Introduction
The peritrophic membrane/matrix (PM) is a chitin-protein matrix that surrounds the food bolus in the midgut of the majority of insects (Bolognesi et al., Reference Bolognesi, Terra and Ferreira2008). In some insects, however, the PM is absent and is replaced by an extra-cellular lipoprotein membrane called the perimicrovillar membrane (PMM) (Silva et al., Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004). This PMM allows insects to exploit restricted ecological niches during all postembryonic stages (Terra & Ferreira, Reference Terra, Ferreira, Gilbert, Latrou and Gill2005; Damasceno-Sá et al., Reference Damasceno-Sá, Carneiro, DaMatta, Samuels, Terra and Silva2007). The PMM is present only in some members of the superorder Paraneoptera, including some species of medical and economic importance such as the Reduviidae, vectors of Trypanosoma cruzi (Lane & Harrison, Reference Lane and Harrison1979), the parasite that causes Chagas disease. Chagas disease (American trypanosomiasis) is a human disease endemic in large areas of Latin America.
This review provides an overview of the digestive tract in insects and changes that have occurred in different phylogenetic groups with an emphasis on the midgut of the Paraneoptera. Subsequently, we discuss the presence of the PMM and its interaction with pathogens and how the PMM may become a target to reduce populations of insects of economic and medical importance.
Insect digestive system
General morphology
The digestive system in insects comprises the salivary glands and the alimentary canal, which are involved in digestion, absorption and feces elimination (Terra & Ferreira, Reference Terra, Ferreira, Resh and Cardé2009). The salivary glands open into the cibarium and the saliva lubricates the mouthparts (Terra et al., Reference Terra, Ferreira, Baker, Lehane and Billingsley1996). The alimentary canal, moreover, can broadly be divided into foregut, midgut and hindgut (Billingsley & Lehane, Reference Billingsley, Lehane, Lehane and Billingsley1996).
The foregut begins at the mouth and includes the buccal cavity; the esophagus, crop and proventriculus. The crop is a storage organ in many insects although it also serves as a site for digestion in some species. The proventriculus is a triturating organ and in most insects serves as valve controlling the entry of food into the midgut (Chapman, Reference Chapman1998). The midgut is a tube with a ventriculus (anterior and posterior ventriculus) and sacs (gastric or midgut cecae) usually at the anterior end (Billingsley & Lehane, Reference Billingsley, Lehane, Lehane and Billingsley1996). The midgut is the principal site for digestion and absorption of nutrients but there is, however, considerable variation in midgut arrangement and structure depending on the insect order and on the diet (Billingsley & Lehane, Reference Billingsley, Lehane, Lehane and Billingsley1996; Terra & Ferreira, Reference Terra, Ferreira, Resh and Cardé2009). The last section of the digestive system is the hindgut which includes the ileum, colon and rectum. This is the section where water absorption and excretion occur (Billingsley & Lehane, Reference Billingsley, Lehane, Lehane and Billingsley1996; Chapman, Reference Chapman1998). In the region of the sphincter (pylorus) separating the midgut from the hindgut, Malpighian tubules used for excretion and osmoregulation, end between midgut and hindgut (Terra, Reference Terra1988).
The midgut epithelium of insects comprises three cell types: cells involved in enzyme secretion and absorption (digestive cells), those with endocrine functions (endocrine cells) and those that play a role in replacement of epithelium (regenerative cells) (Billingsley & Lehane, Reference Billingsley, Lehane, Lehane and Billingsley1996; Pinheiro et al., Reference Pinheiro, Quagio-Grassiotto and Gregório2008; Teixeira et al., Reference Teixeira, das, Marques-Araújo, Zanuncio and Serrão2015). Unlike many other animal groups, insects do not produce glycosylated proteins such as mucins to separate the meal from the epithelium (Tellam et al., Reference Tellam, Wijffels and Willadsen1999), but have developed different membranes or matrices, often referred to as the PM, or PMM, that cover the surface of the intestinal tract and function as a protective lining for the epithelium.
PM
The PM was described by Balbiani (Reference Balbiani1890) as a membranous sac that directly surrounds the food in the lumen. The PM is not a cell component, but forms a cylindrical film or sheet that lines the lumen of the midgut between the midgut columnar cells and the ingested or digested food (fig. 1a). The PM is made up of a matrix of chitin, glycosaminoglycans and proteins (Eisemann & Binnington, Reference Eisemann and Binnington1994; Liu et al., Reference Liu, Zhang, Li, Zhu, Ma and Zhang2012; Moussian, Reference Moussian2013). Although it impedes direct contact of food with the striated border of columnar cells, this membrane permits the passage of digestive enzymes in the direction of the midgut lumen and the absorption of products resulting from digestion that are subsequently eliminated with the feces, preventing mechanical injury and hindering or impeding the entry of pathogens (Lehane & Billingsley Reference Lehane and Billingsley1996; Pinheiro et al., Reference Pinheiro, Quagio-Grassiotto and Gregório2008). Furthermore, the PM hinders the free movement of molecules, dividing the midgut lumen into two compartments: endoperitrophic space and ectoperitrophic space (Dow, Reference Dow1987; Terra & Ferreira, Reference Terra, Ferreira, Resh and Cardé2009; Kuraishi et al., Reference Kuraishi, Binggeli, Opota, Buchon and Lemaitre2011; Moussian, Reference Moussian2013; Teixeira et al., Reference Teixeira, das, Marques-Araújo, Zanuncio and Serrão2015).

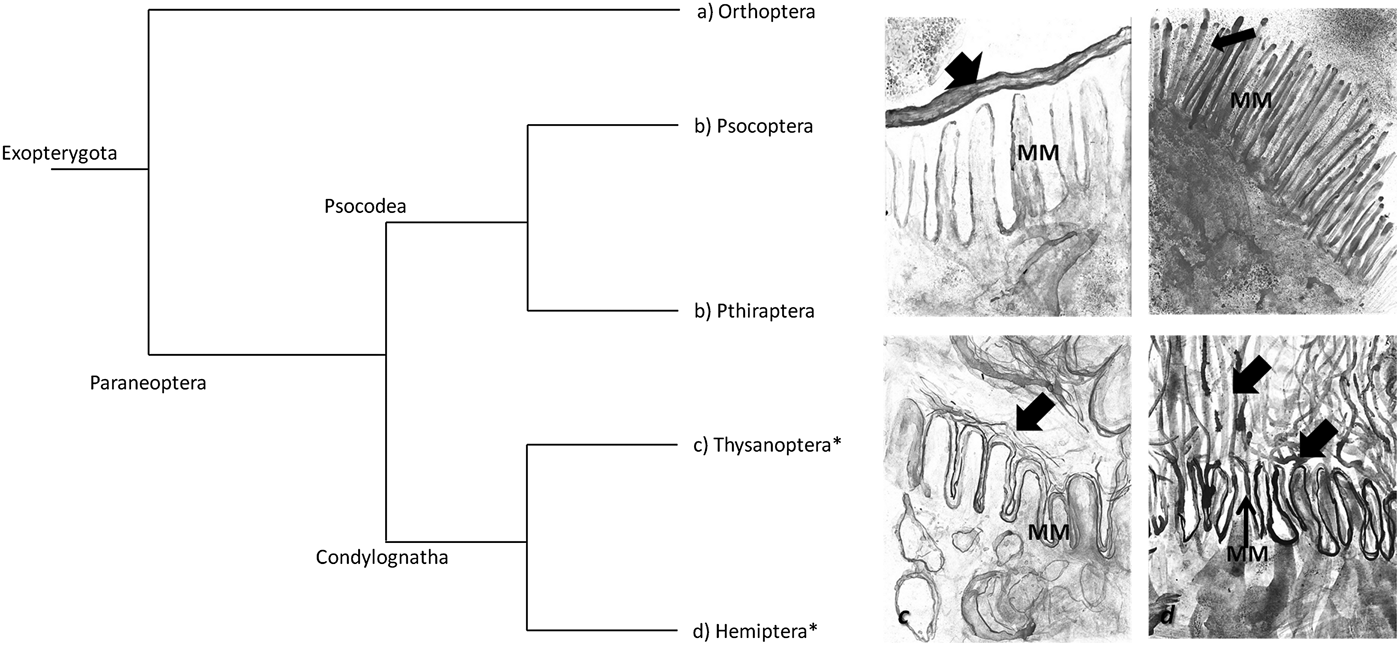
Fig. 1. Phylogeny of Paraneoptera and schematic representation of the different membranes in Exopterygoya insect orders. The Paraneoptera group includes two superorders Psocodea, which comprises the orders Psocopetra (booklice, bark lice and psocids) plus Phthiraptera (sucking and biting lice) and the superorder Condylognatha that includes the order Thysanoptera (thrips) and Hemiptera (suborders Homoptera and Heteroptera). The schematic representation of different membranes to separate the meal from the epithelium are: (a) peritrophic membrane in insects belonging to the Polyneoptera group; (b) peritrophic gel in Psocoptera (booklice) and Phthiraptera (lice); (c) kind of perimicrovillar membrane in Thysanoptera order; and d) perimicrovillar membrane in Hemiptera. Arrows = membranes of the midgut in the different orders of insects; DM = microvilli; asterisks show the orders which have perimicrovillar membrane.
