Background
According to the most recent international guidelines,Reference Thygesen, Mair and Mueller 1 , Reference Yancy, Jessup and Bozkurt 2 natriuretic peptides, and in particular the peptides related to the B-type cardiac peptide hormone – such as brain natriuretic peptide (BNP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) – are considered first-line biomarkers for the diagnosis of both acute and chronic heart failure in adult patients. In adult patients, the quality of the clinical evidence is in establishing that the measurements of BNP and NT-proBNP are useful in validating clinical judgement to confirm or exclude chronic ambulatory heart failure or acute decompensated heart failure.Reference Yancy, Jessup and Bozkurt 2 The value of natriuretic peptide testing is particularly significant when the aetiology of dyspnoea is unclear. Indeed, all international guidelines, dating from the beginning of the 21st century, state that lower levels of BNP or NT-proBNP actually exclude the presence of heart failure and higher levels have a reasonably high positive predictive value to diagnose heart failure in adult patients.Reference Thygesen, Mair and Mueller 1 – Reference Emdin, Clerico and Clemenza 7
Although interest in the measurement of BNP and NT-proBNP for the management and follow-up of children with acquired and CHD has progressively increased in recent years, no evidence-based guidelines or even expert consensus recommendations exist for this clinical setting at present.Reference Cantinotti, Giovannini, Murzi and Clerico 8 – Reference Cantinotti, Law and Vittorini 10 Several studies, however, do support the use of BNP/NT-proBNP in the screening, management, and follow-up of children with CHD, as recently reviewed in detail.Reference Cantinotti, Giovannini, Murzi and Clerico 8 – Reference Cantinotti, Law and Vittorini 10 Although all of these studies indicate that BNP/NT-proBNP is a reliable prognostic biomarker after paediatric cardiac surgery, there are some limitations, which may adversely affect the scientific validity and clinical implications of these data. Some of these studies are relatively small and underpowered, and large-scale multi-centre studies are limited. As yet, there are no prospective randomised clinical trials specifically designed to evaluate the cost-effectiveness of BNP/NT-proBNP use in paediatric cardiology/paediatric cardiac surgery.Reference Cantinotti, Giovannini, Murzi and Clerico 8 – Reference Cantinotti, Law and Vittorini 10
The goal of this review article is to provide an update of the evidence related to the use of BNP/NT-proBNP in paediatric cardiology and cardiac surgery with particular focus on CHD. The most important biochemical characteristics and the pathophysiological role of natriuretic peptides in paediatric cardiology and cardiac surgery are considered in the first section. In the second section, the clinical usefulness of the BNP/NT-proBNP assay for the diagnosis, prognosis, and follow-up of paediatric patients with CHD will be discussed in accordance with established evidence-based principles.Reference Sackett and Haynes 11 , Reference Price and Christensen 12
Biochemical characteristics of the B-type cardiac natriuretic peptide system
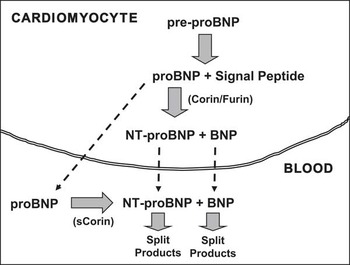
The human BNP gene in the cardiomyocyte nucleus encodes for a 134 amino-acid pre-proBNP1–134 molecule. This pre-proBNP molecule is split into a 108 amino-acid proBNP1–108 molecule – usually termed proBNP – as well as a 26 amino-acid signal peptide. Before being secreted from the cardiomyocyte into the bloodstream, proBNP is split by proteolytic enzymes, corin and/or furin, into two more peptides: a biologically inactive NH2-terminal peptide fragment called NT-proBNP1–76 and the COOH-terminal peptide fragment proBNP77–108, which is the biologically active 32 amino-acid peptide usually designated simply as BNPReference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 (Fig 1). In addition to the biologically active peptide hormone BNP and the inactive peptide NT-proBNP, a large number of circulating fragments, derived from proBNP, can be identified by chromatographic techniques in human plasma, including the intact and glycosylated forms of the precursor proBNP.Reference Liang, O'Rear and Schellenberger 15 – Reference Semenov, Tamm and Seferian 33 Some recent studies demonstrate that the intact and glycosylated forms of proBNP constitute a significant percentage of the immunoreactive B-type-related peptides circulating in the plasma of patients with heart failure.Reference Liang, O'Rear and Schellenberger 15 – Reference Semenov, Tamm and Seferian 33 These data, therefore, suggest an additional mechanism for synthesis of the biologically active BNP and inactive NT-proBNP that are found in the bloodstream. That is, these two peptides can also be produced from proBNP that is released into the bloodstream through enzymatic cleavage by plasma proteases, such as corin (Fig 1).Reference Jiang, Wu and Wang 34 – Reference Semenov, Seferian and Tamm 37
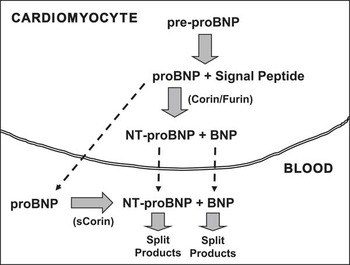
Figure 1 Schematic representation of production/secretion pathways of B-type natriuretic hormone and its related peptides. Human brain natriuretic peptide (BNP) is synthesised as a 134 amino-acid (aa) precursor protein (pre-proBNP) and is subsequently processed during secretion to form the 108-aa peptide, proBNP. The pro-peptide hormones of the cardiac natriuretic peptides can be enzymatically cleaved by at least two pro-protein convertases produced in the cardiomyocytes, such as corin and furin. In particular, proBNP is processed to form the 76-aa N-terminal peptide (NT-proBNP), and then the biologically active 32-aa C-terminal peptide called proBNP (or abbreviated BNP), which has a shorter plasma half-life of about 15–20 minutes versus 1–2 hours for NT-proBNP. There are, consequently, lower plasma concentrations of proBNP (BNP) compared with NT-proBNP. Moreover, the intact proBNP 108-aa peptide is also present in plasma, especially in the plasma of patients with heart failure, in both glycosylated and non-glycosylated forms.
