Introduction
Information on population dynamics of organisms are of fundamental importance for understanding the distribution of species within landscapes (Levin, Reference Levin1992; Turchin, Reference Turchin1998; Morales & Ellner, Reference Morales and Ellner2002). Analysis of movement can improve the understanding of patterns of species presence at multiple scales (Turchin, Reference Turchin1991; Crist et al., Reference Crist, Guertin, Wiens and Milne1992; Johnson et al., Reference Johnson, Milne and Wiens1992; Morales & Ellner, Reference Morales and Ellner2002; Samu et al., Reference Samu, Sziranyi and Kiss2003), for instance within woodlands in fragmented landscapes. At the landscape scale, habitat environments typically display a high level of heterogeneity, which can influence the movement and dispersal ability of species (Johnson et al., Reference Johnson, Milne and Wiens1992; Doak, Reference Doak2000; Hein et al., Reference Hein, Gombert, Hovestadt and Poethke2003; Schtickzelle et al., Reference Schtickzelle, Joiris, Van Dyck and Baguette2007) and their pattern of distribution. Analysis of movement patterns displayed at small spatio-temporal scales provide a valuable first insight that can help explain distribution patterns and presence observed at larger scales (Turchin, Reference Turchin1991; Wiens et al., Reference Wiens, Stenseth, Horne and Ims1993; Samu et al., Reference Samu, Sziranyi and Kiss2003).
A range of approaches is available for studying movement patterns of species. A particularly powerful way to quantify movement is directly observing and following individuals when moving through the environment (Turchin, Reference Turchin1998). By recording movement paths and behaviour, possible strategies that may account for the movement pattern can be quickly analysed and tested (Turchin, Reference Turchin1998). Individual movement paths have been recorded and analysed for a variety of species across a range of spatial scales (Benhamou, Reference Benhamou1990; Cain, Reference Cain1990; Vernes & Haydon, Reference Vernes and Haydon2001; Bowlby et al., Reference Bowlby, Hanson and Hutchings2007; Dai et al., Reference Dai, Shannon, Slotow, Page and Duffy2007; Hapca et al., Reference Hapca, Crawford, MacMillan, Wilson and Young2007). Individual-based movement data in such studies are often compared with uncorrelated random walk (i.e. simple diffusion) and/or correlated random walk models, primarily because these data can be used to infer an overall rate of population spread of the species under investigation (Turchin, Reference Turchin1998). Testing the applicability of these models to observational data is relatively straightforward; and, where these models are intuitive in terms of population spread (Crist et al., Reference Crist, Guertin, Wiens and Milne1992; Turchin, Reference Turchin1998), they prove useful in interpreting ecological relevant processes and distribution patterns.
For invertebrates, these types of analyses have been used for relatively mobile species, such as butterflies, and for species that move by walking, such as carabid beetles. In these studies, movement patterns and strategies were related to a range of physical and ecological characteristics of the individual species, including dispersal ability (Doak, Reference Doak2000; Samu et al., Reference Samu, Sziranyi and Kiss2003; Conradt & Roper, Reference Conradt and Roper2006; Schtickzelle et al., Reference Schtickzelle, Joiris, Van Dyck and Baguette2007), hunger level (Wallin & Ekbom, Reference Wallin and Ekbom1988; Wallin, Reference Wallin1991; Wallin & Ekbom, Reference Wallin and Ekbom1994) and food availability (Root & Kareiva, Reference Root and Kareiva1984; Turchin, Reference Turchin1998). Furthermore, for individual species, differences in movement patterns have been recorded across temporal (Johnson et al., Reference Johnson, Milne and Wiens1992; Morales & Ellner, Reference Morales and Ellner2002) and spatial scales (Johnson et al., Reference Johnson, Milne and Wiens1992; Samu et al., Reference Samu, Sziranyi and Kiss2003), between life-stages (With, Reference With1994; Doak, Reference Doak2000), and when moving through different habitat environments (Baars, Reference Baars1979; Wallin & Ekbom, Reference Wallin and Ekbom1988; Crist et al., Reference Crist, Guertin, Wiens and Milne1992; Fownes, Reference Fownes2002; Hein et al., Reference Hein, Gombert, Hovestadt and Poethke2003).
Previous studies mainly have been undertaken for species associated with open habitats, such as agricultural fields and meadowland, and only very few studies have been undertaken with woodland-associated species (Brouwers & Newton, Reference Brouwers and Newton2009c). Understanding the population dynamics of woodland species is of particular importance in highly fragmented landscapes (Bailey, Reference Bailey2007), for example to determine the impacts of fragmentation on the dispersal ability and distribution of individual species, and the functioning of ecological corridors or habitat networks (Vos et al., Reference Vos, Baveco, Grashof-Bokdam and Gutzwiller2002; Bennett, Reference Bennett2003; Crooks & Sanjayan, Reference Crooks and Sanjayan2006). However, information on the population dynamics of woodland-associated invertebrate species, particularly for those with a high conservation concern, is severely lacking.
