Pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries (pulmonary artery/ventricular septal defect/major aortopulmonary collaterals) is a complex congenital malformation characterised by heterogeneous pulmonary artery anatomy.Reference Bull, Somerville, Ty and Spiegelhalter 1 , Reference Leonard, Derrick, O’Sullivan and Wren 2 Complete repair of this anomaly consists of unifocalisation of all major collateral vessels and partitioning of the circulation by eliminating intracardiac shunts (ventricular septal defect closure) and establishing right ventricle–pulmonary artery continuity.
The ultimate goal of surgical therapy is to achieve a complete repair with a low-resistance pulmonary vascular bed. Adequate unifocalisation of major aortopulmonary collaterals is a key strategy to achieving this goal. Although several unifocalisation techniques have been reported, the two main approaches include single-stage unifocalisation by median sternotomy and staged unifocalisation procedures by bilateral thoracotomies. Although both unifocalisation strategies continue to be practiced widely, some groups strongly advocate the use of one approach over the other,Reference Duncan, Mee and Prieto 3 – Reference Reddy, Liddicoat and Hanley 5 whereas some even favour repair without unifocalisation.Reference d’Udekem, Alphonso and Norgaard 6 The major advantage of multi-stage unifocalisation is the ability to perform anastomoses into hilar pulmonary arteries, which may be less prone to restenosis, as compared with anastomosis of the muscularised, arterial-like portion of collateral segments. Furthermore, distal stenosis within the major aortopulmonary collateral may be difficult to address through a median sternotomy approach.Reference Duncan, Mee and Prieto 3 , Reference Gupta, Odim, Levi, Chang and Laks 7 , Reference Mei, Ding, Zhu, Bao, Xie and Zhang 8 On the basis of this premise, it has been theorised that performing hilar anastomosis via thoracotomy results in fewer reinterventions on the branch pulmonary arteries. However, proponents of single-stage repair claim that branch pulmonary artery stenosis is not a significant problem,Reference Reddy, Liddicoat and Hanley 5 and early complete unifocalisation is necessary to prevent the development of pulmonary microvascular disease and degeneration of unrepaired collateral vessels.Reference Malhotra and Hanley 4 , Reference Reddy, Petrossian, McElhinney, Moore, Teitel and Hanley 9
The choice of unifocalisation strategy is largely driven by preoperative assessment of the highly heterogeneous pulmonary vascular tree. Conventional assessment tools, such as the Nakata IndexReference Nakata, Imai and Takanashi 10 and MacGoon Ratio,Reference Piehler, Danielson, McGoon, Wallace, Fulton and Mair 11 quantify the degree of mediastinal pulmonary artery hypoplasia. However, a key deficiency of contemporary surgical planning is the inability to accurately characterise the collateral vasculature. The functional status of collateral vessels has important prognostic and therapeutic implications. Accurate and reproducible assessment of major aortopulmonary collateral calibre and function could provide important prognostic information regarding the overall burden of pulmonary vascular disease. This may help inform a more patient-specific approach to surgical decision-making.
The purpose of this study was to review our centre’s experience in the surgical management of infants with pulmonary artery/ventricular septal defect/major aortopulmonary collaterals and introduce an imaging-based collateral vessel assessment tool.
Materials and methods
Clinical and anatomic data
The Institutional Review Board approved this retrospective study. Patients were identified using the cardiac surgery database. Medical records, baseline angiographic studies, operative notes, and catheterisation reports of all patients with a diagnosis of pulmonary artery/ventricular septal defect/major aortopulmonary collaterals who underwent unifocalisation procedure(s) were reviewed. Patients who did not undergo unifocalisation were excluded. All study patients underwent either single-stage (Group 1) or multi-stage unifocalisation repair (Group 2). Single-stage unifocalisation was defined as one operation that included unifocalisation of all significant collaterals, including bilateral vessels, into a central confluence through median sternotomy. Group 1 patients may or may not have undergone ventricular septal defect closure and/or right ventricle–pulmonary artery conduit insertion during the same procedure. Multi-stage unifocalisation was defined as unifocalisation approached through the left and right lateral thoracotomies, performed in separate procedures.
