Palygorskite is a hydrous Mg-aluminosilicate with a layered structure and a fibrous or rod-like morphology. The structure of palygorskite consists of channels and tunnels formed by discontinuous octahedra due to inversion of Si-tetrahedra (Liu et al., Reference Liu, Yao, Cheng and Frost2012; Nasedkin et al., Reference Nasedkin, Vasiliev, Boeva and Belousov2014). The channels and tunnels host zeolitic water and octahedral Mg-coordinated water and impart a large specific surface area to palygorskite. Theoretically, the specific surface area can be as large as 800 m2 g–1, but due to its limited accessibility the measured value does not exceed 350 m2 g–1 (Moore & Reynolds, Reference Moore and Reynolds1989). Exchanged cations, water molecules and organic molecules of a certain size can be directly adsorbed into the palygorskite tunnels. Natural palygorskite mostly exists in the form of crystal bundles due to hydrogen bonding and van der Waals interactions between the individual crystals.
Palygorskite is of either sedimentary or hydrothermal origin (Singer, Reference Singer1979). Sedimentary palygorskite is generally formed in an arid to semi-arid palaeoclimate and in alkaline to semi-alkaline sedimentary media rich in Mg2+ (Galan, Reference Galan1996; Khademi, Reference Khademi1999) in marine, lagoonal and inland lake sediments (Singer, Reference Singer1979; Callen, Reference Callen1984). The occurrence and genesis of palygorskite in arid soils have been widely investigated (Daoudi, Reference Daoudi2004; Ali & Selahattin, Reference Ali and Selahattin2006; Owliaie et al., Reference Owliaie, Abtahi and Heck2006; Tlili et al., Reference Tlili, Felhi and Montacer2010; Kadir et al., Reference Kadir, Eren, Kulah, Onalgil, Cesur and Gurel2014), and a formation mechanism through direct precipitation from Mg-rich solutions has been proposed. It has been suggested that the pre-existing smectite, interstratified illite/smectite (Yaalon & Wieder, Reference Yaalon and Wieder1976; Chahi et al., Reference Chahi, Clauer, Toulkeridis and Bouabdelli1999; Chen et al., Reference Chen, Xu, Lu, Xu, Peng and Yue2004) and chlorite (Hong et al., Reference Hong, Yu, Xue, Zhu, Xiang and Zhang2007) can be transformed into palygorskite via energy input (Chahi, Reference Chahi1993; Khormali & Abtahi, Reference Khormali and Abtahi2003; Romero et al., Reference Romero, Barrios and Revuelta2004). Hydrothermal palygorskite is formed via alteration of igneous rocks due to hydrothermal activity, whereby it replaces the pre-existing minerals of wall rocks (Singer, Reference Singer1979; Chen, Reference Chen1991).
The palygorskite clay in this study occurs in the middle Neogene Baiyanghe Formation in the Yangtaiwatan Basin in the north of Linze County, Gansu Province, China. The age of palygorskite in this area is consistent with most continental palygorskite deposits in the world. However, the controlling factors for the formation of palygorskite in the Baiyanghe Formation have not been systematically investigated. Zhang et al. (2021) studied the genesis of palygorskite in this area. Although the enrichment characteristics of rare earth elements and trace elements indicated that palygorskite formed from the weathering of detrital clay minerals, the specific alteration process was not presented. Moreover, the source of the detrital clay minerals and the specific palaeoclimatic characteristics also were not clarified.
In the present study, we used the mudstones from the lower part of the palygorskite bed as a comparison to study the environmental factors impacting palygorskite formation and to determine the formation mechanism of palygorskite claystones and mudstones in the Yangtaiwatan Basin, northwest China. This would allow us to: (1) characterize various palygorskite claystones and mudstones; (2) determine the controlling factors for the formation of palygorskite claystones and mudstones; and (3) provide new insights into the formation of continental palygorskite deposits in the Neogene.
