.51; controls: M = 5.71, SD = 5.42; F (1, 215) = 3.39, p = .03.
Associations between maternal and child's biomarkers
In both CT and sAA, significant correlations emerged between maternal and child biomarkers at all assessments: Pearson r values for maternal and child CT were as follows: baseline, r = .35, following challenge, r = .37, and recovery, r = .28, ps < .01. Correlations between maternal and child sAA were as follows: baseline, r = .58, following challenge, r = .51, and recovery, r = .34, ps < .001. No significant correlations emerged between CT and sAA at any observation.
Maternal mental health and relational factors
Maternal PTSD symptoms
A univariate analysis of covariance with group and child gender as the between-subject factors and child age as the covariate assessed group differences in mothers’ PTSD symptoms. A main effect for group was found, F (1, 229) = 11.36, p < .001. As seen in Table 1, exposed mothers reported significantly more PTSD symptoms than did controls. However, mothers of children diagnosed with PTSD had more PTSD symptoms than did mothers of exposed children not diagnosed with the disorder.
Mother–child reciprocity
A similar analysis of covariance examined group differences in mother–child reciprocity. A main effect was found for group, F (1, 229) = 4.27, p < .01. Differences were found among the three groups, and means are presented in Table 1. As seen, control mothers and children showed higher reciprocity than did exposed dyads. However, mothers and children in the exposed-no-PTSD group showed higher levels of reciprocity as compared to mothers and children diagnosed with PTSD.
Child temperamental factors
MANCOVA assessing the three temperamental factors: negative emotionality, child comfort seeking, and child withdrawal, with group and child gender as between-subject factors and child age as covariate showed an overall effect for group, F (1, 225) = 5.18, p = .02, ES = 0.05. Exposed children exhibited more negative emotionality compared to controls. However, whereas exposed-no-PTSD children showed the highest levels of comfort seeking among all groups, children diagnosed with PTSD exhibited the highest levels of withdrawal (Table 1).
Predicting children's stress response
Prior to computing the regression models, correlations between predictor variables were examined with Pearson correlations. The results indicated that more maternal PTSD symptoms were associated with lower child CT (r = –.18, p < .01), lower mother–child reciprocity (r = –.15, p < .05), and higher child withdrawal (r = .16, p < .05). Higher reciprocity correlated with lower child CT (r = –.20, p < .01) and lower maternal CT (r = –.19, p < .01). In contrast, higher reciprocity correlated with greater child sAA (r = .21, p < .01) and greater maternal sAA (r = .19, p < .01). More child negative emotionality correlated with higher child CT (r = .22, p < .01), and more child withdrawal was related to lower child CT (r = –.19, p < .01). Higher child comfort seeking was related to higher child sAA (r = .27, p < .001) and higher maternal sAA (r = .15, p < .05).
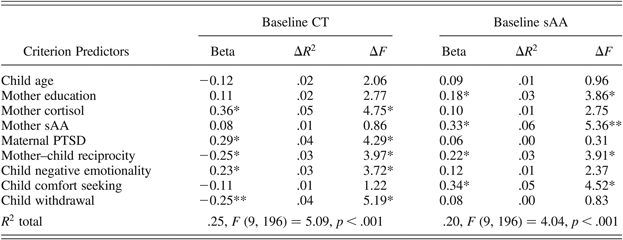
Finally, two hierarchical regression equations were computed predicting children's baseline CT and sAA. Variables were entered in nine blocks in a theoretically determined order. In the first two blocks, child age in months and mother education were entered to partial out variance due to these demographic factors. In the third and fourth blocks, maternal biomarkers (CT and sAA) were entered as predictors of the child's biomarkers. In the fifth and sixth blocks, maternal factors, including maternal PTSD symptoms and mother–child reciprocity, were entered. In the last three blocks, child temperamental factors, including negative emotionality, comfort seeking, and withdrawal, were entered. The results are presented in Table 2.
Table 2. Predicting CT and sAA

Note: CT, child cortisol; sAA, salivary alpha amylase; PTSD, posttraumatic stress disorder.
*p < .05. **p < .01.
The results of the first model indicated that child CT was independently related to maternal CT, higher maternal PTSD symptoms, lower mother–child reciprocity, greater child negative emotionality, and lower withdrawal. The results of the second model showed that maternal sAA, higher mother–child reciprocity, and more child comfort seeking were each independently linked with child sAA. Overall, these models suggest that these two biomarkers of the stress response in children are related to components of the child's ecology, including maternal biomarkers, PTSD symptoms, and reciprocal parenting, to children's emotional reactivity, and to the strategies children use to regulate fear.
Discussion
The results of the current study are the first to address neuroendocrine markers of stress in infants and young children exposed to political violence and to describe biological, relational, maternal mental health, and child temperamental factors that correlate with children's stress response under such conditions. Overall, the findings point to differences between war-exposed children and controls on all factors tested. War-exposed children showed a different physiological stress response, as indexed by both CT and sAA, and tended to react with more negative emotions to the elicitation of fear. Mothers living in war zones reported more PTSD symptoms, were less reciprocal during interactions, and their stress physiology showed higher levels of CT and sAA. However, whereas some war-exposed children were diagnosed with PTSD, others did not develop the disorder, and differences in child hormones, temperamental dispositions, and maternal well-being and relational behavior emerged between these two groups. In this context, it is important to note that because the study is cross-sectional and not longitudinal, no causal effects regarding the impact of prolonged war exposure on mother or child can be ascertained.