The structure of the PM is thought to result from chitin fibrils being interlocked with chitin-binding domains of peritrophins (Moussian, Reference Moussian2013). Mucin-like domains line the ectoperitrophic and endoperitrophic sides of the PM (Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012). As these domains are highly hydrated, they lubricate the surface of the PM, easing the movement of food inside the PM and in the ectoperitrophic fluid outside the PM (Terra, Reference Terra2001; Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012). The PM is classified into two types (Peters, Reference Peters1992; Marques-Silva et al., Reference Marques-Silva, Serrão and Mezêncio2005). Type I PM (primarily studied in lepidopteran larvae and dipteran adults) is produced along the midgut epithelium (Marques-Silva et al., Reference Marques-Silva, Serrão and Mezêncio2005; Teixeira, et al., Reference Teixeira, das, Marques-Araújo, Zanuncio and Serrão2015) and is induced by the distension of the gut caused by food ingestion (Terra, Reference Terra2001). This type of PM is found in Coleoptera, Dictyoptera, Ephemeroptera, Hymenoptera, Odonata, Orthoptera, Phasmida, larval Lepidoptera and adult hematophagous Diptera with subtle differences (Peters, Reference Peters1992; Hegedus et al., Reference Hegedus, Erlandson, Gillott and Toprak2009). Type II PM is produced in a restricted region called the cardia that separates the foregut from the midgut (Marques-Silva et al., Reference Marques-Silva, Serrão and Mezêncio2005; Teixeira, et al., Reference Teixeira, das, Marques-Araújo, Zanuncio and Serrão2015). This PM is found in some orders of Polyneoptera, such as Dermaptera, Isoptera and Embiodea and some species of Lepidoptera, and the larvae of Diptera (Hegedus et al., Reference Hegedus, Erlandson, Gillott and Toprak2009). PM production control is poorly understood; in some insects (i.e. mosquitoes), ingestion of a meal induces PM production, but whether this effect is direct (food in the midgut) or indirect (via endocrine pathways) is unknown (Peters, Reference Peters1992). The protein components of PM I and PM II are similar. PM proteins have been classified into four types: class 1 proteins, thought to be digestive enzymes and food proteins loosely absorbed at the PM surface; class 2 proteins, proteins enclosed in membrane vesicles trapped between PM sheets; class 3 and 4 proteins, integral proteins of the PM named peritrophins, characterized by the presence of chitin-binding domains and mucin-like domains (Tellam et al., Reference Tellam, Wijffels and Willadsen1999; Geer et al., Reference Geer, Domrachev, Lipman and Bryant2002). Furthermore, Tellam et al. (Reference Tellam, Wijffels and Willadsen1999) proposed four classes of PM proteins based on the ease with which they can be removed from the PM. Class I proteins are removed with physiological buffers and represent loosely associated proteins, likely digestive enzymes and food remnants. Class II proteins are extractible with mild detergents, such as sodium dodecyl sulfate, that disrupt weak ionic interactions, whereas Class III proteins are released only with strong denaturants, such as urea. Class IV proteins cannot be removed by any means and are likely covalently linked to other proteins or chitin.
The PM has multiple functions which are associated with its ability to compartmentalize the gut. The compartmentalization increases the efficiency of the digestion of polymeric molecules (Silva et al., Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004). The characteristics of the PM can be grouped according to their role in digestion: semipermeability, enzyme immobilization, counter-current flow, water and ion movement, all of which facilitate absorption (Ferreira et al., Reference Ferreira, Capella, Sitnik and Terra1994; Terra & Ferreira, Reference Terra and Ferreira1994; Agrawal et al., Reference Agrawal, Kelkenberg, Begum, Steinfeld, Williams, Kramer, Beeman, Park, Muthukrishnan and Merzendorfer2014); and protection of the midgut epithelium: food abrasion and invasion by microorganisms (Lehane, Reference Lehane1997; Bolognesi et al., Reference Bolognesi, Terra and Ferreira2008; Hegedus et al., Reference Hegedus, Erlandson, Gillott and Toprak2009; Kuraishi et al., Reference Kuraishi, Binggeli, Opota, Buchon and Lemaitre2011). The class III proteins known as peritrophins (nonmucin peritrophins and invertebrate intestinal mucins) are involved in the protection of the midgut epithelium (Hegedus et al., Reference Hegedus, Erlandson, Gillott and Toprak2009) Thus, insects lacking a PM may have the midgut cells damaged and may be subject to invasion by microorganisms (Tellam, Reference Tellam, Lehane and Billingsley1996; Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012). Although protection is thought to be the ancestral function of PM, new functions have been added during the evolution of insect digestive tracts (Terra, Reference Terra1988). These may include: (a) prevention of nonspecific binding of undigested material onto midgut membrane hydrolases and/or transport proteins; (b) prevention of enzyme excretion by permitting the endo-ectoperitrophic circulation of digestive enzymes; (c) mechanisms to ensure that monomers produced from food remain close to the surface of the midgut cells (Terra, Reference Terra1988; Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012). These functions not only guarantee protection but also an effective digestive machinery. The presence of the PM is characteristic of almost all insects (Lehane, Reference Lehane1997; Hegedus et al., Reference Hegedus, Erlandson, Gillott and Toprak2009; Terra & Ferreira, Reference Terra, Ferreira, Resh and Cardé2009).
