Introduction
Obesity and mood disorders are highly prevalent conditions, affecting an estimated 500 million and 350 million individuals worldwide, respectively. 1 , Reference Marcus, Taghi Yasamy, van van Ommeren, Chisholm and Saxena 2 Furthermore, they are highly comorbid, as mood disorders are associated with a 1.5 to 2.0 times greater risk of obesity, with similarly increased risk of mood disorders in obese individuals. 3-5 Obesity and depression are associated with adverse long-term outcomes in both psychosocial and physical health, such as low self-esteem, decreased quality of life, increased risk for cardiovascular events, and early mortality. 6-9 In addition, there is a high economic burden of disease associated with both obesity and depression due to increased use of services and loss of productivity.Reference Finkelstein, Ruhm and Kosa 10 , Reference Tremmel, Gerdtham, Nilsson and Saha 11
Despite the common co-occurrence of obesity and depression, it remains unclear whether obese individuals are more (or less) likely to improve with monoamine-based antidepressant therapy relative to nonobese individuals. For example, Papakostas and colleaguesReference Papakostas, Petersen and Iosifescu 5 found that relative body weight, and not BMI, predicted nonresponse to an 8-week trial of fluoxetine. A separate study reported that higher baseline body weight and body mass index (BMI) predicted less favorable improvement in functional and symptom measures in adults with major depressive disorder (MDD) after a 6-week trial of fluoxetine.Reference Lin, Chen, Wong and McIntyre 12 Individuals with higher BMI may also exhibit significantly slower time to response, as suggested by a preliminary study showing slower time to response (but not attenuated response) in adults with MDD who were categorically obese.Reference Kloiber, Ising and Reppermund 13
Conversely, Jha and colleaguesReference Jha, Wakhlu, Dronamraju, Minhajuddin, Greer and Trivedi 14 found that individuals with class II obesity (ie, BMI ≥ 35 kg/m2) were more likely to experience remission of depressive symptoms with combination bupropion-selective serotonin reuptake inhibitor (SSRI) therapy when compared to overweight or normal-weight individuals. It was also observed in this study that overweight/normal-weight individuals preferentially responded to SSRI monotherapy and venlafaxine-mirtazapine combination therapy when compared to individuals with class II obesity.Reference Jha, Wakhlu, Dronamraju, Minhajuddin, Greer and Trivedi 14 The observation that adjunctive anti-inflammatory treatments may be effective in adults with MDD and obesity provides mechanistic hints that inflammatory processes associated with obesity may be moderating antidepressant response.Reference Papakostas, Shelton and Zajecka 15
While BMI is a poor predictor of response to typical monoamine-based antidepressants, converging lines of evidence suggest that BMI may be a positive predictor of response to ketamine, an N-methyl-D-aspartate receptor antagonist. Ketamine differs from conventional antidepressants insofar as it is hypothesized to primarily target the glutamate system as opposed to the monoaminergic system. Intravenous (IV) ketamine has been shown to have rapid antidepressant effects in double-blind placebo-controlled trials and in real-world clinical settings in patients with depression. 16-20 Unlike monoamine-based antidepressants, individuals with higher BMI experience greater depressive symptom relief from ketamine treatment than individuals with lower BMIs. 21-23
