Coarctation of the aorta is a CHD occurring in approximately 3 of 10,000 live births.Reference Torok, Campbell, Fleming and Hill 1 This defect may be accompanied by varying degrees of aortic arch hypoplasia. The diagnosis is based on echocardiographic studyReference Al Balushi, Sunny and Al Senaidi 2 and usually is an indication for an urgent surgery in the neonatal period. Coarctation of the aorta repair is an example of operation demanding a substantial experience in vascular anastomosis creation in time-limited neonatal left lateral thoracotomy conditions, as there is reduction of a blood flow in the patient’s lower body under passive mild hypothermia. The necessity to perform an extended end-to-end anastomosis on a delicate neonatal aortic tissue increases the difficulty of the procedure further.
Repetitio est mater studiorum. Success in performing any procedure originates from tedious process of learning and practicing. Surgeons have very few opportunities to train, aside from operation on living patient. It takes many attempts to acquire all technical skills necessary to perform safely and independently an operation without cardio-pulmonary bypass, such as coarctation of the aorta repair. Insufficient availability of courses exploiting human cadavers and ethical problems concerning operating on animals result in operating on patient being the most common way to gather essential experience and know-how. As learning process is inseparably bounded with making mistakes, culture of focusing on outcomes and general emphasis on patient’s safety stand in opposite to this approach. Using appropriate simulators may address mentioned issues.
A plethora of different medical simulators have been created with the purpose of training the next-generation physician in different fields of medicineReference Azzie, Gerstle and Nasr 3 – Reference Macfie, Webel, Nesbitt, Fann, Hicks and Feins 5 including cardiac surgery.Reference Ito, Shimamoto, Sakaguchi and Komiya 6 – Reference Shaikhrezai, Khorsandi and Brackenbury 8 Multiple repetitions of operative sequences during the simulation improve the trainees’ manual skills, increase self-confidence during these manoeuvres, and help to reduce stress during the first surgery on a real patient. All this accounts for potentially better clinical outcomes. During the development of aortic coarctation simulator, we hypothesised that deliberate practice with this training device could facilitate acquisition of aware automatism regarding safe manipulation of delicate aortic wall tissue and handling of fine monofilament suture (6-0, 7-0).