PMM
Among the few insects that lack a PM are adult ants (Hymenoptera), most adult moths and butterflies (Lepidoptera) and Bruchidae (Coleoptera) (Peters, Reference Peters1992). One possible reason for this absence of PM in Hymenoptera and Lepidoptera is that these animals feed almost exclusively on low-molecular weight substances such as sugar, which does not require luminal digestion (Terra, Reference Terra2001; Waniek, Reference Waniek2009). Rather than a PM, some insects have a peritrophic gel in their midgut which is the case of Bruchidae (Terra, Reference Terra2001). Based on these data, it was concluded that the PM should be absent in insects lacking luminal digestion (Terra, Reference Terra2001). However, in insects with a diluted diet and lacking a PM, they produce a second external membrane to the microvillar membrane (MM), which may have an analogous function and is called the PMM. This is the case of some members of Paraneoptera group (figs 1 and 2)

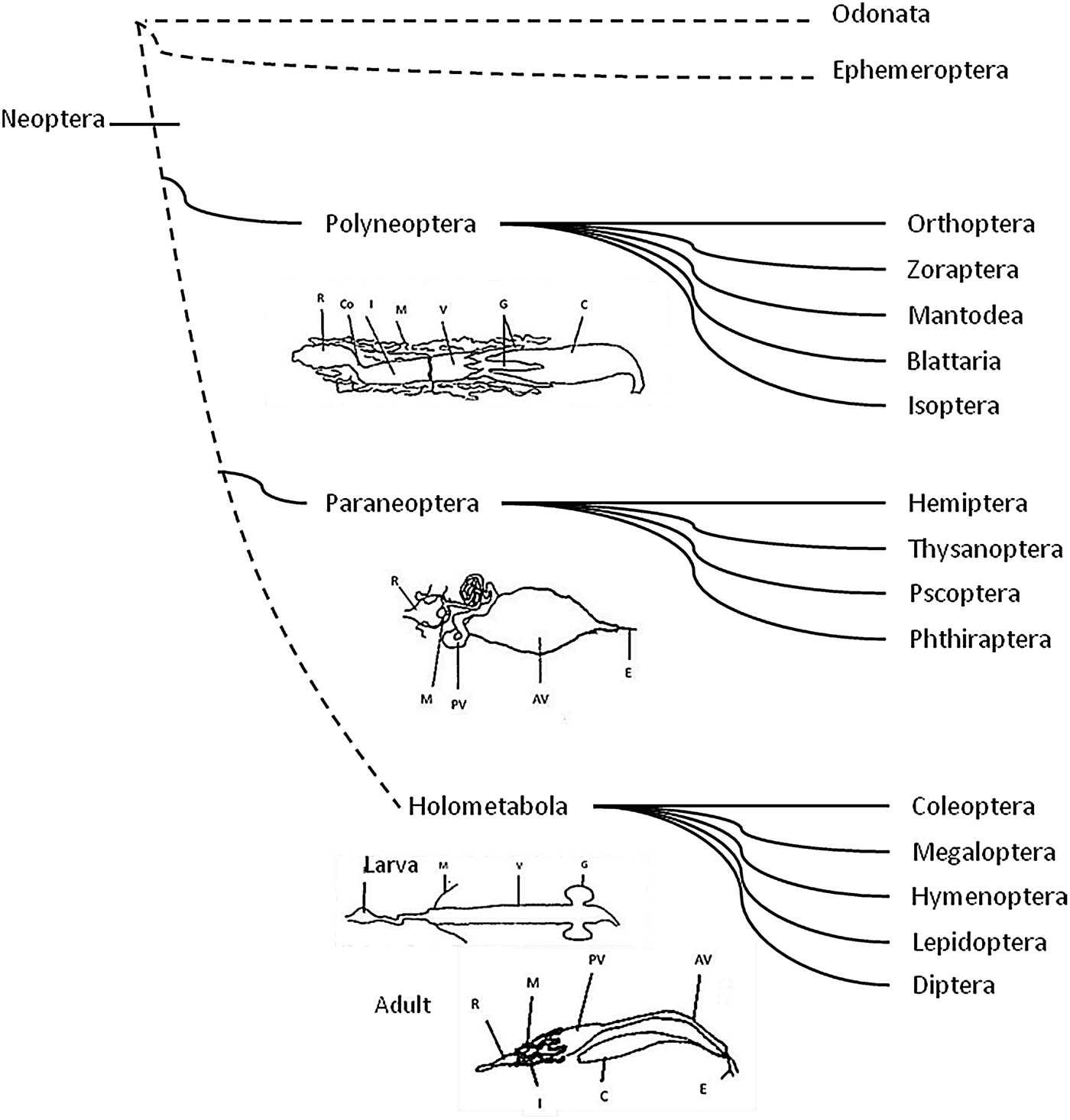
Fig. 2. Organization of gut compartments in the major insect orders. Neopteran insects are the common ancestor and include all the winged insects, except Ephemeroptera and Odonata. Polyneoptera, Paraneoptera and Holometabola have different features in the digestive physiology. C = crop; G = gastric cecae; V = ventriculus; M = Malpighian tubules; I = ileum; Co = colon; R = rectum; E = esophagus; AV = anterior ventriculus; PV = posterior ventriculus (midgut). (Intestinal representations were taken and modified from Terra, Reference Terra1988).
The PMM refers to a double membrane covering the MM of the intestine epithelial cells forming an outer microvillar (perimicrovillar) membrane which maintains a constant distance from the inner, or true, MM and projecting themselves towards the intestine lumen (fig. 1d) (Reger, Reference Reger1971; Burgos & Gutiérrez, Reference Burgos and Gutiérrez1976; Billingsley & Downe, Reference Billingsley and Downe1988). The PMM production depends on factors such as abdominal distention, diet content, activation of the neuroendocrine system leading to the release of prothoracicotropic hormone (PTTH) and ecdysone production (Billingsley & Downe Reference Billingsley and Downe1988; Azambuja et al., Reference Azambuja, Feder and Garcia1993; Nogueira et al., Reference Nogueira, Gonzales, Garcia and de Souza1997; Garcia et al., Reference Garcia, Azambuja, de Souza, Feder, Nogueira and Gonzalez1998; Albuquerque-Cunha et al., Reference Albuquerque-Cunha, Mello, Garcia, Azambuja, de Souza, Gonzalez and Nogueira2004).
The presence of the PMM is related to a modification of the alimentary canal that enables direct absorption of nutrients, such as essential amino acids, which occur in very low concentrations (Terra, Reference Terra1990). Such modifications are believed to deal rapidly with large amounts of dilute fluid food to prevent hemolymph dilution. The functions of the PMM are similar to those of the PM, such as compartmentalization of the digestive process and a mechanical barrier to immobilize molecules and protection of intestinal epithelium. Unlike the PM, however, the PMM increases the absorption capacity of nutrients from diluted diets (Billingsley & Downe, Reference Billingsley and Downe1988; Ferreira et al., Reference Ferreira, Ribeiro, Garcia and Terra1988; Billingsley, Reference Billingsley1990; Terra & Ferreira, Reference Terra, Ferreira, Gilbert, Latrou and Gill2005).
Based on the results from immunolocalization of the PMM-bound-α-glucosidase which was found in both MM and PMM (Albuquerque-Cunha et al., Reference Albuquerque-Cunha, Gonzalez, Garcia, Mello, Azambuja, Almeida, de Souza and Nogueira2009, Allahyari et al., Reference Allahyari, Bandani and Habibi-Rezaei2010), it has been suggested that PMM is formed by budding from the trans area of the Golgi complex, migrating as the internal membrane of double membrane vesicles, fusing their outer membranes with the MM and their inner membranes with the PMM (Silva et al., Reference Silva, Ribeiro, Gulbenkian and Terra1995; Damasceno-Sá et al., Reference Damasceno-Sá, Carneiro, DaMatta, Samuels, Terra and Silva2007). Despite these developmental differences, Silva et al. (Reference Silva, Ribeiro, Gulbenkian and Terra1995) and Terra et al. (Reference Terra, Costa and Ferreira2006) proposed that the MM and PMM of posterior midgut cells have the same origin. Furthermore, MM and PMM share some biochemical properties as both are rich in sterols, Mg2+-ATPase and Na+k+-ATPase reaction, glycoconjugates and carbohydrate-binding molecules (Ferreira et al., Reference Ferreira, Ribeiro, Garcia and Terra1988; Albuquerque-Cunha et al., Reference Albuquerque-Cunha, Gonzalez, Garcia, Mello, Azambuja, Almeida, de Souza and Nogueira2009). However, once both have fully developed, MM and PMM show some functional differences which may reflect the different intestinal microenvironments and the enzymatic dispersion to which they have been exposed (Albuquerque-Cunha et al., Reference Albuquerque-Cunha, Gonzalez, Garcia, Mello, Azambuja, Almeida, de Souza and Nogueira2009). In relation to this, Bittencourt-Cunha et al. (Reference Bittencourt-Cunha, Silva-Cardoso, de Oliveira, da Silva, da Silveira, Kluck, Souza-Lima, Gondim, Dansa-Petretsky, Silva, Masuda, da Silva and Atella2013) demonstrated that after a meal, Rhodnius prolixus, uses fatty acids from the lumen for the synthesis of different lipid and phospholipid classes that were organized into PMM.