A single interventional cardiologist blinded to the surgical strategy independently reviewed the available preoperative angiographic studies. Angiographic measurements were recorded by calibration of imaging software to catheters of known size. Nakata Index was calculated for all patients in whom confluent mediastinal pulmonary arteries were visualised.Reference Nakata, Imai and Takanashi 10 The presence of right and left hilar pulmonary arteries was noted in patients with discontinuous pulmonary arteries. A new scoring system termed a collateral complexity score was devised in an effort to objectively assess baseline functionality of the pulmonary vasculature. The score is the sum of two components. The first component is the minimum number of arterial branches, regardless of vessel origin, requiring unifocalisation in order to provide flow to all lung segments – counted as 1 if confluent mediastinal pulmonary arteries were present and supplied all perfused segments. All arterial segments counted in the first component provided sole supply to a given lung segment. The second component is the number of angiographically detectable stenoses, defined as distal vessel larger than proximal lesion, at the lobar or segmental level located within arterial segments counted in the first component. Stenoses located at the origin of systemic arteries or the aorta were not counted (Fig 1).

Figure 1 Example angiograms from a cohort patient used to define collateral complexity score (CCS). ( a ) Four collaterals must be unifocalised (white arrows). A large collateral from base of the right innominate artery supplies the right upper lobe; two hypoplastic collaterals from the descending aorta supply the remainder of the right lung. A single hypoplastic collateral from the descending aorta ramifies to supply the entire left lung. ( b ) and ( c ) Distal stenoses involving branches to the left upper lobe ( b ) and superior segment left lower lobe ( c ), indicated by white arrows. ( d ) In the lateral projection of selective injection in the most inferior collateral, two distal stenoses (white arrows) in branches to the right middle and lower lobes are observed. In this case, CCS=8 (four collaterals+ distal stenoses).
Surgical approach
Single-stage unifocalisation, whereby all significant collaterals are detached from the aorta and implanted into a central confluence at a single operation through median sternotomy, has been well described.Reference Reddy, Liddicoat and Hanley 5 , Reference Carotti, Di Donato, Squitieri, Guccione and Catena 12 , Reference Lofland 13 Similarly, the multi-stage repair technique, consisting of staged unifocalisation procedures performed by bilateral thoracotomies, has been reported previously.Reference Gupta, Odim, Levi, Chang and Laks 7 , Reference Iyer and Mee 14
Study end points
The primary study end point was the number of branch pulmonary artery reinterventions – surgical or catheter-based – after initial unifocalisation procedure and after complete unifocalisation. Secondary end points were ventricular septal defect status and right ventricle/left ventricle pressure ratio at latest follow-up, and overall mortality.
Statistical analysis
Independent binary proportions were compared using Fisher’s exact test. Branch pulmonary artery reinterventions were compared using non-parametric Mann–Whitney U-test. Adjusted Poisson regression analysis assessed the association between collateral complexity score and number of branch pulmonary artery reinterventions. Pearson’s χ2 test was used to compare the percentage of patients in the two groups at each catheterisation. Generalised linear modelling and the likelihood ratio test were used to assess for differences in the number of reinterventions. Survival was determined by the Kaplan–Meier method and log-rank test. Analyses were performed with SPSS 23.0 (IBM Corporation, Armonk, New York, United States of America). p<0.05 was considered statistically significant.
Results
Baseline pulmonary vascular anatomy
Preoperative status of the native pulmonary arteries and aortopulmonary collaterals is described in Table 1. Pulmonary angiography allowed for assignment of a Nakata Index in 15 of 23 Group 1 patients and 16 of 22 Group 2 patients with documented native mediastinal pulmonary arteries. Outcomes of the blinded angiographic analysis are presented in Table 2. Group 2 had a greater number of lobar/segmental stenoses and a higher collateral complexity score compared with Group 1 (p = 0.02, Table 2).
Table 1 Patient characteristics.

ASD=atrial septal defect; MAPCA=major aortopulmonary collateral; PA=pulmonary artery
Data are presented as number (%) or median (range)
The bold values are all statistically significant with a p value < 0.05
Table 2 Pre-unifocalisation angiographic analysis of pulmonary vasculature.

PAs=pulmonary arteries
Data are presented as number (%) or median (range)
* Includes only arterial segments that provide sole supply to a given lung segment
** Number of distal stenoses identified within arterial segments counted in (i)
Unifocalisation strategy
From January 1996 to July 2015, 84 consecutive pulmonary artery/ventricular septal defect/major aortopulmonary collaterals patients underwent unifocalisation repair. In all, 41 patients underwent single-stage unifocalisation (Group 1), and 43 were treated by the multi-staged approach (Group 2). In Group 1, six patients had initial palliation with a right ventricle–pulmonary artery conduit before unifocalisation and one (2%) underwent aortopulmonary shunt. In Group 2, initial palliative procedures were performed in 15 patients – right ventricle–pulmonary artery conduit in 13, aortopulmonary shunt in 1, and modified Blalock-Taussig shunt in 1.