From an analytical chemistry point of view, the marked heterogeneity of the B-type natriuretic peptides that are circulating in human blood may explain the large differences seen in the results reported for the different immunoassay methods that are considered specific to the biologically active peptide hormone BNP. On the other hand, one sees greater consistency in the results observed using the various immunoassay techniques for the NT-proBNP, perhaps because there is less heterogeneity in the circulating forms of that biologically inactive peptide.Reference Rawlins, Owen and Roberts 38 – Reference Franzini, Masotti and Prontera 41 For example, a recent study, using standardised protocols and quality control techniques, demonstrated that the IRMA method (Shionogi’s Diagnostic Division, Osaka, Japan), the ADVIA method for the Centaur platform (Siemens Health Care Diagnostics, Tarrytown, New York, United States of America), and the ST AIA-PACK method for the AIA platform (TOSOH Corporation, Tokyo, Japan) yielded significantly lower, as much as 50%, BNP levels compared with other immunoassays, such as the point-of-care test Triage method (Alere Diagnostics, Waltham, Massachusetts, United States of America), the BNP Triage Biosite for Access and UniCell DxI platforms (Beckman Coulter Diagnostics, Beckman Coulter Inc., Brea, California, United States of America), the MEIA method for the AxSYM platform (Abbott Diagnostics, Abbott Park, Lake Forest, Illinois, United States of America), and the chemiluminescent microparticle immunoassay for the ARCHITECT platform (Abbott Diagnostics).Reference Franzini, Masotti and Prontera 41 Luckenbill et alReference Luckenbill, Christenson and Jaffe 42 reported that many of these systematic differences between the various BNP immunoassay systems could be due to cross-reactivity between the glycosylated and non-glycosylated forms of the precursor proBNP. These data suggest that clinicians should be cautious when comparing results obtained from different laboratories using different BNP immunoassay methods. The reference levels for “normal” natriuretic peptide (BNP/NT-proBNP) levels in children differ according to age.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 , Reference Clerico, Zucchelli, Pilo, Passino and Emdin 43 – Reference Soldin, Soldin, Boyajian and Taskier 55 Plasma BNP/NT-proBNP concentrations are highest during the first 4 days of life, possibly due to the stress of the birth process and possibly due to adaptation of the neonate to postnatal circulation. These levels then rapidly fall during the 1st week, with a further slower progressive reduction throughout the 1st month of life (Table 1 and Fig 2). After the 1st month of life, BNP/NT-proBNP concentrations remain steady, without any significant change up to 12 years of age. There are generally no gender-related differences in “normal” BNP/NT-proBNP levels in children up to the beginning of adolescence.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 , Reference Nir, Lindinger and Rauh 44 , Reference Mir, Flato, Falkenberg, Haddad, Budden and Weil 45 , Reference Mir, Laux and Hellwege 48 , Reference Nir, Bar-Oz, Perles, Brooks, Korach and Rein 49 , Reference Maffei, Del Ry, Prontera and Clerico 56 Throughout adolescence, fertile girls show progressively higher circulating BNP/NT-proBNP levels than boys, probably because of a direct positive action of female sex steroid hormones on production of the cardiac natriuretic hormones by cardiomyocytes and a negative action of male sex hormones on this process.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 , Reference Maffei, Del Ry, Prontera and Clerico 56 – Reference Saenger, Dalenberg, Bryant, Grebe and Jaffe 58

Figure 2 Plasma brain natriuretic peptide (BNP) levels in healthy newborns throughout the first days of life. Plasma BNP values were measured in 188 healthy newborns throughout the first days of life. Plasma BNP concentrations are very high during the first 4 days of life; then values fall rapidly during the 1st week with a further slower progressive reduction throughout the 1st month of life. Plasma BNP was measured in the authors’ laboratory with the automated Access platform (Triage BNP reagents, Access Immunoassay Systems, REF 98200, Beckman Coulter Inc., Fullerton, California, United States of America). The trend was indicated by a continuous line, assessed by smooth spline analysis (data modified from referencesReference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Storti, Parri, Murzi and Clerico 54 , Reference Holmgren, Westerlind, Lundberg and Wahlander 78 ).
Table 1 Distribution of BNP values (ng/L) grouped according to four time periods from birth to 12 years of life measured in 253 healthy newborns and infants in the authors’ laboratory with the BNP triage biosite for access platform (by Beckman Coulter Diagnostics).

Range: minimum and maximum values
* Significantly higher than all subsequent time period values
** Significantly higher than the values observed throughout the subsequent time periods (i.e., 2–12 months and 2–12 years)
From a clinical standpoint, it is important to note that BNP/NT-proBNP levels may be affected by several physiological or clinical conditions. For example, neonatal twins usually have higher BNP/NT-proBNP concentrations than singletons. Moreover, babies of mothers with type 1 diabetes, prematurity, intrauterine growth retardation, caesarian section following uterine contraction, and intrauterine stress usually have higher BNP/NT-proBNP levels compared with neonates unaffected by these conditions.Reference Hammerer-Lercher, Puschendorf and Sommer 59 – Reference Kanbe, Maeno and Fujino 62
The pathophysiological role of BNP
The natriuretic peptides – atrial natriuretic peptide and BNP – are synthesised and secreted mainly by cardiomyocytes;Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 , Reference Goetze 16 however, there is evidence, especially in patients with chronic cardiac diseases, that atrial natriuretic peptide is preferentially synthesised in the atria, whereas BNP is preferentially synthesised in the ventricles.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 Synthesis and secretion of atrial natriuretic peptide and BNP may be regulated differently in atrial versus ventricular myocytes and, most likely also, during neonatal versus adult life.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 , Reference De Bold, Ma, Zhang, Kuroski de Bold, Bensimon and Khoshbaten 63 As a consequence, it is conceivable that two separate cardiac endocrine systems exist, one in the atrium, where atrial natriuretic peptide and its related peptides are preferentially produced, and the other in the ventricle, predominantly secreting BNP and its related peptides.