Geological background and sampling
Regional geological background
The Yangtaiwatan Basin is a Cretaceous–Tertiary-faulted basin located in Linze County, Gansu Province, China (Fig. 1a). The sedimentary sequence in this basin is subdivided into the Cretaceous Miaogou Formation, the Neogene Baiyanghe Formation and Quaternary sediments. The Cretaceous Miaogou Formation (K1) outcrops around the basin, which consists of sandy conglomerate, sandstone, mudstone and muddy limestone (Fig. 1b). The Neogene Baiyanghe Formation (N1b) is widespread and unconformably overlies the basement rocks of the Cretaceous Formation (Fig. 1c). The Baiyanghe Formation consists of a set of clastic rocks, claystone and gypsum (Zhou & Dong, Reference Zhou and Dong2004; Wang et al., Reference Wang, Cui and Zhang2005; Wei & Cui, Reference Wei and Cui2005). The occurrence of palygorskite clay in the Neogene Baiyanghe Formation in the basin is controlled by the Zhengbeishan syncline (Fig. 1).

Fig. 1. Geological map and part of the stratigraphic column of the Yangtaiwatan Basin (modified from Zhang et al., Reference Zhang, Liu, Liu, Zhang, Qiao and Teppen2021). (a) Location map of the study area in China; (b) geological map of the study area; (c) the stratigraphic column.
Sample collection
The samples were collected from the top profile of the Baiyanghe Formation in the Kaimao and Yangtai mining areas of the Yangtaiwatan Basin (Fig. 2). Eight samples were collected at various depths in the middle of the Baiyanghe Formation (N1b4) in the Kaimao mining area, and these were labelled as KM-1 to KM-8 in sequence. Among them, KM-1, KM-3 and KM-6 consist mainly of gypsum, whereas the remaining samples were palygorskite claystone samples (Fig. 2). Seven mudstone samples and two gypsum samples were also collected from the lower part of the Neogene Baiyanghe Formation (N1b1–3) in the Yangtai mining area, which were labelled as YT-1 to YT-9 in sequence (Fig. 2).

Fig. 2. Field profile photographs of the middle Baiyanghe Formation in the Kaimao (KM) mining area and the lower Baiyanghe Formation in the Yangtai (YT) mining area of the Yangtaiwatan Basin.
Methods
X-ray diffraction (XRD) was used to determine the mineral composition of bulk rocks and the clay fractions of the specimens. The <2 μm clay mineral fractions in the bulk rocks were separated by sedimentation after dispersion for 4 h in deionized water, followed by centrifugation of the suspension. Oriented thin films of the clay fractions were prepared via air drying of the dispersions of the clays on XRD slides. Subsequently, the films were subjected to the following treatments: solvation with ethylene glycol (EG) at 30°C for 8 h and heating at 450°C for 2.5 h. One XRD trace was recorded after each treatment.
All XRD traces were recorded on a D/max-2500PC automatic powder X-ray diffractometer in continuous scan mode with Cu-Kα radiation operated at 40 kV and 100 mA at a scan speed of 4° min–1 using 1° divergence and antiscatter slits and a 0.3 mm receiving slit. The XRD traces of the bulk rock powder specimens were collected from 2.5 to 70°2θ, while the XRD traces of the oriented air-dried clay films were recorded in the range 2.5–15°2θ, and the XRD traces of the EG films were recorded in the range 2.5–30°2θ. This XRD analysis was performed at the State Key Laboratory of Coal Resources and Safe Mining, China University of Mining and Technology (Beijing). The quantitative analysis of the mineral composition of the bulk rocks was carried out using the method of Brindley (Brindley & Brown, Reference Brindley and Brown1980). The relative abundances of clay minerals were determined using their basal reflections and the mineral intensity factors of Moore & Reynolds (Reference Moore and Reynolds1989).
The minerals in the bulk rock were examined under a CX40P polarizing microscope (Shunyu Optical Technology Co., Ltd). Fresh broken surfaces of the bulk rocks were examined using a Hitachi SU8020 field emission scanning electron microscope (SEM) to record the morphologies and associations of the clay minerals.