Treatment details and early outcomes
In all, seven patients in Group 1 had delayed ventricular septal defect closure by the central approach; median time to complete repair was 1.6 years – ranging from 5 months to 9.3 years. Of the 15 patients in Group 2 who underwent eventual ventricular septal defect closure, median time to complete repair was 1.7 years – ranging from 3 days to 7.8 years.
Survival outcomes
Overall mortality was 6 (15%) versus 9 (21%) for Groups 1 and 2, respectively. A period of 5-year actuarial survival was similar (Group 1: 85%; Group 2: 79%, p = 0.49 (Fig 2)). In Group 1, two deaths occurred within 30 days, two died within 1 year, and the remaining two deaths occurred at 2.8 and 6.8 years post unifocalisation, respectively. In Group 2, four patients died in-hospital, another four died within 1 year of initial unifocalisation, and one patient died 2.9 years after initial unifocalisation.
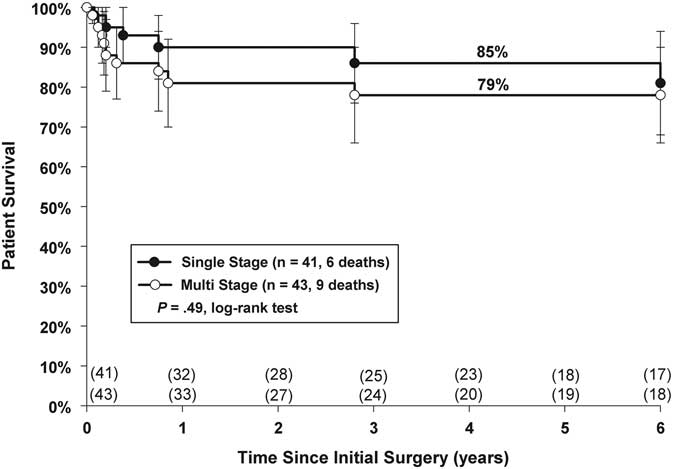
Figure 2 Kaplan–Meier actuarial survival analysis of single-versus multi-stage repair.
Reinterventions and follow-up
Median follow-up was 4.8 years, ranging from 6 months to 21 years, in Group 1 compared with 5.7 years, ranging from 5 months to 20 years, in Group 2, p = 0.65. Group 2 underwent a significantly higher number of catheter-based branch pulmonary artery reinterventions (Figs 3 and 4 and Table 3). Among patients who underwent at least one catheterisation after initial unifocalisation (Group 1, n = 34; Group 2, n = 39), the median number of branch pulmonary artery balloon dilations in Group 1 was 5 (inter-quartile range from 2 to 10) per patient compared with 9 (inter-quartile range from 5 to 17) in Group 2, p = 0.009, Figure 3. After complete unifocalisation, the median number of branch pulmonary artery reinterventions was 5 (a range from 1 to 17) versus 6 (a range from 1 to 42) in single-stage and multi-stage patients (p = 0.035). Baseline collateral complexity score correlated positively with the number of late branch pulmonary artery reinterventions (Spearman’s correlation=0.55, p⩽0.001; Fig 4). Patients who underwent one to five balloon dilation/stenting procedures had a median collateral complexity score of 3 (a range from 1 to 9), and those with six or more reinterventions had a median collateral complexity score of 6 (a range from 3 to 9), p = 0.004. Poisson regression modelling showed that the number of branch pulmonary artery reinterventions increase by a factor of 1.24 times per 1-unit increase in collateral complexity score, independent of the unifocalisation group.

Figure 3 Box–Whisker plot demonstrates median number of branch pulmonary artery (BPA) reinterventions in patients who underwent at least one catheterisation procedure after initial unifocalisation; BPA reinterventions include balloon dilation or stent procedures. PA=pulmonary artery.

Figure 4 Scatter-plot displaying the correlation between total number of branch pulmonary artery reinterventions and baseline collateral complexity score. PA=pulmonary artery.
Table 3 Catheter-based reinterventions on the pulmonary vasculature.

PA=pulmonary artery; VSD=ventricular septal defect
Data are presented as number (%) or median (range)
The bold values are all statistically significant with a p value < 0.05
Table 4 Ventricular septal defect (VSD) status and right ventricle (RV)/left ventricle (LV) pressure ratio among survivors at latest follow-up.

