Introduction
Foot-and-mouth disease virus (FMDV) is a member of family Picornaviridae, genus Aphthovirus (Bachrach, Reference Bachrach, Diener and Romberger1977; Rodrigo and Dopazo, Reference Rodrigo and Dopazo1995; Rueckert, Reference Rueckert1996). FMDV was the first virus of vertebrates to be identified, i.e., Loeffier and Frosch (Reference Loeffier and Frosch1897) collected vesicular fluid, passed it through ceramic filters impermeable to bacteria, and reproduced clinical signs in cattle exposed to the filtrate. FMDV consists of a single-stranded, positive-sense RNA genome of approximately 8500 bases organized in three major regions (5′ non-coding regulatory region, polyprotein coding region, and 3′ non-coding regulatory region), with a polyadenylated 3′-end and a small, covalently linked protein (VPg) at the 5′-end. Polyproteins are post-translationally cleaved by viral protease into four structural proteins (VP1, VP2, VP3, and VP4) and eight non-structural proteins (NSPs; L, 2A, 2B, 2C, 3A, 3B, 3C, and 3D) (Ryan et al., Reference Ryan, Belsham and King1989). Structural proteins VP1, VP2, and VP3 assemble to form an icosahedral structure that is internally bound by VP4. NSPs function in virus replication and interactions with host cell factors and for processing of the structural proteins (Domingo et al., Reference Domingo, Baranowski, Escarmís and Sobrino2002; Grubman and Baxt, Reference Grubman and Baxt2004).
The classic clinical signs of FMDV infection (vesicles on the mouth and feet) were first described by Hieronymous Fracastorius (1546) after observing an outbreak in cattle near Verona, Italy (Mahy, Reference Mahy2005). FMDV is infectious for most animals in the order Artiodactyla (even-toed ungulates), but especially cattle, buffalo, swine, sheep, and goats (Burrows, Reference Burrows1968; Gibbs et al., Reference Gibbs, Herniman, Lawman and Sellers1975a, Reference Gibbs, Herniman and Lawman1975b; Bastos et al., Reference Bastos, Boshoff, Keet, Bengis and Thomson2000; Kitching, Reference Kitching2002a, Reference Kitching2002b; Alexandersen and Mowat, Reference Alexandersen, Mowat, Compans, Cooper, Honjo, Melchers, Olsnes and Vogt2005). In addition, more than 70 wildlife species are known to be susceptible to FMDV, including white-tailed deer (Odocoileus virginianus) (Snowdon, Reference Snowdon1968; Fenner et al., Reference Fenner, Gibbs, Murphy, Rott, Studdert, White, Fenner, Bachmann and Gibbs1993; Moniwa et al., Reference Moniwa, Embury-Hyatt, Zhang, Hole, Clavijo, Copps and Alexandersen2012). FMDV in wildlife species is a serious concern because of the problems entailed in eradicating the virus from such populations. In the USA, 20,000 mule deer (Odocoileus hermionus) were killed in Stanislav National Forest to control the 1924–1926 FMDV outbreak in California.
The virus is highly contagious and, depending on the route of exposure, ≤10 tissue culture infectious doses are sufficient to infect and produce clinical disease in susceptible ruminants (Sellers et al., Reference Sellers, Herniman and Mann1971; Alexandersen et al., Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b). Although incubation time can be considerably longer, depending on dose and route of infection, viremia typically appears 24–48 h post-exposure with vesicles in the mouth and on the feet, thereafter (Yilma, Reference Yilma1980; Baxt and Mason, Reference Baxt and Mason1995). In an FMDV outbreak, transmission within and between populations can be rapid due to the short in vivo replication cycle (4–6 h) and acute onset of shedding (1–3 days) (Donaldson et al., Reference Donaldson, Gibson, Oliver, Hamblin and Kitching1987; Grubman and Baxt, Reference Grubman and Baxt2004; Grau et al., Reference Grau, Schroeder, Mulhern, McIntosh and Bounpheng2015). The most common route of FMDV transmission is direct contact, however, transmission can occur over significant distances due to aerosol and mechanical dissemination of virus through water, feed, and fomites (Brooksby, Reference Brooksby1982; Thomson et al., Reference Thomson, Vosloo and Bastos2003). Clinically healthy FMDV carriers (reported up to 3.5 years in cattle, 9 months in sheep, and 4 months in goats) occur in both naïve and vaccinated ruminants, complicating control and eradication efforts (Pereira, Reference Pereira and Gibbs1981; Kitching, Reference Kitching1998; Alexandersen et al., Reference Alexandersen, Zhang and Donaldson2002a, Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b).
Infection elicits a rapid immune response, but as a result of extensive antigenic variation, immunity against one FMDV isolate does not necessarily protect against others (Bedson and Maitland, Reference Bedson and Maitland1927; Galloway et al., Reference Galloway, Henderson and Brooksby1948; van Bekkum et al., Reference Van Bekkum, Frenkel, Frederiks and Frenkel1959; Gebauer et al., Reference Gebauer, De La Torre, Gomes, Mateu, Barahona, Tiraboschi, Bergmann, De Mello and Domingo1988; Salt, Reference Salt1993; Sutmoller et al., Reference Sutmoller, Barteling, Olascoaga and Sumption2003).Variation in VP1, VP2, and VP3 proteins made it possible for early investigators to use cross-neutralization tests to classify serotypes. In 1922, Vallée and Carré reported the presence of what is known today as serotype O in France and serotype A in Germany. Shortly thereafter, Waldmann and Trautwein (Reference Waldmann and Trautwein1926) reported what is now identified as serotype C in Germany (Brown, Reference Brown2003). Three more serotypes (South African Territories; SAT 1, SAT 2, and SAT 3) were discovered in South Africa by Brooksby (Reference Brooksby, Smith and Lauffer1958) and Asia 1 was identified in Pakistan in 1957 (Brooksby and Rogers, Reference Brooksby and Rogers1957). Antigenic variation is a challenge to FMDV control because it has the potential to complicate vaccinology and diagnostics.