The study described here focused on the woodland-associated invertebrate wood cricket (Nemobius sylvestris) (Orthoptera: Gryllidae). Wood cricket is a small (∼1 cm) non-flying cricket species that has a semi-voltine (two-year) life-cycle in the UK. After overwintering, eggs hatch in June/July and nymphs develop and grow throughout the summer and autumn by means of moulting up to five times from the 1st to the 5th instar stage. Moulting ceases completely in September when the nymphs prepare to overwinter. In the second year, nymphs continue to develop (5th–8th instar) from April onwards until they reach sexual maturity (i.e. become adults) in July/August and are reproductively active through to September/October until they die (Gabbutt, Reference Gabbutt1959; Brown, Reference Brown1978). The species is strongly associated with deciduous woodland and is typically found in relatively large woodlands that lie in close proximity to each other within the landscape (Brouwers & Newton, Reference Brouwers and Newton2009a, Reference Brouwers and Newtonb). Within woodlands, the species can be found in open areas, such as clearings and in edge habitat along woodland tracks, footpaths, railway lines and woodland peripheries (Richards, Reference Richards1952; Morvan & Campan, Reference Morvan and Campan1976; Beugnon, Reference Beugnon1980; Brouwers & Newton, Reference Brouwers and Newton2009b). The species lives on the ground and prefers a well-developed leaf litter layer, which serves as shelter, food and breeding ground (Richards, Reference Richards1952; Brown, Reference Brown1978; Proess & Baden, Reference Proess and Baden2000; Brouwers & Newton, Reference Brouwers and Newton2009b). In the UK, the species has the national status of a ‘Species of Conservation Concern’ (NBN Gateway, 2009). The main habitat requirements identified for this species at the local scale were presence of a thick leaf litter layer, an open canopy and low levels of ground vegetation (Brouwers & Newton, Reference Brouwers and Newton2009b). To date, no detailed study has been undertaken on the movement patterns displayed by different life-stages of wood cricket through different habitat environments. Therefore, this study addressed the following aims: (i) to analyse the movement patterns of wood cricket juveniles (i.e. nymphs) and adults within different ground surface substrates using random walk models; (ii) to infer the rate of population spread for both nymphs and adults in these different environments; and (iii) to determine the preferred ground substrate of both adults and nymphs when presented with a choice.
Methods
Study context
Cricket species typically go through several life-stages before reaching sexual maturity (Marshall & Haes, Reference Marshall and Haes1988). The juvenile stages generally encompass the biggest part of the life-cycle, making these life-stages equally important in terms of determining the dispersal ability of a species (Diekotter et al., Reference Diekotter, Csencsics, Rothenbuhler, Billeter and Edwards2005). Based on earlier findings for wood crickets (Richards, Reference Richards1952; Gabbutt, Reference Gabbutt1959; Beugnon, Reference Beugnon1979; Brouwers & Newton, Reference Brouwers and Newton2009a, Reference Brouwers and Newtonb, Reference Brouwers and Newtonc; Brouwers et al., Reference Brouwers, Watts, Bailey, Newton, Catchpole, Smithers, Baarda and Eycott2009) and studies of other invertebrate species (e.g. Doak, Reference Doak2000), it was hypothesized that because of the physical differences between adults and nymphs and the habitat preferences of the species: (i) differences in movement patterns would be found between life-stages (because of differences in body size and sexual maturity) and with different ground surface substrates (because of differences in food availability and the shelter it provides); (ii) nymphs would spread more slowly than adults (because of physical differences like body size); and (iii) leaf litter would be the preferred substrate to move through, for both nymphs and adults (because of their preference for leaf litter providing shelter and food).
Study site
Between the 5th and 29th of June and the 30th of July and 7th of September 2007, a series of experiments were conducted using wood cricket nymphs (6–7th instar) and adult males and females, respectively. The experiments were carried out in the Briddlesford area (50°42′41.00″ N, 1°13′30.50″ W) situated on the Isle of Wight, UK, which is owned by ‘The People's Trust for Endangered Species’ (PTES), a non-governmental conservation organisation. The majority of woodlands in this area are classified as ‘ancient woodland’ (Spencer & Kirby, Reference Spencer and Kirby1992) and are dominated by native deciduous tree species, particularly oak (Quercus spp.). Since 2005, extensive new plantings of native tree species have taken place in this area, funded by the ‘JIGSAW’ scheme (Forestry Commission, 2005), in order to increase connectivity between the individual woodland fragments.
Movement experiments
Two separate experiments were undertaken. These focused on recording individual movement paths for the two life-stages in order to analyse movement patterns through different homogeneous ground substrates and to test for preferences among different ground substrates upon release. For the experiments, both the nymphs and adults were caught using a custom designed pooter. For the duration of the experiments, caught individuals were kept in a plastic container with ample supplies of food (bread, various fungi and oak leaf litter) available. To increase the visibility of individual wood crickets that were released during the experiments, individuals were marked by dusting them with fluorescent pigment (UV Gear, Mark SG Enterprises, Surrey, UK; www.uvgear.co.uk) (following Cronin, Reference Cronin2003). Marking was achieved by placing individuals in a plastic container with a small amount of pigment and shaking the container gently until all specimens were marked sufficiently. A control study was performed observing 20 marked and 20 unmarked nymphs and adults for a period of five days. This control study revealed no changes in behaviour and no significant increases in mortality between the marked and unmarked groups. All experiments were conducted on sites where wood crickets were initially not present (i.e. released wood crickets were strangers to the site) and under similar meteorological conditions where mean average daytime temperature at the ground surface did not go below 15°C.
