Introduction
Several species in the genus Macrolophus (Hemiptera: Miridae) are efficient predators of vegetable crops pests (e.g. whiteflies, aphids and thrips) (Riudavets & Castañé, Reference Riudavets and Castañé1998; Hansen et al., Reference Hansen, Brödsgaard and Enkegaard1999; Montserrat et al., Reference Montserrat, Albajes and Castañé2000; Lucas & Alomar, Reference Lucas and Alomar2002; Athanassiou et al., Reference Athanassiou, Kavallieratos, Ragkou and Buchelos2003; Margaritopoulos et al., Reference Margaritopoulos, Tsitsipis and Perdikis2003; Alomar et al., Reference Alomar, Riudavets and Castañe2006). Control strategies that incorporate Macrolophus species are based on both seasonal inoculative releases of commercially produced individuals and habitat management to conserve natural populations and enhance colonization (Alomar et al., Reference Alomar, Goula and Albajes2002). In order to avoid the incorrect use of predator reservoir plants, it is essential to correctly identify the predator species.
Several Macrolophus species are morphologically similar or have highly variable taxonomic characters, which hampers correct identification and questions species-identity status (Josifov, Reference Josifov1992; Kerzhner & Josifov, Reference Kerzhner, Josifov, Aukema and Rieger1999). This is particularly true for the two species that have been most cited as biological control agents in vegetable crops, M. pygmaeus (Rambur, 1839) and M. melanotoma (Costa, 1853) (the last one mostly cited as its junior synonym M. caliginosus Wagner, 1951), and has probably resulted in incorrect attribution of biological control by M. pygmaeus to M. melanotoma (see Martínez-Casales et al., Reference Martínez-Cascales, Cenis, Cassis and Sanchez2006 for a historical review). DNA methods have been employed recently to distinguish M. pygmaeus from M. melanotoma (Perdikis et al., Reference Perdikis, Margaritopoulos, Stamatis, Mamuris, Lykouressis, Tsitsipis and Pekas2003; Martínez-Cascales et al., Reference Martínez-Cascales, Cenis, Cassis and Sanchez2006). Cuticular hydrocarbon analysis could provide an additional method to separate morphologically similar species (Dall'Aglio-Holvorcem et al., Reference Dall'Aglio-Holvorcem, Benson, Gilbert, Trager and Trigo2009; Bagnères & Wicker-Thomas, Reference Bagnères, Wicker-Thomas, Blomquist and Bagnères2010).
The insect cuticle is coated with a thin lipid layer containing a high percentage of hydrocarbons (linear, branched, saturated and unsaturated), one of which main functions is to prevent desiccation and pathogen entrance into the body (Howard & Blomquist, Reference Howard and Blomquist2005). Cuticular hydrocarbons are usually present in high amounts and are easy to extract by quickly rinsing the specimen in non-polar organic solvents (Blomquist et al., Reference Blomquist, Nelson and de Renobales1987). In addition, most cuticular hydrocarbons are chemically stable and not very volatile (Martin et al., Reference Martin, Zhong and Drijfhout2009). The hydrocarbon blend is often species specific, and therefore it is a useful character in insect taxonomy (reviewed by Bagnères & Wicker-Thomas, Reference Bagnères, Wicker-Thomas, Blomquist and Bagnères2010).
In contrast to other insect groups, there are relatively few studies of cuticular hydrocarbon taxonomy in Hemiptera. A recent publication reviews the hydrocarbons of blood-sucking bugs (Juárez & Fernández, Reference Juárez and Fernández2007), and isolated studies compare cuticular hydrocarbons of Orius species (Anthocoridae) (Nakabou & Ohno, Reference Nakabou and Ohno2001) and aphids (Clements et al., Reference Clements, Wiegmann, Sorenson, Smith, Neese and Roe2000). With regard to mirids, analysis of cuticular hydrocarbons has been reported only for Lygocoris pabulinus (L.) (Drijfhout & Groot, Reference Drijfhout and Groot2001).