Depending on the geographic region, serotype-specific, inactivated FMDV vaccines are used to control clinical disease in endemic areas, but have also been used in FMDV eradication campaigns, e.g., Uruguay, Argentina, and Paraguay (Sumption et al., Reference Sumption, Rweyemamu and Wint2008). Outbreaks have occurred in every livestock-containing region of the world with the exception of New Zealand. According to the World Animal Health Organization (OIE, 2017), 66 countries are free of FMDV without vaccination, nine countries are free of FMDV with vaccination, and the remainder are endemically infected or lack reliable data upon which to base their true status.
Originally, FMDV used in vaccine production was derived from fluid collected from vesicular lesions on virus-inoculated cattle, just as was done previously for the production of smallpox vaccine virus (vaccinia virus) (Fenner, Reference Fenner, Fields, Knipe, Chanock, Hirsch, Melnick, Monath and Roizman1990; Sutmoller et al., Reference Sutmoller, Barteling, Olascoaga and Sumption2003). Thus, Vallée et al. (Reference Vallée, Carré and Rinjard1926) attempted to produce a FMDV vaccine using formaldehyde-inactivated fluid and loose epithelial tissues from vesicles on calves. Thereafter, Frenkel (Reference Frenkel1947) used macroscopic slices of tongue epithelium to propagate virus and prepare formaldehyde-inactivated vaccine. This approach was used by Rosenbusch et al. (Reference Rosenbusch, Decamps and Gelormini1948) to produce enough FMDV vaccine to vaccinate more than two million cattle in Argentina (Brown, Reference Brown2003). Over time, various cell lines, e.g., pig kidney (IBRS-2, MVPK-1), porcine kidney (LFBK), or baby hamster kidney fibroblast (BHK-21), were used in diagnostics or for FMDV propagation (Capstick et al., Reference Capstick, Telling, Chapman and Stewar1962; Snowdon, Reference Snowdon1966; Swaney, Reference Swaney1976; Mohapatra et al., Reference Mohapatra, Pandey, Rai, Das, Rodriguez, Rout, Subramaniam, Sanyal, Rieder and Pattnaik2015). Among these cell lines, BHK-21 has been used for large-scale production of FMDV vaccine (Doel, Reference Doel2003). In addition, a variety of contemporary vaccine technologies have been evaluated under experimental conditions, e.g., subunit, vector expression of subunit components, and DNA vaccines.
Protective immunity is directed toward structural proteins (Longjam et al., Reference Longjam, Deb, Sarmah, Tayo, Awachat and Saxena2011). Therefore, elimination of NSPs (L, 2A, 2B, 2C, 3A, 3B, 3C, and 3D) during vaccine production results in vaccinates without antibodies against these proteins, i.e., DIVA (differentiating infected from vaccinated animals) vaccines. That is, DIVA-vaccinated animals produce antibodies against FMDV structural proteins, but not against NSPs, whereas FMDV-infected animals produce antibodies against both structural and NSPs. Implementation of a DIVA strategy based on the detection of antibodies against NSPs in infected animals is used to monitor the ongoing success of FMDV eradication and to maintain ‘FMD-free with vaccination’ status (Bergmann et al., Reference Bergmann, Malirat, Neitzert and Melo2004). However, it has been observed that inadequately purified FMDV vaccines can contain enough residual NSP to induce anti-NSP antibody and produce false-positive enzyme-linked immunosorbent assay (ELISA) results (Uttenthal et al., Reference Uttenthal, Parida, Rasmussen, Paton, Haas and Dundon2010).
Whether the goal is early detection, sustained control, or eradication, diagnostically and analytically sensitive and specific (but affordable) FMDV surveillance tools are mandatory. Herein we review FMDV testing methods, contemporary and alternative diagnostic specimens, and their application in FMDV surveillance in livestock (cattle, swine, sheep, and goats).
Tests and testing
Prior to the development of the complement fixation test (1929), FMDV infection was diagnosed primarily by clinical signs, i.e., the presence of vesicles on epithelial surfaces of the feet, mouth, nasal regions, and mammary glands (Bachrach, Reference Bachrach1968). However, diagnosis based on clinical signs is complicated by the fact that other viral infections, e.g., swine vesicular disease virus (SVDV), vesicular stomatitis virus (VSV), and vesicular exanthema of swine virus (VESV), may produce lesions which are indistinguishable from FMDV. Today, the detection of FMDV infections relies on the detection of FMDV-specific antibody (virus neutralization, antibody ELISA) or on the detection of the virus and/or viral components (virus isolation, antigen-capture ELISA, or reverse transcription-polymerase chain reaction (RT-PCR)). These techniques are reviewed below.
Virus detection
Direct complement fixation test
Prior to the development of techniques for virus isolation, Ciuca (Reference Ciuca1929) showed that the direct complement fixation test could be used to detect FMDV and serotype isolates. The method was based on the fact that guinea pig-derived complement is bound by virus–antibody complexes. If virus–antibody binding does not occur, the free complement will lyse sheep red blood cells (RBC) in the presence of anti-sheep RBC antibody. It was possible to identify FMDV serotypes using the direct complement fixation test because FMDV antibodies are serotype-specific. Later, Traub and Mohlmann (Reference Traub and Mohlmann1943) used the direct complement fixation test to serotype FMDV in cattle. The direct complement fixation test is best used early in infection because it requires a high concentration of virus in the test specimen; thus, it is not useful when vesicles begin to resolve (Rice and Brooksby, Reference Rice and Brooksby1953). Further, serum with pro- or anti-complementary activity will affect the test results (Ferris and Dawson, Reference Ferris and Dawson1988).
Virus isolation
FMDV isolation was first described by Frenkel (Reference Frenkel1947) using primary bovine tongue epithelial cells, but Sellers (Reference Sellers1955) and Bachrach et al. (Reference Bachrach, Hess and Callis1955) adapted primary bovine and swine kidney cells to FMDV diagnostics. Historically, bovine thyroid cells were considered the best primary cells for FMDV isolation, but more recently, continuous cell lines, e.g., IBRS-2, MVPK-1 clone 7, LFBK, BHK21, and BHK21-CT, have been widely used (Dinka et al., Reference Dinka, Swaney and McVicar1977; Nair, Reference Nair1987; House and House, Reference House and House1989; Ferris et al., Reference Ferris, King, Reid, Hutchings, Shaw, Paton, Goris, Haas, Hoffmann, Brocchi and Bugnetti2006a, Reference Ferris, King, Reid, Shaw and Hutchings2006b). Among several stable cell lines, bovine kidney cells expressing β6 and αV and integrin subunits (LFBK-αVβ6) were highly susceptible to all FMDV serotypes (LaRocco et al., Reference LaRocco, Krug, Kramer, Ahmed, Pacheco, Duque, Baxt and Rodriguez2013). The availability of cell culture techniques and the realization that FMDV could be grown in vitro made typing of FMDV isolates more practicable (Rweyemamu et al., Reference Rweyemamu, Pay and Simms1982).