Leaf litter vs. bare soil
A 4×4 m experimental site was created on an open woodland track within a mature open woodland dominated by oak. At this site, a grid was constructed using garden twine with each grid cell measuring 20×20 cm. This experimental site was divided equally with one half having a layer of leaf litter (2 cm deep) and one half with bare soil (Fig. 1). Individual wood crickets were released at the centre of the grid at the edge of the contrasting substrates. To avoid directional bias, individuals were released in alternating directions. The released individuals were observed from a position outside the grid. For each individual, choice of substrate was recorded after the release. To avoid a directional bias related to the experimental set-up, movement paths were recorded only after individuals had made their choice for one substrate and had moved from the centre-line of the experimental plot into one of the homogeneous substrates on either side (see Fig. 1). This strategy guaranteed unbiased movements through either one of the homogeneous substrates where the individuals were unable to see or otherwise sense the contrast between the substrates. The movement paths were recorded on gridded paper by drawing the exact route made by each individual after release. Furthermore, to relate movement with time, the exact position within the experimental plot was recorded for each individual after every minute and highlighted on the individual paths. Observations were terminated either after individuals were observed exiting the grid or remained stationary for more than six minutes. Only unbiased movement paths made entirely within one homogeneous substrate (i.e. for individuals that were not influenced by the centre-line within the experimental plot) were used for further analyses (see Fig. 1).
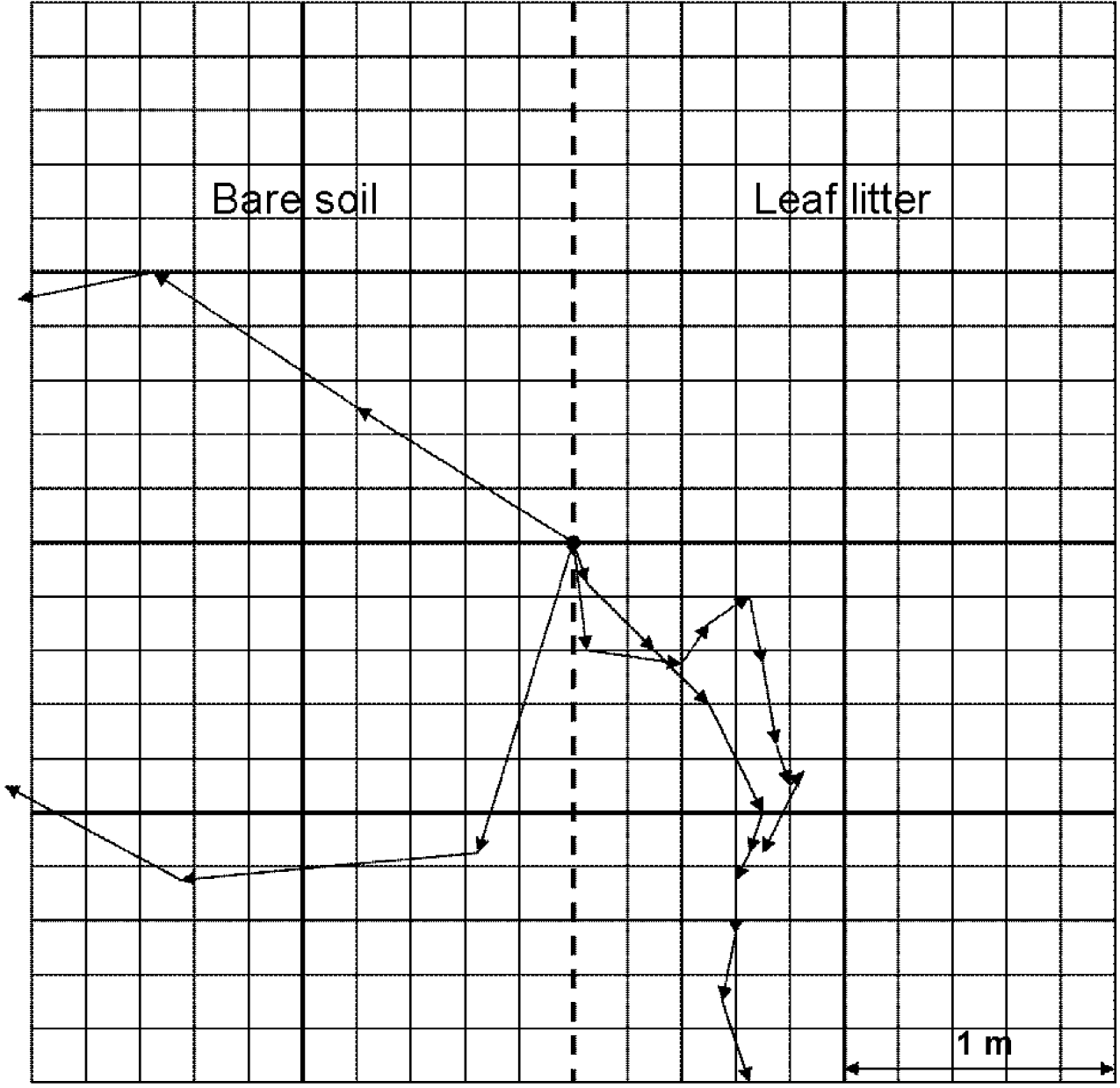
Fig. 1. Movement paths recorded for four wood cricket adults, each revealing a different pattern of movement. Experimental grid containing bare soil (left half) and leaf litter (right half) divided into 20×20 cm cells.