A normal ventricular myocardium may produce only a limited amount of BNP in response to an acute event such as a sudden rise in the end-diastolic pressure, probably through increased secretion of BNP that is already synthesised and is being stored in secretory granules in the cardiomyocyte cytoplasm. On the other hand, in the presence of chronic heart failure, an upregulation of the actual synthesis of BNP and its subsequent secretion from the cardiomyocyte into the bloodstream seems to be triggered by neuro-hormonal and immunological signals to the myocardium.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14
Wall distension is generally considered to be the primary mechanical stimulus for BNP production by ventricular tissue. This occurs in clinical conditions that are associated with electrolyte and fluid retention with consequent expansion of the effective plasma volume, as seen in primary or secondary hyperaldosteronism that accompanies cardiac, renal, and liver failure.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 Several studies, however, indicate that BNP synthesis/secretion may be regulated differently in normal versus diseased ventricular myocardium. Indeed, ventricular hypertrophy, especially associated with fibrosis, can stimulate BNP production.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 , Reference Sakata, Yamamoto and Masuyama 64 – Reference Walther, Klostermann, Hering-Walther, Schultheiss, Tschope and Stepan 66 Furthermore, some experimental and clinical studies indicate that myocardial ischaemia and, perhaps, hypoxia could induce the synthesis/secretion of BNP and its related peptides by ventricular cardiomyocytes, even if they are isolated and cultured.Reference Toth, Vuorinen and Vuolteenaho 67 – Reference Chun, Hyun and Kwak 72 Accordingly, BNP plasma concentrations can fluctuate widely depending on the surrounding pathophysiological stimuli and cardiovascular haemodynamics, both in healthy individuals and in patients with heart failure.Reference Clerico, Zucchelli, Pilo, Passino and Emdin 43 , Reference Clerico 73 , Reference Clerico, Zucchelli, Pilo and Emdin 74 The biologically active hormone BNP, however, has a shorter plasma half-life compared with its biologically inactive sibling, NT-proBNP. Consequently, BNP typically has a lower plasma concentration.Reference Clerico, Zucchelli, Pilo, Passino and Emdin 43 , Reference Clerico 73 , Reference Clerico, Zucchelli, Pilo and Emdin 74 In particular, it is estimated that BNP has a plasma half-life of about 15–20 minutes, whereas that of NT-proBNP is more than 60 minutes in healthy individuals.Reference Clerico, Zucchelli, Pilo, Passino and Emdin 43 , Reference Clerico 73 , Reference Clerico, Zucchelli, Pilo and Emdin 74 Furthermore, it is likely that the intra-individual biological variability for BNP and NT-proBNP ranges from 30 to 70% both in healthy individuals and in patients with heart failure.Reference Clerico, Zucchelli, Pilo, Passino and Emdin 43 , Reference Clerico 73 , Reference Clerico, Zucchelli, Pilo and Emdin 74 For these reasons, only variations in serial measurements of circulating levels of BNP and NT-proBNP higher than 30% should be considered to have clinical relevance in the follow-up of patients with heart failure.Reference Clerico, Fontana, Ripoli and Emdin 75 , Reference Januzzi and Troughton 76
BNP levels depend upon the type of CHD
Circulating BNP levels are more related to the type of CHD than to disease severity. On average, patients with complex CHD, such as a functionally univentricular heart and transposition of the great arteries, show higher BNP concentrations compared with those with simpler cardiac defects.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Storti, Parri, Prontera, Murzi and Clerico 77 – Reference Holmgren, Westerlind, Berggren, Lundberg and Wahlander 80 Generally speaking, BNP is higher in CHD that primarily involves the left rather than the right ventricle. Several studiesReference Cantinotti, Storti, Parri, Murzi and Clerico 54 , Reference Cantinotti, Storti, Parri, Prontera, Murzi and Clerico 77 , Reference Holmgren, Westerlind, Lundberg and Wahlander 78 , Reference Koch, Zink and Singer 81 , Reference Cowley, Bradley and Shaddy 82 have indicated that higher BNP concentrations are observed in patients with CHD characterised by left ventricular volume overload. For example, defects such as ventricular septal defect, patent ductus arteriosus, truncus arteriosus, and atrioventricular septal defects are associated with higher BNP levels than those found in patients with right ventricular volume overload – for example, atrial septal defect, anomalous pulmonary venous return – or pressure overload – for example, tetralogy of Fallot, pulmonary stenosis.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 Furthermore, defects characterised by left ventricular pressure overload, such as aortic stenosis or coarctation of the aorta, are associated with higher BNP levels compared with defects with right ventricular pressure overload. Furthermore, children with uncorrected pulmonary stenosis or tetralogy of Fallot usually show BNP levels only slightly higher than those of normal children.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10
From a clinical point of view, it is important to emphasise that several pathophysiological mechanisms and clinical conditions may affect plasma BNP levels, including associated cardiac defects; complex haemodynamic interactions between the ventricular chambers; the presence of extracardiac co-morbidity; systemic diseases; and the age and general status of the child.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10
BNP levels in children with cardiomyopathies