Digestive pattern related to phylogeny of the insects
All insects can be grouped according to their digestive physiology and organization of the digestive system (fig. 2) (Terra, Reference Terra1988; Lehane & Billingsley, Reference Lehane and Billingsley1996). Neopteran insects are the common ancestor and include all the winged insects, except Ephemeroptera (mayflies) and Odonata (dragonflies) and evolved along three main lines: Polyneoptera (including Orthoptera, Zoraptera, Mantodea, Blattaria, Isoptera); Paraneoptera (including Hemiptera, Thysanoptera, Psocoptera and Phthiraptera) and Holometabola (including Coleoptera, Megaloptera, Hymenoptera, Lepidoptera and Diptera) (fig. 2) (Wheeler, Reference Wheeler, Whiting, Wheeler and Carpenter2001; Misof et al., Reference Misof, Liu, Meusemann, Peters, Donath, Mayer, Frandsen, Ware, Flouri, Beutel, Niehuis, Petersen, Izquierdo-Carrasco, Wappler, Rust, Aberer, Aspöck, Aspöck, Bartel, Blanke, Berger, Böhm, Buckley, Calcott, Chen, Friedrich, Fukui, Fujita, Greve, Grobe, Gu, Huang, Jermiin, Kawahara, Krogmann, Kubiak, Lanfear, Letsch, Li, Li, Li, Lu, Machida, Mashimo, Kapli, McKenna, Meng, Nakagaki, Navarrete-Heredia, Ott, Ou, Pass, Podsiadlowski, Pohl, Reumont, Schütte, Sekiya, Shimizu, Slipinski, Stamatakis, Song, Su, Szucsich, Tan, Tan, Tang, Tang, Timelthaler, Tomizuka, Trautwein, Tong, Uchifune, Walzl, Wiegmann, Wilbrandt, Wipfler, Wong, Wu, Wu, Xie, Yang, Yang, Yeates, Yoshizawa, Zhang, Zhang, Zhang, Zhang, Zhao, Zhou, Zhou, Ziesmann, Zou, Li, Xu, Zhang, Yang, Wang, Wang, Kjer and Zhou2014).
The Neoptera is the ancestor of Polyneoptera and evolved to Paraneoptera and Holometabola (fig. 2). Based on Terra et al (Reference Terra, Ferreira, Baker, Lehane and Billingsley1996), the neopteran ancestors have different features in the digestive physiology: digestive enzymes may pass forward from midgut to crop; the hydrolases are free and small, able to pass through the PM; the endo-ecto peritrophic circulation of digestive enzymes is driven by the secretion of fluid by Malpighian tubules and fluid absorption in the midgut cecae; and finally, there is differentiation of an acid anterior midgut (with carbohydrase activity) and an alkaline posterior midgut (with protease activity). Generally this group has a large crop and relatively short midgut with diverticula (midgut caeca) at the anterior end.
Polyneoptera
This group has retained characteristics of their neopteran ancestor but has reduced the size of the crop; some insects have lost caeca and have a differentiation of hindgut structures associated with the utilization of refractory material (Terra et al., Reference Terra, Ferreira, Baker, Lehane and Billingsley1996; Terra & Ferreira, Reference Terra, Ferreira, Resh and Cardé2009). The majority of digestion is carried out in the crop by digestive enzymes propelled by antiperistalsis from the midgut. Then, there is a transfer of digestive enzymes and partially digested food towards the ventriculus. The anterior ventriculus is acidic and has high carbohydrase activity, whereas the posterior ventriculus is alkaline and has high proteinase activity (Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012). In the midgut, the food bolus moves backwards by peristalsis. When the polymeric molecules have been digested they pass through the PM. Once the molecules pass through the PM (fig. 2a), they diffuse, along with digestive enzymes, into the ectoperitrophic space. Subsequently, with a countercurrent flux caused by secretion of fluid by the Malpighian tubules, the enzymes and nutrients are moved towards the midgut cecae where final digestion is completed and nutrient absorption occurs (Terra & Ferreira, Reference Terra, Ferreira, Resh and Cardé2009, Reference Terra, Ferreira and Gilbert2012). The Polyneoptera group includes different orders such Dictyoptera (Blattaria and Mantodea), Orthoptera, Phasmatodea, which gave rise to Paraneoptera and Holometabola (fig. 2).
Paraneoptera
In this group of insects the PM is absent and is replaced by a variety of membranes on the MM that performs the same role of absorption of essential nutrients from a diluted diet (i.e. amino acids that are present in very low concentrations) (Silva et al., Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004). The ancestral origin of Paranoptera group (see ‘Adaptation of the midgut Paraneoptera Insects’ below) may have had adaptations to deal rapidly with large amounts of dilute fluid food. This group have an alimentary tract in the form of a simple tube (Kollien et al., Reference Kollien, Waniek, Pröls, Habedank and Schaub2004) distinguished by modifications of the crop, anterior caeca and endo-ectoperitrophic circulation of digestive enzymes and polymer and oligomer hydrolases, all associated with the lack of midgut luminal digestion (Terra et al., Reference Terra, Ferreira, Baker, Lehane and Billingsley1996). Therefore, the fluid food is stored essentially undigested in the anterior part of the midgut before concentration and partial enzymatic hydrolysis, passing rapidly through the narrow posterior part of the midgut into the hindgut (Kollien et al., Reference Kollien, Waniek, Pröls, Habedank and Schaub2004; Waniek, Reference Waniek2009).
Holometabola
This group has similar water fluxes and circulation of enzymes as does the Polyneoptera, except that the fluid secretion occurs in the posterior ventriculus instead of the Malpighian tubules. The posterior midgut fluid does not contain waste, as does the Malpighian tubular fluid (Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012). It should be noted, however, that there may be considerable variation in the digestive systems of insects: Holometabolous (lower Holometabola- Coleoptera, Hymenoptera; and higher Holometabola- Diptera, Lepidoptera, Trichoptera), or between larvae and adults of the same groups. The compartmentalization, however, seems to be conserved, facilitating the digestion of polymeric food in more restricted environments (Terra et al., Reference Terra, Valentin and Santos1987, Reference Terra, Ferreira, Baker, Lehane and Billingsley1996).
Adaptations of the midgut in Paraneoptera insects
The Paraneoptera group is subdivided in Psocodea and Condylognatha that have marked differences in their digestive tracts (Terra, Reference Terra1988).
Psocodea
This group includes the orders Psocoptera (booklice) and Phthiraptera (lice). As a particular feature of the midgut, they have a peritrophic gel (fig. 2b) that covers the midgut columnar cells instead of a PM (Terra, Reference Terra2001; Silva et al., Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004). The absence of the PM in this group is thought to be related to their type of food and the countercurrent flows and their small size that allows for easy and efficient diffusion of digested products to the midgut surface (Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012). The digestion in Psocodea insects occurs in a few hours, implying serine proteases for digestion and an alkaline pH value for the midgut lumen (Waniek et al., Reference Waniek, Hendgen-Cotta, Stock, Mayer, Kollien and Schaub2005). The absence of PM was confirmed by Silva et al. (Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004) who observed, via transmission electron microscopy, dark material among midgut MM of booklice, which corresponds to the remains of a peritrophic gel that separates cells and midgut contents and an absence of α-glucosidase bound to the peritrophic gel demonstrated by immunocytolocalisation tests Essentially it can be said that Psocodea does not have a PMM but a primitive membrane form, the peritrophic gel (fig. 1).
Condylognatha
This group gave rise to the Thysanoptera and Hemiptera, but there are few general characteristic of the midgut in these two orders.