The chemical compositions were obtained for the five palygorskite claystone specimens and the seven mudstone samples ground with a tungsten carbide mill and sieved using 200 mesh sieves. The major element composition of the bulk rocks was determined using X-ray fluorescence (XRF) spectrometry. The trace element composition of the samples was determined using inductively coupled plasma mass spectrometry (ICP-MS) at the Analytical Laboratory of Beijing Research Institute of Uranium Geology, China. The test methods were based on the standards GB/T 14506.28-2010 and GB/T 14506.30-2010, respectively.
Results
Petrographical and mineralogical analyses
The XRD traces of the bulk rocks and clay fractions of the claystones collected in the middle of the Baiyanghe Formation (N1b4) in the Kaimao mining area are presented in Figs 3 & 4. The major minerals in the claystones are quartz, orthoclase, plagioclase, dolomite, gypsum, halite and clay minerals. The clay minerals mainly consist of palygorskite and illite with small amounts of kaolinite and chlorite. The clay mineral fraction accounted for 30–50% of the bulk claystones (Table 1), except for gypsum samples KM-1, KM-3 and KM-6. The quartz content in palygorskite claystones (KM-2, KM-4, KM-5, KM-7 and KM-8) accounted for 20–40% of the mineral composition. Moderate amounts of dolomite (10–20%) and feldspar, including orthoclase and plagioclase (10–20%), also occur in the samples. Calcite, halite and gypsum constitute <10% of the claystones.

Fig. 3. XRD traces of bulk rocks from Kaimao (KM) and Yangtai (YT).

Fig. 4. XRD traces of oriented clay fractions from samples KM-7 and YT-1.
Table 1. Quantitative mineralogical compositions of the palygorskite claystones and mudstones collected from the middle and lower Baiyanghe Formation.

The mudstones collected in the lower part of the Neogene Baiyanghe Formation (N1b1–3) in the Yangtai mining area are also interbedded with gypsum beds. However, the bulk rocks are free of orthoclase and contain analcime (Fig. 3 & Table 1). The clay fractions of the mudstones in the lower Baiyanghe Formation are composed mainly of mixed-layer illite/smectite and illite with small amounts of chlorite and kaolinite (Fig. 4 & Table 2).
Table 2. Quantitative analysis of the clay mineral fractions of the palygorskite claystones and mudstones collected from the middle and lower Baiyanghe Formation.

Ch = chlorite; I = illite; I/Sm = mixed-layer illite/smectite; Kaol = kaolinite; Pal = palygorskite.
Figure 5 shows optical microscope images of samples under cross-polarized light. The interference colour of the clay minerals is yellowish-brown. The clay minerals fill the space between the detrital minerals of low roundness and poor sorting (Fig. 5a–d). In addition, the bulk rocks of palygorskite claystones also contain a certain amount of quartz and feldspar. The detrital quartz is generally chemical stable during weathering, and it displayed some evidence of minor abrasion (Fig. 5a). Some quartz and plagioclase crystals of volcanic origin were angular and generally intact (Fig. 5b,d), although some quartz crystals displayed corrosion embayments (Fig. 5c). However, in general, terrigenous clastic minerals in the mudstone samples of Yangtai are scarce (Fig. 5f). Sample YT-3 contained large amounts of cryptocrystalline calcite and dolomite (Fig. 5e), which is consistent with the XRD results.

Fig. 5. Optical microscope images of samples under cross-polarized light: (a) terrigenous clastic quartz; (b) volcaniclastic quartz; (c) quartz with corrosion embayment; (d) volcaniclastic plagioclase; (e) cryptocrystalline calcite and dolomite of YT-3; and (f) mudstones of Yangtai area.
The morphology of palygorskite and its relationship with other minerals in the bulk rocks were observed using SEM. The rod-like palygorskite crystals were intertwined with each other and mixed with other lamellar clay minerals (Fig. 6a,c). The length of the palygorskite rods was 1–3 μm and the width was ~0.05 μm (Fig. 6b,d). The individual palygorskite rods were almost wholly intact, while the edges of the lamellar clay minerals were well rounded (Fig. 6e,f).