For example, an analysis of four separate studies by Niciu and colleaguesReference Niciu, Luckenbaugh and Ionescu 22 found that at both 230 minutes (n = 108; 50% male) and 1 day (n = 82) following a single 0.5 mg/kg IV dose of ketamine, participants with treatment-resistant depression (TRD; MDD or bipolar disorder [BD]) with a higher BMI experienced a significantly greater reduction in depressive symptoms (mean BMI = 30.5 kg/m2, SD = 6.9). Notwithstanding, BMI was not a predictor of sustained ketamine response at 7 days after infusion (n = 71). Machado-Vieira and colleaguesReference Machado-Vieira, Gold and Luckenbaugh 21 observed an inverse relationship between baseline adiponectin levels and rapid response to a single ketamine infusion (N = 80) in individuals with MDD (n = 49) or BD (n = 31) with an average BMI of 29.2 kg/m2 (SD = 5.8). Adiponectin is an anti-inflammatory cytokine secreted by the adipose tissue, and is typically low in both individuals with high BMI and with depression.Reference Cizza, Nguyen and Eskandari 24 Furthermore, participants who received IV ketamine experienced a significant decrease in resistin, a pro-inflammatory adipokine associated with both high BMI and depression.Reference Machado-Vieira, Gold and Luckenbaugh 21 , Reference Ahima and Lazar 25
Mechanistically, it has been hypothesized that ketamine’s efficacy in adults with either obesity and/or laboratory evidence of pro-inflammatory balance is a consequence of the interplay between glutamate and central inflammatory processes.Reference Haroon, Chen and Li 26 Reduction in suicidal ideation (SI) with a single dose of IV ketamine treatment (N = 128) has not been found to be associated with BMI.Reference Ballard, Yarrington and Farmer 27 A synthesis of data from two open-label repeat-dose 0.5 mg/kg IV ketamine studies (N = 22) found that patients with a higher BMI (M = 30.7 kg/m2, SD = 6.6) were significantly more likely to experience remission of depressive symptoms, regardless of whether BMI was analyzed as a continuous measure or as a categorical construct.Reference Singh, Bobo and Rasmussen 28 However, the association between categorical BMI and response to IV ketamine was only present when categorical obesity was split into obese I and obese II categories, and not when obesity categories were not differentiated. In addition, when controlling for baseline depression symptom severity, percent change in depressive symptoms following treatment was not significantly associated with log (BMI). Most recently, in a post hoc analysis of a randomized, double-blind, placebo-controlled trial (N = 80) of single-dose ketamine (0.1, 0.2, 0.5, or 1.0 mg/kg) or placebo, both categorical and continuous BMI were positively associated with greater antidepressant response. However, only 12 study participants were obese, limiting the reliability of these findings.Reference Freeman, Hock and Papakostas 29 Conversely, a recent studyReference Dale, Bryant and Thompson 30 reported that BMI as a categorical measure did not significantly predict response to repeat-dose IV ketamine (3-6 infusions, every other day, followed by maintenance infusions) at 0.5 mg/kg (N = 150). However, the presence of metabolic syndrome negatively predicted remission probability of depressive symptoms.Reference Dale, Bryant and Thompson 30 Notably, Dale and colleaguesReference Dale, Bryant and Thompson 30 did not differentiate between obesity I and obesity II categories.
The extant literature has conventionally defined treatment response as a reduction in overall depressive symptomatology or SI, rather than improvement in functioning, anxiety, or anhedonic severity. Herein, we sought to determine whether categorical BMI moderates response to repeat-dose IV ketamine in a large, well-characterized sample of adults with MDD or BD receiving care at a community-based treatment center. We defined response not only as improvement in depressive symptom severity, but we also separately evaluated the association between BMI and change scores of SI, anxiety, anhedonia, and general functioning.