Thysanopterans have particular modifications at the midgut cells. Current studies suggest that the MM of midgut cells have two different types of glycocalyx, not seen in other insects. In the anterior region of the midgut the MM are surrounded by a myelin-like membrane that encloses several MM in a bundle. This structure is similar to a PMM and provides a form of protection for the MM in a region where cells are involved in secretory activity (Kitajima, Reference Kitajima1975). In the posterior region of the midgut the MM have numerous rod-like projections arranged to form continuous layers characterized by dense material in the intercellular space that are more similar to a PMM (fig. 1c, table 1) (Del Bene et al., Reference Del Bene, Dallai and Marchini1991).
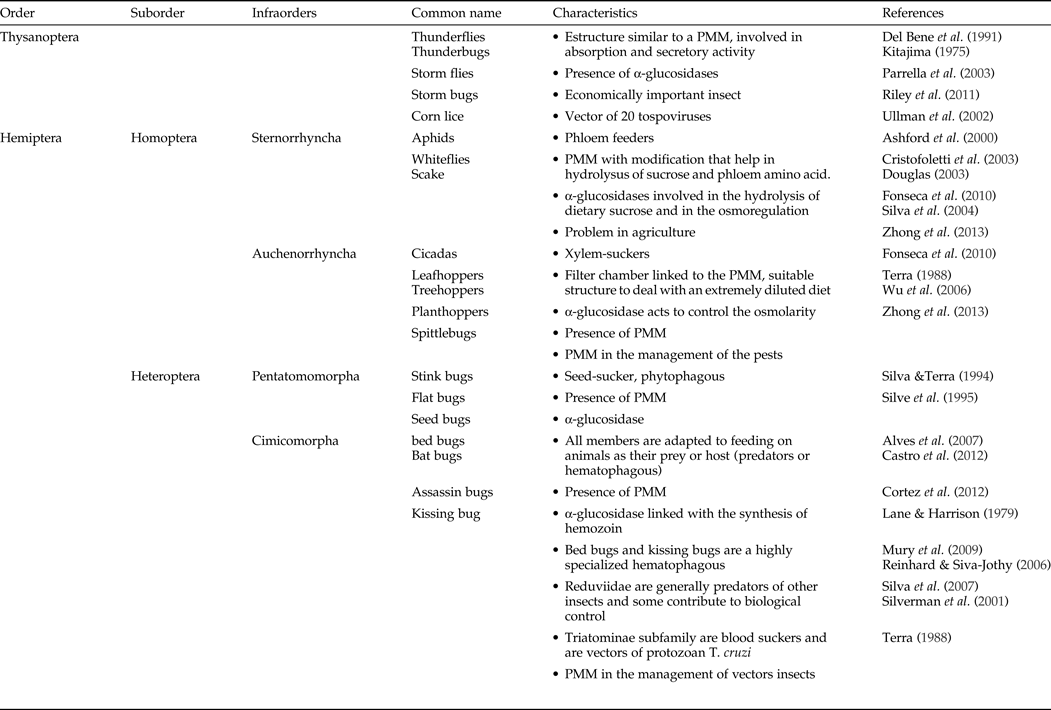
Table 1. Characteristics of the PMM in Thysanoptera and Hemiptera.

Features of the PMM in Hemiptera
The order Hemiptera comprises two suborders: Homoptera and Heteroptera (table 1). The Homoptera includes: Sternorrhyncha (Coccidae, scale insects; Aphididae, aphids; Aleyrodidae, whitefly) and Auchenorrhyncha (Fulgoroidae, planthopper; Cercopidae, spittle bugs; Cicadidae, cicadas; Cicadellidae, leafhoppers; Membracidae, treehoppers), all terrestrial plant feeders (Cranston & Gullan, Reference Cranston, Gullan, Resh and Cardé2009). The Heteroptera comprises Pentatomomorpha (Lygaeoidae, seed bugs; Pyrrhocoroidea, cotton strainers; Coreoidae, squash bugs; Pentatomidae, shield bugs, chust bugs and stink bugs) and Cimicomorpha (Cimicidae, bed bugs; Reduviidae, assassin bugs) that have different feeding strategies that include predation, sap sucking and hematophagy (Terra et al., Reference Terra, Ferreira, Baker, Lehane and Billingsley1996; Misof et al., Reference Misof, Liu, Meusemann, Peters, Donath, Mayer, Frandsen, Ware, Flouri, Beutel, Niehuis, Petersen, Izquierdo-Carrasco, Wappler, Rust, Aberer, Aspöck, Aspöck, Bartel, Blanke, Berger, Böhm, Buckley, Calcott, Chen, Friedrich, Fukui, Fujita, Greve, Grobe, Gu, Huang, Jermiin, Kawahara, Krogmann, Kubiak, Lanfear, Letsch, Li, Li, Li, Lu, Machida, Mashimo, Kapli, McKenna, Meng, Nakagaki, Navarrete-Heredia, Ott, Ou, Pass, Podsiadlowski, Pohl, Reumont, Schütte, Sekiya, Shimizu, Slipinski, Stamatakis, Song, Su, Szucsich, Tan, Tan, Tang, Tang, Timelthaler, Tomizuka, Trautwein, Tong, Uchifune, Walzl, Wiegmann, Wilbrandt, Wipfler, Wong, Wu, Wu, Xie, Yang, Yang, Yeates, Yoshizawa, Zhang, Zhang, Zhang, Zhang, Zhao, Zhou, Zhou, Ziesmann, Zou, Li, Xu, Zhang, Yang, Wang, Wang, Kjer and Zhou2014).
Sternorrhyncha (Homoptera) species are phloem feeders and most studies have evaluated the digestive systems in aphids, whiteflies and scale insects (Ashford et al., Reference Ashford, Smith and Douglas2000; Douglas, Reference Douglas2003; Fonseca et. al., Reference Fonseca, Silva, Samuels, Da Matta, Terra and Silva2010; Zhong et al., Reference Zhong, Zhang and Wei2013). The data suggest modifications associated with the PMM, that originate from multimembrane vesicles from Golgi (Cristofoletti et al., Reference Cristofoletti, Ribeiro, Deraison, Rahbé and Terra2003) and which help in the hydrolysis of sucrose and absorbing dilute phloem amino acids (Terra, Reference Terra1990).
Auchenorryncha (Homoptera) species, principally cicadas, leafhoppers, treehoppers, planthoppers and spittlebugs, are xylem-feeders. This group of insects acquired a filter chamber linked to the PMM, in the conical segment and anterior and posterior extensions of the midgut (Zhong et al., Reference Zhong, Zhang and Wei2013). These filter chambers with the PMM provide a suitable mechanism to deal with an extremely diluted diet (Terra, Reference Terra1988; Fonseca et al., Reference Fonseca, Silva, Samuels, Da Matta, Terra and Silva2010). Xylem fluid is a diet poor in organic nutrients and hypotonic to the hemolymph; these insects require obligate bacterial symbionts to synthesize essential amino acids (Wu et al., Reference Wu, Daugherty, Van Aken, Pai, Watkins, Khouri, Tallon, Zaborsky, Dunbar, Tran, Moran and Eisen2006).
The compartmentalization of digestion in Heteroptera is known mainly from the Pentatomorpha: Dysdercus peruvianus a seed-sucker bug; and Cimicomorpha: R. prolixus a hematophagous bug (Lane & Harrison, Reference Lane and Harrison1979; Silva & Terra, Reference Silva and Terra1994). Despite having different diets, these insects share digestive system similarities including the presence of the PMM (Lane & Harrison, Reference Lane and Harrison1979). The anterior midgut of these insects is used to store food and absorb water and also absorbs glucose in Pentatomomorpha (Bifano et al., Reference Bifano, Alegria and Terra2010). The digestion and protein absorption of amino acids occurs in the posterior midgut. Most protein digestion in Heteroptera occurs in the lumen with the aid of cysteine and/or aspartic proteinases, mainly cathepsins and ends in the perimicrovillar space under the action of aminopeptidases and dipeptidases (Terra & Ferreira, Reference Terra and Ferreira1994; Balczun et al., Reference Balczun, Siemanowski, Pausch, Helling, Marcus, Stephan, Meyer, Schneider, Cizmowski, Oldenburg, Höhn, Meiser, Schuhmann and Schaub2012).