The objective of the study was to perform preliminary tests of aortic coarctation repair trainer, including evaluation of its surgical properties, as well as to assess and enhance residents’ skills by monitoring intraoperative surgical behaviour at different simulation difficulty levels.
Methods
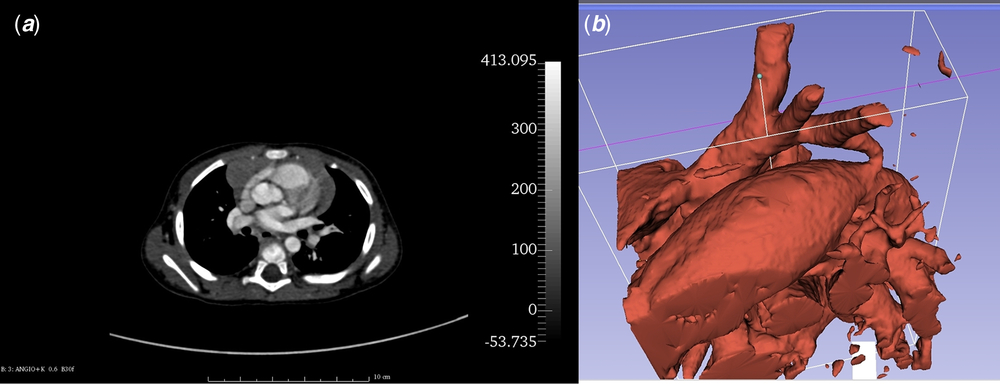
Patient’s anatomical data were collected from angio-CT scan obtained during ELECTROCARDIOGRAM-gated protocol (128-slice device; Siemens Somatom Definition AS, Siemens, Berlin, Germany) with the use of intravenous contrast agent (Visipaque; GE Healthcare AS, Oslo, Norway; dosage: 1.5 mg/kg, flow: 0.8 ml/second). Scan was performed after transthoracic echocardiogram turned out to be insufficient for surgical approach planning. Digital Imaging and Communications in Medicine (DICOM) images (Fig 1a) were processed by the open-source DICOM processing software – Slicer 3D (The Brigham and Women’s Hospital, Inc., Boston, Massachusetts, United States of America) (version 4.5.0, http://www.slicer.org).Reference Fedorov, Beichel and Kalpathy-Cramer 9 The volume representing aorta was segmented with Crop Volume and Threshold Effect Tools (Fig 1b) – only voxels with specific tissue density were selected and merged into a solid structure corresponding to the aorta and its branches. Then, the surface of the structure was translated into a mesh and saved as a stereolitography file. The mesh was processed using open-source 3D software: Blender (Stichting Blender Foundation, Amsterdam, Netherlands) (version 2.76; Blender Foundation, http://www.blender.org) and MeshLab (Istituto di Scienza e Tecnologie 78 dell’Informazione – Consiglio Nazionale delle Ricerche, Pisa, Italy) (version 2016.12; Istituto di Scienza e Tecnologie dell’Informazione – Consiglio Nazionale delle Ricerche, http://www.meshlab.net)Reference Cignoni, Callieri, Corsini, Dellepiane, Ganovelli and Ranzuglia 10 giving the final, magnified virtual model of coarctation of the aorta with hypoplastic aortic arch (Fig 2a).
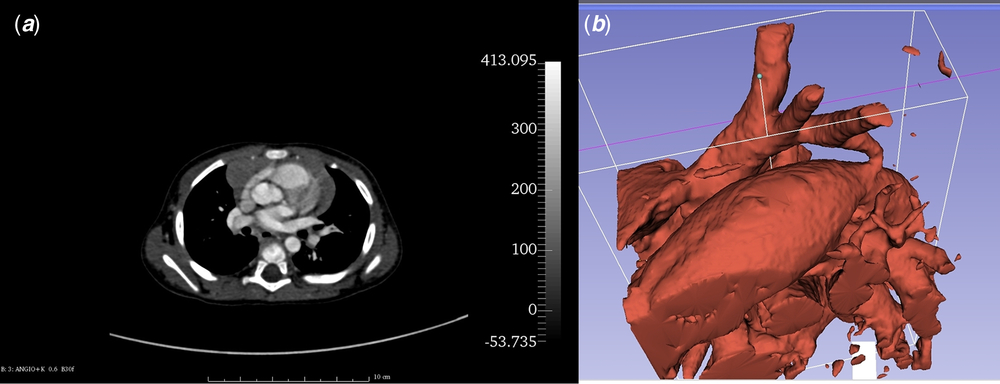
Figure 1. Imaging of the coarctation of the aorta with the use of angio-CT. ( a ) – patient’s angio-CT source image. ( b ) –Threshold Effect Tool allows to extract voxels representing given shade of grey. Image shows dilated patent ductus arteriosus, hypoplastic aortic arch with its three vascular branches, and the coarctation of the aorta.

Figure 2. Consecutive stages of silicone phantom creation. ( a ) Virtual model – result of angio-CT data processing. ( b ) 3D-printed, rigid hollow model. ( c ) Wax core ready to apply silicone coating. ( d ) Hollow elastic phantom before simulation.