Leaf litter vs. short grass
A 5×3 m experimental site was created within a grass/hay field adjacent to a mature woodland edge dominated by oak (Quercus spp.). At this site, a grid was constructed using garden twine with each grid cell measuring 20×20 cm. The experimental site was equally divided with one half having a layer of leaf litter (2 cm deep) and the other half short grass (10 cm high). Single wood cricket nymphs and adults were released in the centre of the plot, at the edge of the contrasting substrates, further following the methods described above. Additionally, several individuals of both nymphs and adults were released within the short grass, where their movement paths and one-minute positions were recorded.
Analyses
For the analyses described in the following paragraphs, all data extracted from the individual registered paths were pooled. Therefore, these analyses represent the overall dynamics of the nymph population and adult population of wood crickets.
Paths made entirely within one homogeneous substrate, being either leaf litter, bare soil or short grass, were visually analysed to divide the continuous paths into distinct moves. This visual analysis revealed that the one-minute positions that were recorded for each individual matched the discrete moves relatively well. Therefore, the one-minute step positions were used to represent discrete moves for wood crickets in further analyses. In this case, moves represented the straight-line distances between each location as recorded over the one-minute interval. These coordinates combined with standard rules for right-angled triangles and trigonometry (cosine rule) were then used to calculate move distance, the mean speed (cm min−1) and turning angles between moves with functions available in Excel (Microsoft Office XP).
Initially, the individual paths made within the three individual substrates were tested for presence of a release effect. This was established by comparing the speed displayed in the first move of each individual path with the speed of subsequent moves. After exploration of the speed data and testing for normality (Kolmogorov-Smirnov test), Mann-Whitney and t-tests (for independent samples) were used for the analyses. Furthermore, chi-square ‘goodness of fit’ tests were performed to test for substrate preference for each life-stage and Fisher's exact probability tests (appropriate when using small samples) were used to test for difference in substrate preference between the sexes. Finally, Mann-Whitney tests were used to test for speed differences within the different substrates, and between sexes and life-stages. All of the statistical analyses were performed using SPSS (Version 14.0, SPSS Inc., Chicago, Illinois, USA).
Walk analysis
The individual paths were further analysed following the steps described in Turchin (Reference Turchin1998). The movement paths over bare soil and through leaf litter were analysed for deviances with a simple uncorrelated random walk (URW) and a correlated random walk (CWR) model (from Turchin, Reference Turchin1998). These models are suitable for analysis of paths made inside homogeneous environments and can be used as a tool to infer the rate of population spread within these substrates as a measure of dispersal for wood crickets (see Turchin, Reference Turchin1998). Uncorrelated random walk (URW) models assume that organisms move through the environment without any correlation between moves (i.e. no directional persistence or any other kind of correlation between successive displacements) and, therefore, do not include a parameter that accounts for a directional persistence within the equation. CRW models assume that there is a certain level of directional persistence in the movement of organisms and, therefore, include parameters that account for this persistence based on absolute turning angles between moves. Therefore, under the CRW formulation, a species is predicted to spread quicker compared to URW models. Not enough data was gathered to perform this analysis for grass, and data for adults were pooled regardless of sex.
When using URW or CRW models as a way to analyse recorded movement paths and patterns, a series of different statistical approaches need to be adopted in order to test if the data accurately fit the models and, consequently, if the models can accurately describe the population spread of a species. The series of tests that were performed, therefore, do not stand alone, and their results need to be interpreted together to draw conclusions for the species under study.
Both models assume that move duration, speed and turning angles within each path are not serially (or auto)correlated (Turchin, Reference Turchin1998). Particularly when speed and turning angle between moves show autocorrelations, this will affect and be reflected in the model outcomes and should be interpreted accordingly. Furthermore, the indication for the applicability of a CRW model over the URW model is when turning angles show an overall positive correlation (i.e. a symmetric distribution around 0° (range −180°/180°)). These criteria were tested as follows. To check the primary assumption to apply the CRW model, first the distribution of the absolute turning angle (range −180°/180°) was explored. Second, serial correlations for the individual paths were examined. Where move duration in our approach was perfectly correlated (using one-minute time steps to quantify moves), only speed (cm min−1) and turn angles were tested for presence of serial or autocorrelations between subsequent moves. Autocorrelation is a method specifically designed to examine correlations within time-series data. For movement paths, it correlates one move with move values (i.e. distance) lagged by one (first-order) or more (higher-order) previous moves. For speed, individual paths were analysed for presence of first-order and second-order autocorrelations. Turn angles were analysed by defining them as right (R) or left (L) turns relative to the previous move direction. To test for autocorrelation, subsequent turns for each individual path were paired relative to each previous turn and defined as RR, RL, LR and LL. Deviations from a random sequence were tested by applying a chi-square test of association for these turn pairs. Furthermore, chi-square ‘goodness of fit’ tests were performed to test for turn direction preference (even (LL, RR) vs. alternating (LR, RL)). Finally, Spearman's correlations between speed and absolute (positive) turn angles (0–180 degrees) between moves were performed for all registered paths. All analyses were performed using functions available in SPSS (Version 14.0, SPSS Inc., Chicago, Illinois, USA).
