Introduction
Soils constitute the major carbon reservoir of the terrestrial system (Silva & Mendonça Reference Silva and Mendonça2007), hence the study of carbon dynamics at different soil types, even in natural environments and in areas under anthropogenic influence, has been an important issue due to its relation to the greenhouse gas effect (Batjes Reference Batjes1996). In contrast with other cold regions (Arctic and alpine environment), the Antarctic continent has been comparatively less studied, although it represents an opportunity for exploring phenomena. Terrestrial Antarctic ecosystems are restricted in the ice-free zone, distributed basically along the coast or isolated mountains chain, representing less than 0.5% of the total area of that continent (Campbell & Claridge Reference Campbell and Claridge1987).
The severe climate and low water availability are important factors driving the Antarctic soils formation (Campbell & Claridge Reference Campbell and Claridge1987), and soil losses by periglacial erosion influences the amount of organic matter in Antarctic soils, which are highly variable (Michel et al. Reference Michel, Schaefer, Dias, Simas, Benites and Mendonça2006, Carvalho et al. Reference Carvalho, Mendonça, Barbosa, Reis, Seabra and Schaefer2010).
Soil C stock is a result of the combination between the primary production by autotrophic organisms and the organic matter decay promoted microbial activity (Silva & Mendonça Reference Silva and Mendonça2007). For this reason, the monitoring of soil C stocks with emphasis on soil temperature may indicate future changes in stocks due to changes in the terrestrial environment. The Maritime Antarctic has experienced significant temperature increases in the last 50 years (Vaughan et al. Reference Vaughan, Marchall, Connolley, King and Mulvaney2001, Quayle et al. Reference Quayle, Peck, Peat, Ellis-Evans and Harrigan2002, Steig et al. Reference Steig, Schneider, Rutherford, Mann, Comiso and Shindell2009). Higher temperatures may increase the soil organic matter (SOM) decomposition due to the great exposure of formerly frozen soil (La Scala et al. Reference La Scala, Mendonça, Carvalho, Panosso, Simas and Schaefer2010, Mendonça et al. Reference Mendonça, La Scala, Panosso, Simas and Schaefer2011), resulting in decreasing soil C stocks (Carvalho et al. Reference Carvalho, Mendonça, Barbosa, Reis, Seabra and Schaefer2010, La Scala et al. Reference La Scala, Mendonça, Carvalho, Panosso, Simas and Schaefer2010).
The organic matter mineralization process affects primarily the less persistent SOM forms, resulting in a gradual accumulation of organic materials with higher recalcitrance in substances such as chitin, uric acid and humic substances (Myrcha et al. Reference Myrcha, Pietr and Tatur1983). This process is part of the C cycle and promotes, simultaneously, enhanced CO2 in the atmosphere and reduction of soil C stocks. The microbial activity in Antarctic soils, like elsewhere, is dependent on multiple factors, such as nutrient availability, pH, moisture, salinity, besides temperature.
The prevailing lower soil temperatures in Antarctica reduce SOM mineralization. Cyanobacteria, lichens, algae, bryophytes and a few higher plants present on the soil surface are capable of fixing atmospheric C in Antarctic soils (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006). However, the majority of global warming models predict an increase in temperature especially in periglacial regions, which will greatly affect CO2-C production and SOM content (La Scala et al. Reference La Scala, Mendonça, Carvalho, Panosso, Simas and Schaefer2010). In addition, increasing soil temperature results in higher soil CO2-C emissions according to different vegetation covers (Mendonça et al. Reference Mendonça, La Scala, Panosso, Simas and Schaefer2011). There is a consensus that the characterization of C dynamics in Antarctic soils is fundamental to establish the relation between soil properties and climatic changes (Beyer et al. Reference Beyer, White, Pingpank and Bölter2004).
To examine the consequences of increasing soil temperature in Antarctica the understanding of mineralization rate of SOM, including an enhanced knowledge of C quality and soil microbial activity, is necessary. The aim of this study was to analyse the influence of these characteristics on the mineralization process of SOM in selected, representative Maritime Antarctic soils.