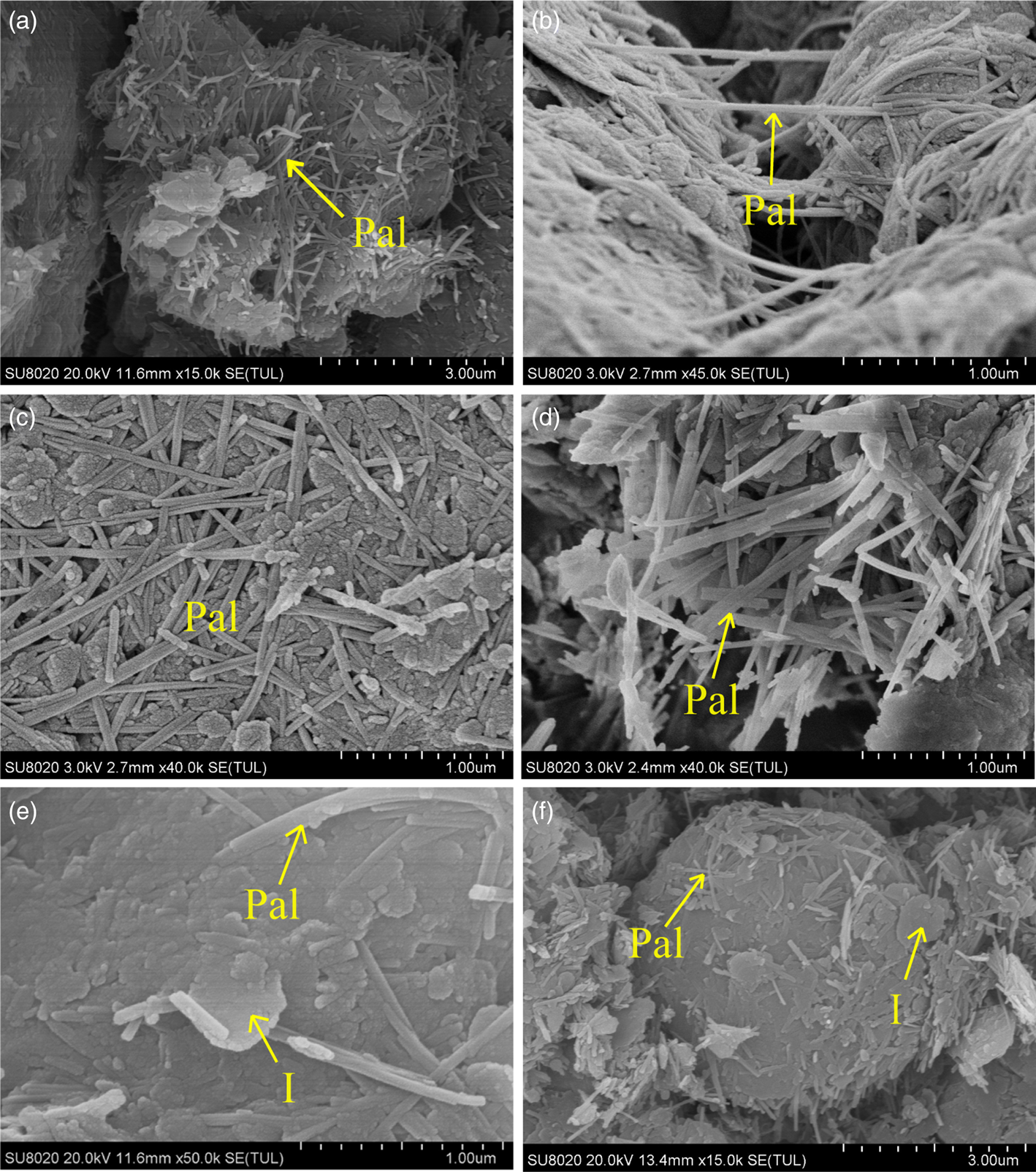
Fig. 6. SEM micrographs of fresh surfaces of Pal claystones: (a,c,d) rod-like Pal crystals intertwined with each other; (b) rod-like Pal crystals interwoven with other minerals; (e) the well-rounded edges of lamellar clay minerals; (f) Pal mixed with other laminar clay minerals coating the detrital minerals. I = illite; Pal = palygorskite.
