Postural orthostatic tachycardia syndrome, a dysautonomia, is continuing to become a more commonly recognised disorder.Reference Arnold, Ng and Raj 1 Nevertheless, despite the relative lack of severity of the symptoms, as compared with other disorders of the autonomic nervous system such as multiple system atrophy or pure autonomic failure, postural orthostatic tachycardia syndrome can still be highly debilitating and disruptive to patients, such that they are unable to perform routine activities of daily living.Reference Sandroni, Opfer-Gehrking, McPhee and Low 2 Patients may be bedridden with light-headedness, nausea, fatigue, or headaches and may not be able to function appropriately in routine activities, including school, sports, work, or normal daily social interactions owing to cognitive dysfunction or gastrointestinal symptoms. In order to help these patients achieve clinical and symptomatic improvement to be able to perform normal daily activities, various treatment modalities are required. These modalities can include non-pharmacologic interventions, such as high-volume fluid and sodium loading, elevation of the head of the bed, good sleep hygiene, and exercise.Reference Stewart, Boris and Chelimsky 3 However, these approaches, although fundamental and necessary, are not always sufficient to effectively improve or control these symptoms. The initial goals in the care of these patients are to be able to perform activities of daily living, such as bathing, dressing, and toileting, with subsequent progression to higher level goals, such as school attendance, participation in recreational or competitive athletics, college attendance, or functional independence to successfully maintain an occupation. There is a general agreement that exercise, consisting of aerobic activity combined with leg and core strengthening, may be the most efficacious therapy in being able to control, or even eliminate, postural orthostatic tachycardia syndrome.Reference George, Bivens, Howden, Saleem, Hendrickson, Fu and Levine 4 Yet, for some patients, the act of performing their activities of daily living can be completely overwhelming and exhausting. Thus, the adjunctive use of medical therapy to further suppress and control disabling symptoms may be necessary to support patient functionality.
Several articles describe the use of medications and their outcomes in the management of postural orthostatic tachycardia syndrome. Some are retrospective reviews of the utilisation of specific pharmaceuticals in the treatment of specific associated symptomsReference Kanjwal, Saeed, Karabin, Kanjwal and Grubb 5 or are single-dose trials of medications.Reference Raj, Black, Biaggioni, Paranjape, Ramirez, Dupont and Robertson 6 Some are overall review articles of the diagnosis and management of this disorder that also identify the medical therapies used.Reference Grubb 7 , Reference Boris 8 Despite these previous publications, there are relatively few articles describing specific approaches to medication management of symptoms or symptom complexes seen in these patients.
The Postural Orthostatic Tachycardia Syndrome Program at the Children’s Hospital of Philadelphia was officially established in January 2014, although patients have been evaluated and cared for since 2007 with increasing frequency and volume. We endeavoured to use our patient database to provide an evaluation of medications used in our programme, including therapies for specific symptoms, the diversity of medications used for these symptoms, and their clinical outcomes and efficacy.
Materials and methods
The Postural Orthostatic Tachycardia Syndrome Program database consists of a REDCap (Research Electronic Data Capture) database hosted at the Children’s Hospital of Philadelphia with demographic and clinical features of patients seen in both initial and subsequent clinic visits.Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde 9 The diagnosis was made on the basis of a combination of historical symptoms indicating chronic orthostatic intolerance for at least three months along with other typical symptoms, in addition to a heart rate increase of 30 or more beats per minute during a 10-minute standing test after supine position. Patients aged 18 years and younger at the time of initial evaluation and who were subsequently treated with medications for the symptoms of postural orthostatic tachycardia syndrome were included in the study. All patients received initial treatment with increased oral fluid and sodium loading without improvement of symptoms. We used the names, dates of birth, and medical record numbers from the database to then identify patients in our clinical data warehouse populated by our electronic health record, Epic (Epic Systems, Verona, Wisconsin, United States of America). Data were extracted for all medications ordered for these patients from their initial visit between November 2007 and June 2016, including all product formulations, doses, and refills. One of the authors (J.R.B.) filtered out medications that were not used as primary therapies for these patients, such as therapies for asthma, allergies, infections, and other disease processes not directly related to this disorder. Data were further classified by specific symptom or symptom complex, based on specific utilisation in our programme. Some symptoms were treated simultaneously, with a graded approach to therapy. That is, patients were treated with the lowest dose of medication necessary to control symptoms, as reported by the patient. Medications would be increased to maximally effective dosing, as tolerated, or until side effects either required discontinuation or lowering of the dose. If clinically indicated, such as with incomplete or ineffective control of symptoms, more than one medication would be used to try to incrementally achieve effective symptomatic improvement. Certain combinations of medications were not used: for example, verapamil, a calcium channel blocker, would not be used concomitant with beta blockade (metoprolol, atenolol, nebivolol), as co-administration of these could cause life-threatening atrioventricular block.
