Introduction
Huntington’s disease (HD) is a devastating, progressive, familial, neurodegenerative disease with dominant autosomal inheritance. It has a major impact on the patients’ quality of life (QOL) and daily function. Main clinical manifestations include involuntary movements, psychiatric features, and progressive cognitive impairment.Reference Ross and Tabrizi 1 Many HD patients experience dysphagia,Reference Ross and Tabrizi 1 , Reference Heemskerk and Roos 2 which seems to worsen with disease progression and may cause life-threatening complications, such as malnutrition, dehydration, and aspiration pneumonia.Reference de Tommaso, Nuzzi and Dellomonaco 3
The literature on specific swallowing disturbances of HD patients and their possible correlation to other manifestations of HD is limited. According to a recent study by de Tommaso et al.,Reference de Tommaso, Nuzzi and Dellomonaco 3 dysphagia may be related to a combination of motor deficits and behavioral changes which may affect food intake and increase feeding dependency. Those authors observed that the majority of the HD patients experienced dysphagia symptoms and oral motor impairment, specifically, tongue protrusion, while cognitive decline did not seem to correlate with dysphagia severity. According to our clinical experience, the cognitive decline of HD patients interferes with their ability to follow swallowing therapy recommendations and to use compensatory maneuvers to improve their dysphagia.
To address the question of whether there are specific characteristics of dysphagia in HD patients, we assessed the oropharyngeal and pharyngeal stages of swallowing objectively by means of the bed side swallowing evaluation (BSE) and the fiberoptic endoscopic evaluation of swallowing (FEES). We also used subjective patients’ self-report questionnaires to acquire a detailed characterization of the swallowing dysfunction of HD patients. We specifically looked for correlations between swallowing dysfunction as apparent in the patients’ FEES studies, as reported by the Swallowing Related Quality Of Life (SWAL-QOL) questionnaire and the Pleasure of Eating–Visual Analog Scale (POE-VAS) measurements.
Methods
Participants
Fourteen genetically confirmed HD patients who were followed at the Movement Disorders Unit at Tel Aviv Sourasky Medical Center were recruited for this retrospective study. To be included, patients needed to be able to fill in the questionnaires and be willing to tolerate the FEES protocol. Exclusion criteria included a history of another neurological or medical disorder that interferes with swallowing and inability to perform the required tasks. The study was approved by the institutional Helsinki Committee.
Patient assessment
All patients were assessed by a speech and language pathologist (SLP) who performed a BSE including oral motor examination. All patients were examined by an otolaryngologist specializing in swallowing disturbances and underwent a flexible fiberoptic examination of the nasal cavity, pharynx, and larynx, and a FEES study. All patients filled in 2 self-report questionnaires: SDQ and SWAL-QOL and responded to the POE scale.Reference Manor, Giladi, Cohen, Fliss and Cohen 6
The SDQ consists of 5 questions related to the oral phase of swallowing and 10 questions related to the pharyngeal phase of swallowing. A score of ≥11 indicates that the patient should be referred to a speech and language pathologist (SLP) for a swallowing evaluation.Reference Manor, Giladi, Cohen, Fliss and Cohen 6 The SWAL-QOL questionnaire is a 44-item tool that assesses 10 QOL domains: eating desire (2 items), fatigue (3 items), food selection (2 items), communication skills (2 items), fear of eating (4 items), burden (2 items), social functioning (5 items), sleep (2 items), mental health (5 items), and symptom frequency. All the responses are rated on a scale of 0–4. The lower the SWAL-QOL scores, the better the QOL. The POE-VAS was designed to measure the patient’s degree of enjoyment from food (1 = “no enjoyment” and 10 = “much enjoyment”). The VAS score is determined by measuring in millimeters from the left end of the line to the point that the patient marks.Reference Nasreddine 9
All patients were evaluated by a neurologist specializing in movement disorders who graded their clinical performance and cognition according to the Unified Huntington’s Disease Rating Scale (UHDRS)Reference Fletcher 7 and the Montreal Cognitive Assessment (MoCA),Reference Kremer 8 respectively.