Relatively few data are available on BNP levels in children with cardiomyopathies;Reference Price, Thomas and Grenier 83 – Reference Saji, Takatsuki and Fujiwara 93 however, the usefulness of BNP as a diagnostic and/or prognostic biomarker has been tested in some cardiomyopathies, including dilated,Reference Price, Thomas and Grenier 83 , Reference Mir, Marohn, Läer, Eiselt, Grollmus and Weil 86 left ventricular non-compaction,Reference Price, Thomas and Grenier 83 Duchenne muscular dystrophy,Reference Price, Thomas and Grenier 83 inflammatory,Reference Price, Thomas and Grenier 83 , Reference Saji, Takatsuki and Fujiwara 93 ischaemic,Reference Price, Thomas and Grenier 83 oncologic,Reference Price, Thomas and Grenier 83 , Reference Kervancioglu 89 , Reference Aggarwal, Pettersen, Bhambhani, Gurczynski, Thomas and L'Ecuyer 92 , and mitochondrialReference Aggarwal, Pettersen, Bhambhani, Gurczynski, Thomas and L'Ecuyer 92 cardiomyopathies. Children with dilated cardiomyopathy usually have higher BNP levels compared with those with hypertrophic and restrictive forms.Reference Mir, Marohn, Läer, Eiselt, Grollmus and Weil 86 Several studies have reported that echocardiographic parameters, such as ventricular systolic and diastolic function, volume, and wall thickness, are significantly related to BNP/NT-proBNP levels.Reference Price, Thomas and Grenier 83 – Reference Mir, Marohn, Läer, Eiselt, Grollmus and Weil 86 Furthermore, the BNP and NT-proBNP assay is very useful in the early detection of left ventricular impairment in children with doxorubicin-induced cardiomyopathy,Reference Häusermann, Fasnacht, Hersberger, Gessler and Bauersfeld 87 – Reference Aggarwal, Pettersen, Bhambhani, Gurczynski, Thomas and L'Ecuyer 92 as well as in those with iron-overload cardiomyopathy in beta thalassemia major.Reference Phil, Khan and Tuyyab 91
BNP as a prognostic biomarker in paediatric cardiac diseases
The criteria for the determination of clinical utility and cost-effectiveness of a biomarker have been recently revised.Reference Pletcher and Pignone 94 – Reference Hlatky, Greenland and Arnett 96 These criteria require a specific and complex statistical approach,Reference Pletcher and Pignone 94 – Reference Hlatky, Greenland and Arnett 96 which is difficult to achieve in studies involving paediatric cardiac surgery. Measuring biomarker levels alone in fact is not sufficient for establishing clinical utility.Reference Pletcher and Pignone 94 – Reference Hlatky, Greenland and Arnett 96 Demonstrating a statistically significant association between a specific biomarker level and a given clinical outcome using regression analysis is necessary, but not sufficient, to demonstrate that particular biomarker’s predictive value according to these evidence-based criteria.Reference Pletcher and Pignone 94 – Reference Hlatky, Greenland and Arnett 96 Indeed, at least three other criteria, in addition to statistical significance, have been proposed for the rigorous evaluation of new biomarkers: discrimination, calibration, and reclassification.Reference Pletcher and Pignone 94 – Reference Hlatky, Greenland and Arnett 96 Unfortunately, of the studies performed thus far on the use of BNP/NT-proBNP assay in CHD, none have met these criteria.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 As discussed further in more detail, the existing evidence available on BNP-NT-proBNP in patients with CHD is derived from single-centre studies using multivariable models. Although these data are able to establish a statistically significant association between the BNP levels and certain clinical outcomes or events, they have not been shown to be superior to other clinical indicators derived from cardiac imaging or haemodynamic investigations.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10
BNP as a diagnostic biomarker in CHD
Several recent studies have demonstrated that BNP levels are associated with reasonable diagnostic accuracy (area under the curve values from receiver operating characteristic analysis ranging from 0.75 to 0.97) in differentiating between children with and those without CHD, when appropriate cut-off levels are used.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 As theoretically expected, the highest diagnostic accuracy was found when CHD patients were compared with a group of healthy individuals rather than with paediatric patients with respiratory or other non-cardiac diseases with similar symptomatology, such as dyspnoea.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 Several studies have used adult BNP clinical guidelinesReference Thygesen, Mair and Mueller 1 – Reference Hunt 6 to evaluate the usefulness of the BNP assay to differentiate between acute and chronic cardiac and respiratory diseases in children, in particular those admitted to a paediatric intensive care unit.Reference Davlouros, Karatza and Xanthopoulou 97 – Reference Law, Hoyer, Reller and Silberbach 102 These studies are not comparable because of a lack of homogeneity related to important differences in the populations of patients enrolled and because of the methods employed for BNP and NT-proBNP measurements, including the upper limit of normal.Reference Davlouros, Karatza and Xanthopoulou 97 – Reference Law, Hoyer, Reller and Silberbach 102 The reliability of the statistical analyses of several studies is also adversely affected by small sample sizes, with <50 CHD patients enrolled.Reference Maher, Reed and Cuadrado 100 , Reference Cohen, Springer and Avital 101 , Reference Flynn, da Graca, Auld, Nesin and Kleinman 109 Furthermore, the results of some studies are limited by significant differences in the clinical severity of the CHD in the enrolled patients. From a statistical point of view, this lack of clinical homogeneity makes it difficult to perform an accurate meta-analysis of data reported in the literature. For example, Koulouri et alReference