Stem cell research has become a major topic, widely discussed not only in the scientific society but also in politics. Regarding the heart, scientific research is usually motivated by the goal to treat ischaemic cardiac failure with cardiac progenitor or stem cells.1, 2 This raises the question whether paediatric cardiologists should also become more interested in this field of research.
Definitions
By definition, stem cells are undifferentiated cells characterised by the ability, at the level of the single cell, both to self-renew and to differentiate into mature progeny, including both non-renewing progenitors and terminally differentiated cells (Fig. 1). As a rule, gain of differentiated function is accompanied by loss of plasticity. The fertilized oocyte, and the cells of the preimplantation embryonic stage from 8 to 16 cells, are totipotent, since they are able to become a functional embryo, including all embryonic and extra-embryonic cell types. During further development, the formation of a blastula gives rise to the inner cell mass, made up again of pluripotent cells which are able to differentiate into all cell types of the embryo, but not its morphology. Pluripotency is also typical of the embryonic stem cells that are cultured from the inner cell mass. These cells, however, can no longer give rise to trophoectodermal cells that subsequently would give rise predominantly to the placenta.3 Multipotent stem cells still differentiate to a larger subset of oligopotent stem cells which have a more restricted subset of cell lineages. Unipotent stem cells, in contrast, are able to contribute only to one mature cell type. Whereas both ends of the spectrum, made up of totipotent and differentiated cells, can readily be defined, the potency of cells that have been considered pluri-, multi-, oligo-, or unipotent had to be revised in the past.

Figure 1. Cells able to give rise to cardiomyocytes. The fertilized egg and the morula are totipotent and can give rise to every cell line. The inner cell mass of the blastocyst, from which embryonic stem cells are isolated can give rise to all cell lines of the embryo proper, ectoderm, endoderm and mesoderm. Cardiac progenitor cells or cardioblasts can only develop into fully differentiated cardiomyocytes.
Mechanisms of action
There is evidence that all structures of the heart can be addressed by stem cell therapy (Fig. 2). Pacemaker-like cardiomyocytes have been generated from embryonic stem cells, and cardiomyocytes derived from stem cells have been used and investigated experimentally as a biological pacemaker.4–6 Similar approaches might improve the prospects for valvar heart disease, by replacing current bioprostheses, with their limited durability, and need for lifelong anticoagulation, by tissue engineered, living, completely autologous biological structures, for example scaffolds populated by mesenchymal stem cells obtained from the bone marrow of the patient.7, 8 In addition, stem cells might be a source for populating bioartificial vessels (Fig. 2).9, 10

Figure 2. Cardiac structures amendable to therapeutic strategies involving stem cells.
Cell types
During the recent years, an increasing diversity of stem cells or progenitor cells have been identified and were suggested for cardiac cell therapy (Table 1), and the number of potentially useful cell types is still increasing.11, 12 Nowadays, even for experts in the field, it is hardly possible to judge the clinical potential of these numerous and newly identified cell types. At the moment, therefore, it is more helpful to define important criterions for judging the relevance of a cell type for cardiac stem cell therapy, and to identify potential risks associated with such treatment in order to allow a more systematic approach (Table 2). Even if some therapeutic strategies aim to create cells that are even superior to normal cardiac cells, for example with respect to their tolerance to ischaemia, such as myoblasts, a more intuitive and realistic goal of replacement strategies is to generate physiological cardiac tissue. Criterions for the assessment of a cell type, therefore, are the quality of electromechanical integration, as well as the degree of cardiac differentiation, since these are probably major determinants of the contractile force that can be achieved. Electrophysiological characteristics of these cells before and after implantation determine the arrhythmogenic potential. For clinical purposes, it is mandatory to show that a sufficient number of cells is available within a relevant time. Possible side effects, for example immunological rejection or formation of tumours, and ethical concerns, must also be taken into account.
Table 1. Cell types suggested for cardiomyoplasty.

Table 2. Criterions to judge the clinical relevance of stem cells or progenitor cells.