Assessment tools
Bedside swallowing evaluation (BSE)
The BSE was performed by the SLP. It includes an oromotor examination and the provision of a score of normal/distorted functions. Volitional cough strength and the ability to initiate volitional swallow were evaluated on a scale of 0–2 (0 = normal, 1 = slightly distorted, 2 = significantly distorted). In addition, the patients were asked to perform a diadochokinetic (DDK) task, an assessment tool used by SLPs that measures how quickly an individual can accurately produce a series of rapid, alternating soundsReference Nasreddine 9 (production of 10 “PATAKA” as fast as possible), and the performance time was measured. For adult Hebrew speakers, the mean oral-DDK rate performance was found to be 6.4 syllables/seconds±0.8 with a mean performance time of 15.6 seconds for 10 repeats.Reference Icht and Ben-David 10
FEES study
The FEES was performed by an otolaryngologist and a SLP specializing in swallowing disorders. The patients were instructed to swallow 3 food consistencies (pureed, solid, and liquid) under visualization. FEES scoring includes 5 swallowing function parameters: bolus flow time, bolus location when the swallowing reflex is triggered, residue location of the 3 food consistencies, laryngeal penetration, and aspiration before/after swallowing (Table 1). Each parameter is scored on a scale of 0–3 (0 = normal, 3 = abnormal). The measurement of bolus flow time was evaluated on a scale of 0–3 (0 = 0–1 sec, 1 = 2–4 sec, 2 = 5–7 sec, and 3 = >8 sec). Each function parameter was tested for the 3 food consistencies, and the total score of each swallowing function measurement was the sum of the 3 scores. All 5 swallowing function parameters were measured and scored while the patients swallowed 3 teaspoons (5 cc) of applesauce, 3 bites of a biscuit, a sip of milk from a cup, and, if no aspiration is noted, 3 more sips of milk from a cup. The applesauce and milk were tinted by a blue food coloring powder. A swallowing disorder is diagnosed when one of the swallowing function measurements was scored more than 0.
Table 1 The fiberoptic endoscopic evaluation of swallowing scoring parameters for pureed, solid, and liquid bolusesReference Manor, Mootanah, Freud, Giladi and Cohen 5
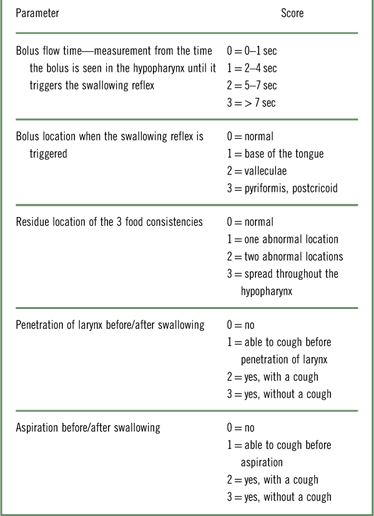
The level of difficulty in performing the FEES is graded 0–3 (0 = no difficulty, 3 = very difficult) by the otolaryngologist according to the patient’s level of cooperation, ability to maintain adequate positioning, and ability to perform the swallowing instructions.
Besides the swallowing evaluation, patients responded to the SDQ,Reference Manor, Giladi, Cohen, Fliss and Cohen 6 the SWAL-QOL questionnaire,Reference McHorney, Robbins and Lomax 4 and the POE-VAS that was designed to measure the patient’s degree of enjoyment from food (1 = “no enjoyment” and 10 = “much enjoyment”).Reference Wallerstein 11 .
Statistical analysis
The statistical analysis was performed using SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY). The Spearman correlation test was used, and P values < 0.05 were considered significant.
Results
Fourteen HD patients participated in the study (Table 2). The oral motor assessment results are presented in Table 4. The oral phase was characterized by impaired tongue, lips, and jaw movements in 9 patients and they were involuntary, impulsive, inconsistent, and limited in range. There was a reduced rate in the DDK task (mean score of 7.22±3.9 seconds, normal range (10/ptk/in <5 seconds).
Table 2 Clinical characteristics of the study Huntington’s disease patients (n = 14)

Four patients tolerated the endoscope during the FEES and performed the swallowing tasks with no difficulty, 8 patients had mild difficultly, and 2 had moderate difficulty. The FEES results demonstrated an adequate oral transit time for all food consistencies in 8 patients and adequate chewing in 10 patients. The pharyngeal phase was characterized by a weak volitional cough and abnormal volitional swallow. The main pharyngeal phase dysfunction detected by FEES (Figure 1) included delay in initiation of the swallowing reflex with the initiation of swallowing only when the food bolus reached the vallecula in 7 patients, solid food residues in different pharyngeal locations in 8 patients, pre-swallowing spillage in 12 patients, and post-swallowing spillage in 12 patients. Liquid and/or solid laryngeal penetration and/or aspiration were observed in only 2 patients. A significant positive correlation was found between the volitional cough and the ability to initiate volitional swallow scores (P < .01) and between volitional cough and disease duration (P < .05); negative correlation was observed between the volitional cough and the cognitive status scores (P < .05).