Koulouri, Acherman, Wong, Chan and Lewis 99 reported a BNP cut-off value of 40 pg/ml for the diagnosis of heart failure versus respiratory diseases (sensitivity 91% and specificity 77%) in a group of 49 children with acute respiratory distress. By way of comparison, Choen et alReference Cohen, Springer and Avital 101 compared a group of 17 children with heart failure with another group of 18 children with acute lung disease. In this study, plasma NT-proBNP levels were significantly higher for infants with heart failure (median: 18452 ng/L; range: 5375–99,700 ng/L) than for infants with lung disease (median: 311 ng/L; range: 76–1341 ng/L). In a prospective study, Maher et alReference Maher, Reed and Cuadrado 100 enrolled 33 children with newly diagnosed congenital or acquired heart disease and 70 children with respiratory and infectious diseases. BNP levels were significantly higher in patients with cardiac diseases (mean 3290±1609 ng/L; range: >521–5000 ng/L) than in those with non-cardiac diseases (mean 17.4±20 ng/L; range: <5–174 ng/L).Reference Maher, Reed and Cuadrado 100 The only prospective and blinded study in the literatureReference Law, Hoyer, Reller and Silberbach 102 enrolled 100 children who presented to the paediatric intensive care unit. These authors divided the patients enrolled in the study into two groups according to age –42 neonates and 58 children – and then calculated different BNP cut-off levels for the two groups as follows: one for neonates 0–7 days of age (170 ng/L with sensitivity of 94% and specificity of 73%) and the other for older children (41 ng/L with sensitivity of 87% and specificity of 70%).Reference Law, Hoyer, Reller and Silberbach 102
BNP as a screening biomarker of CHD
From a clinical point of view, it is important to more accurately evaluate the usefulness of the BNP assay in screening for CHD. Recently, several authors recommended the use of pulse oximetry to screen for CHD in the neonatal and paediatric age groups.Reference Zhao, Ma and Ge 103 , Reference Thangaratinam, Brown, Zamora, Khan and Ewer 104 It is well known, however, that pulse oximetry may give inaccurate results in detecting some potentially life-threatening CHDs, such as coarctation of the aorta and lesions associated with left-to-right shunts in neonates. The addition of a BNP assay may increase the accuracy of neonatal screening programmes for the diagnosis of CHD,Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 , Reference Cantinotti, Vittorini and Storti 105 especially given the fact that is it now possible to measure BNP using a point-of-care test method with blood samples collected by pricking the heel or fingertip of neonates and children. This technique yields BNP levels that are comparable to those measured using an automated platform (Fig 3). The use of a point-of-care test method and blood samples collected by venipuncture allows for easier and less invasive BNP measurements, and thus it is more conducive for screening programmes in neonates and children, even in an ambulatory setting.
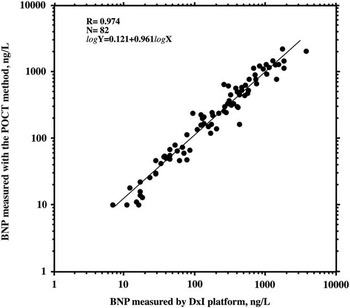
Figure 3 Linear regression between log-transformed brain natriuretic peptide (BNP) values measured with the automated DxI 800 platform (Triage BNP reagents) in 82 venous plasma samples (X-axis, log-scale) and BNP values measured with the point-of-care test method in the respective finger blood samples (Y-axis, log-scale). A very close linear regression was found between the BNP values measured with these two methods, as indicated by the results of the regression analysis reported in the figure.
BNP in the management of uncorrected CHD
Several studies support the usefulness of BNP/NT-proBNP measurements in the follow-up of patients after surgical correction/palliation of CHD, as well as in the follow-up of children with cardiomyopathies of various aetiologies.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 In particular, the clinical relevance of the BNP/NT-proBNP assay in the surgical ligation of neonatal patent arterial ducts was evaluated by several authors.Reference Czernik, Lemmer, Metze, Koehne, Mueller and Obladen 106 – Reference Kalra, DeBari, Zauk, Kataria, Myridakis and Kiblawi 111 Indeed, BNP levels after the first 3 days of life were able to identify the patients requiring patent ductus arteriosus surgical ligation.Reference Czernik, Lemmer, Metze, Koehne, Mueller and Obladen 106 , Reference Farombi-Oghuvbu, Matthews, Mayne, Guerin and Corcoran 107 – Reference Flynn, da Graca, Auld, Nesin and Kleinman 109 BNP/NT-proBNP levels also correlate with the magnitude of shunt, pulmonary artery pressure, pulmonary vascular resistance, and end-diastolic volume in the presence of a patent arterial duct.Reference Holmgren, Westerlind, Lundberg and Wahlander 78 , Reference Koch, Zink and Singer 81 , Reference Czernik, Lemmer, Metze, Koehne, Mueller and Obladen 106 – Reference Tosse, Pillekamp and Verde 110 From a clinical point of view, BNP levels may be helpful especially when it is difficult to decide whether it is necessary to treat and how to treat neonates with CHD associated with a left-to-right shunt.Reference Cantinotti, Assanta, Murzi and Lopez 112 BNP/NT-proBNP levels are higher in patients with left and right heart obstructive lesions than they are in normal individuals, and these biomarker levels usually correlate with oxygen saturation in cyanotic patients where BNP levels are higher.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 No correlation was found, however, between BNP levels and some functional or structural parameters, such as haemodynamic gradients or the degree of hypertrophy in patients with CHD characterised by left and right heart obstruction,Reference Koch, Zink and Singer 81 and therefore, further studies are needed to better clarify the role of BNP in these CHDs.