The aortic coarctation prototype was 3D-printed using Selective Laser Sintering technology (EOS Formiga P110, material PA 2200; EOS, Monachium, Germany) – Figure 2b. This printed model was used to create a mould that served for wax copies casting (Fig 2c). The wax cores were painted with six layers of stretch resistant silicone (GUMOSIL M, Zakład Chemiczny, Silikony Polskie Ltd, Nowa Sarzyna, Poland) and melted after complete polymerisation yielding the ultimate elastic phantom (Fig 2d), which was magnified 2.4 times compared to patient’s aorta. The final diameter of the model’s transverse arch was 14.2 mm at the level of brachiocephalic trunk, 9.6 mm at the level of left common carotid artery, 8.6 mm at the level of left subclavian artery, and 5.5 mm at the level of isthmus. The phantom was fixed in the custom made mounting and placed on the operative table.
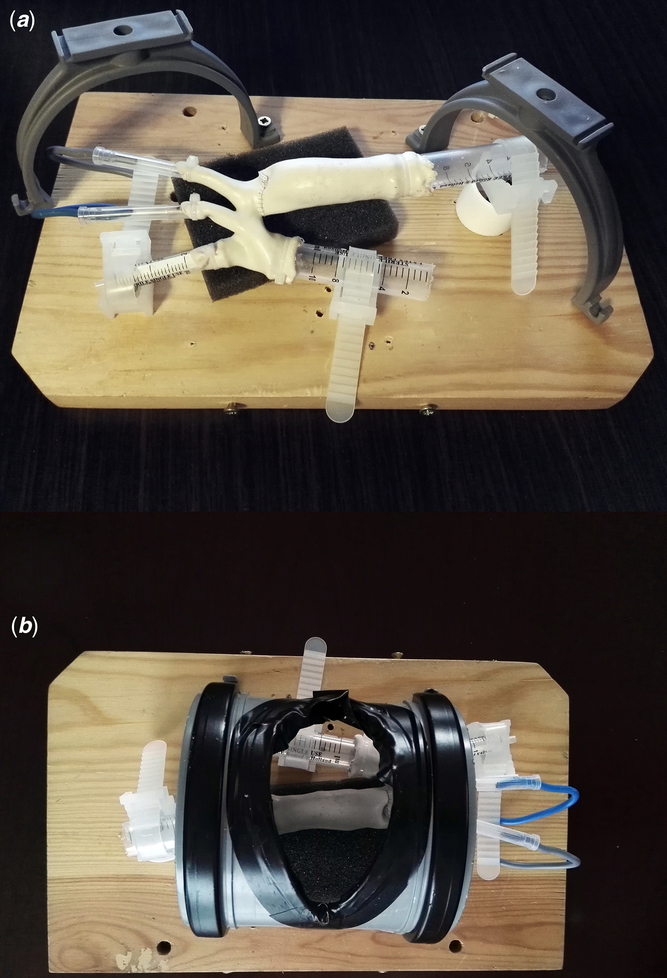
The simulator mounting was composed of a wooden plank of 30 cm × 18 cm × 2 cm, two drainage pipe holders to support a detachable sector of poly-vinyl pipe imitating thoracic wall during lateral thoracotomy and a piece of sponge supporting the descending aorta. The phantom was fixed with elements of disposable syringes, cable ties, and insulated copper wire (Fig 3).