Major element geochemistry
The palygorskite claystones have a greater SiO2 content than the mudstones (57.5% and 43.4% on average, respectively), but their Al2O3 content was lower (10.4% and. 15.6% on average, respectively) (Tables 3–5). The Fe2O3, MgO and CaO contents of the palygorskite claystones was slightly lower than that of the mudstones (3.8%, 4.5% and 4.8% on average, respectively, vs 6.4%, 5.0% and 6.4% on average, respectively). However, the contents of other major elements were similar. The major element contents of the palygorskite claystones were compared with those of the upper continental crust (UCC) (Taylor & McLennan, Reference Taylor and McLennan1985) and post-Archean Australian shale (PAAS); the contents of SiO2, Al2O3, Fe2O3, K2O and TiO2 of the claystones were lower, while those of MgO and CaO were generally greater than their UCC and PAAS counterparts.
Table 3. Major element compositions (wt.%) of the palygorskite claystones and mudstones.

LOI = loss on ignition.
Table 4. Trace element concentrations (μg g–1) and elemental ratios of the palygorskite claystones collected from the middle Baiyanghe Formation.

Table 5. Trace element concentrations (μg g–1) and elemental ratios of the mudstones collected from the lower Baiyanghe Formation.

Trace element geochemistry
The palygorskite claystones had relatively high concentrations of most trace elements compared with the corresponding averages for the UCC and PAAS. The concentration coefficients (CCs), or the ratio of elemental concentrations in the investigated palygorskite claystones vs the corresponding averages for the UCC (Taylor & McLennan, Reference Taylor and McLennan1985), are shown in Fig. 7. The CCs were comparable to their counterparts from the mining area in the Yangtaiwatan Basin (Zhang et al., Reference Zhang, Liu, Liu, Zhang, Qiao and Teppen2021), indicating that the whole mining area has a relatively constant composition. Compared with the UCC, the palygorskite claystones are significantly enriched in Sb and B (CC > 5). The Sr, Cd, W, Bi and U contents are also enriched (2 < CC < 5), while the contents of Nb and Ta are considerably lower than those for the UCC (CC < 0.5).

Fig. 7. CCs of trace elements in palygorskite claystones. Data for UCC adopted from Taylor & McLennan (Reference Taylor and McLennan1985).
Discussion
The abundances of trace elements in sediments are controlled by the mobility of elements themselves and the sedimentary environment and sedimentation processes; thus, there are various dispersion and accumulation rules for trace elements in various sedimentary environments (Coker & Nichol, Reference Coker and Nichol1975, Cao et al., Reference Cao, Jia, Wang and Cheng2021). The redox environment, palaeosalinity and palaeoclimate during deposition of the studied sedimentary rocks can be revealed by applying appropriate parameters and classification standards.
Palaeosalinity
Palaeosalinity is an important indicator for the analysis of palaeosedimentary environments, which is usually regarded as an important parameter for identifying land and sea changes in geological history (Abanda & Hannigan, Reference Abanda and Hannigan2006). Some trace elements, such as Sr, Ni, B and the ratios of Sr/Ba and B/Ga, are sensitive to palaeosalinity; therefore, these are generally used as discriminant indices reflecting the palaeosalinity of the sedimentary medium. The solubility of barium (Ba2+) compounds is lower than that of strontium (Sr2+) compounds, and Ba2+ is more likely to react with SO42− to form sulfate precipitates than Sr2+. As the salinity of lake/sea water increases, SO42− concentration increases, and Ba2+ precipitates first in the form of BaSO4. Then Sr2+ precipitates as SrSO4 after the SO42− concentration in lake/sea water reaches a critical level (Panahi et al., Reference Panahi, Young and Rainbird2000; Abanda & Hannigan, Reference Abanda and Hannigan2006). Consequently, the Sr/Ba ratio may effectively reflect the relative strength of evaporation in continental deposits. A Sr/Ba ratio > 1.0 indicates saline water, 0.5 < Sr/Ba < 1.0 represents a brackish water phase and Sr/Ba < 0.5 reflects a freshwater phase (Liu & Zhou, Reference Liu and Zhou2007; Peng et al., Reference Peng, Wang and Jiang2012). The Sr/Ba ratio of palygorskite claystones in the middle Baiyanghe Formation ranged from 0.29 to 4.30 with a mean value of 1.71. After removing the apparently outlier value of 0.29, the variation in range was from 0.74 to 4.30 (mostly >0.5) with a mean value of 2.07 (Table 4), indicating a salt lake depositional environment.