Method
This study was registered at clinicaltrials.gov under the identifier NCT04209296 and was approved by a community institutional review board (IRB#00000971). The methods of this study as well as safety and tolerability of IV ketamine have been previously described in detail.Reference Rodrigues, McIntyre and Lipsitz 31 , Reference McIntyre, Rodrigues and Lee 32
In brief, adults (18+) with TRD received four infusions of IV ketamine over 1 to 2 weeks at a community-based outpatient clinic, the Canadian Rapid Treatment Center of Excellence (CRTCE). In the initial two infusions, all patients received a dose of 0.5 mg/kg of ketamine hydrochloride diluted in 0.9% saline solution. The total dose of ketamine was determined at each infusion based on the patient’s actual body weight. If a patient had a BMI greater than 35 kg/m2, ideal body weight (IBW) was used to calculate their dose (males: IBW = 50 + 2.3 kg/inch over 5 feet; females: IBW = 45.5 + 2.3 kg/inch over 5 feet). Following the second ketamine infusion, patients who experienced insufficient response (ie, ≤20% reduction in total score on the Quick Inventory for Depressive Symptomatology-Self-Report 16 [QIDS-SR16]Reference Rush, Trivedi and Ibrahim 33) were eligible for a dose optimization to 0.75 mg/kg for subsequent infusions. Approximately 2 days after each infusion and 1 week after the fourth infusion, participants completed the QIDS-SR16. At baseline, after infusion 3, and at the post-initiation treatment visit, patients completed the General Anxiety Disorder 7-Item Scale (GAD-7),Reference Spitzer, Kroenke, Williams and Löwe 34 the Snaith–Hamilton Pleasure Scale (SHAPS),Reference Snaith, Hamilton, Morley, Humayan, Hargreaves and Trigwell 35 and the Sheehan Disability Scale (SDS).Reference Sheehan, Harnett-Sheehan and Raj 36
Data analysis
Data were retrospectively analyzed using IBM SPSS Version 23 for Mac (SPSS, Inc., Chicago, Illinois) and GraphPad Prism 8.0. The primary aim of this analysis was to identify if there were differences in response to IV ketamine treatment across four infusions between individuals with obesity and without obesity. To accommodate for missing data, we fit the data to a mixed model using a compound symmetry covariance matrix and restricted maximum likelihood with the alpha level set to 0.05. Follow-up pairwise comparisons were conducted for significant main effects and interactions, and Bonferroni corrections were applied to adjust for multiple comparisons.
Data were stratified by BMI category as follows: normal (18.5-24.9 kg/m2; n = 72), overweight, (25.0-29.9 kg/m2; n = 76), obese I (30.0-24.9 kg/m2; n = 47), or obese II (≥25.0 kg/m2; n = 35). Participants who were categorically underweight were removed from the analyses, since the sample size was small (n = 11).
Seven repeated measures hierarchical models were conducted to evaluate changes in each of the outcome measures across infusions. The model terms were group, infusion, and a group by infusion interaction. The dependent variables were total QIDS-SR16 , QIDS-SR16 SI, GAD-7, SDS work/social/family, and SHAPS scores. Sex, age, level of treatment resistance (ie, number of past antidepressant trials), and baseline depressive symptom severity were included as covariates in all models. Baseline severity of the symptom of interest was also included in each model, respectively (eg, baseline anxiety severity was included in the anxiety model).
Categorical partial response, response, remission, and clinically significant improvement were calculated for each group. Partial response was operationalized as a 20% to 50% decrease in depressive symptoms (QIDS-SR16 score) from baseline compared to post-infusion 4, response was operationalized as a 50% or greater decrease in symptoms, and remission was defined as a QIDS-SR16 score of 5 or lower following four infusions. Clinically significant improvement was defined as a 20% or greater improvement in symptoms following four infusions, compared to pretreatment. Response and remission rates were compared using a Chi-square test for trend in GraphPad Prism 8.0, in order to determine if rates of response differed between groups.
Results
A total of 290 participants received IV ketamine infusions at the CRTCE between July 2018 and July 2020. Twenty-four participants were missing baseline BMI data. Twenty-five participants were excluded due to missing data from four or more timepoints excluded. Eighteen visits across 11 participants were removed due to assessments being completed more than 4 days after the infusion (for infusions 1-3), or due to the post-infusion 4 assessment being completed more than 14 days after the fourth infusion. In total, 230 participants were included in this study. Demographic information for the included sample is described in Table 1.
Table 1. Baseline Demographics of Included Participants, with Two-Tailed Kruskal-Wallis or Chi-Square Tests to Compare Groups

a Secondary diagnosis was missing for some participants.