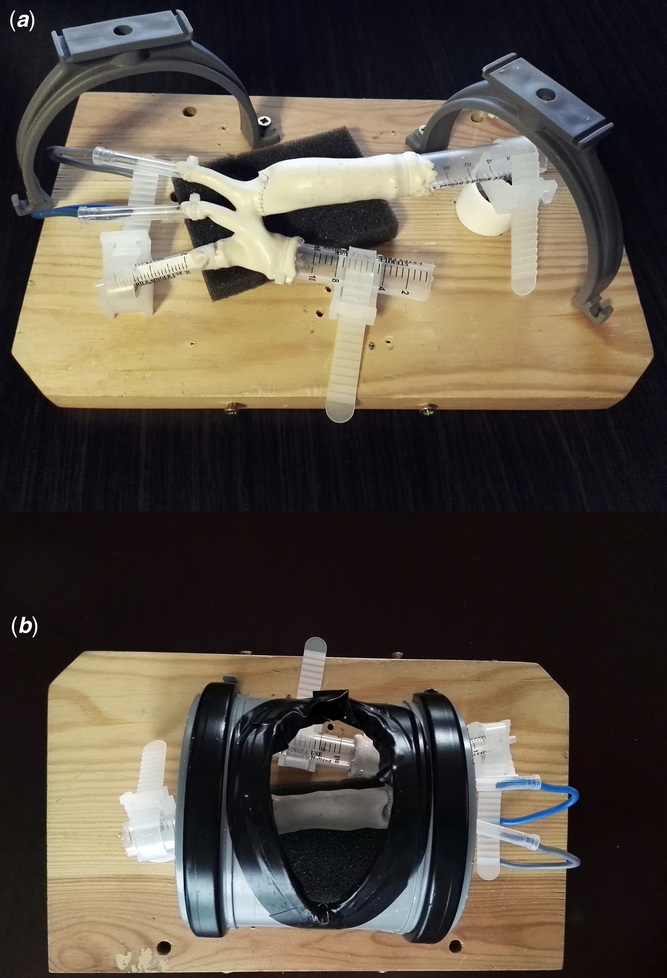
Figure 3. Simulator’s construction. ( a ) Phantom mounted inside the simulator’s basis – after simulation run – perspective of the assistant. Thoracic wall removed. ( b ) Phantom mounted inside the simulator’s basis, thoracic wall attached – surgeon’s perspective.
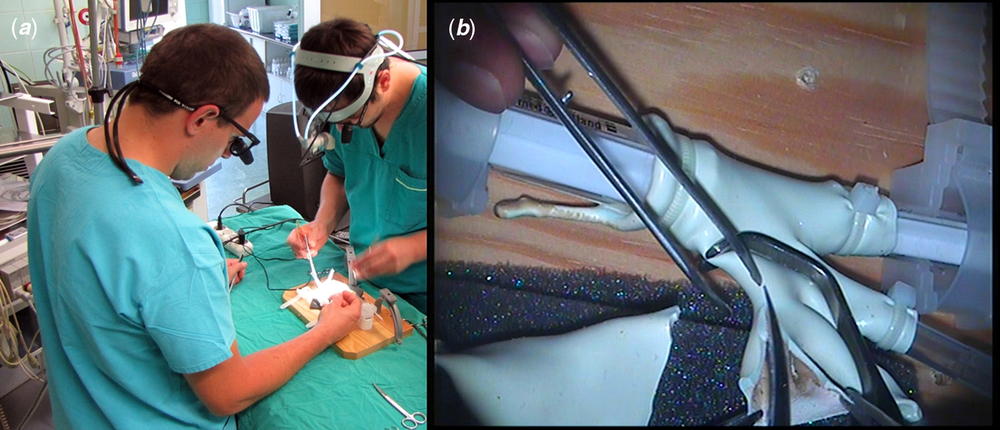
All simulations took place in an operating theatre environment (Fig 4a). Residents were equipped with surgical telescopes, and primary operator also held head-mounted flashlight. Proximal and distal aortic arch clamping, excision of patent ductus arteriosus, incision of lesser curvature of the arch as well as extended end-to-end anastomosis (with the use of Yavo Polipropylen 7-0 suture; Yavo, Bełchatów, Poland, http://yavo.com.pl) were simulated. Initial simulations were performed without the detachable part imitating the thoracic wall, greatly increasing manoeuvrability in the operative field (Fig 3a). In order to increase the difficulty level of the training, the thoracic wall element was mounted back on during final simulation runs (Fig 3b).

Figure 4. Simulation runs. ( a ) Simulation in operating theatre environment – two residents equipped with surgical telescopes and headlight during the simulation. ( b ) Operator’s view recorded with head-mounted camera – video frame showing incision of lesser curvature of aortic arch. The phantom is clamped proximally and distally (out of the cadre).