Ga and B are preferably enriched in fluvial facies and salt lake mudstones, respectively. Therefore, the ratio of B/Ga may be used to identify fluvial and salt lake mudstones. Generally, the B/Ga ratio in fluvial mudstones is relatively low, while in salt lake mudstones this value is greater and increases with the palaeosalinity of the sedimentary environment. The B/Ga ratio in the palygorskite claystones in the present study ranged from 7.64 to 8.71 with a mean value of 8.04, indicating that the claystones were highly enriched in B and also implying the formation of the palygorskite claystones in saline water. Nevertheless, the Sr/Ba ratios of mudstones in the lower Baiyanghe Formation varied from 0.32 to 1.08 with a mean value of 0.66, and those of B/Ga varied from 4.24 to 6.46 with a mean value of 5.21. The salinity of the depositional environment of mudstones in the lower Baiyanghe Formation was significantly lower than that of the palygorskite claystones in the middle Baiyanghe Formation during the formation of the strata (Fig. 8). Thus, the greater salinity of the depositional medium was favourable for the formation of palygorskite.

Fig. 8. Trace element diagram indicating the palaeosalinity, palaeoclimate and redox conditions of the palygorskite claystone and mudstone depositional environments.
Palaeoclimate
In continental environments, the sediments of closed basins (lakes) often contain records of hydrochemical, hydrological and climatic variations. Even small changes in evaporation or precipitation rates may lead to significant changes in lake level and salinity (Battarbee, Reference Battarbee2000). The trace elements in closed salt lake sediments are sensitive to hydrological changes in the sedimentary environment. In warm and humid climates, Fe, V, Ni, Ba and Co are readily dissolved (Ruiz et al., Reference Ruiz, Kastner, Torres, Gamiz, Moreno and Huertas2015; Bussan et al., Reference Bussan, Ochs, Jackson, Anumol, Snyder and Cizdziel2017). When the climatic conditions change from warm to dry, the evaporation of water gradually increases and the dissolved elements precipitate in the form of salts, resulting in relatively large contents of these elements in the sedimentary rocks (Li et al., Reference Li, Shi, Li and Zhang2013). Thus, the sedimentary environment at a particular time can be analysed based on these trace elements. The concentration coefficients of V, Ni and Co in the palygorskite claystones varied between 1 and 2 (Fig. 7). These coefficients indicate that the contents of these elements were high in the palygorskite claystones, suggesting a dry climate.
The Sr/Cu ratio is commonly used as indicator of palaeoclimatic conditions. The Sr/Cu ratios from 1.3 to 5.0 indicate a warm and humid climate, while Sr/Cu ratios >5 indicates a dry and hot climate. The Sr/Cu ratio in the palygorskite claystones ranges from 4.25 to 133 with an average of 47.16, which is far greater than 5, indicating a dry and hot climate in the depositional environment. The Sr/Cu ratio in the mudstones of the lower Baiyanghe Formation ranged from 4.79 to 9.84 with an average of 6.53. These values are much lower compared to those of the palygorskite claystones in the middle Baiyanghe Formation, suggesting a less dry and hot climate.