Data are presented as number (%) or median (range)
The bold values are all statistically significant with a p value < 0.05
Group 1 patients underwent a median of 2, a range from 1to 4, surgical procedures compared with 3, a range from 1 to 7, in Group 2 (p = 0.012). Both groups had similar numbers of right ventricle-pulmonary artery conduit revisions (Group 1, n = 25; Group 2, n = 26, p = 1.00), percutaneous PV replacement (Group 1, n = 5; Group 2, n = 4, p = 0.74), and late pulmonary arterioplasty procedures (Group 1, n = 16; Group 2, n = 17, p = 1.00). Among patients who achieved complete repair, 1.6 and 1.7 years after initial unifocalisation in Groups 1 and 2, the median right ventricle/left ventricle pressure ratio was 0.48 – a range from 0.38 to 1.01 – in Group 1 compared with 0.78 – a range from 0.39 to 1.15 – in Group 2, p = 0.03, Table 1. Late right ventricle/left ventricle pressure ratio of <0.5 was achieved in 14/22 (63%) single-stage patients, compared with 1/15 (7%) patients in Group 2, p = 0.0006.
Discussion
In this study, we evaluated all pulmonary artery/ventricular septal defect/major aortopulmonary collaterals patients who underwent unifocalisation by either single- or multi-stage approach at a single institution. Our analysis suggests that patients who received multi-stage unifocalisation had worse baseline pulmonary vascular anatomy. Specifically, the multi-stage cohort had a significantly greater number of lobar/segmental stenoses within collateral vessels compared with patients who underwent repair by the single-stage approach. Furthermore, patients selected for single-stage repair achieved good mid-term outcomes, with significantly fewer late reinterventions for branch pulmonary artery restenosis and lower right ventricle pressures compared with patients who underwent multi-stage repair.
Development of a preoperative imaging-based evaluation tool to accurately characterise the collateral circulation would help identify the optimal unifocalisation strategy for each individual patient. To date, several classification systems have been proposed. The Nakata IndexReference Caspi, Zalstein and Zucker 15 , Reference Di Donato, Jonas, Lang, Rome, Mayer and Castaneda 16 and McGoon ratioReference Hadjo, Jimenez and Baudet 17 , Reference Li, Zhang and Li 18 have been used to evaluate the central/intrapericardial pulmonary arteries. The total neopulmonary artery index, a combined index for all major aortopulmonary collaterals and the central pulmonary artery, has been shown to correlate with postoperative right ventricle/left ventricle pressure ratio.Reference Reddy, Petrossian, McElhinney, Moore, Teitel and Hanley 9 Moreover, others have attempted to classify pulmonary blood supply based on arbitrarily defined size parameters for native pulmonary vessels and major aortopulmonary collaterals.Reference Murthy, Krishnanaik, Coelho, Punnoose, Arumugam and Cherian 19 , Reference Griselli, McGuirk and Winlaw 20 A key limitation of existing evaluation tools is that preoperative anatomic findings do not uniformly correlate with functionality. For example, a whole lung field may be perfused by a single, unobstructed major aortopulmonary collateral; however, resistance within that lung field may vary considerably, depending on factors such as duration of exposure to high pressures and adaptive mechanisms within the lung vasculature.
We sought to overcome these limitations by developing a novel angiography-based scoring system to evaluate the calibre and functionality of the aortopulmonary collateral circulation. The collateral complexity score helped us to characterise the anatomic phenotype of the two surgical cohorts and thereby enabled a more enlightened interpretation of the late clinical outcomes observed in this study. Importantly, we found that a higher baseline collateral complexity score is associated with a greater number of late reinterventions for branch pulmonary artery stenosis, irrespective of unifocalisation strategy. The collateral complexity score may be a useful tool to identify the overall burden of pulmonary vascular disease and inform surgical decision-making regarding the optimal unifocalisation strategy in patients with pulmonary artery/ventricular septal defect/major aortopulmonary collaterals. Prospectively, we intend to evaluate the utility of the collateral complexity score as a surgical decision-making tool by including this in the preoperative assessment of patients who present for surgical repair at our institution.