To further test the applicability of the URW or CRW formulation for the species moving through different substrates, net-squared displacements (R n2) were calculated (Equation 1). Under the URW formulation, net-squared displacements (R n2) typically increase linearly with time. Therefore, R n2 (Equation 1a) was plotted against time (n) and linear regression analyses was used to assess the overall fit. For testing the CRW model, comparisons were made between (theoretical) predicted and (actual) observed displacements. For these analyses, all paths with more than two recorded consecutive moves were examined. Predicted and observed displacements were calculated as net-squared displacements (R n2) (Equation 1b), employing mean observed values for move length and turn angle. These values were plotted against the number of consecutive moves observed for each recorded path made by the individually released specimens. To further asses the appropriateness of the CRW formulation for wood cricket movement, the 95% confidence interval for the predicted net-squared displacement was included for comparison with the observed values (following Turchin, Reference Turchin1998).
Equation 1. Net-squared displacement (R n2) formulation for (a) Uncorrelated Random Walk (URW) and (from Turchin, (Reference Turchin1998) ) (b) Correlated Random Walk (CRW) (corrected from Turchin (Reference Turchin1998) and Kareiva & Shigesada (Reference Kareiva and Shigesada1983) ).
L 1=mean move length (here in cm)
L 2=mean squared move length (here in cm2)
n=number of consecutive moves
c=mean cosine of the turn angle
Furthermore, to calculate the rate of population spread for wood cricket nymphs and adults, estimations for net displacement (R n) (in cm min−1) were calculated using the appropriate correction factor (z) (![]() : with mean absolute turning angle >30°, z=0.89; see Byers (Reference Byers2001) ).
: with mean absolute turning angle >30°, z=0.89; see Byers (Reference Byers2001) ).
Results
When looking at the individual movement paths of released nymph and adult wood crickets, a variety of movement patterns were revealed, indicating heterogeneity in movement among individuals within the species (Fig. 1). The data of all individual paths, therefore, were pooled to uncover the overall dynamics and movement patterns that prevail within the populations. The following findings, therefore, indicate the predominant movement patterns for wood cricket nymphs and adults.
Release effect
For nymphs, both in leaf litter and on bare soil, no significant difference was found between the speed generated in the first one-minute move and subsequent moves after release (Mann-Whitney test: n=299, z=−0.580, P=0.562; independent samples t-test: t 153=1.018, P=0.310, respectively). For adults, speed during the first move on leaf litter did not significantly differ from that during subsequent moves (Mann-Whitney test: n=142, z=−1.125, P=0.260). However, over bare soil, the speed generated during the first move was significantly higher than during the following moves (independent samples t-test: t 111=3.150, P=0.002), indicating an initial release effect. The first move of each path made on bare soil by adults for both males and females, therefore, was omitted from further analyses.
Speed through different substrates
For both life-stages, the frequency distribution of speed (cm min−1) through leaf litter showed a high amount of variation but was generally skewed towards relatively low values (Fig. 2a, b). Speed over bare soil was similarly highly variable (Fig. 2c, d) but was more evenly distributed around the mean value (Table 1), showing a normal distribution. No differences were found between the sexes (i.e. adult males and females). For grass, not enough observations were made to provide a clear frequency distribution.

Fig. 2. Frequency distribution of speed (cm min−1) generated through leaf litter by (a) nymphs, (b) all adults together, and on bare soil for (c) nymphs and (d) adults. For (b) and (d), one outlier was excluded from the graph for clarity (100.1 cm min−1 for (b) and 185.1 cm min−1 for (d)). Interval range=5 (a, b) and 10 (c, d) cm min−1. n=n s, see Table 1.
Table 1. Mean speed (cm min−1) recorded for nymphs and adults generated within different substrates
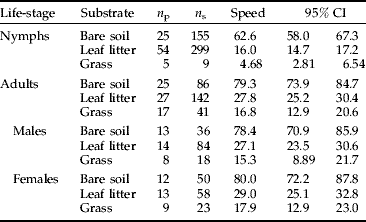
n p, number of paths (or individuals); n s, number of one-minute steps (or moves) taken by all individuals (for all paths) used to calculate the mean speed; Speed, mean speed (cm min−1); 95% CI=95% confidence interval around the mean speed.
Speed of nymphs on bare soil was higher than their speed through leaf litter (Mann-Whitney test: n=454, z=−15.31, P<0.001). No tests were performed for nymphs moving through grass because of the small number of observations made (n s=9; Table 1). Speed of adults was higher on bare soil than in leaf litter and grass (Mann-Whitney test: n=228, z=−11.57, P<0.001; n=127, z=−8.840, P<0.001, respectively; Table 1). Furthermore, speed within leaf litter was higher than within grass (Mann-Whitney test: n=183, z=−4.260, P<0.001; Table 1). No differences were found between adult males and females with respect to speed over bare soil, through leaf litter or through grass (independent samples t-test: t 84=−0.287, P=0.775; Mann-Whitney test: n=142, z=−1.163, P=0.245 and n=41, z=−0.911, P=0.363, respectively; Table 1). Between adults and nymphs, adults were significantly faster than nymphs when moving over bare soil and through leaf litter (independent samples t-test: t 239=−4.472, P<0.001; Mann-Whitney test: n=441, z=−8.462, P<0.001, respectively; Table 1).
Substrate preference
Both nymphs and adults preferred moving through leaf litter when offered a choice between leaf litter and bare soil or grass (chi-square test: P<0.001–0.039; Table 2), and no difference was observed in substrate preference between adult males and females (Fisher's exact probability test: P=0.486–1.000; Table 2).