Residents’ intraoperative surgical behaviour was monitored by the analysis of video recorded from a head-mounted camera (Integra Luxtec DLX UltraLite Pro Camera, Plainsboro, New Jersey, United States of America) – Figure 4b. Parameters such as anastomosis time and mean one suture bite time were registered by frame-by-frame analysis of each simulation footage by DaVinci Resolve software (version 12.5; Blackmagic Design, Port Melbourne, Australia, https://www.blackmagicdesign.com/products/davinciresolve).
The robustness of anastomoses was assessed by the following water test. Each phantom was fixed to the mounting with the use of closed tip needle protectors or disposable syringes of different sizes occluding all five vascular ends in a water-tight manner. This enabled a connection of the phantom to a test hydraulic system composed of infusion set, three-way stop cock, IV bag with saline, and disposable 50 ml syringe. This system was connected to cardio-monitor (Infinity Delta XL, Dräger, Lübeck, Germany) in order to maintain a perfusion pressure of 100 mmHg generated by the syringe plunger. Every imperfection of suture line was revealed by leaking water and subsequently marked and counted. Leakage from areas where suture passed through silicone wall (suture holes) was not taken into consideration.
All variables analysed in this study were expressed as median with ranges. Intergroup comparisons were performed with the use of non-parametric Mann–Whitney U-test. The p-value not exceeding 0.05 was considered statistically significant. Statistical analyses were performed with the use of open-source R project statistical software (R Foundation, Vienna, Austria) (R: A Language and Environment for Statistical Computing, version 3.3, https://www.r-project.org).
Results
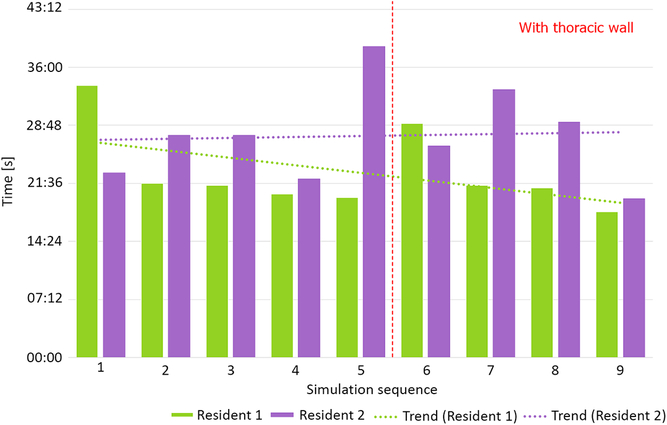
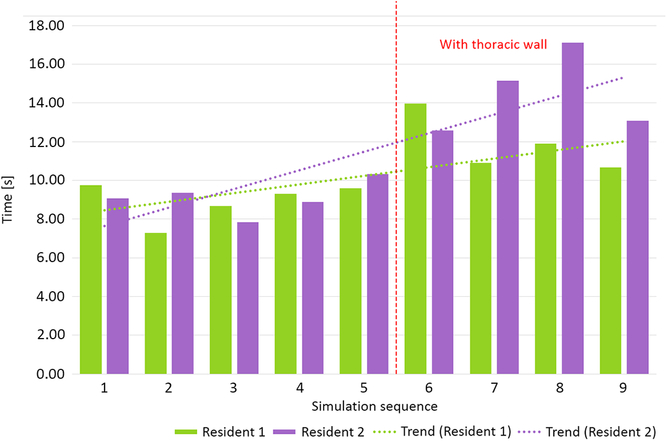
Two residents performed nine simulation runs each. The last four runs were performed with thoracic wall attached. The phantoms performed well, enabling tissue-like handling and cutting, excellent suture retention, and satisfactory elasticity. Median anastomosis time during simulation without thoracic wall was 23′33″ (20′50″–39′35″). Simulations with attached thoracic wall yielded a median anastomosis time of 24′47″ (18′5″–33′14″). This difference was not statistically significant (p = 0.63, Fig 5). Median suture bite times were 9″ (7″–10″) and 13″ (11″–17″) for simulations, respectively, without and with the thoracic wall (p < 0.001, Fig 6). Similarly, there were significantly more suture bites applied during simulation without (163.5, range from 124 to 224) compared to with the thoracic wall (111.5, range from 90 to 131, p = 0.0013).