In phloem feeding Sternorrhyncha, α-glucosidases are involved in the hydrolysis of dietary sucrose and in the osmoregulation in the midgut lumen through their transglycosidase activity (Ashford et al., Reference Ashford, Smith and Douglas2000). Members of Auchenorrhyncha, feed on xylem contents, that have low concentrations of organic compounds and it is possible that the membrane-bound α-glucosidase acts to control the osmolarity, following the passage of the meal into the midgut (Terra & Ferreira, Reference Terra, Ferreira, Gilbert, Latrou and Gill2005; Fonseca et al., Reference Fonseca, Silva, Samuels, Da Matta, Terra and Silva2010). Silva et al. (Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004) confirmed the existence of an integral protein α-glucosidase in the PMM that works as a biochemical marker for the PMM. This protein was first described in the seed-sucker bug D. peruvianus (Silva & Terra, Reference Silva and Terra1995; Silva et al., Reference Silva, Ribeiro, Gulbenkian and Terra1995) and kissing bugs R. prolixus and Triatoma infestans (Burgos & Gutiérrez, Reference Burgos and Gutiérrez1976; Terra, Reference Terra1988). Subsequently, α-glucosidases have been described in different groups of hemipteran insects and they are involved in different functions other than digestion (Allahyari et al., Reference Allahyari, Bandani and Habibi-Rezaei2010; Fialho et al., Reference Fialho, Terra, Moreira, Zanuncio and Serrão2013). In the hematophagus bug, R. prolixus, the perimicrovillar-associated α-glucosidase has been linked with the synthesis of hemozoin that protects the bug from the oxidative stress caused by the release of hemin, a product of the hemoglobin digestion (Oliveira et al., Reference Oliveira, Silva, Dansa-Petretski, de Souza, Braga, Masuda and Oliveira2000; Silva et al., Reference Silva, Mury, Oliveira, Oliveira, Silva and Dansa-Petretski2007; Mury et al., Reference Mury, da Silva, Ferreira, Ferreira, dos, de Souza-Filho, de Souza-Neto, Ribolla, Silva, do Nascimento, Machado, Berbert-Molina and Dansa-Petretski2009).
The presence of integral proteins in the Paraneoptera group confirmed the presence of a PMM in Hemipterans and Thysanopterans (fig. 1), but not in Psocopterans and Phthirapterans, suggesting that α-glucosidases and PMM of Thysanoptera and Hemiptera are homologous (Silva et al., Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004). Despite a common origin and composition of the PMM (Terra, Reference Terra1990; Albuquerque-Cunha et al., Reference Albuquerque-Cunha, Mello, Garcia, Azambuja, de Souza, Gonzalez and Nogueira2004, Reference Albuquerque-Cunha, Gonzalez, Garcia, Mello, Azambuja, Almeida, de Souza and Nogueira2009), the synthesis of the PMM can vary among species, which can be explained by different feeding behaviors (Damasceno-Sá et al., Reference Damasceno-Sá, Carneiro, DaMatta, Samuels, Terra and Silva2007; Azevedo et al., Reference Azevedo, Neves, Mallet, dos, Gonçalves, Zanuncio and Serrão2009; Fialho et al., Reference Fialho, Zanuncio, Neves, Ramalho and Serrão2009, Reference Fialho, Moreira, Zanuncio, Ribeiro, Terra and Serrão2012). In the hematophagous Cimex hemipterus (Hemiptera: Cimicidae), Triatoma pallidipennis and others triatomine species (Hemiptera: Reduviidae), of Cimicomorpha infraorder, the PMM is evident 20 and 15 days post feeding, respectively (Billingsley & Downe, Reference Billingsley and Downe1986; Azevedo et al., Reference Azevedo, Neves, Mallet, dos, Gonçalves, Zanuncio and Serrão2009; Gutiérrez-Cabrera et al., Reference Gutiérrez-Cabrera, Alejandre-Aguilar, Hernández-Martínez and Espinoza2014). In the phytophagous cotton stainer, D. peruvianus (Hemiptera: Pyrrhocoridae) and sunn pest, Eurygaster integriceps (Hemiptera: Scutelleridae), both of the Pentatomomorpha infraorder, the PMM covers all the cells 30 and 20 h post-feeding, respectively (Damasceno-Sá et al., Reference Damasceno-Sá, Carneiro, DaMatta, Samuels, Terra and Silva2007; Mehrabadi & Bandani, Reference Mehrabadi and Bandani2011). While in the zoophytophagous Brontocoris tabidus (Hemiptera: Pentatomidae), also of Pentatomomorpha infraorder, the PMM is evident in both the starved and fed condition (Fialho et al., Reference Fialho, Zanuncio, Neves, Ramalho and Serrão2009, Reference Fialho, Terra, Moreira, Zanuncio and Serrão2013).
Therefore, the development of PMM in hemipterans varies according to how frequently the animal has access to food: in phytophagous and zoophytophagous species access to food seems frequent (Damasceno-Sá et al., Reference Damasceno-Sá, Carneiro, DaMatta, Samuels, Terra and Silva2007; Fialho et al., Reference Fialho, Zanuncio, Neves, Ramalho and Serrão2009), while in blood-suckers such access is less frequent as hosts are harder to find (Nogueira et al., Reference Nogueira, Gonzales, Garcia and de Souza1997; Azevedo et al., Reference Azevedo, Neves, Mallet, dos, Gonçalves, Zanuncio and Serrão2009).
Economic and public health importance of species with PMM
The Thysanoptera is a worldwide order of nearly 6000 species, many of which are economically important insects: they cause direct damage to plants as a result of their feeding and indirect damage as vectors of plant pathogens (Ullman et al., Reference Ullman, Westcot, Hunter and Mau1989; Shipp et al., Reference Shipp, Hao, Papadopoulos and Binns1998). For example, at least 14 species of thrips transmit 20 tospoviruses (genus Tospovirus, family Bunyaviridae), a major group of plant viruses affecting >1000 host-plant species many of them important for human use (table 1) (Ullman et al., Reference Ullman, Meideros, Campbell, Whitfield, Sherwood and German2002; Parrella et al., Reference Parrella, Gognalons, Gebre-Selassie, Vovlas and Machoux2003; Riley et al., Reference Riley, Joseph, Srinivasan and Diffie2011).
The Hemiptera contains many species of medical and economic importance. This order has a high biodiversity, is adapted to a large number of habitats, exploits different diets such as phloem and xylem sap, seed sucking, predation and hematophagy. Most are vectors of viruses, bacteria and protozoa and as such are a serious problem in agriculture and public health (Dedryver et al., Reference Dedryver, Le Ralec and Fabre2010; Fonseca et al., Reference Fonseca, Silva, Samuels, Da Matta, Terra and Silva2010). Some hemipterans of economic importance to humans include: aphids and whiteflies as phloem-suckers; planthoppers, cicadas, cercopids and leafhoppers as xylem-suckers; seed bugs, cotton strainers and squash bugs that are phytophagous terrestrial bugs and include many plant pests; and finally, bed bugs and assassin bugs that include ectoparasites and vectors of human parasites and pathogens.