Table 2. Substrate preference (or choice) of wood cricket nymphs and adults with related chi-square ‘goodness of fit’ tests and Fisher's exact tests for differences in preference between sexes.
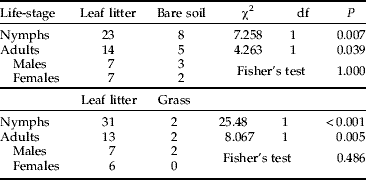
Leaf litter/Bare soil or Leaf litter/Grass=number of individuals choosing either substrate.
Turning angle analyses
The overall frequency distribution for turning angles both for leaf litter and over bare soil show an even distribution around 0° (Fig. 3), indicating directional persistence displayed by the species. This indicates a violation of the primary assumption (i.e. no correlations) made under the URW formulation, however, meets the assumption made under the CRW formulation.

Fig. 3. Frequency distribution of turning angles made when moving through leaf litter and over bare soil for (a, c) nymphs and (b, d) adults, respectively. Values on the x-axis represent the mid-point of the turning angle interval. Interval range=30°.
Autocorrelation for speed
First- and second-order autocorrelations for speed were found for nymphs moving through leaf litter and over bare soil (autocorrelation: P=0.025–0.043), indicating a violation of the URW/CRW criteria. This result indicates that for wood cricket nymphs the discrete moves made in each path were possibly oversampled by using the arbitrarily chosen move duration of one-minute, and this needs to be taken into consideration. For adults, analyses of the individual paths did not reveal any autocorrelations for speed through leaf litter habitat (autocorrelation: n=7, first-order: df=1, P=0.093–0.982; second-order: df=2, P=0.160–0.851) or over bare soil (autocorrelation: n=7, first-order: df=1, P=0.275–0.777; second-order: df=2, P=0.099–0.553), indicating no violation of the URW or CRW criteria. This result indicates that for adult wood crickets the moves that were made in each path were accurately defined using the one-minute time step.
Autocorrelation for turning angle
Nymphs moving through leaf litter revealed autocorrelations in their turning angle between consecutive moves (Table 3). Furthermore, alternating directions were favoured over even turns, indicating a linear persistence in their movement (i.e. directed movement pattern) (Table 4). Nymphs showed (weak) uncorrelated movement over bare soil (Table 3); however, grouping even and alternating turning pairs together revealed that even turns were favoured over alternating turns (Table 4). Furthermore, even pairs turning left were favoured over even pairs turning right (chi-square test: χ12=10.26, n LL=32, n RR=11, P=0.001), indicating a circling movement pattern for nymphs on this substrate. For adults moving through leaf litter, turn pairs were evenly distributed (Table 3 and 4), indicating no autocorrelation between consecutive moves. However, over bare soil, movements were autocorrelated (Table 3) where (like nymphs) even turns were favoured over alternating turns (Table 4). Both for nymphs and adults, no significant correlations were recorded between speed and turn angle for movement paths made in leaf litter and over bare soil (Table 5). Together, these results indicated that only adult wood crickets moving through leaf litter met all of the criteria under the CRW formulation.
Table 3. Contingency tables for turning angles of subsequent moves made by nymph and adult wood crickets moving over bare soil or through leaf litter with related chi-square tests of association.
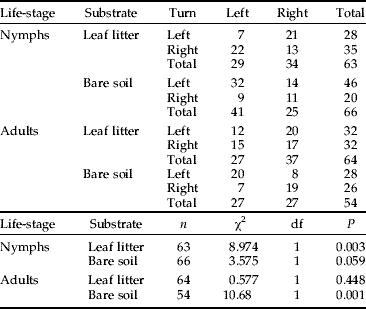
Turn=turn direction relative to the direction of the previous move.
Table 4. Results of chi-square ‘goodness of fit’ tests for the displayed movement patterns of nymphs and adults moving over bare soil or through leaf litter based on turning angles grouped in even turns left/left+right/right (LL+RR) and alternating turns left/right+right/left (LR+RL); see also Table 3.

Table 5. Spearman's rank correlation between speed and turn angle of all individual paths made by nymphs and adults over bare soil or through leaf litter.

Net-squared displacement (R n2)
When using the URW model (Equation 1a), linear regression analyses revealed a significantly positive relationship between net-squared displacement (R n2) and time for adult wood crickets moving through leaf litter and over bare soil (regression: R 2=0.93; F=40.7, df=4, P=0.008; R 2=0.86; F=19.1, df=4, P=0.022, respectively). For nymphs, no linear relationship was found for both leaf litter and bare soil (regression: R 2=0.52; F=3.25, df=4, P=0.169; R 2=0.49; F=3.79, df=5, P=0.123, respectively).
Under the CRW model (Equation 1b), when observed values exceed the predicted values, more directed movement by the species is suggested; and, where the observed values are below the predicted values, more random movement is suggested. Within leaf litter, for nymphs, six out of 11 observed values do not correspond with the predicted R n2 values (Fig. 4a). Overall, the observed R n2 shows a more rapid displacement than predicted until three consecutive moves, after which a gradual decrease is shown towards lower values than predicted (Fig. 4a). For adults, however, observed values through leaf litter display a good overall fit with the predicted R n2 values (Fig. 4b), confirming the results from the autocorrelation analyses (see above). Over bare soil, observed R n2 for nymphs reveal a relative good fit with the predicted values; however, generally the CRW formulation seems to overestimate the observed displacement (Fig. 4c). Adults moving over bare soil reveal a similar trend with a general overestimation of the predicted displacement compared to the observed values (Fig. 4d). Overall, these results indicate that only adult wood crickets moving through leaf litter can accurately be modelled using the CRW formulation. Furthermore, the adult population is predicted to spread quicker through leaf litter and over bare soil than the nymph population (Fig. 4a, predicted displacement R n=10.1 cm min−1; Fig. 4b, predicted R n=17.9 cm min−1; Fig. 4c, predicted R n=37.2 cm min−1; and Fig. 4d, predicted R n=43.2 cm min−1, respectively).