Virus isolation is the only way to confirm the presence of live FMDV, despite well-recognized challenges: (1) working with infectious FMDV presents a significant biosafety risk; (2) cell cultures lose susceptibility to the virus over time; (3) cell lines lose permissiveness to the virus over passages; (4) antibodies present in samples from infected animals may completely or partially neutralize FMDV; (5) virus isolation is much less analytically sensitive than RT-PCR (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a); (6) cytopathic effect can be caused by a variety of factors, not just FMDV, thus positive results must be confirmed using other methods.
Propagating virus on cell culture requires technical skill, adequate laboratory facilities, and more time than molecular assays. The diagnostic sensitivity of FMDV isolation varies among laboratories, virus serotype, and the cells used in the procedure (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a). Ferris et al. (Reference Ferris, King, Reid, Hutchings, Shaw, Paton, Goris, Haas, Hoffmann, Brocchi and Bugnetti2006a) evaluated test performance using a set of vesicular samples from FMDV-infected cattle (serotypes O, A, Asia 1, and SAT 2), SVDV-infected pigs, and negative control samples from cattle and pigs. Based on the results obtained from five European FMDV reference laboratories, bovine thyroid primary cells provided the highest rate of FMDV isolation (94%) when compared with primary lamb kidney cells (69%). The rate of isolation also varied among continuous cell lines: 69% for IBRS-2, 56% for BHK21 and 25% for BHK21-CT. In addition, primary bovine thyroid cells and IBRS-2 cells were susceptible to all FMDV serotypes, whereas primary lamb kidney cells, BHK21, and BHK21-CT cells were not susceptible to FMDV serotype SAT2. Data from more recent studies suggested that newer cell lines are highly susceptible to FMDV, but only partial comparisons among cell lines have been done. Brehm et al. (Reference Brehm, Ferris, Lenk, Riebe and Haas2009) compared primary bovine thyroid cells, IBRS-2, BHK21, and ZZ-R 127 (fetal goat) cell lines using FMDV isolates representing all seven serotypes. Although less sensitive than primary bovine thyroid cells, cell line ZZ-R 127 was more sensitive than the other cell lines included in the comparison. Similarly, LaRocco et al. (Reference LaRocco, Krug, Kramer, Ahmed, Pacheco, Duque, Baxt and Rodriguez2013) found the LFBK-αVβ6 continuous cell line to be more susceptible to FMDV than primary lamb kidney, IBRS-2, and BHK21 cells.
Antigen-capture ELISA
The OIE (2012) recommends the use of FMDV antigen-capture ELISA for the detection of viral antigen and identification of viral serotype in clinical specimens and culture isolates (Roeder and Le, Reference Roeder and Le1987; Ferris and Donaldson, Reference Ferris and Donaldson1992). Crowther and Abu-El Zein (Reference Crowther and Abu-El Zein1979) and Crowther and Elzein (Reference Crowther and Elzein1979, Reference Crowther and Elzein1980) initially reported the use of antigen-capture ELISA to detect FMDV in cell culture and later applied the test to the detection of FMDV in cattle epithelial tissues. Currently, antigen-capture ELISAs based on polyclonal antibodies or various monoclonal antibodies targeting structural or NSPs are available (Hamblin et al., Reference Hamblin, Armstrong and Hedger1984; Roeder and Le, Reference Roeder and Le1987; Ferris and Dawson, Reference Ferris and Dawson1988). Antigen-capture ELISA is capable of rapidly testing large numbers of samples, i.e., results can be obtained in 3–4 h (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a; Grubman and Baxt, Reference Grubman and Baxt2004). However, the antigenic variability within and between serotypes further compromises the limited analytical sensitivity of the antigen-capture ELISA format. Studies showed that 70–80% of cell culture-positive samples and 63–71% of RT-PCR-positive oral/nasal swabs were detected by antigen-capture ELISA (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a; Morioka et al., Reference Morioka, Fukai, Sakamoto, Yoshida and Kanno2014).
Antigen-capture lateral-flow assay
FMDV antigen-capture lateral-flow assays or rapid chromatographic strip tests allow rapid on-site diagnosis in areas where the disease is endemic and in reference laboratories when a rapid result is needed. These assays detect FMDV antigens in vesicular fluids or epithelial suspension from infected animals using monoclonal or polyclonal antibodies (Reid et al., Reference Reid, Ferris, Brüning, Hutchings, Kowalska and Åkerblom2001; Ferris et al., Reference Ferris, Nordengrahn, Hutchings, Reid, King, Ebert, Paton, Kristersson, Brocchi, Grazioli and Merza2009, Reference Ferris, Nordengrahn, Hutchings, Paton, Kristersson, Brocchi, Grazioli and Merza2010; Oem et al., Reference Oem, Ferris, Lee, Joo, Hyun and Park2009; Jiang et al., Reference Jiang, Liang, Ren, Chen, Zhi, Qi and Cai2011). Oem et al. (Reference Oem, Ferris, Lee, Joo, Hyun and Park2009) reported that a monoclonal antibody-based lateral-flow assay showed 87% diagnostic sensitivity and 99% diagnostic specificity for the detection of FMDV serotypes O, A, Asia1, and C when testing epithelial suspension specimens.