Abbreviations: ADHD, attention deficit hyperactivity disorder; BD, bipolar disorder; BMI, body mass index; BPD, borderline personality disorder; GAD, generalized anxiety disorder; Max, maximum; Min, minimum; MDD, major depressive disorder; OCD, obsessive-compulsive disorder; PD, personality disorder; PTSD, post-traumatic stress disorder; SAD, social anxiety disorder.
No significant differences in response to IV ketamine were reported for overall depressive symptoms (Figure 1), anxiety, anhedonic severity (Figure 2), workplace, or family function (Table 2 and Figure 3). Participants with a BMI in the obese I category reported lower difficulty with social function overall (Table 3). While a significant interaction effect was observed between categorical BMI and number of IV ketamine infusions, follow-up pairwise comparisons did not show statistically significant differences based on BMI (Table 3 and Figure 2).

Figure 1. Changes in depressive symptoms (A) and suicidal ideation (B) (ie, measured by the Quick Inventory for Depressive Symptomatology-Self-Report 16 total score and suicidal ideation score) with repeated IV ketamine infusions, adjusted for baseline symptom severity, age, sex, and level of treatment resistance.

Figure 2. Changes in symptoms of anxiety (A; measured by Generalized Anxiety Disorder-7 scale) and anhedonic severity (B; measured by the Snaith–Hamilton Pleasure Scale) with repeated intravenous ketamine infusions, by BMI category, adjusted for baseline depression severity, baseline symptom severity, age, sex, and level of treatment resistance.