Utilisation of a specific medication for an individual patient was considered successful if the medication at a consistent dose was ordered at least five times in chronological sequence. This was subsequently confirmed by manual review of the patient’s chart to ensure documentation of successful therapy. Because no validated method of quantification exists for postural orthostatic tachycardia syndrome (POTS)-related symptoms, we assessed symptomatic improvement by qualitative review of patient notes, based on patient report of improvement in symptoms after both initiation of therapy and with maintenance of the same dose at subsequent visits. Patients were evaluated at clinic visits with a standard symptom questionnaire used at all subsequent follow-up evaluations that queried the presence or absence of specific symptoms and recorded qualitative data as appropriate (e.g. partial improvement, specific side effects, and so on). Patients were excluded if they had a sole single medication event with no further refills or medications utilised, despite still being subsequently seen in our programme. If a medication did not produce effective clinical improvement of symptoms or caused side effects that the patient could not tolerate, the medication was discontinued.
The percentage of successful therapies by specific medication was calculated by dividing the number of patients with successful therapy with a specific medication by the total number of patients for whom that medication was ordered. The percentage of successful therapy for a specific symptom was calculated by dividing the sum of the number of patients with any successful therapy for a symptom by the total number of patients treated for that symptom. Statistical analysis was performed using Microsoft Excel plus the website, Social Science Statistics (http://www.socscistatistics.com/Default.aspx). t-Test analysis was used to assess for differences between means in normally distributed continuous variables, and χ2 was used to assess for differences between specific categorical variables. Mann–Whitney U-test was used for differences between non-normally distributed variables. The level of significance was set at p<0.05.
A waiver of consent was granted by the Institutional Review Board of the Children’s Hospital of Philadelphia, as this was a review of data collected through the electronic health record for routine clinical management with intent to treat, and it was a retrospective assessment of data for which it would have been impractical to obtain consent.
Results
A total of 722 patients with postural orthostatic tachycardia syndrome were treated between November 2007 and June 2016 and were entered in the database for study. In all, 98% (708) of patients met entry criteria for 18 years of age or younger at the time of initial evaluation to be included in the study. Demographic information for the study patients is summarised in Table 1. The symptoms treated and the medications used to treat those symptoms, dosing ranges, and side effects are shown in Table 2.
Table 1 Patient cohort characteristics, n (%).

Joint hypermobility and gender data are against all joint hypermobility
Table 2 Medications used for management of POTS symptoms: dosing and side effects.

BID=twice daily; qAM=every morning; qHS=at bedtime; TID=three times daily
Mood changes include irritability, anxiety, or depression
Utilisation of medications grouped by symptom is shown in Table 3. The rates of efficacy, by medication and by symptom, are displayed in Table 4. Overall, all medications used demonstrated some efficacy in the management and control of symptoms. Some therapies, or combinations of therapies used for specific symptoms, demonstrated greater overall efficacy, such as for light-headedness (52.2%). Others, including therapies for GI dysmotility, demonstrated efficacy for patients who were able to tolerate the medications, but often caused intolerable side effects that led to discontinuation of therapy. Other therapies showed minimal efficacy, although with a small cohort of patients reporting that their symptoms were clinically improved.
Table 3 Utilisation of medications, by symptom, n (%).

Percentages of total patients for a specific symptom are against the overall total of 708 patients. Percentages of total patients for a specific medication are against the total patients within the specific symptom. Percentages within gender are against total patients receiving treatment
Table 4 Efficacy rates of medical therapy, by symptom.