Reverse transcription-polymerase chain reaction
Relative to other virus detection methods, RT-PCR is considered to offer shorter turn-around time plus higher diagnostic and analytical sensitivity and specificity (Callens et al., Reference Callens, De Clercq, Gruia and Danes1998; Reid et al., Reference Reid, Forsyth, Hutchings and Ferris1998, Reference Reid, Hutchings, Ferris and De Clercq1999, Reference Reid, Ferris, Hutchings, Samuel and Knowles2000; Moss and Haas, Reference Moss and Haas1999; Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a; Shaw et al., Reference Shaw, Reid, King, Hutchings and Ferris2004; King et al., Reference King, Ferris, Shaw, Reid, Hutchings, Giuffre and Beckham2006). Although FMDV is highly resistant to degradation in the environment, RT-PCR can detect nucleic acid from both infectious or inactivated virus, thereby reducing the impact of sample-handling deficiencies on virus detection (Cottral, Reference Cottral1969; Longjam et al., Reference Longjam, Deb, Sarmah, Tayo, Awachat and Saxena2011). The FMDV genome is heterogeneous. To avoid false-negative results, RT-PCR primers and probes must target nucleic acid sequences that are broadly conserved across all serotypes. For surveillance, RT-PCR can be used in parallel with virus isolation to achieve a more complete epidemiological picture (Laor et al., Reference Laor, Torgersen, Yadin and Becker1992; Höfner et al., Reference Höfner, Carpenter and Donaldson1993; Rodríguez et al., Reference Rodríguez, Dopazo, Saiz and Sobrino1994; Marquardt et al., Reference Marquardt, Straub, Ahl and Haas1995; Callens et al., Reference Callens, De Clercq, Gruia and Danes1998; Callens and De Clercq, Reference Callens and De Clercq1999).
Realtime RT-PCR
Realtime RT-PCR (rRT-PCR) has been widely used in FMDV diagnosis because it offers improved analytical sensitivity and a simpler testing format, i.e., electrophoresis is not required. The first universal FMDV rRT-PCR used primers and probes specific to a highly conserved region within a polypeptide gene (P3) and achieved an analytical sensitivity for all FMDV serotypes estimated at 1 × 102 TCID50 (Meyer et al., Reference Meyer, Brown, House, House and Molitor1991). Carrillo et al. (Reference Carrillo, Tulman, Delhon, Lu, Carreno, Vagnozzi and Rock2005) compared whole-genome sequences of 113 FMDV isolates and found that the 5′UTR and 3D (RNA-dependent RNA polymerase gene) regions shared a high degree of nucleotide identity among FMDV isolates, i.e., 83% (5′UTR) and 91% (3D) homology. Further studies showed that primers and probes based on 5′UTR or 3D were analytically specific, i.e., no false positives were observed when testing specimens containing SVDV, VSV, or VESV (Callahan et al., Reference Callahan, Brown, Osorio, Sur, Kramer, Long, Lubroth, Ellis, Shoulars, Gaffney and Rock2002; Reid et al., Reference Reid, Ferris, Hutchings, Zhang, Belsham and Alexandersen2002; Ferris et al., Reference Ferris, King, Reid, Hutchings, Shaw, Paton, Goris, Haas, Hoffmann, Brocchi and Bugnetti2006a, Reference Ferris, King, Reid, Shaw and Hutchings2006b; Shaw et al., Reference Shaw, Reid, Ebert, Hutchings, Ferris and King2007). Although OIE currently recommends the use of ‘universal’ primers and probes targeting conserved sequences within the 5′UTR or 3D regions, serotype-specific assays have also been created (Reid et al., Reference Reid, Mioulet, Knowles, Shirazi, Belsham and King2014; Bachanek-Bankowska et al., Reference Bachanek-Bankowska, Mero, Wadsworth, Mioulet, Sallu, Belsham, Kasanga, Knowles and King2016).
Several studies have evaluated the diagnostic performance of 5′UTR and 3D FMD RT-PCRs. Using a variety of specimens containing viruses representing O, A, and Asia-1 serotypes plus serum and vesicular samples from FMDV-negative animals, Reid et al. (Reference Reid, Mioulet, Knowles, Shirazi, Belsham and King2014) reported no false-positive results and detection rates of 91 and 96% for 3D and 5′UTR rRT-PCRs, respectively.
Hindson et al. (Reference Hindson, Reid, Baker, Ebert, Ferris, Tammero and Hullinger2008) evaluated 5′UTR, 3D, or both rRT-PCRs using vesicular epithelium samples containing FMDV (serotypes O, C, Asia-1, SAT1, SAT2, SAT3), SVDV, or VESV. The diagnostic sensitivities of the 5′UTR and 3D rRT-PCRs were 87 and 97%, respectively. Combining the two methods resulted in a diagnostic sensitivity of 98%. King et al. (Reference King, Ferris, Shaw, Reid, Hutchings, Giuffre and Beckham2006) compared the diagnostic sensitivities of the 5′UTR and 3D FMDV rRT-PCRs using 394 FMDV clinical specimens (serum, vesicular epithelium). Approximately 94% of samples (367 of 392) were positive on one of the two rRT-PCRs, with 88.1% (347 of 394) positive on both assays. Sequence analyses showed that all false-negative tests were the result of nucleotide substitutions within the region targeted by the primers or probes (King et al., Reference King, Ferris, Shaw, Reid, Hutchings, Giuffre and Beckham2006). Therefore, laboratories may need to provide both 3D and 5′UTR RT-PCR testing, to reduce the likelihood of false-negative results caused by nucleotide changes in the 3D or 5′UTR target areas (Moniwa et al., Reference Moniwa, Clavijo, Li, Collignon and Kitching2007).
Antibody detection
FMDV antibody detection methods are routinely used for several purposes; e.g., to certify animals or animal by-products are free from FMDV infection prior to import or export, to demonstrate previous exposure to FMDV or vaccination, or to evaluate antigenic matching of vaccines.
Indirect complement fixation test
The indirect complement fixation test was the first in vitro test developed for the detection of FMDV-specific antibody (Rice and Brooksby, Reference Rice and Brooksby1953). The assay was further developed to detect FMDV antibodies from multiple FMDV serotypes (Nordberg and Schjerning-Thiesen, Reference Nordberg and Schjerning-Thiesen1956; Sakaki et al., Reference Sakaki, Suphavilai and Tokuda1977, Reference Sakaki, Suphavilai and Chandarkeo1978). At present, use of the indirect complement fixation test is only recommended by the OIE if FMDV ELISA testing is not available (OIE, 2012).