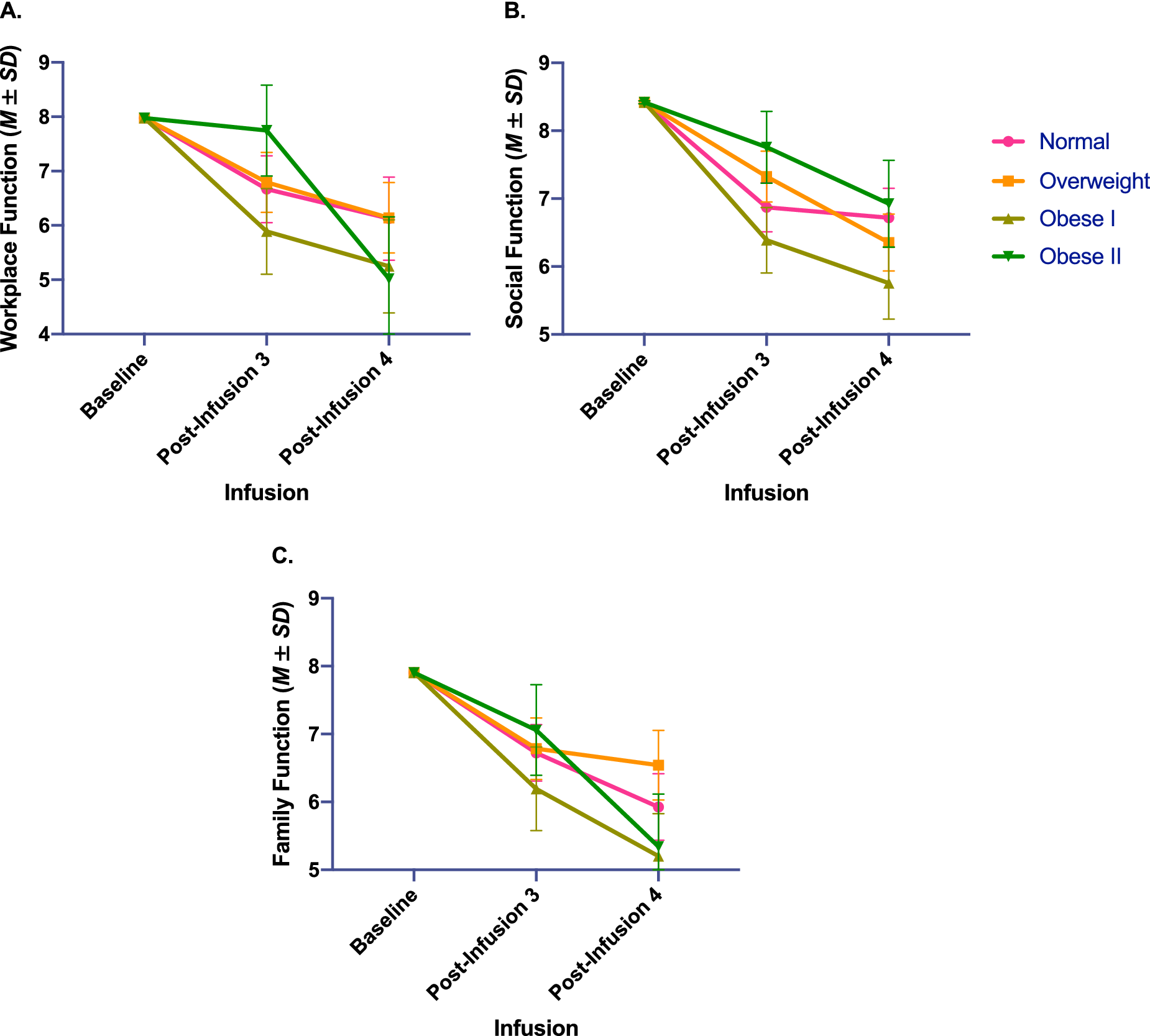
Figure 3. Changes in work (A) and psychosocial function (B and C; measured by Sheehan Disability Scale) with repeated intravenous ketamine infusions, by body mass index category, adjusted for baseline depression severity, baseline symptom severity, age, sex, and level of treatment resistance.
Table 2. Model Effects of All Outcome Measures, Controlling for Baseline Symptom Severity, Baseline Depression Severity, Age, Sex, and Level of Treatment Resistance (ie, Number of Past Antidepressant Trials)
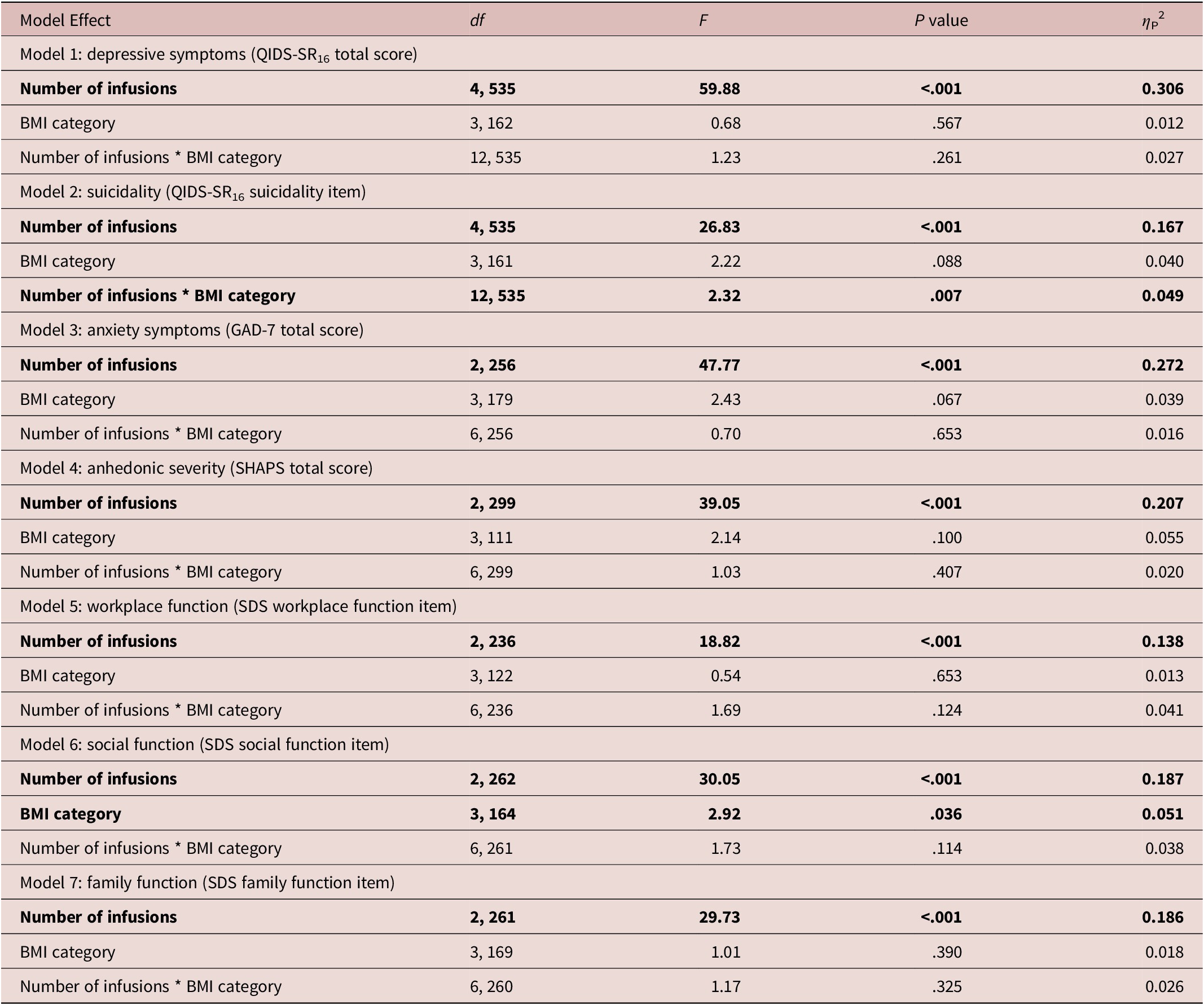
Abbreviations: BMI, body mass index; df, degrees of freedom; GAD-7, generalized anxiety disorder-7; QIDS-SR16, Quick Inventory of Depressive Symptomatology-Self-Report 16; SDS, Sheehan Disability Scale; SHAPS, Snaith–Hamilton Pleasure Scale.
Notes. Significant main effects and interaction effects, at a P < .05 threshold, are denoted in bold typeface.
Table 3. Pairwise Comparisons of Main Effect of BMI Category on Social Function Scores, and Interaction Effect Between BMI Category and Ketamine Infusion Number on Suicidal Ideation

Abbreviation: BMI, body mass index.
Note. All pairwise comparisons were two-tailed, and Bonferroni corrections were applied to all P values to correct for multiple comparisons.
a Compared to the overweight group, when adjusting for covariates.
In addition, similar rates of categorical response, remission, and clinically significant symptomatic improvements were observed between BMI groups following four infusions (Table 4).
Table 4. Categorical Response Outcomes to Repeat-Dose Intravenous Ketamine

Abbreviations: BMI, body mass index; QIDS-SR16, Quick Inventory for Depressive Symptomatology-Self-Report 16.
Note. Participants who were missing baseline data were excluded from the categorical analyses. Where post-infusion 4 data were missing but post-infusion 3 data were available, post-infusion 3 data were used to calculate response and remission.
Discussion
Individuals with TRD, regardless of baseline BMI, responded favorably to repeat-dose IV ketamine. Across BMI categories, between 55% and 66% of participants reported a clinically significant improvement in depressive symptoms following four infusions. Overall, a meaningful differential response to ketamine based on categorical BMI was not observed. This finding is in accordance with the results of Ballard and colleaguesReference Ballard, Yarrington and Farmer 27 from a smaller sample, wherein no association between obesity and improvement in SI was observed. While a significant interaction effect was observed in our study between categorical BMI and IV ketamine infusions on symptoms of SI, follow-up analyses did not reveal significant differences in SI between groups.