Exposed-no-PTSD children exhibited the highest levels of CT at each of the three assessments (baseline, following challenge, and recovery) as well as consistently high sAA in these same assessments. Such co-occurrence of high CT and high sAA has been linked with lower adaptation and higher externalizing and internalizing symptoms in studies that assessed the interactive effects of the two biomarkers (e.g., El-Sheikh et al., Reference El-Sheikh, Erath, Buckhalt, Granger and Mize2008). Nevertheless, “adaptation” is a context-specific construct that receives its meaning only in relation to the demands of specific ecologies. In the context of repeated missile attacks that leave only a few seconds to enter sheltered spaces and continue unpredictably over the course of several years, it is possible that a consistently aroused physiology may be a more adaptive by-product of the allostatic process. As seen, higher CT and higher sAA were also found in the exposed mothers, who needed to maintain constant vigilance for the safety of themselves and their child. In addition to highly aroused stress response, exposed-No-PTSD children employed more approach, comfort seeking emotion regulation strategies as compared to all other groups and had mothers who were more reciprocal as compared to mothers of children with PTSD. Possibly, within the limited resources available to mothers during extended periods of war, children who turn more naturally for help and seek the mother's assistance more actively would receive more involved parenting, which in turn may be associated with greater resilience. At the same time, it is also possible that when the mother is better able to provide reciprocal parenting, the child learns to use the mother for the regulation of distress. Within a cross-sectional design, it is not possible to determine whether the child's approach tactics shaped the mother's reciprocal style or vice versa.
In contrast to the exposed-no-PTSD group, children diagnosed with PTSD showed hypocortisolism, expressed in low baseline CT levels and blunted reactivity. These findings are consistent with the description of hypocortisolism in young children exposed to severe trauma by Gunnar and Vazquez (Reference Gunnar and Vazquez2001), with the findings that following the 9/11 attack children diagnosed with PTSD showed blunted CT reactivity (Pfeffer et al., Reference Pfeffer, Altemus, Heo and Jiang2007), and with the results that young children growing in highly stressful environments show hypocortisolism and that such low CT was considered a marker of allostatic load (Badanes et al., Reference Badanes, Watamura and Hankin2011). Although the mechanisms leading from early adversity to HPA hypoactivity are not fully understood, these authors suggest that hypocortisolism may have been the body's response to periods of very high HPA reactivity and that the allostatic process functioned to maintained equilibrium by hypoactivity, similar to the findings reported for animals following prolonged maternal deprivation (Hofer, Reference Hofer, Goldberg, Muir and Kerr1995). Both exposed groups showed minimal CT reactivity, perhaps suggesting that continuous war may be associated with reduced responsiveness to momentary environmental stressors possibly as a result of lengthy exposure to highly stressful challenges that upregulate the system's responsivity. Among the children with PTSD, there was an additional and significant decrease in physiological and behavioral responsivity, which was not observed in the exposed-no-PTSD group. Although these children displayed high negative emotionality during the fear paradigm, they used withdrawal tactics to regulate emotions, expressed in gaze aversion, termination of verbal dialogue, and self-soothing behavior. It is of interest that although these children exhibited at least two hyperarousal symptoms in order to receive a diagnosis of childhood PTSD, their stress physiology and regulatory tactics reflected hypoactivation and withdrawal. In parallel, mothers of children with PTSD reported the highest levels of PTSD symptoms and engaged in the lowest levels of reciprocal interactions as compared to all other groups. It is possible that these children did not learn to turn to their mothers for regulating distress or adapted the posttraumatic avoidant strategies of their mothers, but future longitudinal studies are required to examine this issue in depth.
The two types of physiological and behavioral response in children echo the writings of Spitz (Reference Spitz1946) and Bowlby (Reference Bowlby1969), similarly formulated on the basis of their experience with war-exposed children during World War II. According to these authors, during periods of prolonged war, when the attachment system is in jeopardy, children first react by increasing physiological and behavioral arousal (the “separation” protest response) in order to force the mother back into her regulatory role. When the mother's physical or emotional absence continues, the separation response, which is typically short-lived, turns into “loss,” characterized by hypoactivity of physiological and behavioral systems that support engagement with the world. The work of Hofer (Reference Hofer, Goldberg, Muir and Kerr1995) in animal models details a similar process. When the mother's physical proximity, which functions to regulate the pup's physiology in a system-specific fashion, is removed, pups first react by increasing arousal and agitation. As separation continues, this hyperactivity turns into hibernation-like disengagement. Although our study is cross-sectional and we do not suggest that the children diagnosed with PTSD experienced a period of high arousal prior to the period of hyporesponsivity, these perspectives provide a conceptual framework to the findings that children's response to prolonged periods of war may be observed in either hyper- or hypoactivation of the child's physiological and behavioral systems.