Serum-virus neutralization test
The FMDV serum-virus neutralization test (SVN) is a serotype-specific assay for the detection of neutralizing antibodies elicited by vaccination or infection (Golding et al., Reference Golding, Hedger and Talbot1976). Post-vaccination sero-surveys for FMDV are a major indicator in the assessment of preventive vaccination programs (Sobrino et al., Reference Sobrino, Sáiz, Jiménez-Clavero, Núñez, Rosas, Baranowski and Ley2001). The existence of circulating neutralizing antibody is associated primarily with resolution of viremia (Pacheco et al., Reference Pacheco, Arzt and Rodriguez2010). The test may be performed on various cell lines, although Moonen and Schrijver (Reference Moonen and Schrijver2000) found that BHK or IBRS-2 cells provided better results than PK-2 cells. The test is more specific than the indirect complement fixation test and is recommended for international trade by OIE, but the slow throughput (72 h to perform the test) is incompatible with rapid response and/or routine commerce. In addition, the assay's requirement for infectious virus mandates that testing be performed in a high-level biocontainment facility; often a difficult and expensive hurdle to clear.
Enzyme-linked immunosorbent assay
Elzein and Crowther (Reference Elzein and Crowther1978) developed the first indirect FMDV antibody ELISA. Subsequently, various FMDV ELISAs have been developed for the detection of antibodies and for serotyping of viruses (Rai and Lahiri, Reference Rai and Lahiri1981; Ouldridge et al., Reference Ouldridge, Barnett and Rweyemamu1982; Hamblin et al., Reference Hamblin, Armstrong and Hedger1984; Ouldridge et al., Reference Ouldridge, Barnett, Parry, Syred, Head and Rweyemamu1984; Roeder and Le, Reference Roeder and Le1987; Pattnaik and Venkataramanan, Reference Pattnaik and Venkataramanan1989). ELISAs are highly repeatable, cost-effective, and compatible with a variety of sample types, e.g., milk, probang, and oral fluid specimens (Burrows, Reference Burrows1968; de Leeuw et al., Reference De Leeuw, Van Bekkum and Tiessink1978; Blackwell et al., Reference Blackwell, Wool and Kosikowski1981; Longjam et al., Reference Longjam, Deb, Sarmah, Tayo, Awachat and Saxena2011; Senthilkumaran et al., Reference Senthilkumaran, Yang, Bittner, Ambagala, Lung, Zimmerman, Giménez-Lirola and Nfon2017).
Structural protein ELISAs
FMDV structural protein ELISAs are serotype-specific tests designed to detect antibodies elicited by vaccination or infection. Several blocking or competitive ELISAs have been developed based on serotype-specific polyclonal or monoclonal antibodies against capsid protein (VP1, VP2, and VP3), 146S particle, or 12S subunit epitopes (Cartwright et al., Reference Cartwright, Chapman and Brown1980; Roeder and Le, Reference Roeder and Le1987; Sáiz et al., Reference Sáiz, Cairó, Medina, Zuidema, Abrams, Belsham, Domingo and Vlak1994). These assays provide faster throughput than SVN and avoid the need for tissue culture and live FMDV.
NSP ELISAs
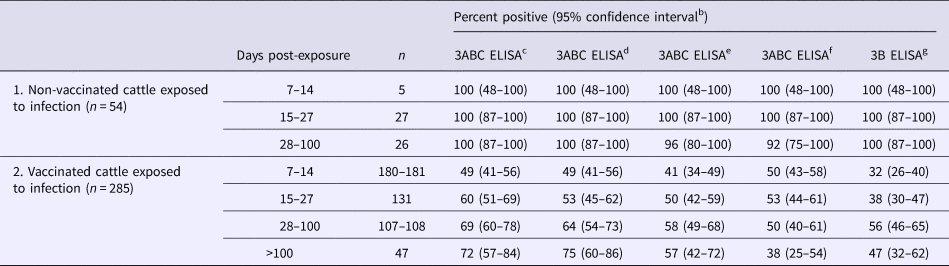
Several FMDV-recombinant NSPs, e.g., 3ABC, 3AB, 3A, 3B, 3C, 2A, 2B, and 2C, have been used as target antigens in FMDV blocking and indirect ELISAs. Among these, antibodies against the 3ABC polyprotein are the most sensitive indicator of FMDV replication (Grubman, Reference Grubman2005; Henderson, Reference Henderson2005). Brocchi et al. (Reference Brocchi, Bergmann, Dekker, Paton, Sammin, Greiner, Grazioli, De Simone, Yadin, Haas and Bulut2006) compared four commercial NSP ELISAs and the OIE index screening assay using serum samples (n = 3551) from vaccinated and unvaccinated cattle, pigs, and sheep exposed to FMDV (Table 1). Diagnostic specificity was adequate for all tests (97–98%) and all tests displayed excellent diagnostic sensitivity (100%) when testing samples from recently exposed, unvaccinated animals. However, detection rates were much lower when testing vaccinated or exposed animals. As discussed previously, NSP antibody ELISAs can play a key role in verifying the status of countries considered FMD-free with vaccination.
Table 1. Detection of FMDV infection in cattle using non-structural protein-based ELISAs (modified from Brocchi et al., Reference Brocchi, Bergmann, Dekker, Paton, Sammin, Greiner, Grazioli, De Simone, Yadin, Haas and Bulut2006)a

a Cattle serum samples obtained from experimental and known-status field animals.
b 95% confidence intervals calculated from proportional data given in Brocchi et al. (Reference Brocchi, Bergmann, Dekker, Paton, Sammin, Greiner, Grazioli, De Simone, Yadin, Haas and Bulut2006).
c NCPanaftosa-screening (Panaftosa, Pan American Health Organization, Rio de Janeiro, Brazil).
d Ceditest® FMDV-NS (Cedi diagnostics B.V., Lelystad, The Netherlands. Currently produced and marketed as Priocheck® FMDV-NS by Thermo Fisher Scientific Prionics Lelystad B.v., Lelystad, The Netherlands).
e SVANOVIR™ FMDV 3ABC-Ab ELISA (Svanova, Upsala, Sweden).
f CHEKIT-FMD-3ABC (Bommeli Diagnostics/Idexx, Bern, Switzerland).
g UBI® FMDV NS ELISA (United Biomedical Inc., New York, USA).