Material and methods
Characterization of the studied sites
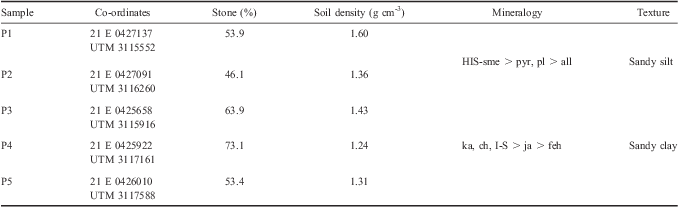
Admiralty Bay is located at King George Island (62°03′–62°05′S, 58°23′–58°24′W) which is the largest island in the South Shetland Islands (Simas et al. Reference Simas, Schaefer, Melo, Albuquerque-Filho, Michel, Pereira, Gomes and Costa2007). During the 2008–09 summer, soils were collected in three replicates at 0–10 cm and 10–20 cm in five different soils locations across Keller Peninsula, as shown in Fig. 1.

Fig. 1 Map of King George Island, highlighting Keller Peninsula, where soils were collected (adapted from Birkenmajer Reference Birkenmajer2002).
Based on the physical-chemical, mineralogical and morphological soil characteristics (Table I), soil located in sites 1 and 2 were classified as basaltic/andesitic soils while those in sites 3, 4 and 5 were classified as acid sulphate soils (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006). Prior to analytical procedures, all samples were passed through a 2 mm sieve to quantify the percentage of coarse fragments, larger than 2 mm.
Table I Co-ordinates, physical and mineralogical characteristics of the soils collected in the Keller Peninsula.

HIS-sme = interstratified smectite-hydroxy-interlayered-smectite, pyr = pyroxene, pl = plagioclase, all = allophone, ka = kaolinite, ch = chlorite, I-S = interstratified illite-smectite, ja = jarosite, feh = ferrihydrite.
Total soil organic carbon (TOC) content in all studied locations was determined according to Yeomans & Bremner (Reference Yeomans and Bremner1988). Oxidizable organic C labile and recalcitrant fractions (Clabile and Crecalc) were determined according to Chan et al. (Reference Chan, Bowman and Oates2001), while total nitrogen was determined by the Kejldahl method (Bremner & Mulvaney Reference Bremner and Mulvaney1982). Microbial biomass (Cmic) was determined by the irradiation extraction method (Islam & Weil Reference Islam and Weil1998, Ferreira et al. Reference Ferreira, Camargo and Vidor1999). The Cmic/TOC was used as an indicator of soil microbial biomass pool (Marchiori & Mello Reference Marchiori and Melo1999), and considered a proxy of SOM quality.
Soil humic and fulvic acids fractions were extracted, fractioned and purified according to the International Humic Substances Society (IHSS) (Swift Reference Swift1996), and following, total C content was determined according the method described in Yeomans & Bremner (Reference Yeomans and Bremner1988). Soil bulk density was determined in soil samples with the use of paraffin impregnation method (Embrapa 1997). C stock of surface horizons of soil was calculated using the following formula:
where Cst = C stock (kg m-2), TOC = total soil organic carbon (g C kg-1 soil), E.C. = thickness of the soil (m), and D = soil density (kg soil m-3), expressed in g cm-3 or 10-3 kg m-3.
In situ soil CO2-C emission experiment
The field experiment was conducted in the vicinity of Comandante Ferraz Brazilian Antarctic Station, Keller Peninsula. Soils from locations 1–5 (0–10 cm), with and without vegetation (mixed Deschampsia and mosses), were kept at open environmental conditions. Measurement of CO2-C emission were conducted during two summer seasons, from 24 January to 10 February 2008 and from December 2008 to March 2009, totalling 21 days of readings during these periods, in accordance with weather conditions.