The purpose of the present study is to determine if cuticular hydrocarbons can be used to distinguish adults of two Macrolophus species (M. melanotoma and M. pygmaeus) that cannot be distinguished easily by external morphological characters alone. Since diet and sex may affect insect cuticular hydrocarbon composition (Liang & Silverman, Reference Liang and Silverman2000; Thomas & Simmons, Reference Thomas and Simmons2008; Guerrieri et al., Reference Guerrieri, Nehring, Jorgensen, Nielsen, Galizia and d'Ettorre2009), we controlled for the effect of diet (i.e. host plant and associated prey) and sex on cuticular hydrocarbons. M. costalis Fieber was included in the study as a reference species and to compare our results with a previous study on the taxonomic relationship of the three Macrolophus species based on DNA methods (Martínez-Cascales et al., Reference Martínez-Cascales, Cenis, Cassis and Sanchez2006).
Materials and methods
Insects
Chemical analyses were performed on adults of both sexes. Wild individuals were collected in the proximities of Cabrils (Barcelona, Spain) in the spring of 2008. Macrolophus melanotoma was collected from Dittrichia viscosa (L.) Greuter (Compositae), where it is abundant. Macrolophus costalis, which is readily distinguished by the dark spot on the scutellum, was collected on Cistus aldibus L. (Cistaceae), where it is commonly found. M. pygmaeus was obtained from a >5-year-old laboratory colony originated from tomato fields, and maintained on tobacco plants and frozen Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) egg prey. This colony is refreshed annually with individuals collected in local tomato fields. Examination of the length and shape of the respiratory horns of eggs (Perdikis et al., Reference Perdikis, Margaritopoulos, Stamatis, Mamuris, Lykouressis, Tsitsipis and Pekas2003) was used to confirm the identity of collected females of M. melanotoma and M. pygmaeus. Some of those individuals were frozen (−20 °C) immediately or within seven days of being collected (in which case they were maintained in their respective diet) and are referred to as the ‘field’ individuals. Additionally, in order to evaluate the effect of diet on the cuticular hydrocarbon profile, 4th-instar M. pygmaeus nymphs taken from the colony, and the progeny of field-collected M. melanotoma and M. costalis, were reared individually on tobacco leaves and E. kuehniella eggs. The resulting adults, referred to as the ‘laboratory’ individuals, were frozen when 3–6 days old and analyzed at the same time as the ‘field’ individuals described above.
Cages used for oviposition and nymphal development (7-cm diameter × 3.5-cm high with a ventilated lid) had a layer of 0.5% agar and a tobacco leaf disc placed on top of it, with the abaxial surface facing upwards, and were kept at 25 ± 2 °C and 85 ± 5%RH, under a 16:8 light:dark photo regime. Individuals were moved to new cages as needed and were provided with frozen E. kuehniella egg prey twice per week.
Chemical analyses
Individuals were taken out of the freezer one at a time, let to defrost and submerged in 20μl of Hexane (HPLC grade, Sigma-Aldrich, Madrid, Spain) containing 50ng of pentadecane (98% pure, Sigma-Aldrich, Madrid, Spain) in a conical-bottom hexane-rinsed glass vial. The use of internal standard allows minimization of any differences in injection volume and in the daily response of the equipment. Previous analyses indicated that pentadecane is not present in significant amounts in cuticular extracts of these species. Five minutes after immersion in the solvent the insect was removed and the solution was either analysed immediately or returned to the freezer (−20 °C) for later analysis.
For chemical analyses the volume of the extract was reduced immediately before injection to 2–4μl with a gentle nitrogen stream, and 1μl was injected manually in a gas chromatograph (GC) in the splitless mode (split valve opened after 1min). Samples were analysed in either a GC-FID or a GC-MSD (Agilent Technologies (Madrid, Spain) 6890NGC and 5973 Network quadrupole MSD), each equipped with a DB-5 column (30m × 0.32mm ID × 0.25μm, Agilent Technologies) and run with the same temperature program: start at 60 °C for 1min, then increase to 320 °C at 10 °C min−1, and maintain at this temperature for 25min. Carrier gas was Helium at constant flow (1mlmin−1), the injector was set a 250 °C and the detector at 280 °C.