Sampling and sample types
Serum
Transmission of FMDV can occur via respiratory, oral, or percutaneous exposure (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a). The initial replication of virus usually occurs at the site of entry followed by spread to regional lymph nodes through the circulatory system (Henderson and Brooksby, Reference Henderson and Brooksby1948). Viremia appears as soon as 24 h post-exposure (Cottral and Bachrach, Reference Cottral and Bachrach1968; Alexandersen et al., Reference Alexandersen, Zhang and Donaldson2002a, Reference Alexandersen, Zhang, Donaldson and Garland2003a, Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b; Kitching, Reference Kitching2002a; Murphy et al., Reference Murphy, Bashiruddin, Quan, Zhang and Alexandersen2010). Viremia typically lasts 4–5 days in ruminants and 2–10 days in pigs, although the level of viremia is usually higher in pigs than in ruminants (Alexandersen et al., Reference Alexandersen, Oleksiewicz and Donaldson2001, Reference Alexandersen, Brotherhood and Donaldson2002b, Reference Alexandersen, Zhang, Reid, Hutchings and Donaldson2002c, Reference Alexandersen, Zhang, Donaldson and Garland2003a, Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b; Alexandersen and Donaldson, Reference Alexandersen and Donaldson2002; Hughes et al., Reference Hughes, Mioulet, Kitching, Woolhouse, Alexandersen and Donaldson2002; Murphy et al., Reference Murphy, Bashiruddin, Quan, Zhang and Alexandersen2010; Stenfeldt et al., Reference Stenfeldt, Pacheco, Smoliga, Bishop, Pauszek, Hartwig, Rodriguez and Arzt2016).
Serum specimens are useful for the detection of FMDV during viremia, i.e., serum samples collected ≤7 days post-infection (DPI) can be used for FMDV detection by virus isolation, rRT-PCR, and antigen-capture ELISA, with later samples useful for antibody detection. In cattle and pigs, Alexandersen et al. (Reference Alexandersen, Zhang and Donaldson2002a, Reference Alexandersen, Brotherhood and Donaldson2002b, Reference Alexandersen, Zhang, Reid, Hutchings and Donaldson2002c) reported the appearance of ELISA-detectable FMDV serum antibody by 5 DPI and neutralizing antibodies ≤2 days later (Alexandersen et al., Reference Alexandersen, Zhang and Donaldson2002a, Reference Alexandersen, Zhang, Donaldson and Garland2003a). In sheep, ELISA-detectable serum antibody appeared by 9 DPI and neutralizing antibody between 6 and 10 DPI (Armstrong et al., Reference Armstrong, Cox, Aggarwal, Mackay, Davies, Hamblin and Paton2005). Coincident with the first detection of antibody is the progressive clearance of virus from circulation and a reduction of virus in most tissues, with the exception of the pharyngeal region of ruminants (McCullough et al., Reference McCullough, Pullen and Parkinson1992; Alexandersen et al., Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b). Paired serum samples collected 7–14 days apart may be used to diagnose FMDV on the basis of rising antibody levels in response to infection. Serum antibody remains at high levels for several months post-infection and is detectable for years, with the exception that FMDV-specific antibody may be detected for only a few months in young pigs (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a). The use of filter papers for antibody detection or FTA cards for nucleic acid detection has been reported as a method to achieve diagnosis without the need to refrigerate or freeze serum samples (OIE, 2008).
Vesicular epithelium and fluid
During viremia, FMDV is distributed to secondary replication sites, i.e., tongue epithelium, nasal mucosa, salivary glands, coronary band epithelium, myocardium, kidney, spleen, and liver (Alexandersen et al., Reference Alexandersen, Oleksiewicz and Donaldson2001, Reference Alexandersen, Zhang, Donaldson and Garland2003a). Viral amplification occurs mainly in cornified stratified squamous epithelium, e.g., feet, teats, dental pad, gum, tongue, and lips, resulting in the formation of liquid-filled vesicles (Alexandersen et al., Reference Alexandersen, Oleksiewicz and Donaldson2001; Oleksiewicz et al., Reference Oleksiewicz, Donaldson and Alexandersen2001; Arzt et al., Reference Arzt, Baxt, Grubman, Jackson, Juleff, Rhyan, Rieder, Waters and Rodriguez2011a, Reference Arzt, Juleff, Zhang and Rodriguez2011b). FMDV replication in pharyngeal epithelial and lymphoid tissues of cattle, sheep, and goats occurs in both the acute and persistent phases of disease (Alexandersen et al., Reference Alexandersen, Oleksiewicz and Donaldson2001, Reference Alexandersen, Zhang, Donaldson and Garland2003a).