Fig. 4. Relationship between the net-squared displacement (R n2) (cm2) for CRW and the number of consecutive one-minute moves made within leaf litter (a) (n=114), (b) (n=116) and over bare soil (c) (n=124), (d) (n=76) for (a, c) nymphs and (b, d) adults, respectively. –□–, predicted square displacement; –▴–, observed square displacement; - - - - - -, 95% confidence interval of the predicted square displacement.
Discussion
The relationship between net-squared displacement (R n2) and time that was recorded in this study was found to be close to linear for adult wood crickets moving through leaf litter. In contrast, the relationship was non-linear for nymphs. Furthermore, the estimated rate of population spread as calculated from the predicted R n2 was found to be considerably lower for nymphs than for adults (10.1 vs. 17.9 cm min−1). This was also reflected in the differences in speed recorded through leaf litter, where nymphs were found to move more slowly than adults. Similar differences were also found between life-stages of a moth (Doak, Reference Doak2000) and a grasshopper (With, Reference With1994) species, where juveniles showed a lower rate of spread through their natural habitat than adults. A likely explanation for these differences could be the physical constraints (e.g. body size) related to the different life-stages. For Orthoptera, juvenile life-stages tend to be smaller and less mobile than the adults (Marshall & Haes, Reference Marshall and Haes1988), and this will consequently influence their ability to move through the environment. Wood cricket adults, therefore, can be considered more powerful dispersers than nymphs and the adult life stage most important in terms of dispersal success.
The wood cricket has a two-year life-cycle in the UK, of which two-thirds is spent as a nymph and one-third as an adult (Gabbutt, Reference Gabbutt1959; Brown, Reference Brown1978). To fully understand the dispersal ability of the species, it is, therefore, important to consider both life-stages (Diekotter et al., Reference Diekotter, Csencsics, Rothenbuhler, Billeter and Edwards2005). This is demonstrated by the results of this study. At the scale of this investigation, the movement pattern found for wood crickets moving through their preferred substrate (i.e. leaf litter) differed considerably between life-stages. When using a fine temporal scale, nymphs displayed a movement pattern that changed from a more directed walk to random movements. The pattern demonstrated by adult wood crickets was strikingly different. For adults moving through leaf litter, the CRW model described the observed movements relatively well, indicating a gradual spread of adults over time. Thus, compared to nymphs, adults showed a higher tendency to spread out. Doak (Reference Doak2000) found similar differences in movement patterns between life-stages for a moth species. Caterpillars of this species were found to move randomly, compared to a more directed movement recorded for the flightless female adult (Doak, Reference Doak2000). One of the reasons for these differences is likely to be a consequence of the sexual maturity of the species. Compared to juvenile life-stages, adults are influenced by factors related to reproduction and mating. For instance, responses to reproductive pheromones and finding suitable breeding locations are likely to influence their behaviour and related movement pattern. Together, this indicates that, compared to adults, nymphs tend to settle down operating within a specific home range. For the majority of their life cycle, wood crickets, therefore, will stay within a fixed area, where dispersal events are likely to take place only during the relatively short period of the adult phase of their life cycle.
Wood cricket adults and nymphs consistently preferred to move through leaf litter rather than over bare soil (or through grass). This is consistent with the previously documented association of the species with a well-developed leaf litter layer (Richards, Reference Richards1952; Brown, Reference Brown1978; Brouwers & Newton, Reference Brouwers and Newton2009b). This preference was also reflected in the movement pattern and speed observed in both environments (i.e. leaf litter vs. bare soil). When considering adult movements only, the movement pattern through leaf litter was accurately described as a CRW. This result is similar to that recorded for two woodland-associated carabid beetles, Pterostichus melanarius and Carabus nemoralis, moving through their preferred woodland habitat environment (Wallin & Ekbom, Reference Wallin and Ekbom1988). However, the movement pattern of adult wood crickets over bare soil (i.e. unfavourable habitat) was less accurately described by the CRW formulation. Similar results were also observed for P. melanarius and another woodland beetle, Pterostichus niger, moving through less favourable habitat (i.e. a cereal field) (Wallin & Ekbom, Reference Wallin and Ekbom1988). Furthermore, these and other carabid species were found to move considerably more rapidly through ‘unfavourable’ environment than their preferred habitat (Baars, Reference Baars1979; Wallin & Ekbom, Reference Wallin and Ekbom1988). This difference in the rate of movement was also observed for wood crickets, where the estimated speed over bare soil (i.e. unfavourable habitat) was 2.6–4.3 times greater than that recorded through leaf litter. For these species, a possible explanation for the observed differences could be the level of safety that is provided by a certain substrate. For wood crickets, the amount of cover provided by leaf litter compared to bare soil, therefore, is likely to have a significant influence on their movement pattern and behaviour. These similar results suggest that woodland invertebrate species tend to display a different movement pattern and move with less velocity within their preferred woodland habitat than when moving through less favourable environments like arable and grassland. This might further indicate that wood crickets and similar species are unlikely to disperse through non-woodland habitat environments, making this group particularly sensitive to woodland habitat fragmentation.