The mean total SDQ score was 13.21±7.5, with a mean oral phase score of 3.86±3.34 and a mean pharyngeal phase score of 8.64±4.74. A score of ≥11 is an indicator of a marked swallowing disturbance, and it was noted in 10 patients: specifically, 12 patients reported coughing while drinking liquid and 9 reported difficulty in chewing solids. One patient reported a history of aspiration pneumonia.
The participants’ SWAL-QOL was assessed according to 10 domains (Table 3). The SWAL-QOL fear of eating score (8.07±4.3) correlated significantly with the SWAL-QOL mental health (2.53±1.19) and SWAL-QOL social functioning scores (6.53±4.62, P < .05). The mean POE-VAS score was 8.71±1.54.
Table 4 Means and standard deviations of the swallow quality of life questionnaire parameters

Significant correlations were found between the SWAL-QOL fear of eating score and the SDQ oral score (3.86±3.34, P < .05), pharyngeal score (8.64±4.74, P < .05), and total score (13.21±7.5, P < .05). The SWAL-QOL fear of eating score (8.07±4.3) also significantly correlated with the FEES parameters of pureed and solid food bolus flow time (8.07±4.3, P < 0.05).
Table 3 Oral motor assessment results (n = 13)

There were significant correlations between the total UHDRS score (36.75±17.52) and the volitional cough score (0.58±0.67, P < .05); the total UHDRS score (36.75±17.25) and the SWAL-QOL disease burden score (10.46±6.17, P < .05); and the UHDRS mental health score (2.53±1.19) and both the SWAL-QOL social functioning score (6.53±4.62) and the SWAL-QOL fear of eating score (8.07±4, P < .05).
Discussion
This study aimed to characterize the swallowing disturbances of HD patients. Dysphagia severity was assessed both objectively (BSE, FEES) and subjectively (SWAL-QOL, POE-VAS, SDQ). Swallowing disturbances were present in both oral and pharyngeal phases of swallowing and were characterized by discoordination between the swallowing functions during the various phases of swallowing (eg, impaired and involuntary tongue, lips, and jaw movements, a weak volitional cough, abnormal volitional swallow, delay in initiation of the swallowing reflex that occurred only when the food bolus reached the vallecular, solid food residues, and pre- and post-swallowing spillage), as demonstrated by FEES. This swallowing pattern may be defined as oropharyngeal dyssynergia. A similar distortion in the swallowing pattern of HD patients was also described by Kagel and Leopold,Reference Kagel and Leopold 12 who assessed their patients by videofluoroscopy. Those authors observed reductions in speed and range of movements of the tongue and a lack of coordination of the lips, tongue, mandible, hyoid, and larynx, as well as unpredictable forceful inspiratory phonation during swallowing. In our study, 5 of 14 patients had a weak volitional cough and 5 of 14 abnormal volitional swallow, which could be related to a lack of coordination of the hyoid and larynx and an unstable inspiratory pattern. We hypothesize that this dyssynergia may be the result of 2 factors: (1) a disturbance of central swallowing controlReference Lee, Lee and Kim 13 and (2) motor dysfunction as manifested by slow performance of the diadochokinetic task, and reduction in oral motor functionReference Atkinson and McHanwell 14 and buccolingual chorea, all resulting in involuntary food transfer.
The importance of motor control and of the efficiency of the mouth–tongue aperture in determining dysphagia had been reported by Van Lieshout et al,Reference Van Lieshout, Steele and Lang 15 who noted a correlation between dysphagia and dysarthria.
The swallowing characteristics in HD as demonstrated by the FEES study included delay in the initiation of the swallowing reflex with initiation of the reflex only when the food bolus reached the vallecula, solid food residues, pre- and post-swallowing spillage to the pharynx, and rarely laryngeal penetration and/or aspiration These findings are in accordance with a recent study by Alves et al,Reference Alves, Cola, Santos, Motonaga and Silva 16 who reported posterior oral spillage of liquids and nectar, small amounts of pharyngeal residues, and no laryngeal penetration or aspiration in 2 HD patients undergoing FEES studies. Food residues in HD patients were also noted in other studies that utilized videofluoroscopy to evaluate dysphagia.Reference Kagel and Leopold 12 , Reference Lee, Lee and Kim 13 , Reference Hamakawa, Koda and Umeno 17 We suspect that the reduced coordination of the muscles involved in the swallowing process might be the cause for the food residues and spillage.