Cimicidae (bed bugs) are a highly specialized hematophagous taxon that parasitizes primarily humans, birds and bats (Reinhardt & Siva-Jothy, Reference Reinhardt and Siva-Jothy2007). Bed bugs are capable of carrying different infectious agents such as bacteria, protozoa and viruses that may cause diseases such as typhus, anthrax, plague, relapsing fever, tularemia, Q fever, leishmania, hepatitis B virus and HIV (Burton, Reference Burton1963; Ryckman et al., Reference Ryckman, Bentley and Archbold1981); however, the cimicids rarely transmit them to their hosts (Goddard & deShazo, Reference Goddard and deShazo2009; Silverman et al., Reference Silverman, Qu, Blow, Zitron, Gordon and Walker2001). These insects also can harbor trypanosomes (Bower & Woo, Reference Bower and Woo1981; Gardner & Molyneux, Reference Gardner and Molyneux1988), including T. cruzi the causative agent of Chagas disease (Chang & Chao, Reference Chang and Chao1999). Although the trypanosomes such as Trypanosoma (Megatrypanum) incertum (Gardner & Molyneux, Reference Gardner and Molyneux1988) and Trypanosoma (Schizotrypanum) hedricki (Bower & Woo, Reference Bower and Woo1981) have been found in bed bugs, this taxon had not been considered a major vector of any human parasite. However, recently Salazar et al (Reference Salazar, Castillo-Neyra, Tustin, Borrini-Mayorí, Náquira and Levy2015) showed that bed bug (Cimex lecturarius) seems to be a competent vector of T. cruzi. Bed bugs efficiently acquired T. cruzi on feeding on infected mice and then transmitted the parasite back to susceptible hosts both during cohabitation and through contaminated feces placed on broken host skin by researchers (Salazar et al., Reference Salazar, Castillo-Neyra, Tustin, Borrini-Mayorí, Náquira and Levy2015).
In contrast, the Reduviidae (assassin bugs) is one of the largest families of the Hemiptera and many are predators of other insects and some contribute to biological control. However, some species of the Triatominae sub-family are blood feeders and are important vectors of the protozoan T. cruzi, the causative agent of Chagas’ disease that affects an estimated 6–7 million people, mainly in Latin America (WHO, 2015). The interactions between T. cruzi and the PMM of the triatomine vectors are keys to the success of the parasite in the midgut (Alves et al., Reference Alves, Albuquerque-Cunha, Mello, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007). Bed bugs and triatomine bugs, share many similarities. Besides belonging to the same infraorder, both are exclusively hematophagous and develop the PMM, although the blood sucking lifestyle of each insect has evolved independently.
Despite their importance in agriculture and public health, there are few studies of these insects on the morphology and ultrastructure of their midguts with particular attention to the interaction with the pathogens that could lead to novel control measures.
Importance of the PMM in the control of insects of economic importance: the case of vectors of Chagas disease
Understanding the function and structure of the PMM may provide us with a target to control insects of economic and/or health importance. As an example, we will use the case of vectors of Chagas disease. This disease is caused by the protozoan T. cruzi which proliferates and multiplies in the insect vector especially in the rectum. During the blood meal some bugs defecate and deposit the infected feces on the skin or mucosa. The bite causes a skin wound that allows the parasite to enter underneath the skin (Brenière et al., Reference Brenière, Aznar, Hontebeyrie, Telleria and Tibayrenc2010). Although this transmission is complex to facilitate transmission of the protozoan (Takano-Lee & Edman, Reference Takano-Lee and Edman2002), Chagas disease is highly dependent on the interaction of triatomines and the parasite (Kollien & Schaub, Reference Kollien and Schaub2000; Azambuja et al., Reference Azambuja, Ratcliffe and Garcia2005).
The life cycle of T. cruzi in the vector has three stages of development (Kollien & Schaub, Reference Kollien and Schaub2000; Azambuja et al., Reference Azambuja, Ratcliffe and Garcia2005). The first stage occurs when the triatomine ingests blood containing trypomastigotes from the vertebrate host. In the second stage, a few hours after ingestion, the trypomastigotes transform into epimastogotes in the anterior midgut (Azambuja et al., Reference Azambuja, Ratcliffe and Garcia2005); thus establishing the infection in the insect (Garcia et al., Reference Garcia, Genta, Azambuja and Schaub2010). Subsequently, the epimastigotes pass to the midgut where they attach to the PMM and multiply by binary division (Garcia & Azambuja, Reference Garcia and Azambuja1991: Kollien et al., Reference Kollien, Schmidt and Schaub1998; Alves et al., Reference Alves, Albuquerque-Cunha, Mello, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007). The posterior midgut is the region of greatest digestive activity and where the greatest concentration of metabolites should occur (Schaub, Reference Schaub1989). In the final stage, the epimastigotes pass to the rectum, adhere to the rectal cuticle by hydrophobic interactions and multiply to very large numbers and transform into the metacyclic trypomastigotes which are eliminated with the feces and urine and are able to infect the vertebrate hosts (Kollien et al., Reference Kollien, Schmidt and Schaub1998; Kollien & Schaub, Reference Kollien and Schaub2000; Azambuja et al., Reference Azambuja, Ratcliffe and Garcia2005).
Experimental studies highlight the importance of the interactions between the parasite and the PMM, because PMM disruption is correlated with a blockage not only of epimastigote multiplication but also of T. cruzi development in the triatomine vector (Garcia et al., Reference Garcia, Gonzalez, Azambuja and Rembold1989; Gonzalez & Garcia, Reference Gonzalez and Garcia1992; Cortez et al., Reference Cortez, Gonzalez, Cabral, Garcia and Azambuja2002, Reference Cortez, Provençano, Silva, Mello, Zimmermann, Schaub, Garcia, Azambuja and Gonzalez2012). In regions where PMM is absent or poorly developed, the parasite rarely comes in contact with the MM of the intestine and thus fail to multiply (Gonzalez et al., Reference Gonzalez, Nogueira, Mello, de Souza, Schaub, Azambuja and Garcia1999; Kollien & Schaub, Reference Kollien and Schaub2000; Azambuja et al., Reference Azambuja, Ratcliffe and Garcia2005). These inhibitory effects could be counteracted by either head transplantation or ecdysone therapy. Furthermore, a simple oral ingestion of PMM is able to rescue T. cruzi development in either decapitated or azadirachtin-treated insects (Gonzalez et al., Reference Gonzalez, Nogueira, Mello, de Souza, Schaub, Azambuja and Garcia1999; Cortez et al., Reference Cortez, Gonzalez, Cabral, Garcia and Azambuja2002, Reference Cortez, Provençano, Silva, Mello, Zimmermann, Schaub, Garcia, Azambuja and Gonzalez2012), indicating that the PTTH–ecdysone pathway interferes with T. cruzi survival and development in its vectors (Nogueira et al., Reference Nogueira, Gonzales, Garcia and de Souza1997; Gonzalez et al., Reference Gonzalez, Nogueira, Mello, de Souza, Schaub, Azambuja and Garcia1999). These results demonstrate the importance of the insect's endocrine system and the PMM in establishing T. cruzi infections in the vector.
According to Albuquerque-Cunha et al. (Reference Albuquerque-Cunha, Mello, Garcia, Azambuja, de Souza, Gonzalez and Nogueira2004, Reference Albuquerque-Cunha, Gonzalez, Garcia, Mello, Azambuja, Almeida, de Souza and Nogueira2009), the PMM in triatomines is composed of glycoconjugates. The carbohydrates attached to the proteins of the insect cells are usually involved in insect–pathogen interactions (Pereira, Reference Pereira, Andrade and Ribeiro1981; Nogueira et al., Reference Nogueira, Gonzalez, Gomes, de Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007). In triatomines a variety of glycoconjugates including mannose, glucose, galactosamine, N-acetyl-galactosamine, N-acetyl-glucosamine and sialic acid (Gutiérrez-Cabrera et al., Reference Gutiérrez-Cabrera, Alejandre-Aguilar, Hernández-Martínez and Espinoza2014) have been identified. These sugar residues attached to the PMM proteins play an important role in the binding of epimastigotes to the surface of intestinal epithelial cells that allows the parasite to complete its life cycle in the vector (Garcia et al., Reference Garcia, Ratcliffe, Whitten, Gonzalez and Azambuja2007, Reference Garcia, Genta, Azambuja and Schaub2010). In R. prolixus mannose and sialic acid are essential in the interaction with T. cruzi (Alves et al., Reference Alves, Albuquerque-Cunha, Mello, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007; Albuquerque-Cunha et al., Reference Albuquerque-Cunha, Gonzalez, Garcia, Mello, Azambuja, Almeida, de Souza and Nogueira2009).