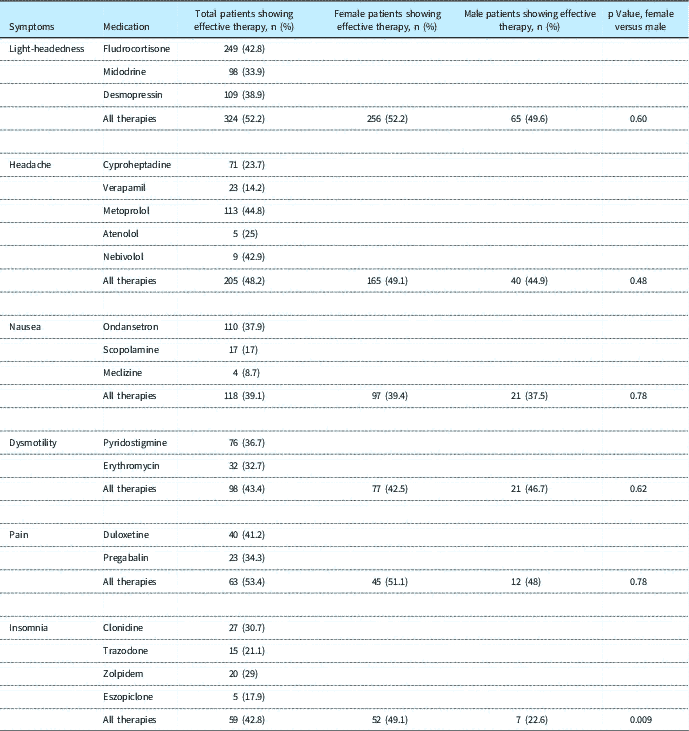
Totals for successful use of All Therapies may not equal the totals of successful use of the individual therapies, as therapies may have been used in tandem
The median number of medications prescribed per symptom was often greater than 1 for each symptom reviewed, with light-headedness, headache, and insomnia requiring a median of two medications in both genders before achieving therapeutic success (Table 5).
Table 5 Median number of therapies used per patient per symptom, n (range).

Discussion
A wide variety of medications has been used in therapeutic approaches to control and manage symptoms of postural orthostatic tachycardia syndrome.Reference Freitas, Santos, Azevedo, Costa, Carvalho and de Freitas 10 – Reference Kanjwal, Karabain, Sheikh, Elmer, Kanjwal, Saeed and Grubb 23 Most of these studies are retrospective in nature, with patient volumes that are relatively small or that include adults and children in their assessment. This study entails a comprehensive assessment of the use and efficacy of medications in a large paediatric population of patients with postural orthostatic tachycardia syndrome. Therapeutic efficacy defined by five or more refills of a medication at a consistent dose coupled with documentation of symptom improvement was modestly varied by medication and by symptom. Therapy for pain appeared to be the most effective, with the next most successful therapy being for light-headedness. Therapy for nausea was least successful overall. It is interesting to note that the therapeutic efficacy of medications used for the management of headaches is approximately 48%, which is similar to a recent study of the use of amitriptyline (52%) and topiramate (55%) for headache prophylaxisReference Powers, Coffey, Chamberlin, Ecklund, Klingner, Yankey, Korbee, Porter and Hershey 24 , as well as to the incidence of placebo effect in a general treatment study (40%).Reference Finniss, Kaptchuk, Miller and Benedetti 25 This suggests that the treatment and management of these symptoms can be difficult, and choosing the most effective medication is not empirically straightforward, leading to a trial-and-error approach. Therapeutic success did not exceed much more than 50% for any medication, confirming a high degree of patient variability in therapeutic response and tolerability. Placebo effect may be an important factor in the success of therapy for these symptoms. That said, considering that many patients required trials of more than one medication for a specific symptom, this could be considered similar to a crossover trial, except that the trial would stop when successful therapy was achieved. It is important to note that this study evaluated medications prescribed by providers in our specific programme at the Children’s Hospital of Philadelphia. The treatment paradigm within the programme, though, was that if the patient did not tolerate therapies for certain symptoms, such as headache or gastrointestinal symptoms, they were referred for further evaluation and management to providers in the divisions of Neurology/Headache Clinic and Gastroenterology/Children’s Hospital of Philadelphia Motility Centre, respectively. Other medications and treatments were then used, such as amitriptyline, topiramate, gabapentin, lidocaine nerve blocks, or botulinum toxin injections for headaches, and amitriptyline, hyoscyamine, polyethylene glycol, H-2 blockers and/or proton pump inhibitors, intrapyloric injection of botulinum toxin, or nasogastric, nasoduodenal, gastrostomy, or jejunal tube feeds for GI symptoms. Cognitive behavioural therapy was used in both of these centres to try to alleviate symptoms. These lists of subsequent, speciality-based therapies are not exhaustive. The effects of these additional therapies and interventions were not assessed, and some of these demonstrated varying degrees of success that further alleviated symptoms and allowed the patients to be more functional with regard to the activities of daily living.