Redox state
In sedimentary rocks, the abundances of trace elements such as Ni, Co, U, Th, V, Cr, Cu and Zn vary under diverse redox conditions (Jones & Manning, Reference Jones and Manning1994). The sensitivity of different trace elements to redox in different environments also varies significantly (Tribovillard et al., Reference Tribovillard, Algeo, Lyons and Riboulleau2006). Some trace elements are easily dissolved under oxic conditions, but some are stable under anoxic conditions (Jenkyns et al., Reference Jenkyns, Dickson, Ruhl and van den Boorn2017). For instance, U, V, Cr, Co, Ni and Mo are soluble in oxidizing environments, but they are insoluble in reducing environments. The abundances of these trace elements and their corresponding ratios can be used to analyse the redox conditions of sedimentary environments (Jones & Manning, Reference Jones and Manning1994). Jones & Manning (Reference Jones and Manning1994) summarized a set of trace elemental ratios in the bottom water of sediments to distinguish the oxidation–reduction environment from the lithofacies palaeogeography in a large number of late Jurassic dark mudstones in northwest Europe. The elemental ratios of V/Cr > 4.25, Ni/Co > 7.00, V/(V + Ni) > 0.84 and U/Th > 1.25 reflect that the depositional medium is stratified with strong reduction. The elemental ratios of 2.00 < V/Cr < 4.25, 5.00 < Ni/Co < 7.00, 0.60 < V/(V + Ni) < 0.84 and 0.75 < U/Th < 1.25 indicate that the depositional medium is anaerobic and moderately stratified. The elemental ratios of V/Cr < 2.00, Ni/Co < 5.00, V/(V + Ni) < 0.60 and U/Th < 0.75 represent weak stratification of an oxygen-rich environment. Iron has two ionic valence states in nature – Fe2+ and Fe3+ – which are sensitive to the oxidation–reduction conditions of the sedimentary medium. A Fe2+/Fe3+ ratio of >1 reflects a reducing environment and a Fe2+/Fe3+ ratio of <1 indicates an oxidizing environment (Rimmer, Reference Rimmer2004). In the present study, the mean value of the V/Cr, Ni/Co, V/(V + Ni), U/Th and Fe2+/Fe3+ ratios in the palygorskite claystones in the middle Baiyanghe Formation were 1.29, 2.63, 0.72, 0.95 and <1, respectively. The mean values of these elemental ratios of the mudstones in the lower Baiyanghe Formation were 1.73, 2.35, 0.76, 0.28 and <1, respectively (Fig. 8). These rations indicate that the palygorskite claystones and the Yangtai mudstones in the study area were formed in a similar oxidation environment.
Formation mechanism of palygorskite
In modern continental environments, climate change has a profound impact on sedimentary processes. Arid climates promote evaporation, sparse vegetation, intense wind activity and active windborne transportation. Conversely, humid climates promote abundant surface water, causing more active waterborne sediment transportation. The clay mineral assemblage may effectively reflect the palaeoclimate and sedimentary medium conditions (Manalt et al., Reference Manalt, Beck, Disnar, Deconinck and Recourt2001; Perederij, Reference Perederij2001; Merriman, Reference Merriman2002; Xie et al., Reference Xie, Chen, Ji, Chen, Xu and Xu2005). The presence of illite and chlorite represents dry climatic conditions, while smectite represents seasonal warm or humid climate conditions and an alkaline sedimentary medium (Hong et al., Reference Hong, Yu, Xue, Zhu, Xiang and Zhang2007). Palygorskite is generally formed under arid and semi-arid palaeoclimate conditions from Mg2+-rich, alkaline and semi-alkaline porewaters (Colson et al., Reference Colson, Cojan and Thiry1998).