Conclusion and future considerations
Digestion of insects takes place in initial, intermediate, and final phases. These phases are separated spatially and temporally by morphological features of the gut. The morphological features may have evolved by adapting to different diets. However, the dietary approach alone cannot explain the presence or absence of some structures and cannot provide a clear explanation for the absence of peritrophic matrix in all Hemiptera, despite their contrasting dietary habits. However, based on the digestive events of different taxa and their diets, the insects may be grouped according to their digestive physiology and phylogenetic position, with regards to a common ancestor.
With regard to the origin and evolution of PMM, the Condylognathan ancestor of the Thysanoptera and Hemiptera likely fed on phloem sap obtained from plant tissues pierced by oral stylets, the so-called ‘punch and suck’ mechanism (Silva et al., Reference Silva, Silva, Vasconcelos, Petretski, Damatta, Ribeiro and Terra2004). This diet would be low in proteins, low carbohydrate polymers, relatively poor in free amino acids but rich in sucrose. These condylognathan ancestors may have lost the PM and the enzymes involved in initial and intermediate protein digestion due to the lack of luminal digestion. When very low concentrations of essential amino acids are present, absorption may be maximized by the PMM (Terra, Reference Terra1988; Terra & Ferreira, Reference Terra and Ferreira1994). The PMM would transport actively potassium ions from the perimicrovillar space into the midgut cells, generating a concentration gradient between the sap in the lumen and that in the perimicrovillar space. This concentration gradient would be used as a driving force for the absorption of organic substances, such as amino acids, through carriers in the PMM. The organic substances, once in the perimicrovillar space, would be absorbed through carriers at the surface of the MM. The α-glucosidase bound to PMM efficiently cleaves sap sucrose without being excreted.
The hemipteran ancestor acquires piercing-sucking mouthparts adapted to suck xylem and phloem sap (Gillott, Reference Gillott1995), thus becoming able to obtain liquids directly from the plant's vascular system. Organic compounds obtained from the xylem and phloem must be concentrated to be absorbed by the perimicrovillar system. Therefore, the evolution of Heteroptera was associated with the ability to digest proteins after losing the appropriate digestive enzymes and maintaining a compartmentalization of digestion by PMM as a substitute of PM (Terra & Ferreira, Reference Terra, Ferreira and Gilbert2012).
Many thysanopterans and hemipterans are species of medical and economic importance and the PMM may be targeted to control insects or to regulate and modulate interactions with pathogens. The development of the PMM varies according to species and depends on how frequently the insect has access to food (Nogueira et al., Reference Nogueira, Gonzales, Garcia and de Souza1997; Damasceno-Sá et al., Reference Damasceno-Sá, Carneiro, DaMatta, Samuels, Terra and Silva2007; Azevedo et al., Reference Azevedo, Neves, Mallet, dos, Gonçalves, Zanuncio and Serrão2009; Fialho et al., Reference Fialho, Zanuncio, Neves, Ramalho and Serrão2009). Therefore, it is necessary to carry out morphological and biochemical studies to obtain detailed information on the insect digestive tract, to identify fundamental parameters we might target to develop novel strategies to control agriculturally and medically-important insects.
In the specific case of triatomine vectors of Chagas disease, there are several mechanisms that regulate the interactions between host and T. cruzi in the alimentary tract of the insect vector. The PMM could be an excellent target to reduce the susceptibility of the insect vector to parasites because T. cruzi epimastigotes interact intimately with these membranes and this is essential for parasite development in the gut (Burgos et al., Reference Burgos, Gutierrez, Lammel and Isola1989; Gonzalez et al., Reference Gonzalez, Nogueira, Mello, de Souza, Schaub, Azambuja and Garcia1999; Gutiérrez-Cabrera et al., Reference Gutiérrez-Cabrera, Alejandre-Aguilar, Hernández-Martínez and Espinoza2014).
Several authors have studied the effects of antisera produced against antigens from the vector gut on parasite infections in various insect orders, highlighting, for example, antibodies that recognize the PM that can also block its development (East et al., Reference East, Fitzgerald, Pearson, Donaldson, Vuocolo, Cadogan, Tellam and Eisemann1993; Eisemann et al., Reference Eisemann, Pearson, Donaldson, Cadogan and Vuocolo1993; Tellam & Eisemann, Reference Tellam and Eisemann1998; Wijffels et al., Reference Wijffels, Hughes, Gough, Allen, Don, Marshall, Kay and Kemp1999; Otranto & Stevens, Reference Otranto and Stevens2002; Foy et al., Reference Foy, Magalhaes, Injera, Sutherland, Devenport, Thanawastien, Ripley, Cárdenas-Freytag and Beier2003). Based on these observations, Gonzalez et al. (Reference Gonzalez, Hamedi, Albuquerque-Cunha, Nogueira, de Souza, Ratcliffe, Azambuja, Garcia and Mello2006) used an antiserum against PMM and observed changes in the PMM organization in the posterior midgut of R. prolixus that had ingested the antisera. Those changes were not important for triatomine survival but the antiserum acted as a transmission-reducing compound that significantly reduced T. cruzi infection in the vector. It is possible that the PMM proteins become unavailable for the parasite after recognition by the antiserum consequently disrupting T. cruzi development. A better understanding of the PMM composition and function can clarify fundamental steps of the process of triatomine digestion, hemozoin formation and the T. cruzi life cycle.
Different studies have shown that glycoconjugates and carbohydrate-binding molecules are associated with the plasma membrane of insect cells and are usually involved in the interactions with pathogens (Rudin & Hecker, Reference Rudin and Hecker1989; Jacobson & Doyle, Reference Jacobson and Doyle1996; Dinglasan & Jacobs-Lorena, Reference Dinglasan and Jacobs-Lorena2005). The PMM contains a variety of glycoconjugates, such as mannose, as the major sugar residues (Albuquerque-Cunha et al., Reference Albuquerque-Cunha, Gonzalez, Garcia, Mello, Azambuja, Almeida, de Souza and Nogueira2009; Gutiérrez-Cabrera et al., Reference Gutiérrez-Cabrera, Alejandre-Aguilar, Hernández-Martínez and Espinoza2014). It has been proposed that these sugar-binding molecules are involved in the binding of T. cruzi epimastigotes to the midgut epithelial cell surface (Pereira et al., Reference Pereira, Andrade and Ribeiro1981; Bonay et al., Reference Bonay, Molina and Fresno2001; Nogueira et al., Reference Nogueira, Gonzalez, Gomes, de Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007). However, much remains to be learned about the biochemical composition of the PMM and the mechanisms of interaction with parasites.
There is an increasing need to develop either new vector control methods or alternative strategies to block transmission of parasites such as T. cruzi. Studies evaluating immunization using components from midguts must be extended by refining the native antigens and expanding our knowledge of the proteins from the digestive tract and the PMM of epidemiologically important triatomines to identify target molecules essential to parasite development in the insect vector. This requires a further understanding about the origin, biogenesis, function, composition and development of PMM in the Thysanoptera and Hemiptera and in more detail, to recognize which molecules are present in the digestive tract and the PMM and how they may modulate insect physiology and parasite development and multiplication.
Acknowledgements
A. E. G.-C. was supported by doctoral and postdoctoral grants from CONACyT and DGAPA-UNAM, respectively. The authors thank the visual artist Aaron J. Morales-Vázquez for the drawing of fig. 1.