Contrary to our observations, Niciu and colleaguesReference Niciu, Luckenbaugh and Ionescu 22 reported a positive correlation between BMI and response to ketamine treatment. It has been conjectured that this preferential response may be confounded by dosing calculations, as patients with higher body weight receive a higher drug dose. A follow-up study by the same group suggests that baseline adiponectin, which is typically aberrant in individuals with depression and obesity, may be a predictor of ketamine response as opposed to BMI.Reference Machado-Vieira, Gold and Luckenbaugh 21 However, subsequent research reported that reduction in inflammatory markers with IV ketamine treatment was not associated with reductions in depressive symptomatology.Reference Park, Newman and Gold 37 Our findings are also in contrast to those reported by Freeman and colleagues,Reference Freeman, Hock and Papakostas 29 who reported a significantly greater antidepressant response in patients with a higher BMI. This may be attributed to the small sample size, with only 12 obese participants and 30 overweight participants, and no stratification between obese I and obese II categories. Furthermore, in this trial, 75% of participants who were obese received a dose of either 0.5 or 1.0 mg/kg (vs 0.1 or 0.2 mg/kg), whereas only 47% of overweight and 50% of participants with a BMI in the normal range received a ketamine dose of 0.5 mg/kg or higher. It is, therefore, possible that this pattern contributed to positively skewed findings in terms of drug effectiveness for the obese group.
Toward the aim of affirming (or refuting) the previous findings, Dale and colleaguesReference Dale, Bryant and Thompson 30 conducted an open-label trial of repeat-dose IV ketamine (3-6 doses per patient) to explore the relationship between obesity, depression, and ketamine treatment response (N = 150). Although BMI did not predict acute or sustained treatment response, individuals with metabolic syndrome (ie, diagnosis of hypertension, hyperglycemia, or hyperlipidemia) were 50% less likely to respond to acute IV ketamine treatment compared to patients without metabolic syndrome, after adjusting for age, sex, and baseline depression severity. Metabolic syndrome did not significantly predict sustained treatment response.Reference Dale, Bryant and Thompson 30 Additional research is essential in order to further delineate the relationship between metabolic conditions (eg, obesity and metabolic syndrome), inflammation, and response to IV ketamine treatment. Methodologically, most of the foregoing studies did not clearly identify whether ideal body weight or actual body weight was used to calculate the dose of ketamine for each participant. It has been suggested that ketamine dosing based on ideal body weight may result in underdosing patients in higher BMI categories. As ketamine is a highly lipophilic drug that crosses the blood–brain barrier, along with efficacy considerations, there are also safety concerns that need to be considered when dosing ketamine in patients with excess weight.Reference Singh, Bobo and Rasmussen 28 , Reference Sanacora, Frye and McDonald 38 Ketamine’s lipid-soluble properties may also cause important differences in pharmacokinetics and pharmacodynamics for patients with a high percentage of body fat.
There are several methodological aspects to our study that may affect inferences and interpretations of our findings. Our results represent a retrospective analysis of data from patients receiving care at an outpatient treatment clinic, rather than a randomized controlled prospective study. The inherent bias associated with a retrospective study design limits the interpretation of the reported findings. Moreover, we allowed for the use of concomitant medications, which were heterogeneous and may have influenced our dependent measures. We also used a convenience sample (ie, datapoints reflected clinical visits), and therefore there was missing data and variance in timing between infusions. The missing data pose a limiting factor in the interpretation and generalizability of the findings. Furthermore, our study operationalized obesity according to BMI, which has been criticized as a poor measure of adiposity.Reference Wells 39 We also did not collect biomarkers of inflammatory cytokines, nor did we collect metabolic parameters. Invasive or time-consuming assessments were avoided in order to reduce the burden on patients receiving clinical care at the CRTCE. Moreover, we did not have information related to past childhood trauma, which is associated with not only higher rates of obesity, but also TRD.Reference McIntyre, Soczynska and Liauw 40 In addition, IV ketamine doses were calculated based on actual body weight for most participants, and therefore individuals with greater body weight received a higher dose of ketamine.