A total of 119 insects were analysed by GC-FID, and these are the samples used in the statistical comparisons (ten individuals of each species, sex and diet (laboratory versus field), except for nine field M. melanotoma males). Two additional insects of each sex and species were analysed by GC-MSD, and these data were used in the chemical identification of the compounds. Straight-chain alkanes were identified by comparison of retention times and mass spectra of authentic standards (Fluka, alkane standard solutions 04070 (C8–C20) and 04071 (C21–C40), Sigma-Aldrich, Madrid, Spain). Retention indices (RIs) were calculated according to van den Dool & Kratz (Reference van den Dool and Kratz1963). Linear and methyl branched alkanes were tentatively identified by comparison of their RI and the characteristic ion fragments with those reported in the literature (Blomquist et al., Reference Blomquist, Nelson and de Renobales1987; Juárez & Blomquist, Reference Juárez and Blomquist1993; Carlson et al., Reference Carlson, Ulrich and Sutton1998; Juárez et al., Reference Juárez, Blomquist and Schofield2001; Gomes et al., Reference Gomes, Trigo and Erias2008; Mullen et al., Reference Mullen, Millar, Schal and Shaw2008; Dall'Aglio-Holvorcem et al., Reference Dall'Aglio-Holvorcem, Benson, Gilbert, Trager and Trigo2009).
Chain length of the selected compounds ranged between C24 and more than C40. Since our largest alkane standard was C40, we fitted a curve to the straight chain alkane standards C27–C40 to extrapolate the retention times of C41 and C42 (R2 = 0.99, y = 125.161 − 0.066 × x+1.105 × 10−5x2, where y = estimated RT and x = chain length), and then estimated the RI of sample peaks 27 and 28 (table 1).
Table 1. Chemical structure of 28 cuticular hydrocarbons of three Macrolophus species deduced from the characteristic ions (after GC-MSD) and the retention indices (RI) relative to straight-chain hydrocarbons.

Statistical analyses
Percent relative abundance ((area of the target peak/area of the internal standard peak) × 100) was the parameter used in all the group comparisons. We chose those compounds that showed a high relative abundance combined with a moderate coefficient of variation, relative to other compounds, and that occurred consistently in at least one class (i.e. species, sex or diet). This resulted in the selection of 28 peaks, some of which were abundant in one sex or species but bellow threshold level in others. A zero value was assigned to undetected compounds. A Kolmogorov-Smirnov test was used to assess normality of the data.
We performed a multivariate analysis of variance (MANOVA) in order to study how the 28 cuticular hydrocarbons altogether vary with species, sex and diet. To measure the variability that could be explained by each of these factors, we computed the Pillai-Bartlett statistic (Hand & Taylor, Reference Hand and Taylor1987), which provides a measure of the ratio of effect variance to error variance. We performed principal component analysis (PCA), using the correlation matrix of untransformed data, to obtain uncorrelated scores, visualize aggregation patterns of the different insect groups, and to determine which compounds had the strongest effect in this pattern. To build a prediction model for the species, the main principal components (those adding at least 78% total variability) were used in a quadratic discriminant analysis (QDA), and the prediction error of discriminant functions was evaluated using a ten-fold cross-validation method. The statistical tests were performed with R software (R Development Core Team, 2008). Analysis codes of the prediction model are available as Supplementary Material.
Results
Representative chromatograms indicating the 28 diagnostic peaks (by order of elution) are shown in fig. 1. Linear alkanes, from C21 to C35, were identified in the cuticular samples of the three species, but in very small quantities, and only one of them (C24 or tetracosane, compound 1, table 1) was abundant enough to be included among the 28 selected compounds. The rest of the hydrocarbons consisted of unbranched monoenes and alkanes with one or more methyl branches (table 1). Ion fragmentation suggests that some peaks may contain more than one compound.

Fig. 1. Representative gas chromatograph traces of Macrolophus cuticular hydrocarbons. Each trace corresponds to a different single individual. The most representative peaks were numbered 1 to 28, corresponding with the column elution time. Notice the large difference between M. melanotoma and the other two species, as well as between M. melanotoma males and females. Arrow indicates the characteristic dark spot on the scutellum of M. costalis.
Normality assumption was accepted for all samples (P > 0.0017, Bonferroni-corrected). Significant differences in cuticular hydrocarbon profiles for diet, sex and species were observed (MANOVA, P < 0.00001; table 2, fig. 2). The differences between sexes or diets were much smaller than those between species. Using the approximated F-statistic, we found that variability among species was almost four times higher than between males and females (26.8/6.9 = 3.87) and almost three times higher than between diets (26.8/9.1 = 2.91).

Fig. 2. Cuticular hydrocarbon abundance (relative to the internal standard) in males and females of three Macrolophus species. Field individuals (white bars) were fed on their natural diet; laboratory individuals (black bars) were reared in a standard tobacco and moth-egg diet.