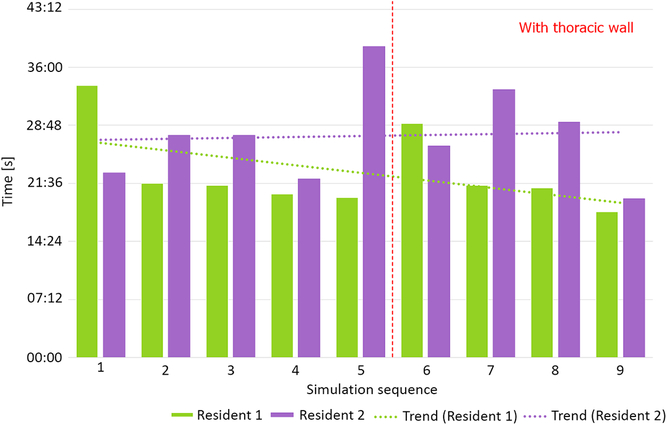
Figure 5. Total anastomosis time without and with thoracic wall.
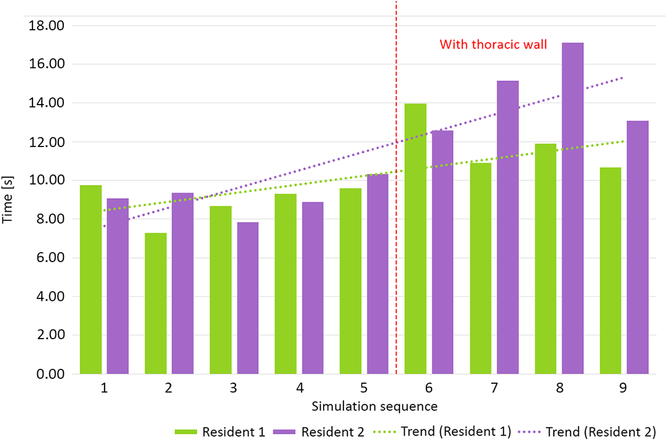
Figure 6. Mean suture bite time without and with thoracic wall.
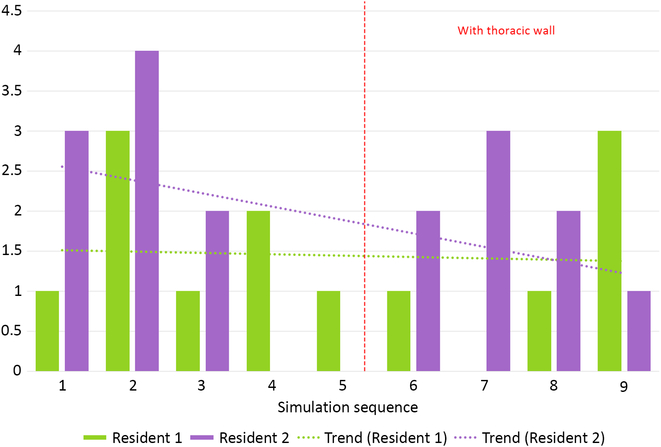
Median number of leakages per phantom was 2 (0–4) for simulation without thoracic wall and 2 (0–3) for more difficult simulation (p = NS, Fig 7). There were no significant inter-resident differences in all assessed parameters.