Notwithstanding the foregoing limitations, the strengths of this analysis are its large sample size of adults with MDD or BD that would be typically encountered in a mood-disorder clinic. Permitting patients with comorbidities as well as various medication combinations also reflects the reality of clinical practice. We used validated measures of treatment outcome and IV ketamine was infused in accordance with best practices. We also found that 69% of patients in our sample were either overweight or obese, reflecting the staggering rate of medical comorbidity in this patient population, and further instantiating the representativeness of our patient population.Reference McIntyre, Soczynska and Konarski 41
The findings of this study also raise multiple further research questions. In addition to greater research on inflammatory biomarkers (eg, cytokines and adipokines), further analysis on features of metabolic syndrome such as waist circumference, lipid profile, and fat percentage is needed to understand how these factors may influence IV ketamine response. In addition, a randomized controlled trial is warranted evaluating the use of ideal body weight compared to total body weight and lean body weight in the dosing of IV ketamine. The role of weight as a factor of treatment response in specific diagnoses, such as BD vs MDD, for example, is also an important area of future research.
Conclusion
Intravenous ketamine is a costly and somewhat invasive treatment compared to oral medications, and identifying predictors of response would allow healthcare providers to counsel patients on the likelihood of response before undergoing treatment. However, predictors of response to IV ketamine are not well characterized and research has reported mixed findings. While BMI, dissociation, a first degree relative with alcohol use disorder, and biomarkers (eg, adipokines and cytokines) have been preliminarily identified as predictors of response, many of these findings have not been reproducible.Reference Machado-Vieira, Gold and Luckenbaugh 21 , 42-47
Identifying treatment response and tolerability markers in mood disorders is a priority research vista.Reference McIntyre 48 Hitherto, no single marker or signature is proven able to improve health outcomes and/or cost-effectiveness at the point of care in adults with mood disorders.Reference Trivedi, Chin Fatt and Jha 49 The results from our analysis do not support the predictive utility of BMI across most outcome measures. This null finding, however, does not militate against the possibility that intermediate measures of metabolic alteration (eg, cytokine profiles, and/or metabolic profiles—eg, insulin resistance) may moderate treatment response to IV ketamine in depression.
Acknowledgments
The authors thank all the team members and co-authors for their contributions to this manuscript, and to all staff at the Canadian Rapid Treatment Center of Excellence for their assistance with data collection. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Disclosures
Orly Lipsitz, Nelson B. Rodrigues, Hartej Gill, Mehala Subramaniapillai, Flora Nasri, and Rodrigo B. Mansur report no conflicts of interest.
Yena Lee received salary support from the Global Alliance for Chronic Diseases/Canadian Institutes of Health Research (CIHR)/National Natural Science Foundation of China's Mental Health Team Grant and the CIHR Frederick Banting and Charles Best Canada Graduate Scholarship; personal fees from Champignon Brands.
Joshua D. Rosenblat has received research grant support from the Canadian Cancer Society, Canadian Psychiatric Association, American Psychiatric Association, American Society of Psychopharmacology, University of Toronto, University Health Network Centre for Mental Health, Joseph M. West Family Memorial Fund and Timeposters Fellowship and industry funding for speaker/consultation/research fees from Janssen, Allergan, Lundbeck, Sunovion, and COMPASS. JDR is the medical director of a private clinic, providing intravenous ketamine infusions and intranasal esketamine for depression.
Roger S. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Minerva, Intra-Cellular, and Abbvie. Dr. Roger McIntyre is a shareholder and CEO of Champignon Brands, which acquired the Canadian Rapid Treatment Center of Excellence in May 2020.
Kevin Kratiuk is the Vice President of Operations at the Canadian Rapid Treatment Center of Excellence, and is a shareholder of Champignon Brands, which acquired the Canadian Rapid Treatment Center of Excellence in May 2020. Champignon Brands was not involved in this study.