Table 2. Results from MANOVA using Pillai-Bartllet's statistic when analysing variability due to species, sex and diet for 28 cuticular hydrocarbons of Macrolophus.

PC1 and PC2 explained 46.28% and 21.73% of the variability, respectively, and clearly segregated M. melanotoma from the other two species, whereas sex and diet differences were only apparent in M. melanotoma (fig. 3). Different compounds contributed in varying degrees to the PCs (fig. 3). Roughly the first half of the 28 compounds (2–15) were more abundant in M. melanotoma than in the other two species, and the second half (16–28) were less abundant in M. melanotoma than in the other species (fig. 2). Compound 7 (2-methyldotriacontane) is clearly characteristic of the M. melanotoma cluster (fig. 3) and is practically absent in the other two species (figs 1 and 2). Compounds 3 to 9 (but not 7) are less abundant in M. melanotoma females than males and contribute to separate the M. melanotoma female cluster, and compounds 11–15 are less abundant in M. melanotoma males than females and contribute to separate the M. melanotoma male cluster (fig. 3). Compounds 17 and 20–28 characterize a cluster enclosing M. pygmaeus and M. costalis, with no clear distinction between species, sexes or diet conditions (fig. 3). Individuals reared in the laboratory in isolation with a homogeneous diet of tobacco and moth eggs had a higher quantity of some hydrocarbons than their ‘field’ relatives, but the species-specific cuticular hydrocarbon pattern was not altered (fig. 2), thus indicating a weak effect of diet.

Fig. 3. Biplot showing individuals projected onto the plane defined by the first two principal components (PC), and cuticular hydrocarbons (vectors 1–28) representing directions and correlations in the same space. PC1 explains 46% of the variability of Macrolophus cuticular hydrocarbons and separates M. melanotoma males (○) from the females (●), and from the other two species (▲, M. pygmaeus; ■, M. costalis). PC2 explains 22% of the variability and separates M. melanotoma females from the males and from the other two species. Laboratory individuals are marked with a asterisk; field individuals are unmarked.
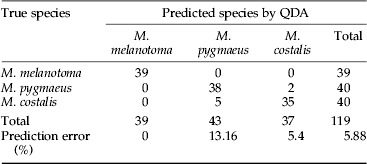
With regard to the prediction model, a QDA based on PC1, PC2 and PC3 (which together explained 78% of total variability) was used to predict the species of each individual (see Supplementary Material files). QDA was able to correctly classify all M. melatonoma individuals, but five M. costalis individuals were classified as M. pygmaeus and two M. pygmaeus were classified as M. costalis (table 3). Therefore the global error was 5.88% when comparing these three species. To further assess the error prediction level, we first run a training and test method using three-quarters of the sample (90 individuals chosen at random) to build a QDA, and the remaining quarter of the sample (29 individuals chosen at random) to test the prediction accuracy, which resulted in a 3.5% global error. Next, we used a ten-fold cross-validation method which indicated a global prediction error of 6.75% (ranging from 0% to 25%), attributed to the separation between M. pygmaeus and M. costalis, given that the error rate when discriminating M. melatonoma from M. pygmaeus or M. costalis was 0% in all cases. We also performed QDA separately for each sex, reaching an improved global error of 1.7% in both cases (only one insect in each sex was erroneously classified; tables S1 and S2, Supplementary Materials). Thus, the model predicted species identity despite the variability contributed by sex and diet, which supports the previous observation that the differences among species outweigh those between sexes and diets. Scripts of the model are available in R as Supplementary Material and can be modified to include other species and compounds.
Table 3. Prediction errors in the classification of three Macrolophus species based on a quadratic discriminant analysis (QDA) using the three main principal components of the cuticular hydrocarbons of males and females. The number in each cell represents the number of individuals of the true species (column) assigned to the three predicted species (rows). A total of 119 insects where analysed.
Discussion
Our study shows that the cuticular hydrocarbon profiles of M. pygmaeus and M. melanotoma are markedly different from each other and can be used as a phenotypic character to discriminate adults of these otherwise practically indistinguishable species. Inclusion of a third species, M. costalis, which has a cuticular hydrocarbon profile that is very similar to that of M. pygmaeus, but different from M. melanotoma, strengthens the validity of the cuticular hydrocarbon method for species separation. Our results agree with sequence variation of mtDNA where M. melanotoma and M. pygmaeus separate in different clusters, whereas M. pygmaeus and M. costalis group in the same cluster, and are therefore considered as sister species (Martínez-Cascales et al., Reference Martínez-Cascales, Cenis, Cassis and Sanchez2006). Crossing experiments between M. pygmaeus and M. melanotoma individuals collected from the same host plants as in this study produced no progeny (O.A. & C. C., personal observation), which is in accordance with the species status of the two taxa.