BNP in children undergoing cardiac surgery for correction/palliation of CHD
Increasing evidence, mainly derived from single-centre studies using regression analysis, supports the use of BNP-NT-proBNP levels as prognostic markers for children undergoing cardiac surgery for the treatment of CHD.Reference Berry, Askovich, Shaddy, Hawkins and Cowley 113 – Reference Heise, Lemmer and Weng 131 In particular, the independent association of BNP levels – especially those measured preoperatively – with important outcome events such as the duration of mechanical ventilation, ICU stay, need for inotropic support, and low cardiac output syndrome were reported.Reference Berry, Askovich, Shaddy, Hawkins and Cowley 113 – Reference Heise, Lemmer and Weng 131
The clinical interpretation of preoperative biomarker levels is relatively easier than the postoperative ones, because the latter may be affected by a huge number of confounding factors, such as patient age and disease severityReference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 , Reference Mir, Haun, Lilje, Läer and Weil 118 , Reference Cantinotti, Clerico and Iervasi 123 , Reference Price and Christensen 12 . In older children, postoperative biomarker levels usually increase compared with preoperative levels, peaking at 12–24 hours after surgery and remaining higher than the preoperative levels at the time of dischargeReference Mir, Haun, Lilje, Läer and Weil 118 – Reference Gessler, Knirsch, Schmitt, Rousson and von Eckardstein 120 , Reference Cantinotti, Lorenzoni and Storti 124 (Fig 4b). Neonates, however, on average show very high preoperative levels of BNP/NT-proBNP with a progressive decline in the biomarker levels after surgeryReference Cantinotti, Clerico and Iervasi 123 , Reference Cantinotti, Lorenzoni and Storti 124 (Fig 4a). This different postoperative pattern of biomarker kinetics between neonates and older children may in part be explained by the generally higher severity of neonatal CHD and by the greater complexity of neonatal surgical procedures; however, age falls out as an independent predictor of biomarker level behaviour in multivariable models that also include surgical complexity, estimated by both Aristotle score and risk adjustment in congenital heart surgery classification.Reference Cantinotti, Clerico and Iervasi 123 , Reference Cantinotti, Lorenzoni and Storti 124 In neonates, biomarker levels usually tend to progressively decline after the initial peak reached within the first 24 hours postoperatively.

Figure 4 Time-course of plasma BNP values in neonates (age<30 days, n=72) ( a ) and in children (age⩾30 days, n=115) ( b ) with congenital heart defects is reported in the figure. The results are expressed as boxes with five horizontal lines, displaying the 10th, 25th, 50th (median), 75th, and 90th percentiles of the variable. All values above the 90th percentile and below the 10th percentile (outliers) are plotted separately (as circles). The p-levels of statistical significance compared with the pre-surgery BNP values by Fisher’s protected least significant difference (PLSD) test after repeated measures ANOVA are also indicated in the figure. Log-transformed BNP values are used for the statistical analysis. Plasma BNP was measured in the authors’ laboratory with the automated Access platform (Triage BNP reagents, Access Immunoassay Systems, by Beckman Coulter).
From a clinical point of view, when biomarker levels do not fall appreciably or when they progressively increase after the early postoperative period, this may suggest complications and/or only partial surgical correction of the CHD.Reference Cantinotti, Clerico and Iervasi 123 , Reference Cantinotti, Lorenzoni and Storti 124 Furthermore, both in neonates and in older children, BNP levels at discharge after surgery remain, on average, higher than those found in normal individuals, thus indicating a slower, progressive recovery towards a “normal” haemodynamic balance, even in patients with well-corrected cardiac defects.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 , Reference Cantinotti, Clerico and Iervasi 123 , Reference Cantinotti, Lorenzoni and Storti 124
Finally, some studies have evaluated the role of BNP in children receiving a heart transplantReference Lindblade, Chun, Darragh, Caldwell, Murphy and Schamberger 125 – Reference Ationu, Sorensen, Whitehead, Singer, Burch and Carter 130 or during mid-term mechanical cardiac support.Reference Heise, Lemmer and Weng 131 These studies indicate that NT-proBNP and BNP are accurate in the prediction and detection of rejection and that biomarker levels decreased after instituting mechanical cardiac support. Further studies are needed to better demonstrate the role of the BNP/NT-proBNP assay in these specific clinical situations.
BNP in the follow-up of corrected/palliated CHD
This section discusses the role of biomarker levels in the follow-up of patients with some major groups of corrected and/or palliated CHD. These diagnoses include a surgically repaired functionally univentricular heart, congenitally corrected transposition of the great arteries, and tetralogy of Fallot.Reference Cantinotti, Giovannini, Murzi and Clerico 8 – Reference Cantinotti, Law and Vittorini 10
BNP in functionally univentricular cardiac circulation
Several studies support the use of biomarker levels in the follow-up of children with a functionally univentricular heart,Reference Holmgren, Westerlind, Berggren, Lundberg and Wahlander 80 , Reference Szymanski, Klisiewicz and Lubiszewska 132 – Reference Chow, Cheung and Chong 154 including those who have undergone staged palliation. Neonates with a functionally univentricular heart usually show the highest levels of BNP/NT-proBNP among all patients with CHD.Reference Cantinotti, Giovannini, Murzi and Clerico 8 – Reference Cantinotti, Law and Vittorini 10 Moreover, adult patients who have undergone Fontan palliation showed higher biomarker levels than those with other corrected/palliated CHD.Reference Ravishankar, Zak and Williams 151 Different morphological types of functionally univentricular hearts are often associated with important differences in biomarker levels, although it is not yet clear whether there is a significant difference in BNP production between functionally univentricular heart defects of a right ventricular versus left ventricular morphology.Reference Koch, Zink and Singer 81 , Reference Szymanski, Klisiewicz and Lubiszewska 132