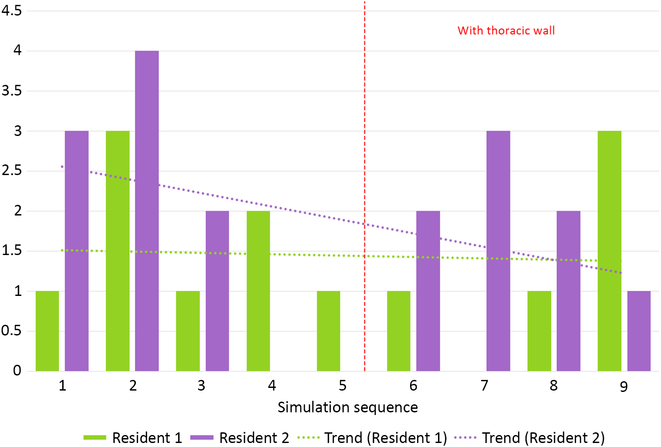
Figure 7. Total number of leakages during all simulation runs performed by two residents.
Discussion
The development of medical technology, the emphasis on patient safety during surgery, and increase of the quality of postoperative care have improved the outcomes of modern paediatric cardiac surgery. This has increased public expectations as well. Therefore, the optimal training of cardio-surgical residents is challenging for all accredited cardiac units. Surgical simulators are interesting and useful tools for future cardiac surgeons’ training. Surgical simulators increase dexterity,Reference Alonso-Silverio, Pérez-Escamirosa and Bruno-Sanchez 11 , Reference Millán, Rey and Lopez 12 which is an indispensable component of surgical skills.
The dynamic development of different medical simulations created a new branch of scienceReference Okuda, Bryson and DeMaria 13 and made surgical simulators a recognised training mean in surgery, especially in cardiac surgery, neurosurgery, general surgery, and vascular surgery.Reference Willaert, Aggarwal, Van Herzeele, Cheshire and Vermassen 14 However, currently, there is no literature description of a surgical simulator dedicated to coarctation of the aorta repair training. PubMed search (dating from 25 June, 2017) with all combinations of the following query: [“surgical” or “blank field”] and [“CoA” or “coarctation of the aorta” or “coarctation of aorta”] and [“repair” or “correction” or “blank field”] and [“simulator” or “trainer” or “simulation”] yielded no results.
Several reports concerning the use of silicone to produce vascular models in neurosurgery,Reference Abla and Lawton 15 – Reference Mashiko, Otani and Kawano 17 paediatric surgery,Reference Cheung, Looi, Lendvay, Drake and Farhat 18 or cardiovascular surgeryReference Sardari Nia, Heuts and Daemen 19 , Reference Håkansson, Rantatalo, Hansen and Wanhainen 20 are available. Similarly, vascular phantoms utilised in our aortic coarctation repair simulator were made of elastic silicone. This material enabled a good surgical performance as well as, thanks to its water-tightness, made leakage tests possible for anastomosis quality assessment.
Currently, 3D printing technology is widely used and being evaluated in modern cardiac surgery. It is possible to create detailed heart and great vessel models that allow surgeons to understand precisely the anatomy and facilitate optimal operation planning.Reference Sardari Nia, Heuts and Daemen 19 – Reference Valverde, Gomez and Gonzalez 23 Valverde et alReference Valverde, Gomez and Gonzalez 23 showed in their paper that using an exact 3D replica of patient’s organ can help to choose the most efficient surgical approach, especially in complex heart diseases. Ma et alReference Ma, Tao and Chen 22 remarked that measures taken on the preoperative 3D models corresponded with actual intraoperative measurements. Shmauss et alReference Schmauss, Haeberle, Hagl and Sodian 21 proved the usability of 3D reconstruction in many various sophisticated cases not only in perioperative planning but also in simulation of procedures and intraoperative orientation as well.
Simulators based on 3D life-like replicas of the human anatomy have serious potential to improve cardiothoracic resident training. Hermsen et alReference Hermsen, Yang and Burke 24 designed curriculum based on 3D-printed model to teach residents septal myectomy. Choice of the procedure was probably non-contingent, as it is very difficult to teach due to limited visibility, remarkably variable anatomy, and significant potential complications. Evaluation after five simulation runs showed that resections performed by residents became as effective as performed by attending surgeon.