The technical advantage of staged unifocalisation in pulmonary artery/ventricular septal defect/major aortopulmonary collaterals is that lateral thoracotomy facilitates better visualisation of the hilar pulmonary vessels and more readily enables performance of unifocalisation anastomoses to the distal thin-walled segment of AP collaterals.Reference Duncan, Mee and Prieto 3 , Reference Gupta, Odim, Levi, Chang and Laks 7 , Reference Iyer and Mee 14 , Reference Ishibashi, Shin’oka, Ishiyama, Sakamoto and Kurosawa 21 Conversely, single-stage midline unifocalisation often requires the use of the more proximal thick-walled, arterial-like portions of the major aortopulmonary collateral for anastomosis to the true pulmonary arteries. It has been theorised that the ability to perform distal anastomoses is key to reducing the long-term risk of branch pulmonary artery restenosis. Yet, in this study, staged unifocalisation did not reduce the risk of late branch pulmonary artery stenosis. In fact, rates of branch pulmonary artery reinterventions were significantly higher in multi-stage patients. One potential explanation for this finding is that staged unifocalisations prolong the exposure of major aortopulmonary collaterals to systemic arterial pressures, resulting in the development of obstructive lesions and local stenosis within unprotected collateral vessels.Reference Murthy, Krishnanaik, Coelho, Punnoose, Arumugam and Cherian 19 , Reference Davies, Mussa and Davies 22 , Reference Watanabe, Mainwaring, Reddy, Palmon and Hanley 23 In contrast, by moving major aortopulmonary collaterals to the low-flow, low-pressure pulmonary circulation at an earlier age, single-stage unifocalisation repair may enable greater long-term stability of collateral vessels. It has also been suggested that single-stage unifocalisation helps minimise the loss of lung segments related to degeneration of stenosed collateral vessels.Reference Malhotra and Hanley 4 , Reference Reddy, Liddicoat and Hanley 5
Nevertheless, it is important to acknowledge that at least part of the observed difference in branch pulmonary artery reinterventions relates to the discordant baseline anatomic substrates of the two surgical cohorts. Our angiographic analysis suggests that both groups had a similar number of functionally significant collaterals – i.e. vessels required to maintain supply to a specific lung segment; however, the number of distal stenoses within each collateral was notably higher in the multi-stage repair group. Furthermore, the presence of more distal stenoses at baseline appears to have been a key factor in surgical decision-making regarding the choice of unifocalisation strategy.
Critical to improving the long-term prognosis of this complex group of patients is achieving ventricular septal defect closure with sustained low right ventricle pressures.Reference Reddy, Petrossian, McElhinney, Moore, Teitel and Hanley 9 , Reference Murthy, Krishnanaik, Coelho, Punnoose, Arumugam and Cherian 19 , Reference Carotti, Albanese, Minniti, Guccione and Di Donato 24 Among patients who achieved complete ventricular septal defect closure, single-stage unifocalisation achieved significantly lower late right ventricle pressures compared with the staged approach. Although our baseline angiographic evaluation warrants a judicious interpretation of these haemodynamic outcomes, the data suggest that patients selected for single-stage unifocalisation can achieve excellent mid-term haemodynamic outcomes. Nonetheless, it remains unclear whether the patients selected for multi-stage repair in this study would have achieved higher rates of ventricular septal defect closure and lower right ventricle pressures at late follow-up if treated by the single-stage approach. In other words, although early, complete unifocalisation of aortopulmonary collaterals appears to facilitate the development of a low-resistance pulmonary vascular bed, and there may be a subset of patients, such as those with prominent distal stenoses, in whom the procedure of choice should be multi-stage unifocalisation. Furthermore, it has also been our institutional approach to treat some pulmonary artery/ventricular septal defect/major aortopulmonary collaterals patients with an initial palliative right ventricle to pulmonary artery conduit, followed by interventional catheterisation balloon dilations to encourage growth of the pulmonary vasculature. It has been our observation that when this approach is undertaken many lung segments are found to have dual arterial supply – i.e. from both the native pulmonary arteries and from a major aortopulmonary collateral. In these situations, occlusion of the major aortopulmonary collateral may be all that is necessary. This patient subset was not included in the current analysis.
This single-institution, retrospective study has several limitations. Angiographic data were unavailable in many patients. More deaths occurred in multi-stage patients – six of nine deaths – who underwent angiographic review compared with the single-stage cohort – one of six deaths. It is possible that we captured a more severe substrate of multi-stage patients and missed those single-stage patients who had worse baseline pulmonary vasculature. Variations in quality of preoperative angiography limit the certainty of the absolute counts of a minimum number of aortopulmonary collaterals needing to be unifocalised, as well as the number of distal stenoses. Furthermore, in many cases it was not possible to elicit the specific indications or rationale for performing single-stage versus staged repair. Surgeon-based preference was probably a major determinant of unifocalisation strategy.
In summary, the present study shows that single-stage unifocalisation is a promising repair strategy in selected patients with pulmonary artery/ventricular septal defect/major aortopulmonary collaterals, achieving lower rates of reintervention for branch pulmonary artery restenosis and superior haemodynamic outcomes compared with multi-stage approach at mid-term. However, it remains unclear whether specific anatomic substrates of pulmonary artery/ventricular septal defect/major aortopulmonary collaterals are better suited to repair by multi-stage unifocalisation. Preoperative evaluation of collateral vessel calibre and function may help inform more patient-specific surgical management and improve long-term outcomes.
Acknowledgements
The authors thank Hua Liu for statistical support.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.