Stem cells can be divided into two broad categories (Table 1): embryonic stem cells and adult stem cells. Embryonic stem cells have the potential to differentiate into cell types of all germ layers, including all types observed in cardiac tissue. In contrast, the majority of adult stem cells, primarily isolated from bone marrow, never gives rise to cardiomyocytes, with the exception of a cardiac precursor cell, a cardioblast.13–16
Below, we offer a more detailed analysis of some selected cell types that have already been characterised extensively, experimentally as well as clinically. Myoblasts serve as an example that most likely can demonstrate an important arrhythmogenic mechanism. Cells derived from the bone marrow have to be discussed, because they are the most easily accessible and most frequently used types. The majority of experimental and clinical data has been collected with these cells. Cardioblasts will be mentioned because of their unique biological properties.16 Embryonic stem cells have to be discussed because of their unique potential.
Myoblasts
Skeletal myoblasts were the first cells to be tested clinically for therapy (for review see Menasché).17 Satellite cells normally lie in a quiescent state under the basal membrane of skeletal muscle fibres, and proliferate following skeletal muscular injury. At this stage they are referred to as myoblasts. Satellite cells, or myoblasts, are precursors rather than stem cells of the skeletal muscle, since they have already reached an advanced stage of differentiation, with myogenic-restricted lineage commitment. Myoblasts are highly resistant to ischaemia, a quality that should make these cells ideal to survive in poorly vascularized tissue. For clinical studies, satellite cells are isolated from a muscle biopsy of the patient's own skeletal muscle, propagated several weeks in cell cultures, and injected directly into the myocardium. Implanted myoblasts differentiate into multinuclear myotubes. Electrophysiologically, these myotubes are characterised by short action potential durations, and they lack electrophysiological coupling to the neighbouring host cardiomyocytes via gap junctions. Clinical studies with myoblasts were associated with an extraordinary high rate of ventricular arrhythmias and sudden cardiac deaths, an observation that might be explained by re-entry circuits resulting from the heterogeneity of electrical membrane properties between donor and recipient cells.18, 19
Stem cells derived from bone marrow
Sex-mismatched cardiac transplants in humans have shown that female hearts in a male host had a significant number of y positive myocytes, coronary vessels, smooth, and endothelial vascular cells.20 These observations support the idea that blood-borne cells might integrate into the myocardium. The bone marrow hosts various stem cell types, primarily haematopoietic stem cells, mesenchymal stem cells, endothelial progenitor cells or angioblasts as well as a yet undefined stem cell population termed ‘multipotent adult progenitor cells’.21 For a thorough description of the diverse adult stem cell types of non-cardiac origin, we refer to recently published reviews by Pittenger and Martin and Yoon et al.22, 23 Under normal conditions some of these bone marrow-derived stem cell lines are suggested to give rise to heart-specific cell types, including endothelial cells and fibroblasts; none of these cells, however, differentiated into cardiomyocytes. Stem cells that can be isolated from adult murine hearts that are characterised by surface markers such as c-kit (c-kit+) or sca-1 (sca-1+) but no markers of haematopoietic differentiation, such as blood lineage negative (lin−) attracted special interest because they were reported to transdifferentiate to fully differentiated cardiomyocytes under pathological or culture conditions.24–29
Stem cells derived from bone marrow – transdifferentiation versus fusion
Transdifferentiation describes the conversion of a cell of one tissue lineage into a cell of a completely distinct lineage, accompanied by a replacement of markers and function of the original cell type with those of the transdifferentiated cell type. Reports on transdifferentiation of cells derived from the bone marrow, especially haematopoietic stem cells,29, 30 are highly questioned by recent experimental data.13–15 Instead, cell-to-cell fusion is suggested as an alternative explanation for the observed results,31 and has been described under various biological conditions.32 Currently, the mechanism of fusion of stem cells is under further investigation. Interestingly, it seems to be associated with a modification of the gene profile of the stem cell.33–35 Whether fusion of such stem cells could contribute to the regeneration of an injured heart is not yet clear.