Depending on the route of introduction, vesicles become visible 1–3 days after exposure (Alexandersen et al., Reference Alexandersen, Oleksiewicz and Donaldson2001, Reference Alexandersen, Zhang, Donaldson and Garland2003a; Murphy et al., Reference Murphy, Bashiruddin, Quan, Zhang and Alexandersen2010; Arzt et al., Reference Arzt, Baxt, Grubman, Jackson, Juleff, Rhyan, Rieder, Waters and Rodriguez2011a). However, subclinical infection is common in small ruminants, e.g., sheep and goats (Cardassis et al., Reference Cardassis, Pappous, Brovas, Strouratis and Seimenis1966; McVicar and Sutmoller, Reference McVicar and Sutmoller1972; Gibson and Donaldson, Reference Gibson and Donaldson1986; Pay, Reference Pay1988; Kitching, Reference Kitching2002a, Reference Kitching2002b). If present, vesicles are generally on the feet of small ruminants, e.g., sheep and goats (Cardassis et al., Reference Cardassis, Pappous, Brovas, Strouratis and Seimenis1966; Littlejohn, Reference Littlejohn1970; Gibson and Donaldson, Reference Gibson and Donaldson1986; Pay, Reference Pay1988). If oral lesions are present in small ruminants, they commonly occur on the dental pad, rather than tongue as occurs in cattle (Geering, Reference Geering1967). Vesicular fluid from unruptured vesicles on the dental pad, gum, tongue, lips, or feet of clinically affected animals is an ideal specimen for FMDV identification, because it contains a high concentration of virus (there are no reports of antibody detection in vesicular fluid) (Alexandersen et al., Reference Alexandersen, Oleksiewicz and Donaldson2001). However, vesicular fluid is generally only present in 1–2 days old lesions before they have ruptured. Alternatively, vesicular epithelium from ruptured lesions can be collected. FMDV can be detected in these samples up to 10–14 days (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a, Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b). These samples should be stored in glycerine containing 0.04 M phosphate buffer saline (pH 7.6) (Ferris and Dawson, Reference Ferris and Dawson1988). In the laboratory, the specimen can be crushed with sterile sand or beads and then mixed with laboratory medium to make a 10% suspension for testing by virus isolation, rRT-PCR, or antigen-capture ELISA (Oliver et al., Reference Oliver, Donaldson, Gibson, Roeder, Le and Hamblin1988; Reid et al., Reference Reid, Ferris, Brüning, Hutchings, Kowalska and Åkerblom2001, Reference Reid, Ferris, Hutchings, Zhang, Belsham and Alexandersen2002; Alexandersen and Donaldson, Reference Alexandersen and Donaldson2002; Sakamoto et al., Reference Sakamoto, Kanno, Yamakawa, Yoshida, Yamazoe and Murakami2002). More recently, it has been reported that FMDV RNA can be detected directly from dry vesicular material by homogenizing the specimen with RNA extraction lysis buffer and then testing by rRT-PCR (Howson et al., Reference Howson, Armson, Madi, Kasanga, Kandusi, Sallu, Chepkwony, Siddle, Martin, Wood and Mioulet2017, Reference Howson, Armson, Lyons, Chepkwony, Kasanga, Kandusi, Ndusilo, Yamazaki, Gizaw, Cleaveland and Lembo2018). Collection of vesicular fluid and epithelium are most appropriate in the acute stage of infection. Both specimens are the sample of choice for FMDV detection using RT-PCR, antigen-capture ELISA, or antigen-lateral-flow device (OIE, 2017).
Buccal samples
FMDV replicates in pharyngeal epithelial tissues and may be detected in esophageal–oropharyngeal fluid by 24 h post-exposure (Salt, Reference Salt1993). In ruminants, FMDV replication in pharyngeal epithelial tissues is protracted, i.e., the virus may be isolated from esophageal–oropharyngeal fluid samples for up to 9 months in sheep and 3.5 years in cattle (McVicar and Sutmoller, Reference McVicar and Sutmoller1969; Straver et al., Reference Straver, Bool, Claessens and Van Bekkum1970; Zhang and Kitching, Reference Zhang and Kitching2001; Juleff et al., Reference Juleff, Windsor, Reid, Seago, Zhang, Monaghan, Morrison and Charleston2008; Arzt et al., Reference Arzt, Baxt, Grubman, Jackson, Juleff, Rhyan, Rieder, Waters and Rodriguez2011a, Reference Arzt, Juleff, Zhang and Rodriguez2011b). In swine, infectious FMDV is present in most buccal samples for <28 days (oral fluid, nasal swab, esophageal–oropharyngeal fluid, tissues of the pharynx, tonsil, tongue, epiglottis, larynx, soft palate, nasopharynx, lung), although FMDV RNA was still detected in the tonsils of the soft palate at 28 DPI (Zhang and Bashiruddin, Reference Zhang and Bashiruddin2009; Arzt et al., Reference Arzt, Juleff, Zhang and Rodriguez2011b; Stenfeldt et al., Reference Stenfeldt, Pacheco, Smoliga, Bishop, Pauszek, Hartwig, Rodriguez and Arzt2016).
Probang sampling was first described as a method to collect esophageal–oropharyngeal fluid from ruminants by Sutmoller and Gaggero (Reference Sutmoller and Gaggero1965). The sample is collected by inserting a small metal cup (‘probang cup’) on a long shaft through the mouth and into the pharyngeal region, thereby allowing the esophageal–oropharyngeal secretions to pool in the cup. Different sizes of probang cups are used, depending on the ruminant species. Probang sampling from pigs has only been reported under research conditions (Parida et al., Reference Parida, Fleming, Oh, Mahapatra, Hamblin, Gloster, Doel, Gubbins and Paton2007; Stenfeldt et al., Reference Stenfeldt, Lohse and Belsham2013). Although esophageal–oropharyngeal fluid samples are the only method that offers a realistic chance of detecting FMDV in late-stage infection and in persistently infected ruminants, probang sampling is labor-intensive (involves several persons), requires technical skill, and necessitates animal restraint during the collection process (Kitching and Alexandersen, Reference Kitching and Alexandersen2002; Kitching and Hughes, Reference Kitching and Hughes2002; Kitching, Reference Kitching2002a, Reference Kitching2002b). Stenfeldt et al. (Reference Stenfeldt, Pacheco, Smoliga, Bishop, Pauszek, Hartwig, Rodriguez and Arzt2013) reported that farmers were reluctant to allow probang sampling because of concerns that the collection process might harm their animals.
Oral fluid samples from pigs and cattle have been used to detect FMDV antibody and nucleic acid (Callens et al., Reference Callens, De Clercq, Gruia and Danes1998; Alexandersen et al., Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b; Parida et al., Reference Parida, Anderson, Cox, Barnett and Paton2006, Reference Parida, Fleming, Oh, Mahapatra, Hamblin, Gloster, Doel, Gubbins and Paton2007; Stenfeldt et al., Reference Stenfeldt, Lohse and Belsham2013; Mouchantat et al., Reference Mouchantat, Haas, Böhle, Globig, Lange, Mettenleiter and Depner2014; Grau et al., Reference Grau, Schroeder, Mulhern, McIntosh and Bounpheng2015; Vosloo et al., Reference Vosloo, Morris, Davis, Giles, Wang, Nguyen, Kim, Quach, Le, Nguyen and Dang2015; Senthilkumaran et al., Reference Senthilkumaran, Yang, Bittner, Ambagala, Lung, Zimmerman, Giménez-Lirola and Nfon2017). Oral fluid samples can be collected from individual animals using various absorbent materials or from groups housed in the same space (pens or corrals) by allowing them to chew on rope suspended in the pen (Alexandersen et al., Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b; Prickett et al., Reference Prickett, Simer, Christopher-Hennings, Yoon, Evans and Zimmerman2008; Kittawornrat et al., Reference Kittawornrat, Prickett, Chittick, Wang, Engle, Johnson, Patnayak, Schwartz, Whitney, Olsen, Schwartz and Zimmerman2010; Stenfeldt et al., Reference Stenfeldt, Lohse and Belsham2013; Mouchantat et al., Reference Mouchantat, Haas, Böhle, Globig, Lange, Mettenleiter and Depner2014; Vosloo et al., Reference Vosloo, Morris, Davis, Giles, Wang, Nguyen, Kim, Quach, Le, Nguyen and Dang2015; Senthilkumaran et al., Reference Senthilkumaran, Yang, Bittner, Ambagala, Lung, Zimmerman, Giménez-Lirola and Nfon2017). Oral fluid collection is simple, non-invasive, rapid and cost-effective; for which reasons it has been widely applied to livestock surveillance, especially swine (Prickett and Zimmerman, Reference Prickett and Zimmerman2010). FMDV can be detected in oral fluid samples by RT-PCR for up to 15 DPI in cattle, 8 DPI in sheep, and more than 27 DPI in pigs (Alexandersen et al., Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b; Parida et al., Reference Parida, Fleming, Oh, Mahapatra, Hamblin, Gloster, Doel, Gubbins and Paton2007).