Simulation makes possible for surgeons to develop new skills by trial and error without risking patient’s health and life. Sardari Nia et alReference Sardari Nia, Olsthoorn, Heuts and Maessen 25 used high-fidelity endoscopic simulator to produce the suturing map for mitral valve annuloplasty. It was then successfully used during European Association for Cardio-Thoracic Surgery (EACTS) endoscopic mitral valve courses as a standardised teaching tool.
Low price, high usability and simplicity of production are desirable features of surgical simulators.Reference Abla and Lawton 15 , Reference Chueh, Wakhloo and Gounis 16 , Reference Ma, Tao and Chen 22 In the presented model, a relatively expensive 3D printing technology was applied to obtain a semi-product that enabled subsequent low-cost single model production. However, end-product – elastic silicone phantom production was time-consuming due to the necessity of repeated silicone painting and drying followed by wax core melting. The direct use of 3D-printed models made of suitable surgical materials for aortic coarctation repair simulation is a potential solution. However, at the time of the development of the simulator, this technology was not available for both technical and financial reasons.
Our aortic coarctation repair trainer was well accepted by the residents willingly taking part in simulation runs. Objective benchmark parameters such as anastomosis time could be assessed and compared between simulation runs revealing, for example, a slight trend towards faster and faster anastomosis at both difficulty levels (Fig 5 – Resident 1). A surgical “obstacle” – the thoracic wall – was a factor that made a simulation more difficult and more realistic (Fig 6). This was reflected by the significant increase of single suture bite time and decrease of suture bite count per anastomosis. These two factors combined gave total anastomosis times similar to those without thoracic wall. It is noteworthy that even less suture bites were taken, the quality of anastomosis did not suffer as shown by incidence of leaks.
The biggest advantage of designed water test is possibility to perform it under physiologic (or supra-physiologic for a neonate) pressure and objectivity of suture line evaluation. Dynamic tests of anastomosis are currently at the design stage.
The presented coarctation of the aorta repair simulator can be a useful training tool for residents thanks to its good surgical properties and the possibility of imitating real anatomical relations of a coarctation of the aorta. What is noteworthy, it allows scaling the complexity of the simulated procedures from the simple procedures (end-to-end) to the complex ones (extended end-to-end) by preparing adequate models. Simulation difficulty degree associated with a size of the phantom and its wall thickness can be modified depending on the level of training advancement – as models used in primary tests were magnified, it is possible to prepare life-sized phantoms for further practice. Addition of the element that simulates the chest wall and restricts the space in the operating field reflects the real difficulties during the operation of a newborn. Moreover, a patient-specific image data from the angio-CT/MRI studies can be used to practice a planned procedure before a real operation.
Our trainer does not replicate the real anatomy of structures surrounding and adhering to the aorta – heart, lungs as well as pericardium and both visceral and parietal pleurae. The phantoms are devoid of intercostal arteries, making the descending aorta more mobile than in reality. Only one pattern of silicone phantoms used in all simulation runs does not represent anatomical variety existing in real patients. As the model was magnified, it does not mimic all technical challenges a surgeon has to manage when dealing with petite organs of a neonate. All these limitations make the trainer a medium-fidelity simulator targeted as vascular anastomosis learning tools in a space constrained environment.
Conclusions
This medium-fidelity CoA repair trainer showed its feasibility in replication of major critical steps of the real operation. Objective surgical efficiency parameters could be obtained from each simulation and compared between trainees and at different adjustable difficulty levels. Further studies taking into account higher number of simulation runs are needed to evaluate trainees’ learning progress.
Acknowledgements
None.
Financial Support
This study was financed from statutory funds of the Department of Paediatric Cardiac Surgery.
Conflicts of Interest
None.