Samples were collected from five sites and were kept in open-air on a 60 x 60 cm wooden board, composed by 21 PVC collars (10 cm diameter each), containing 300 g of bare soil or 200 g of soil + 100 g of natural vegetation (mixed Deschampsia and mosses). An automated soil CO2 flux system (LI-8100, Li-Cor Environmental) was coupled to the collars to measure CO2 emitted at each sample. At measurement mode, three replicates were applied for each collar, performing 63 measurements per day under contrasting soil temperature conditions. The LI-8100 system is based on the infrared absorption spectroscopy that analyse the time changes of CO2 concentration inside the chamber once it is placed onto the soil PVC collars. As the internal chamber is a closed system (internal volume = 854 cm3), with a fixed contact area to the soil (exposed area = 83 cm2), changes in CO2 concentration inside the chamber once it was placed on the collars, was used to calculate emissions at measurement mode, during 1.5 min reading. As soon as emissions were measured, in situ soil temperature was also recorded in all treatments with a field thermometer placed at 10 cm depth.
The relation between CO2 and soil temperature was described by the equation FCO2 = F0 x exp(b x Tsoil), with the natural log (Ln) of the CO2 emission we have Ln(FCO2) = Ln(F0 x exp(b x Tsoil)), the result is Ln(FCO2) =Ln(F0) + b x Tsoil. A linear relation between Ln(FCO2) and the Tsoil is expected in the environments where soil temperature is a limiting factor.
Based on the B coefficients it is possible to derive the Q10 factor, which represents the percentage increase in emission for a 10°C increase in soil temperature. This is derived as Q10 = e10xB.
Statistical analysis
Data was submitted to the students t-test (significance level of 5%) in order to compare the mean values by using the SAEG software (Funarbe 2007). Graphs and linear regressions were performed using the Origin 8.0 software (Origin Lab Corp).
Results
Soil organic matter characterization
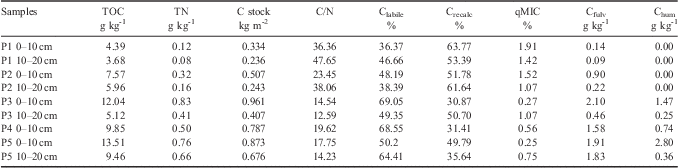
Table II presents the results of SOM characterization in all locations studied for both depths, except for location 4, where the bedrock was found at 10 cm depth and no soil could be collected. The lowest C stocks were found in samples 1 and 2 (basaltic/andesitic soils), both very rocky and shallow. The microbial coefficient (qMIC) ratio indicates higher values for soils 1 and 2, contrasting to different proportion of Clabile and Crecalc fractions. Differences associated with biological and chemical methods can represent different C fractions.
Table II Characterization of the soil organic matter of the soil samples.
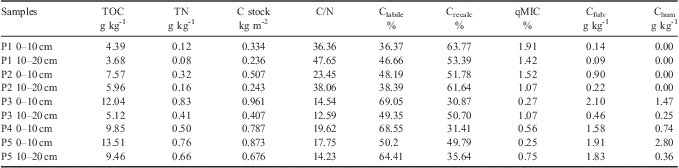
TOC = total organic carbon, TN = total nitrogen, Clabile = C in the labile form, Crecalc = C in the recalcitrant form, qMIC = percentage of the total C in the microbial biomass, Cfulv = C in the fulvic acid fraction, Chum = C in the humic acid fraction.
Soil CO2-C emission
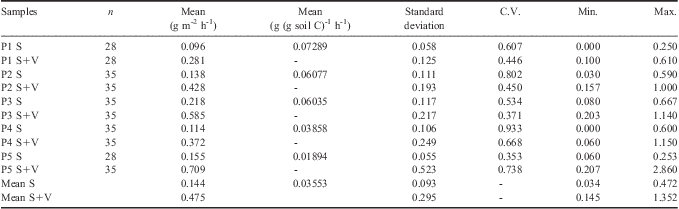
Table III presents the mean CO2-C values from the two in situ summer experiments. The mean CO2-C emissions varied from 0.034–0.472 g of CO2-C m-2 h-1 for soil samples (S) and from 0.145–1.352 g of CO2-C m-2 h-1 for soil plus vegetation samples (S+V). Changes in CO2-C can be related to soil temperature, as shown in Table IV. Significant (P < 0.05) exponential relationship between CO2-C and soil temperature was observed for all locations, in samples either with or without vegetation. The relation FCO2-C = F0 eB x Tsoil, was linearly fit by applying Ln(FCO2-C) = Ln(F0) + B(Tsoil) (Fig. 2). Results indicate that CO2-C emission sensitivity to soil temperature (B coefficient in Table IV) is similar in all soils studied, irrespective of location and presence of vegetation. This is corroborated by the observed B values ± standard errors.