Bare soil can be considered as an environment that does not contain the habitat resources that wood crickets need to maintain their fitness (Richards, Reference Richards1952; Brown, Reference Brown1978). The pattern of movement revealed by the autocorrelation analyses showed that wood crickets tend to circle when moving over this particular substrate. This pattern was found to be more persistent for nymphs than for adults, possibly related to the greater ability of adults to orientate themselves towards external cues like tree trunks or the preferred leaf litter habitat that surrounded the experimental grid (Campan & Gautier, Reference Campan and Gautier1975). A similar circling pattern of movement was observed for the Mexican bean beetle when released in a field without its preferred resource/host plant (Turchin, Reference Turchin1998; pp. 147–150). This pattern was suggested to be related to the availability of their host plant and related search strategy of this species. This explanation could also be valid for wood crickets when moving over bare soil, where they also display a similar search strategy to find their primary resource habitat (i.e. leaf litter).
In this study, moves were defined using a fixed time interval to analyse the movement paths of wood crickets. However, defining discrete moves is often based on expert opinion (Turchin, Reference Turchin1998); and, therefore, their accuracy in representing the actual movements of the organism under study needs to be examined. The strategy for analyses used in this study was similarly used by Kareiva & Shigesada (Reference Kareiva and Shigesada1983), Wallin & Ekbom (Reference Wallin and Ekbom1988) and others (Turchin, Reference Turchin1998). However, this strategy might have affected the outcomes of the walk analysis. For example, the fixed time interval might have had an influence on the observed distribution of the turning angle and speed of the individuals, as shown by Nams (Reference Nams2006), possibly resulting in over- or under-estimations (Turchin, Reference Turchin1998). For nymphs, it was found that this likely had had an impact on the CRW analyses; but, for adults, this was found to have a lesser influence. Therefore, in order to test whether a model describes the movement pattern of a certain species accurately, it is important to analyse the observed movement paths in several different ways with a variety of tests (Turchin, Reference Turchin1998). The individual tests that can be used are complementary and do not necessarily confirm each other (Cain, Reference Cain1990; Turchin, Reference Turchin1998). The analyses of the movement paths that were recorded in the current study clearly showed the validity of this statement. For example, the visual representation of the CRW model showed that the observed net-squared displacement (R n2) roughly matched the predicted displacement for adult wood crickets moving over bare soil (see Fig. 4d), indicating a CRW movement pattern. However, the positive autocorrelation between turning angles indicated that adults tended to move in random circling patterns. This violated the assumptions made under both the URW and CRW formulations that were tested (see Turchin, Reference Turchin1998), indicating that in these cases neither model described the movement pattern of wood cricket adults accurately. Furthermore, for all situations, the first assumption under the URW formulation was violated because of an overall bell-shaped distribution in turning angle around 0°, indicating that a different model for describing wood cricket movement patterns should be applied. Interpretation of all analyses together found that the only movement data passing all individual tests for applying the CRW model were that obtained for adult wood crickets moving through leaf litter.
Although relatively few individuals of both nymphs and adults were used for the analyses, this study provides an important insight into how a specialized woodland species moves through its preferred habitat (i.e. leaf litter). This is particularly important given the limited knowledge of the dispersal ability of many woodland taxa (Dolman & Fuller, Reference Dolman, Fuller, Humphrey, Newton, Latham, Gray, Kirby, Poulsom and Quine2003; Bailey, Reference Bailey2007) and the particular lack of information for dispersal of species in different developmental stages and for those that move by walking (Diekotter et al., Reference Diekotter, Csencsics, Rothenbuhler, Billeter and Edwards2005). The results of this study indicated the difference in movement pattern through homogeneous habitat environments; however, natural environments are typically highly heterogeneous and may include barriers influencing movement and potentially inhibiting dispersal (e.g. Doak, Reference Doak2000). Such factors have been found to have a significant impact on the movement pattern and, consequently, the dispersal ability of a number of invertebrates studied in their natural environment (Johnson et al., Reference Johnson, Milne and Wiens1992; Firle et al., Reference Firle, Bommarco, Ekbom and Natiello1998; Doak, Reference Doak2000; Samu et al., Reference Samu, Sziranyi and Kiss2003). This highlights the need for further investigations on the dispersal ability of wood cricket and similar woodland species, which should focus on movement patterns and population dynamics in natural heterogeneous environments at a range of spatio-temporal scales.
Acknowledgements
We would like to thank the Forestry Commission and the Scottish Forestry Trust for funding this research. Furthermore, we would like to thank the People's Trust for Endangered Species (PTES) (London, UK) for providing us with the opportunity to work in their woodlands, and Sallie Bailey (Forestry Commission, UK), Keith Kirby (Natural England, UK), Kevin Watts (Forest Research, UK) and three anonymous referees for their input and comments on earlier drafts of this manuscript. We would further like to thank Peter Turchin (University of Connecticut, USA) and Nanako Shigesada (Doshisha University, Japan) for pointing out the error and correcting the CRW formula used in this manuscript.