Clinical studies with adult bone marrow
Although recent data questioned the premise that cells derived from the bone marrow are capable of developing into cardiac cells, several clinical studies suggested that injection of adult bone marrow immediately after a heart attack result in a mild improvement of cardiac function.36 Probably this is explained by improvement of capillarization, or matrix structure.
Intrinsic regenerative capacity of the adult heart
Old dogmas claiming that, a few months after birth, cardiomyocytes cannot divide any more. Concepts that growth and adaptation are solely accomplished by changes of cell growth, or hypertrophy, however, have been defeated. Though the number of cells reaches adult values a few months after birth, new cells, such as capillaries, coronary arteries, and probably also cardiomyocytes, constantly form. A constant turnover has been detected in the normal heart at all ages,37, 38 and has been shown to be increased under pathological conditions such as myocardial infarction.24
In other organs, specialised structures called niches have been identified that provide a microenvironment designed to preserve the survival and replication of stem cells.39 Though up to now niches have not been identified in cardiac tissue, most recently, a cardiac progenitor cell, an immature cell type that, as opposed to stem cells, is solely capable of developing into myocytes, was found in the right atrium of human neonates.16 It is characterised by the expression of the homeobox gene islet-1, and is thought to arise from the secondary heart field that originates near the cardiac crest.40 It is reported that these cells can be amplified in vitro, and develop more efficiently into cardiac muscle cells in the presence of other heart cells16 (see Parmacek41 for comment).
Embryonic stem cells
Embryonic stem cells are isolated from the inner cell mass of the preimplantation blastocyst by selective removal of the outer trophoectodermal layer (Figs 2 and 3). Since embryonic stem cells cannot give rise to trophocystic cells, they are by definition not totipotent but pluripotent. They can differentiate into derivatives of all three germ layers, namely the ectoderm, endoderm, and mesoderm. In general, embryonic stem cells have a higher proliferative capacity when compared to adult stem cells.

Figure 3. Generation of cardiomyocytes derived from embryonic stem cells. Undifferentiated embryonic stem cells are isolated from the inner cell mass of the blastocyst by immunological techniques. Under certain culture conditions these cells are able to proliferate without differentiation. If the desired cell number has been reached, culture conditions are changed to promote differentiation. So-called “embryoid” bodies are small cell clusters that contain cells of all three germ layers. In this image the bright areas express myosin heavy-chain indicating areas of cardiac differentiation.
Human embryonic stem cells
Isolation of embryonic stem cells from human blastocysts was described in 1998.42 Since then, numerous lines have been generated, most of them being propagated on non-human feeder layers, which is important in eliminating the risk of zoonosis. A better understanding of the mechanisms regulating proliferation and differentiation of human embryonic stem cells has resulted already in an improvement of propagation without feeder cells.43 In cell cultures, embryonic stem cells form three-dimensional aggregates, termed embryoid bodies, that contain tissue derivatives of all three germ layers.44
Human embryonic stem cells can propagate perhaps indefinitely in culture while maintaining pluripotency, including the ability to differentiate into cardiomyocytes and a stable karyotype. Such human embryonic cardiomyocytes, therefore, may provide an unlimited source for cell-based therapies. Electrically active, donor cardiomyocytes derived from human embryonic stem cells were shown to functionally integrate with otherwise-quiescent, recipient, ventricular cardiomyocytes to induce rhythmic electrical and contractile activities in vitro. Moreover, a functional human embryonic stem cell-derived pacemaker could be implanted in the left ventricle in vivo.5 Cardiac myocytes derived from human embryonic stem cells resemble fetal or embryonic cardiac myocytes with poorly organised sarcomeric structure. Embryonic stem cell-derived cardiac myocytes have spontaneous action potentials, contract spontaneously, respond to hormones and neurotransmitters,45 and will engraft and electrically and mechanically couple to host cardiac myocytes when transplanted into the heart.46 Embryonic stem cells might thus be a suitable source of donor cardiomyocytes for cell transplantation therapies aimed at restoring lost myocardial mass in diseased hearts.