The results of the regression models indicate that children's baseline CT levels were independently related to maternal baseline CT, lower maternal reciprocity, greater maternal psychopathology, higher child negative emotionality, and lower child withdrawal. The concordance between maternal and child CT has been previously reported in infants and young children (Feldman et al., Reference Feldman, Singer and Zagoory2010; Ruttle et al., Reference Ruttle, Serbin, Stack, Schwartzman and Shirtcliff2011; Sethre-Hofstad et al., Reference Sethre-Hofstad, Stansbury and Rice2002), and such “endocrine fit” is thought to stem from genetic, epigenetic, and social interactive sources. The findings relating child CT to maternal psychopathology, lower interactive sensitivity, and greater negative emotionality is consistent with much previous research suggesting that components of the child's ecology are related to the physiological stress response (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001; De Bellis et al., Reference De Bellis, Baum, Birmaher, Keshavan, Eccard and Boring1999; Haley & Stansbury, Reference Haley and Stansbury2003; Tarullo & Gunnar, Reference Tarullo and Gunnar2006). Consistent with Cicchetti and Rogosch (Reference Cicchetti and Rogosch2009), the findings point to the independent associations between the mother and child's biology, relationship, and temperament with children's stress reactivity under conditions of chronic stress. Future research is thus needed to fully understand the neurobiology of stress in the context of political violence and assess multiple sources of influence on the development of psychopathology using longitudinal and experimental designs.
The current findings are the first to assess sAA in war-exposed young children and their mothers and add to the rapidly growing literature on this biomarker and its correlates. Most research on sAA considers it as an index of SAM activity, although this assumption is still controversial and requires further validation. Similar to CT, the data show that maternal and child sAA showed concordance at each assessment, findings consistent with previous research on sAA in adolescence (Gordis et al., Reference Gordis, Margolin, Spies, Susman and Granger2010). Child baseline sAA was related to mother–child reciprocity and the use of comfort-seeking strategies. In addition, maternal sAA similarly correlated with reciprocity and child comfort seeking. Research has pointed to the associations between sAA with more optimal mother–child relationships and with positive emotionality and approach behavior in young children. For instance, Frigerio et al. (Reference Frigerio, Ceppi, Rusconi, Giorda, Raggi and Fearon2009) found that secure attachment, combined with genetic polymorphisms on the serotonin transporter linked polymorphic region gene and gamma-aminobutyric acid receptor subunit alpha-6 gene, predicted sAA in 12- to 18-month-olds; Fortunato, Dribin, Granger, and Buss (Reference Fortunato, Dribin, Granger and Buss2008) showed links between sAA and positive affect and approach behavior in toddlers; and Spinrad et al. (Reference Spinrad, Eisenberg, Granger, Eggum, Sallquist and Haugen2009) reported correlations between sAA and lower dispositional anger in preschoolers. The findings show correlations between sAA and child approach strategies and more positive social interactions, but much further research on sAA is required to assess its biological underpinnings and predictive utility.
Limitations
The limitations of the study are important to consider in the interpretation of the findings. Due to the cross-sectional nature of the study, it is important to emphasize that no causal relationships can be inferred and longitudinal follow-up is required to address predictive relations between components of the young child's ecology and developmental outcome under conditions of political violence. The timing of the saliva collection was set to the reactivity of the HPA axis and did not accommodate the response patterns of sAA, and this should be considered in the interpretation of the sAA results. Children were collected across a wide age range (1.5–5 years), and although age was controlled in the analyses, children were at different developmental stages and a more narrow focus on specific developmental periods would be an important next step. Similarly, children's stress response is shaped by multiple factors, including living conditions, number of children, and availability of caregiving; although we matched war-exposed and control children on multiple demographic factors, some unknown or unmeasured variable could have influenced the results. Taken into consideration life-span and developmental psychopathology perspectives (Chichetti & Rogosch, Reference Cicchetti and Rogosch2009; Juster et al., Reference Juster, Bizik, Picard, Arsenault-Lapierre, Sindi and Trepanier2011), it would be important to follow both groups of exposed children and determine whether the exposed-no-PTSD group is more resilient across development. The lack of father data is an important drawback that precludes a fuller investigation of the child's rearing ecology and functioning during periods of war. In addition, generalizability of the findings to other regions of political violence should be examined, in light of the facts that there were very few casualties across the period of exposure, no father absence or direct exposure to fighting, no real shortage of food or basic supplies, and fighting was limited to a small region of the country. Finally, a more in-depth assessment of the neurobiology of stress, including stress genes and stress-related neural networks, would have provided a more comprehensive picture of the ways in which war exposure shapes the physiological stress response in young children.
Conclusion
Future research is required to assess genetic, brain, and behavioral markers of risk and resilience among children exposed to war-related trauma. Although millions of the world's children are growing up amid armed conflict, research detailing its impact on infants and young children is critically lacking. Such research should chart context-specific pathways of risk and resilience in order to build theoretical models and culturally sensitive interventions that can target the effects of war on children's physiological stress and psychological distress.