The FEES is a sensitive and reliable assessment tool for identifying and detecting silent dysphagia findings, including aspiration, laryngeal penetration, food residue, and pharyngeal spillage.Reference Langmore 18 It is considered a more conservative assessment tool than videofluoroscopy, when there are concerns about aspiration of barium during the videofluoroscopy procedure. Furthermore, FEES has been used previously to describe the dysphagic characteristics of different populations.Reference Leder, Cohn and Moller 19 – Reference Hartley, Harnick, Miller and Willging 21 To the best of our knowledge, our study is the first to describe the dysphagia characteristics in a cohort of HD patients as revealed by the FEES. We found that despite their cognitive and motor dysfunctions, most of the study patients tolerated the insertion of the flexible fiberoptic laryngoscope, underwent the FEES with only mild difficulty, and were able to complete the study protocol. The FEES enabled us to identify dysphagia characteristics that are consistent with findings of other studies that utilized the less commonly available videofluoroscopy to assess dysphagia in HD patientsReference Kagel and Leopold 12 , Reference Lee, Lee and Kim 13 , Reference Hamakawa, Koda and Umeno 17 without subjecting our patients to X-ray radiation.
Symptoms of dysphagia might affect the QOL and spoil the social opportunities and pleasures of mealtimes, so a patient with dysphagia can become isolated, feel excluded by others, and be anxious and distressed at mealtimes.Reference Stringer22 In the current study, swallowing QOL was assessed using the SWAL-QOL questionnaire and POE-VAS questionnaires. The fear of eating measurement, as reported by the patients in the SWAL-QOL questionnaire, significantly correlated with SDQ scores and with bolus flow time of pureed and solid food in the FEES studies. Thus, our findings support the position that dysphagia negatively effects QOL of HD patients. Due to the decrease in POE, patients might experience weight loss, which is a ubiquitous symptom of HD and one that signifies advanced disease. Weight loss may be related to dysphagia and other symptoms, such as fatigue, depression, anxiety, and chorea movements, which require high energy.Reference Rosenblatt, Ranen, Nance and Paulsen23 Metabolism is also affected in HD, and patients with higher Cytosine-Adenosine-Guanine (CAG) trinucleotide repeats tend to lose weight faster due to an increased metabolism compared to those with fewer repeats.Reference Aziz, van der Marck and Pijl24
Our results demonstrated a significant positive correlation between the volitional cough and the ability to initiate volitional swallow. Furthermore, the decrease in quality of the volitional cough also correlated negatively with the level of cognition. Both of these findings make a vital contribution to the design of swallowing therapy for HD patients by recommending that swallowing therapy approaches and swallowing compensatory techniques should be adjusted to the patient’s cognitive level. Given that progressive decline in cognitive level is expected with HD progression,Reference Van Liew, Gluhm, Goldstein, Cronan and Corey-Bloom 25 – Reference Baker and Blumlein 27 frequent visits to the SLP may be indicated.
We are aware of the limitations of our study. The results are based on a small cohort of HD patients who were cognitively impaired, and the patients’ responses to the questionnaires might be affected accordingly. However, the objective FEES swallowing assessment confirmed many of the dysphagia characteristics elicited by the questionnaires. In addition, all of the study patients had dysphagia symptoms; we did not test HD patients who did not exhibit dysphagia.
Conclusion
In conclusion, based on our comprehensive assessment of dysphagia patterns among HD patients, we propose that the main manifestation of dysphagia in HD is oropharyngeal dyssynergia, as demonstrated by FEES. We found that the severity of the decline in both cognition and motor function together with the severity of the disease in general correlated with the severity of dysphagia. We believe that our findings support a proactive approach to the swallowing problems in HD, with early detection and close follow-up of deterioration of the swallowing mechanism in order to cope with and perhaps even reduce complications. In addition, we hope that our findings will assist SLPs in developing the optimal therapeutic approach to HD patients with dysphagia. Management of swallowing disturbances in HD patients is challenging and requires the consideration of such a multifaceted condition.