Indications
Ischaemic heart disease
The vast majority of research regarding cardiac stem cell therapy is focused on either acute or chronic ischaemic heart disease. The reason for focusing on ischaemic heart disease is the importance of this aetiology for adult cardiology. Moreover, the high number of adults that can be considered for clinical studies, the severity of the disease, the still unsatisfactory results achieved by conventional therapies, and practical advantages such as frequent catheterizations that allow application of cells, as well as follow-up, make an application in this field likely. But even if some cell types might be more tolerant to hypoxia, or even metabolic restrictions, minimal nutritional requirements will be a prerequisite for the survival of transplanted cells. The dependence on a successful reperfusion and intact vascular structures to deliver therapeutic cells, therefore, makes heart attacks a challenging but not ideal pathophysiology for cardiac transplantation therapy. Ischaemic heart disease might not be the only cardiac disorder that can be expected to benefit from cell therapy, and ischaemic aetiologies are not very frequent in the setting of paediatric cardiac disease.
Other cardiac disorders
Theoretically, the benefit of cardiac transplantation should be better, and judgement of improvement easier, in chronic disorders where reperfusion therapy is not a prerequisite. A more frequent problem in paediatric cardiology results from remodelling, due to apoptosis induced by cardiac hypertrophy that, in the end, might result in heart failure. In these hearts, a compensatory increase in the diameter of the myocytes not matched by an adequate increase of the number of capillaries is thought to be causal. Similar to the ischaemic heart, pure cardiomyocytic transplantation might be insufficient, since a combination with vasculogenesis would be needed. With the exception of single papers on doxorubicin-induced cardiomyopathy,47 and viral myocarditis,48 no experimental data regarding other indications are available, and research in this area is necessary.
Safety
Formation of tumours and unwanted transdifferentiation
Undifferentiated stem cells are by definition tumourogenic. Human embryonic stem cells injected in immunodeficient mice produce teratomas that consist of cells of all three germ layers.42 Similarly, pluripotent stem cells can still be expected to differentiate into the various cell types that even under normal conditions would be derived from this cell line. For example, mesenchymal stem cells are precursor cells to muscle, bone, and other connective tissues. Cells derived from the bone marrow contain a fraction of mesenchymal stem cells that has the potential to differentiate into other mesenchymal cell types. It seems reasonable, therefore, and has already been reported under experimental conditions, that unselected bone marrow cells that have been injected into acutely infarcted myocardium can result in significant intramyocardial calcifications.49 Theoretically, the likelihood of formation of tumours, or unwanted differentiation, should be reduced by increasing the differentiation of stem cells, and purification of the desired cell type. Since the risk of formation of tumours increases with time, children are more likely to realize this side effect than older patients.
Arrhythmia
Cardiac rhythm can be influenced by cell therapy. Very different electrophysiological characteristics have been observed in stem cell-derived cardiomyocytes. Murine embryonic stem cell-derived cardiomyocytes show different electrophysiological characteristics reflecting different developmental stages and different cell types observed in the human heart, such as pacemaker-like cells or atrial and ventricular myocytes.50 Spontaneous activity, typical of embryonic cardiomyocytes, is frequently observed in cardiomyocytes derived from embryonic stem cells. Therefore, such cardiomyocytes have already triggered experiments suggesting them as a biological pacemaker.4, 46, 51 In contrast, severe arrhythmias that necessitated cardioverter implantation were observed after transplantation of skeletal muscle progenitor cells in humans,17 and are probably caused by a poor electrophysiological communication.52 Lack of connexin 43, the major ventricular gap junction protein, is highly arrhythmogenic, and remodelling of the gap junctions may play a key mechanistic role in arrhythmogenesis.53, 54
Immunology
Human embryonic stem cells are currently derived from embryos surplus to in vitro fertilisation. Though some data suggest that human embryonic stem cells might possess unique immune-privileged characteristics,55 allogeneic rejection must be feared.56 Similar immunological problems would arise from xenogeneic transplantation, for example of murine embryonic stem cell-derived cardiomyocytes.57 Interesting approaches other than immunosuppressive therapies have been suggested.56, 58 Immunomodulatory functions of human mesenchymal stem cells,59 or genetic manipulations modifying immunogenic major histocompatibility complex genes, might be used.60 Another strategy might be ‘therapeutic cloning’, also referred to as nuclear transfer or nuclear cloning.61 It denotes the introduction of a nucleus from an adult donor cell into an enucleated oocyte to generate a cloned embryo (Fig. 4).62, 63 The cytoplasma of this oocyte has the potential to reprogram the differentiated nucleus, and re-establish an embryonic pattern of gene expression in the chromatin of the somatic cell nucleus. If this cloned embryo is transferred to the uterus of a female recipient, this would be termed “reproductive cloning”, when explanted in culture, embryonic stem cells can be generated, called ‘therapeutic cloning’. Biologically, whole major histocompatibility complex regions in the derived embryonic stem cells and other immune molecules might be identical to that of the patient. The influence of the oocyte-derived mitochondrial proteins on the immunological response needs further studies.