BNP/NT-proBNP levels usually tend to decrease throughout the successive stages of Fontan palliation.Reference Lowenthal, Camacho and Lowenthal 134 , Reference Anderson, Sleeper and Mahony 139 Once an asymptomatic Fontan patient reaches school age and adolescence, on average, biomarker levels drop to the reference normal range.Reference Holmgren, Westerlind, Berggren, Lundberg and Wahlander 80 , Reference Heise, Lemmer and Weng 131 – Reference Lowenthal, Camacho and Lowenthal 134 In contrast, adolescents with a Fontan circulation and heart failure demonstrated biomarker levels that are significantly higher than those in patients with a Fontan circulation without heart failure. In these patients, biomarker levels correlate with disease severity as staged by the NYHA or New York University Pediatric Heart Failure scores.Reference Holmgren, Westerlind, Berggren, Lundberg and Wahlander 80 , Reference Lechner, Schreier-Lechner and Hofer 136 , Reference Anderson, Sleeper and Mahony 139 , Reference Wahlander, Westerlind, Lindstedt, Lundberg and Holmgren 147 BNP/NT-proBNP levels also correlate with several echocardiographic parameters including the severity of atrioventricular valve regurgitation,Reference Holmgren, Westerlind, Berggren, Lundberg and Wahlander 80 systolic ventricular dysfunction,Reference Saab and Aboulhosn 152 indices of diastolic dysfunction,Reference Robbers-Visser, Kapusta and van Osch-Gevers 144 and total ventricular mass on MRI.Reference Lechner, Schreier-Lechner and Hofer 136 The data on the relationship between BNP levels and peak oxygen consumption and/or the chronotropic index during exercise testing are conflicting,Reference Holmgren, Westerlind, Berggren, Lundberg and Wahlander 80 , Reference Hjortdal, Stenbog and Ravn 140 because only some, but not all, studies showed significant correlations.Reference Cantinotti, Giovannini, Murzi and Clerico 8 , Reference Cantinotti, Law and Vittorini 10 , Reference Anderson, Sleeper and Mahony 139 Some studies report that higher BNP levels are associated with poor outcomeReference Holmgren, Westerlind, Berggren, Lundberg and Wahlander 80 and worse neuro-developmental outcomes in infants with single-ventricle physiology.Reference Lechner, Schreier-Lechner and Hofer 136 – Reference Lechner, Gitter and Mair 146 , Reference Saab and Aboulhosn 152 From a pathophysiological point of view, it is important to note that the BNP level may help distinguish heart failure due to ventricular dysfunction from that due to isolated failure of a Fontan circulation,Reference Anderson, Sleeper and Mahony 139 since the circulating BNP is produced mainly by ventricular cardiomyocytes rather than by endothelial and vascular tissue.Reference Clerico, Recchia, Passino and Emdin 13 , Reference Clerico, Giannoni, Vittorini and Passino 14 Furthermore, plasma BNP has been shown to be higher in patients with classic atriopulmonary Fontan connection than in those with a total cavo-pulmonary connection.Reference Lechner, Schreier-Lechner and Hofer 136 , Reference Holmgren, Stromvall-Larsson, Lundberg, Eriksson and Wahlander 142 , Reference Man and Cheung 143
BNP in the systemic right ventricle
Several studies report that BNP may be useful as an additional biomarker in the follow-up of patients with a systemic morphological right ventricle after surgical palliation,Reference Schaefer, Tallone, Westhoff-Bleck, Klein, Drexler and Rontgen 155 – Reference Apitz, Sieverding, Latus, Uebing, Schoof and Hofbeck 166 including patients with a systemic morphological right ventricle in a Fontan circulation, in transposition of the great arteries after an atrial switch operation, and after physiological repair of congenitally corrected transposition of the great arteries.Reference Garg, Raman, Hoffman, Hayes and Daniels 158 , Reference Neffke, Tulevski and van der Wall 160 , Reference Dore, Houde and Chan 164 In adults with a systemic right ventricle, biomarker levels were higher than in controls, even in asymptomatic patients.Reference Schaefer, Tallone, Westhoff-Bleck, Klein, Drexler and Rontgen 155 – Reference Apitz, Sieverding, Latus, Uebing, Schoof and Hofbeck 166 In symptomatic patients, BNP levels correlated with the degree of heart failure staged by NYHA functional class.Reference Schaefer, Tallone, Westhoff-Bleck, Klein, Drexler and Rontgen 155 , Reference Larsson, Meurling, Holmqvist, Waktare and Thilen 156
Positive correlations between biomarker levels and some echocardiographic and MRI parameters, including right ventricular function,Reference Schaefer, Tallone, Westhoff-Bleck, Klein, Drexler and Rontgen 155 , Reference Larsson, Meurling, Holmqvist, Waktare and Thilen 156 , Reference Garg, Raman, Hoffman, Hayes and Daniels 158 , Reference Kozelj, Prokselj and Berden 163 , Reference Dore, Houde and Chan 164 end-diastolic volume,Reference Larsson, Meurling, Holmqvist, Waktare and Thilen 156 , Reference Koch, Zink and Singer 157 , Reference Neffke, Tulevski and van der Wall 160 , Reference Vogt, Kuhn, Wiese, Eicken, Hess and Vogel 165 and severity of tricuspid valve regurgitation,Reference Garg, Raman, Hoffman, Hayes and Daniels 158 , Reference Apitz, Sieverding, Latus, Uebing, Schoof and Hofbeck 166 were also demonstrated, whereas negative correlations were usually found with oxygen consumption peak values.Reference Koch, Zink and Singer 157 – Reference Plymen, Hughes and Picaut 159 , Reference Kozelj, Prokselj and Berden 163 , Reference Vogt, Kuhn, Wiese, Eicken, Hess and Vogel 165
BNP in tetralogy of Fallot
The clinical relevance of BNP in the integrated follow-up of patients after tetralogy of Fallot repair has been assessed in several studies.Reference Oosterhof, Tulevski, Vliegen, Spijkerboer and Mulder 167 – Reference Emdin, Vittorini, Passino and Clerico 186 Patients after tetralogy of Fallot correction can demonstrate a series of typical pathological sequelae, such as residual pulmonary regurgitation and/or stenosis, causing right ventricular dilatation, hypertrophy, or both. Patients with these cardiac alterations may be initially asymptomatic, but subsequently deteriorate progressively into having symptomatic heart failure.Reference Oosterhof, Tulevski, Vliegen, Spijkerboer and Mulder 167 , Reference Tulevski, Groenink, Van Der Wall, Van Velduisen, Boomsma and Stoker 168 At present, there are no solid guidelines for the management of these patients, in particular concerning the critical values of elevated right ventricular dimensions to take into account for an eventual re-intervention.Reference Oosterhof, Tulevski, Vliegen, Spijkerboer and Mulder 167 – Reference Emdin, Vittorini, Passino and Clerico 186 The use of BNP may provide important additional information both for the evaluation of asymptomatic individuals as well to grade and monitor the degree of heart failure.Reference Cantinotti, Giovannini, Murzi and Clerico 8 – Reference Cantinotti, Law and Vittorini 10