The choice of medications for these patients was based on the known primary therapeutic use of the medication or a rational use of the secondary effects of these medications. For example, while fludrocortisone was used to increase intravascular volume and midodrine was used as a peripheral vasoconstrictor (primary uses), pyridostigmine was used to increase gut acetylcholine levels, using its procholinergic properties to enhance intestinal motility (secondary effect). Otherwise, pyridostigmine is not known to be a good promotility agent, at least in comparison with metoclopramide.Reference Oigaard 26 Only recently has this feature of pyridostigmine been discussed in the literature.Reference Manini, Camilleri, Grothe and Di Lorenzo 27 While stimulants such as methylphenidate or mixed amphetamine salts are used for patients with attention deficit–hyperactivity disorder in order to reduce their rapid changes in attention, they are used for these patients as central nervous system stimulants to increase activity and concentration. Desmopressin is not thought of as a volume expanding therapy, although for patients with postural orthostatic tachycardia syndrome it is used specifically with that objective to reduce light-headedness.Reference Coffin, Black, Biaggioni, Paranjape, Orozco, Black, Dupont, Robertson and Raj 28
It is evident that multiple medication trials may be needed to suppress or control the symptoms of postural orthostatic tachycardia syndrome. It can be difficult to predict which medications will be successful for specific patients, and thus iterative attempts with various therapies are required to achieve therapeutic goals. This can take time and can be discouraging to the patients, families, and providers. Persistence and patience are necessary. Therapies are not always successful. It is interesting to note that female patients appear to need a greater level of therapeutic interventions for nausea, although the cause of this is unclear. For all other symptoms overall, there was no difference noted between the numbers of therapies needed for female or for male patients.
There are limitations to this study. The use of a definition of therapeutic success as five or more refills of a medication as a threshold level has not been demonstrated to be clinically valid, although we would suggest it has merit from a rational and intent-to-treat approach. One would not continue to refill a medication that either caused intolerable side effects or demonstrated no salutary effects. That said, the success of these therapies was then confirmed by chart review, in which it was documented that the patients reported clinical improvement with these medications. Another limitation of our study is inherent in its retrospective nature, in that it is post hoc reporting of efficacy. A stronger study would evaluate these therapies in a double-blind, placebo-controlled, or crossover manner. We were not able to use a scale to numerically quantify the degree of improvement in symptoms with therapy, so these improvements are qualitatively assessed only. A symptom severity scale specifically for these patients, whether adults or children, does not exist at present. It is hoped that in the future, if such a scale is devised and validated, it can be used to better assess the efficacy of treatments, both medication and non-medication based.
Our retrospective, large-scale study of children demonstrates that a number of diverse therapies are effective to varying degrees in helping to adequately reduce or control the multiple symptoms of postural orthostatic tachycardia syndrome. In the absence of being able to predict which medications will work for specific patients for their specific symptoms, patients are often prescribed more than one medication for any given symptom before achieving a measure of significant clinical improvement. It is also important to note that, even for medications that had a modest success rate, they were still successful for those specific patients. Thus, if the goal is to allow patients to be able to perform activities of daily living, to return to school, and to exercise routinely, then even low-yield therapies still have a place in the therapeutic armamentarium.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation, per the United States Department of Health and Human Services, as well as the Food and Drug Administration, and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee of the Children’s Hospital of Philadelphia.
Acknowledgements
The authors thank Andrew Glatz, MD, MSCE, for his continued support in the editing of this manuscript. The authors also acknowledge Andrea Kennedy, who created our database and was able to help us to link it to the electronic health record.