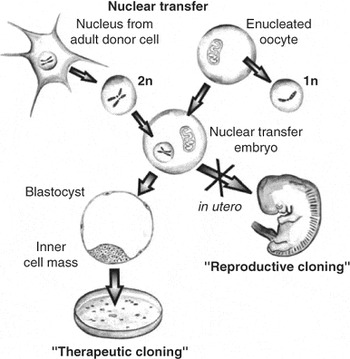
Figure 4. Nuclear transfer or transplantation. As opposed to the normal development where a haploid (1n) sperm and a haploid (1n) oocyte generate a diploid zygote (2n) during cloning the diploid (2n) nucleus of an adult cell is introduced into an enucleated oocyte, which divides after activation. Transferred into a surrogate mother this could give rise to a clone (‘reproductive cloning’), whereas ‘therapeutic cloning denotes the creation of an embryonic stem cell line that has the potential to differentiate in vitro into the desired cell type. These cells are immunologically almost identical to the adult donor cell. However, minor histocompatibility antigens encoded by the DNA of egg-derived mitochondria might differ.
Ethical considerations
The fear that application of human embryonic stem cells will have to involve therapeutic cloning to overcome problems of tissue rejection is one of the major ethical concerns. At the moment, the generation of human cells for stem cell research and therapy is based on cell lines generated from embryos surplus to the needs of in vitro fertilisation. This creation and manipulation of human embryos, that includes the destruction of a human blastocyst, for the purpose of generating therapeutic tissue, is questioned. The theological and ethical dimension of stem cell research is closely related to the definition of the beginning of human life. In addition, many people fear that the distinction between “therapeutic” and “reproductive” cloning is so slight that embryonic stem cells and therapeutic cloning might ultimately lead to reproductive cloning and genetically engineered human beings.64–67 Since our knowledge on stem cells is steadily increasing, and new biological techniques are evolving, this debate will have to continue. Repeated reassessment will be necessary.
Conclusion
Cardiac cell therapy holds the promise to regenerate heart muscle, not only after heart attacks in adults, but also in a variety of paediatric cardiac diseases. Theoretically, stem cells might be useful to generate bioprostheses, or to regenerate lost myocardial tissue, for example after myocarditis. Up to now, experimental data focus on the treatment of ischaemic injury. Clinical data in adults demonstrated a moderate beneficial effect using stem cells derived from bone marrow. The mechanism of improvement, however, is still a matter of debate. Thus, though it can be speculated that cardiac disorders of infancy and childhood might profit from stem cell therapy, direct experimental evidence is sparse. Probably the clinical use of either embryonic or adult stem cell technology in paediatric cardiology is still many years in the future. Nevertheless, in our opinion, the enormous potential of stem cells justifies an intense basic research on the physiology substantiating this therapeutic approach. The development of this option for treatment will need to be accompanied by an ethical discussion, and because of concerns for safety, it should proceed with the same scrupulousness, accuracy and criticism that has become standard today for every other medical innovation. Before this approach is ready to be tested clinically, therefore, far more preclinial experimental data is necessary, and though the proof of principle is done, the practical application still needs to be worked out.