In the last decade, a large number of studies have reported significant relationships between circulating levels of biomarkers and several structural and functional parameters as assessed by echocardiography and MRI.Reference Oosterhof, Tulevski, Vliegen, Spijkerboer and Mulder 167 – Reference Villafañe, Feinstein and Jenkins 185 The majority of these studies have demonstrated a significant association between BNP levels and the degree of pulmonary regurgitation, right ventricular end-diastolic volume, and systolic pressure.Reference Ishii, Harada, Toyono, Tamura and Takada 171 – Reference Knirsch, Dodge-Khatami and Kadner 173 , Reference Tatani, Carvalho, Andriolo, Rabelo, Campos and Moises 177 – Reference Van den Berg, Strengers and Wielopolski 179 , Reference Norozi, Bahlmann and Raab 181 On the other hand, conflicting results were reported concerning the association between BNP levels with right ventricular function.Reference Ishii, Harada, Toyono, Tamura and Takada 171 – Reference Knirsch, Dodge-Khatami and Kadner 173 , Reference Tatani, Carvalho, Andriolo, Rabelo, Campos and Moises 177 – Reference Van den Berg, Strengers and Wielopolski 179 , Reference Norozi, Bahlmann and Raab 181 Increased BNP levels in patients with tetralogy of Fallot may be related not only to right ventricular dysfunction but also to impairment of left ventricular function by the dilated right ventricle.Reference Ishii, Harada, Toyono, Tamura and Takada 171 Several studies have reported a significant association between BNP levels and some cardiopulmonary parameters, including peak oxygen uptake, forced vital capacity, and the minute ventilation/carbon dioxide production ratio.Reference Dodge-Khatami, Büchel and Knirsch 169 , Reference Cetin, Tokel, Varan, Orun and Aslamaci 172 Finally significant reductions of BNP levels after all types of cardiac valve replacements have been well documented.Reference Norozi, Buchhorn and Bartmus 170 , Reference Koch, Zink, Glockler, Seeliger and Dittrich 174
Conclusions and future perspectives
The most recent international guidelines – a report of the American College of Cardiology Foundation and the American Heart Association task force – for the management of heart failure recommend B-type natriuretic peptides as the first-line biomarkers (level I, force of evidence A) for the diagnosis and prognosis of acute and chronic forms of heart failure and for both ambulatory and hospitalised adult patients.Reference Yancy, Jessup and Bozkurt 2 These recommendations are based upon a number of well-designed, randomised clinical trials, as well as on some meta-analyses reported in the literature on the clinical relevance of BNP in adult patients with heart failure.Reference Thygesen, Mair and Mueller 1 – Reference Emdin, Clerico and Clemenza 7 , Reference Emdin, Vittorini, Passino and Clerico 186 , Reference Januzzi 187
Clinical studies evaluating the relevance of BNP as a cardiac biomarker for paediatric cardiac diseases represent <10% of the total number of papers on the clinical usefulness of this cardiac biomarker. As the pathophysiological role of natriuretic peptides is similar in both adult and paediatric cardiac disease, it is conceivable that this discrepancy between clinical studies in the adult and paediatric populations is due to the difficulties related to the design of prospective, randomised clinical trials with an adequate number of patients with CHD, especially neonates. As CHD occurs in <1% of births per year, it is difficult to enrol an adequate number of patients in a single-centre study. Furthermore, some methodological problems and ethical considerations may limit the quality of paediatric clinical research.Reference Baer and Nelson 188 – Reference Ligi, Boubred, Grandvuillemin and Simeoni 190
The authors of this review hope that this gap between adult and paediatric clinical studies of BNP will be reduced in the near future. Certainly, international guidelines and/or expert consensus opinion on the use of BNP in paediatric patients with CHD are long overdue. The use of new point-of-care test methods – which use less invasive samples, such as capillary blood specimens – may contribute to a more widespread use of BNP assays, especially in neonates and infants, and to the development of screening programmes for CHD using this biomarker.
Summary
Increasing evidence supports the use of BNP/NT-proBNP levels as biomarkers in the integrated screening, diagnosis, management, and follow-up of patients with CHD. In particular, the prognostic role of BNP in children undergoing cardiac surgery has been demonstrated by several studies. Similarly, the role of BNP in the follow-up of some broad groups of patients with corrected/palliated CHD, including a functionally univentricular heart at various stages of palliation, systemic right ventricle, and tetralogy of Fallot, is well established. Most of the available evidence, however, derives from single-centre studies, using multivariable models, whereas randomised clinical trials designed to prove the cost-effectiveness of the routine use of BNP/NT-proBNP assays in paediatric cardiology/cardiac surgery are lacking. These prospective and randomised clinical studies are needed to demonstrate the usefulness of the routine use of natriuretic peptide assays in CHD. The results of well-designed clinical trials should promote the development of guidelines and expert consensus recommendations. The use of new point-of-care test methods may contribute to a more widespread use of BNP assays, especially in neonates and infants, as well as to the development of screening programmes for CHD.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None declared for M.C. and A.C. Y.L. has research support from Alere Inc.