Table III Descriptive statistics of CO2-C emission from soil with or without vegetation (S+V or S, respectively).
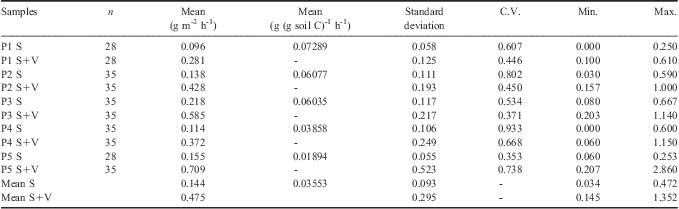
C.V. = coefficient of variation.
Table IV Parameters of the model between CO2-C emissions and soil temperature for the studied soil and Q10 factor.

*Means followed by same letter do not differ significantly by student's t-test at the 5% level of significance.
A and B = linear and angular coefficients, obtained from the linear regression analysis, respectively; R = linear correlation coefficient; P = significance level; and s.e. = standard error.

Fig. 2 Logarithm of CO2-C emission as related to soil temperature for site 2. a. Bare soil, and b. soil with vegetation.
The significant and positive linear correlation of the B factor with C/N ratio and qMIC is illustrated in Figs 3 & 4. These results show how sensitive soil CO2-C emissions are to increasing soil temperature.

Fig. 3 Relationship between the B factor of CO2-C emissions for tests with bare soil (S) versus a. the values of C/N ratio, and b. microbial coefficient (qMIC) for Antarctic soils.

Fig. 4 Relationship between the B factor of CO2-C emissions for tests with bare soil with vegetation (S+V) versus a. the values of C/N ratio, and b. microbial coefficient (qMIC) for Antarctic soils.
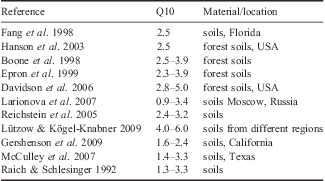
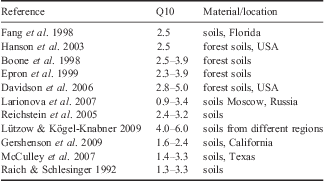
The Q10 values are reported in Table IV. The mean Q10 value in our study (3.792±1.579) is higher than that observed elsewhere by several authors (Table V).
Table V Q10 values for soils from different regions, estimated by several authors.
Discussion
Soil organic matter characterization
The mean C content for all soil samples (7.95±3.47 g kg-1) is within the range of previous studies (Bölter Reference Bölter1995, Bölter et al. Reference Bölter, Blume, Schneider and Beyer1997, Simas et al. Reference Simas, Schaefer, Melo, Francelino, Fernandes Filho and Costa2008, Francelino et al. Reference Francelino, Schaefer, Simas, Fernandes Filho, Souza and Costa2011), but lower than the ones found in Carvalho et al. (Reference Carvalho, Mendonça, Barbosa, Reis, Seabra and Schaefer2010) for mineral soils of King George Island, and other terrestrial studies elsewhere in Antarctica (Campbell & Claridge Reference Campbell and Claridge1987, Simas et al. Reference Simas, Schaefer, Melo, Francelino, Fernandes Filho and Costa2008). However, soil C stocks presented values similar to those reported by Michel et al. (Reference Michel, Schaefer, Dias, Simas, Benites and Mendonça2006), with a range 0.1–0.9 kg m-2 (in the upper 10 cm layer) for Admiralty Bay soils.