The prediction model results in 100% prediction of M. melanotoma, but the same model is less successful at separating M. pygmaeus from M. costalis, as the hydrocarbon profile of these two species are more similar to each other than to M. melanotoma. However, M. costalis has a dark spot on the scutellum that distinguishes it from M. pygmaeus, thus complete separation of all three species is possible. The same level of discrimination between M. pygmaeus and M. melanotoma than the one we have obtained is also achieved with mtDNA sequences (Martínez-Cascales et al., Reference Martínez-Cascales, Cenis, Cassis and Sanchez2006), so both methods are equally suitable to distinguish between the two species. Although there were sex differences in cuticular hydrocarbon profiles, making a separate prediction model for each sex reduces only slightly the prediction error for M. pygmaeus and M. costalis, and thus it is not necessary to analyse males and females separately. However, the two sexes are easily distinguished by eye, and so they could be analysed separately if finer discrimination is needed. Diet also had an effect on the cuticular hydrocarbons, but this effect was smaller than the differences among species and thus diet does not affect the ability of the model to predict species identity. Therefore, the differences among species outweigh those between sexes and diets, and the model will work despite these additional sources of variation.
To our knowledge, there is only one other report of cuticular hydrocarbons in mirids (Drijfhout & Groot, Reference Drijfhout and Groot2001; Drijfhout et al., Reference Drijfhout, Groot, Beek and Visser2003). We did not find any of the reported L. pabulinus compounds in Macrolophus cuticular extracts, neither are they present in several haematophagous triatomide heteroptera (Juárez & Fernández, Reference Juárez and Fernández2007); however, there are similarities between the cuticular hydrocarbons of triatomide bugs and Macrolophus. For example, the majority of compounds in both groups of insects are mostly between 20 and 40 carbons, alkenes are few, and mono and methyl branched alkanes are frequent, often with positions in carbons 11, 13 and 15. In contrast to the pattern observed in triatomide bugs and Macrolophus, in three species of the antocorid bug Orius, straight-chain alkanes, alkenes and single mono-metilated alkanes between 25 and 33 carbons in length comprise the most abundant cuticular hydrocarbons (Nakabou & Ohno, Reference Nakabou and Ohno2001).
M. melanotoma males and females had very different cuticular hydrocarbon profiles, but the hydrocarbon profiles of males and females of the other two Macrolophus species were very similar. Sexual differences are also relatively small in the heteropterans Oncopeltus fasciatus (Lygaeidae) and Rhodnius prolixus (Reduviidae) (Jackson, Reference Jackson1983; Juárez et al., Reference Juárez, Blomquist and Schofield2001). Whether sexual dimorphism of cuticular hydrocarbons plays a role in mediating sexual recognition in Macrolophus is unknown; however, there are many examples in other insects where this is the case (reviewed in Thomas & Simmons, Reference Thomas and Simmons2008). In the mirid L. pabulinus, the leg hydrocarbons (Z)-9-pentacosene and (Z)-9-heptacosene are produced in sex-specific amounts and could be involved in mate location (Drijfhout & Groot, Reference Drijfhout and Groot2001; Drijfhout et al., Reference Drijfhout, Groot, Beek and Visser2003).
The effect of diet on cuticular hydrocarbon composition is well documented in social omnivorous insects such as ants and cockroaches (Liang & Silverman, Reference Liang and Silverman2000; Guerrieri et al., Reference Guerrieri, Nehring, Jorgensen, Nielsen, Galizia and d'Ettorre2009). Although diet had a significant effect on the total quantity of cuticular hydrocarbons of Macrolophus, the species- and sex-specific patterns of cuticular hydrocarbons were not altered by the diet, suggesting that the cuticular hydrocarbon composition of Macrolophus is largely under genetic control and not strongly influenced by diet.
Acknowledgements
We thank Montse Lloveras for technical assistance with the chemical analysis. This research was funded in part by the Spanish Department of Science and Innovation (MICINN), projects AGL2005-03768 and AGL2006-08726 to O.A. and C.C., and AGL2007-62366 to C.G.