The study area has gradually evolved into a semi-closed salt lake in a continental graben basin since the Cretaceous (Wang et al., Reference Wang, Cui and Zhang2005). Previous studies showed that the pH of porewaters during the formation of palygorskite in the present study area was 8.0–9.1 (Wang et al., Reference Wang, Cui and Zhang2005). Such a pH is favourable for the formation of palygorskite. The palygorskite claystones and gypsum beds are interbedded in the middle of the Baiyanghe Formation. Compared with the mudstones in the lower part of the Baiyanghe Formation, which were also interbedded with gypsum beds, the mass percentage of detrital quartz and feldspar in the palygorskite claystones was greater (KM: 40–50%; YT: <15%). Additionally, clastic minerals had poor roundness and sorting (Fig. 5), indicating that they were transported over a short distance. The palygorskite probably formed in the shallow part of the lake in the basin. However, the minerals in the mudstones were mainly cryptocrystalline, and the samples contained large amounts of calcite and dolomite, indicating that they may have formed in the deep part of the lake under weak hydrodynamic conditions.
Al2O3 and SiO2 display greater solubility in an alkaline medium, which was reflected by the highly rounded edges of laminar clay minerals (Fig. 6e,f) in the middle part of the Baiyanghe Formation. However, the interstratified illite/smectite had hair-like edges in the mudstones (Fig. 9a,b). The mudstones in the lower part of the Baiyanghe Formation were also formed in a high-salinity depositional environment, as characterized by the feldspar dissolution (Fig. 9c,d). The crystal morphology indicates that the palygorskite crystallized from the dissolution of detrital aluminosilicates such as clay minerals (illite, smectite and kaolinite) and feldspars.

Fig. 9. SEM images of fresh surfaces of mudstones: (a,b) I/Sm with hair-like edges; (c,d) dissolution of Fsp. Fsp = feldspar; I/Sm = mixed-layer illite/smectite.
In the semi-closed salt lake, the detrital minerals such as quartz and feldspar and the laminar clay minerals such as illite and kaolinite gradually dissolved in the alkaline medium. With the evaporation of water in the basin, the activities of Si4+ and Al3+ increased, thereby raising the salinity of the porewater. The Mg2+ required for the crystallization of palygorskite was derived from the dissolution of smectite or mafic rocks from the surrounding area, which were transported to the basin by flowing water. The palygorskite claystones and mudstones in the middle and lower parts of the Baiyanghe Formation are both interbedded with gypsum layers. Their depositional environment analyses showed that the strata were formed in similar oxidation environments, but the palygorskite claystones were formed under a considerably drier and hotter climate compared to that of the mudstones. Additionally, the palygorskite claystones and mudstones have similar mineral compositions, but the detrital quartz and feldspar contents of the palygorskite claystones were greater, and they had poor roundness and sorting. This indicates that a shallow lake with an oxidative environment may be a favourable terrain for the crystallization of palygorskite (Fig. 10).

Fig. 10. Diagram of the saline basin illustrating the formation and evolution of palygorskite claystones.
Conclusions
The palygorskite claystones and mudstones in the middle and lower sections of the Neogene Baiyanghe Formation in the Yangtaiwatan Basin belong to salt lake facies. The mineralogical composition of the mudstones was similar to that of the palygorskite claystones except that the main clay mineral was mixed-layer illite/smectite rather than palygorskite. The chemical compositional differences between the palygorskite claystones and mudstones and the differences in their crystal morphology indicated that the palygorskite was recrystallized from the dissolution of detrital clay minerals (e.g. illite, smectite and kaolinite) and feldspars in the salt lake. Compared with the depositional environment of the mudstones, the palygorskite claystones were formed in a drier and hotter climate. Additionally, the mineral phases in the mudstones were mainly cryptocrystalline, indicating that they formed in the deep lake of the basin under weak hydrodynamic conditions. The abundance of detrital minerals such as quartz and feldspar with low roundness and sorting in the palygorskite claystones indicates that palygorskite formed in the shallow lake of the basin closer to the provenance area.
Acknowledgements
The authors thank Dr Xiaofang He from China University of Mining and Technology (Beijing) for her assistance with this paper.
Financial support
This work was supported by the Open Project on Development of Attapulgite Industry in Linze County (LZKFKT-1801) and the Open Fund of State Key Laboratory of Coal Resources and Safe Mining (SKLCRSM20KFA01).
Conflict of interest
The authors declare none.

