The high C/N ratio of the soil is usually related to low decomposition rate of soil organic matter (Silva & Mendonça Reference Silva and Mendonça2007). Higher C/N ratio was observed in samples 1 and 2 for both depths, indicating a low decomposition degree of SOM. On the other hand, samples 3, 4 and 5, corresponding to acid sulphate soils, presented intermediate C/N ratios, varying from 12.6–19.6. Considering that all samples are subjected to cold climate conditions, and with a poor vegetation cover (basically Deschampsia antarctica Desv. and mosses), the differences in C/N ratio may be attributed to varying soil moisture conditions. Samples 1 and 2 are loamy sand soils contrasting to sandy clay soils 3, 4 and 5. It is well-documented that higher clay content effectively protects soil C due to colloidal interactions (Silva & Mendonça Reference Silva and Mendonça2007). Under experimental conditions it is expected only limited colloidal interactions between clay and SOM occur since there is little soil structural development. Hence, only resistant structural SOM with high C/N ratio, and recalcitrant fractions, remain.
Soils 1 and 2 have high pH, nutrient contents and cation exchange capacity (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006), which may positively affect soil microbial activity. In these areas erosion is intense, and soils are less developed and have lower SOM content, compared with soils 3, 4 and 5. Our data are supported by the results of Hopkins et al. (Reference Hopkins, Sparrow, Gregorich, Elberling, Novis, Fraser, Scrimgeour, Dennis, Meier-Augenstein and Greenfield2009), which indicated that with permafrost melting, the SOM from Maritime Antarctica can have a relatively fast turnover, which may be related to recent exposure of labile material, protected by the frozen state.
The low or negligible contents of humic and fulvic acid fractions in the soils indicate that inherited humin is the main route for humic substances formation. Low temperature and little clay content of Antarctic soils do not favour the physical protection of SOM, increasing C losses (Silva & Mendonça Reference Silva and Mendonça2007).
Soil CO2-C emission
The presence of vegetation, regardless of the soil studied, resulted in 230% emission increase, or the equivalent of 0.331 g of CO2-C m-2 h-1. In any bare soil condition, the ratio between total emission (or mean emission) and the soil C stock, would be related to the rate of the soil C decay (time-1). In this regard, our results indicate that the higher this ratio, the lower the soil C stock. This means that the soil C losses through CO2 are the main mechanism which controls the soil C stock in Maritime Antarctic soils.
The difference between minimum and maximum mean values for bare soil emissions were as high as 1288%, while in S+V samples differences were 832%. The lowest emissions, with negligible values, were observed in soil 1 (basaltic/andesitic soils, sandy soil), without vegetation, which also had the lowest soil C stocks (Table II). On the other hand higher mean emissions during the two summer periods were obtained for soil 5 with vegetation (2.860 g C-CO2 m-2 h-1, acid sulphate clay soil), which showed higher soil C stocks. The mean emissions suggest that soil C losses through CO2 were greater in soil 5, under D. antarctica plus moss cover. On the other hand higher emissions from bare soils were observed in soil 3, and similar figures were obtained for soils 2 and 4. These results suggest that soil exposure following permafrost melting may enhance the decay of native SOM to CO2, as already suggested by previous studies (Michaelson et al. Reference Michaelson, Dai and Ping2004, Carvalho et al. Reference Carvalho, Mendonça, Barbosa, Reis, Seabra and Schaefer2010, La Scala et al. Reference La Scala, Mendonça, Carvalho, Panosso, Simas and Schaefer2010, Mendonça et al. Reference Mendonça, La Scala, Panosso, Simas and Schaefer2011). Overall, the maximum, minimum and the mean values of C emission from soil plus vegetation were greater than the bare soil emissions, which can be attributed to plant root respiration (Tang & Baldocchi Reference Tang and Baldocchi2005), being influenced by different plant photosynthetic activity (Kuzyakov & Gavrichkova Reference Kuzyakov and Gavrichkova2010).
The CO2-C emission is sensitive to soil temperature and the significance of B values shows three different sensitivity ranges, a higher range for soils 1 and 2, a mean for soils 3 and 4, and a lower range for soil 5, with 0.069 C-1. Soils with higher B factor are those with lower SOM contents and C stocks (soils 1 and 2). These are also loamy sand soils, with lower clay content than the remaining soils 3, 4 and 5. The former soils have higher SOM humification degree, similar SOM content, C/N ratio and soil C stock, whereas soil 5 presented lower labile C content compared to soils 3 and 4. This is related to the lower B factor of soil 5 in relation to the others, since the labile C pool, readily oxidizable, is less at soil 5.
It is known that the sensitivity of soil C to soil temperature is affected by numerous factors that are directly or indirectly related to temperature (Yuste et al. Reference Yuste, Baldocchi, Gershenson, Goldstein, Misson and Wong2007). These relations are better illustrated in Fig. 3 as the B factor, which expresses how sensitive soil CO2-C emission is to increasing soil temperature. We observed a significant and positive linear correlation with C/N ratio and qMIC. These data contrast with the results of Hopkins et al. (Reference Hopkins, Sparrow, Gregorich, Elberling, Novis, Fraser, Scrimgeour, Dennis, Meier-Augenstein and Greenfield2009) that showed positive relation of high C/N ratio to C stocks of Antarctic soils. Nitrogen deficiency has been linked to limiting decomposition of plant-based materials (Jingguo & Bakken Reference Jingguo and Bakken1997). However, highest bacterial biomass is located in surface levels, independent of actual high C/N ratios (Bölter et al. Reference Bölter, Blume and Kuhn1999). It is also well known that N has significant importance in SOM accumulation, since it is effective for organic matter humification routes (Silva & Mendonça Reference Silva and Mendonça2007). These soil characteristics are therefore attributes that may be useful to infer soil C losses through CO2 emission in ice-free zones, during the Antarctic summer. By comparing the data in Fig. 4, a decreasing trend in the coefficient of determination for the soil with vegetation suggests that other factors, besides the C/N ratio and qMIC, are affecting the sensitivity of emissions. This result is corroborated by data obtained by La Scala et al. (Reference La Scala, Mendonça, Carvalho, Panosso, Simas and Schaefer2010), showing that soil temperature exerts a controlling factor on temporal variations in soil CO2-C emissions, and points out that vegetation is an additional important effect.
The experimental results of vegetated soils indicate, after cluster analysis, that B values of soil 3 plus vegetation were intermediate between values for soils 1 and 2 and soil 4 and 5 (Table IV). This is probably due to the higher changes in CO2-C emissions promoted by photosynthetic activity and evolved root exudates (Mendonça et al. Reference Mendonça, La Scala, Panosso, Simas and Schaefer2011), which are more subject to microbial degradation. The studied soils have SOM with greater susceptibility to degradation when exposed to environmental conditions than SOM from other regions (Carvalho et al. Reference Carvalho, Mendonça, Barbosa, Reis, Seabra and Schaefer2010). Results in the present work is in the range of Q10 reported for forest soils (Epron et al. Reference Epron, Farque, Lucot and Badot1999, Davidson et al. Reference Davidson, Janssens and Luo2006), which have much greater SOM contents, hence having high sensitivities.
The above discussion requires a potential scenario in which the regional temperature would significantly increase, consequently reducing the soil C stock by increasing CO2 emissions. However, increasing soil biomass due to greater photosynthesis and primary productivity may counteract this trend (Michel et al. Reference Michel, Schaefer, Dias, Simas, Benites and Mendonça2006). Hence, the SOM balance would be a result of the soil C input/output at this new hypothetical scenario.
Conclusions
Maritime Antarctic soils having lower humification degree showed higher CO2-C emissions. A significant relationship (P < 0.05) between CO2-C emission and soil temperature was observed for both bare soil or vegetated soil.
The emission of CO2-C showed significant exponential relationship (P < 0.05) with temperature, with increasing emission of CO2-C with increasing temperature.
The sensitivity of CO2-C emissions in relation to temperature showed significant correlation with the degree of humification and microbial activity. Thus, further losses of CO2-C with rising temperature are expected in soils with a lower degree of humification.
The average Q10 values for the soils did not differ, but overall values were higher than those observed elsewhere. The high Q10 value is related to the fragility of the SOM with low degree of humification and chemical characteristics of humic substances, becoming more susceptible to microbial degradation.
Acknowledgements
We acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil, for financial support. Carlos Schaefer thanks CAPES agency for granting a sabbatical visiting professorship at Cambridge University. This work is a contribution of INCT-Criosfera TERRANTAR group. The constructive comments of the reviewers are also gratefully acknowledged.