Conventional inactivated FMDV vaccines induce only a systemic antibody response whereas viral replication in infected animals produces both systemic and mucosal immune responses (McCullough et al., Reference McCullough, Pullen and Parkinson1992). Therefore, FMDV infection results in antibody-positive oral fluid or esophageal–oropharyngeal fluid samples, but vaccinated animals remain antibody-negative (DIVA) (Kitching, Reference Kitching2002b; Parida et al., Reference Parida, Anderson, Cox, Barnett and Paton2006). Virus neutralization assays and IgA-specific ELISAs for esophageal–oropharyngeal or oral fluid samples have been developed to detect FMDV-infected animals in vaccinated populations (Archetti et al., Reference Archetti, Amadori, Donn, Salt and Lodetti1995; Salt et al., Reference Salt, Mulcahy and Kitching1996; Amadori et al., Reference Amadori, Haas, Moos and Zerbini2000; Parida et al., Reference Parida, Anderson, Cox, Barnett and Paton2006; Eblé et al., Reference Eblé, Bouma, Weerdmeester, Stegeman and Dekker2007; Biswas et al., Reference Biswas, Paton, Taylor and Parida2008; Mohan et al., Reference Mohan, Gajendragad, Kishore, Chockalingam, Suryanarayana, Gopalakrishna and Singh2008; Pacheco et al., Reference Pacheco, Arzt and Rodriguez2010; Stenfeldt et al., Reference Stenfeldt, Pacheco, Smoliga, Bishop, Pauszek, Hartwig, Rodriguez and Arzt2016). Using an experimental ELISA based on a 3ABC polyprotein, FMDV-specific IgA was detected in oral fluids from pigs by 14 DPI (Senthilkumaran et al., Reference Senthilkumaran, Yang, Bittner, Ambagala, Lung, Zimmerman, Giménez-Lirola and Nfon2017). Earlier workers reported that FMDV-specific IgA could be detected in esophageal–oropharyngeal or oral fluid samples for up to 182 DPI in cattle and 112 DPI in pigs (Eblé et al., Reference Eblé, Bouma, Weerdmeester, Stegeman and Dekker2007; Mohan et al., Reference Mohan, Gajendragad, Kishore, Chockalingam, Suryanarayana, Gopalakrishna and Singh2008).
Mammary secretions
In 1968, Burrows reported that FMDV appeared in the milk of cattle exposed to infected animals an average of 2.2 days before clinical signs. Subsequent experiments showed extensive viral replication in bovine mammary gland parenchyma beginning 8–32 h post-exposure (Burrows et al., Reference Burrows, Mann, Greig, Chapman and Goodridge1971; Alexandersen et al., Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b). FMDV can also be detected in pig, sheep, and goat milk coincident with the appearance of viremia, but higher viral titers are present in sheep milk versus serum, suggesting either FMDV replication in small ruminant mammary gland tissue or the concentration of virus in milk (Burrows, Reference Burrows1968; McVicar, Reference McVicar1977; Arzt et al., Reference Arzt, Baxt, Grubman, Jackson, Juleff, Rhyan, Rieder, Waters and Rodriguez2011a, Reference Arzt, Juleff, Zhang and Rodriguez2011b). Blackwell et al. (Reference Blackwell, Wool and Kosikowski1981) reported that FMDV could be shed in cattle mammary secretions for up to 14 DPI and was detectable in pasteurized whole milk, skim milk, cream, and cellular components in mammary secretions. Using rRT-PCR, FMDV nucleic acids can be detected in bovine milk for up to 23 days. These data justify the testing of bulk tank milk samples by RT-PCR for the early detection of FMDV in dairy herds (Reid et al., Reference Reid, Parida, King, Hutchings, Shaw, Ferris, Zhang, Hillerton and Paton2006). Modeling the concentration of FMDV in bulk milk as a function of the number of cows shedding virus at any point in time, Thurmond and Perez (Reference Thurmond and Perez2006) predicted that FMDV nucleic acids could be detected in bulk tank milk samples between 2.5 and 6.5 days post-exposure, depending on the within-herd transmission rate. Further, it was predicted that nucleic acid could be detected in bulk tank milk before 10% of the cows showed clinical signs.
Individual and bulk tank milk samples have also been tested for FMDV-specific antibody, either for detection or for monitoring the response to vaccination (Armstrong and Mathew, Reference Armstrong and Mathew2001; Rémond et al., Reference Rémond, Kaiser and Lebreton2002; Thurmond and Perez, Reference Thurmond and Perez2006; Fayed et al., Reference Fayed, Abdel-Halim and Shaker2013). Serum antibody is concentrated into mammary secretions by active transport mediated by neonatal Fc receptors on the basolateral surface of the mammary epithelial cells. As a result, mammary secretion collected from FMDV-infected cattle can contain higher levels of antibody than serum (Stone and DeLay, Reference Stone and DeLay1960). FMDV neutralizing antibody can be detected in mammary secretions within 7 days after exposure in cattle (Stone and Delay, Reference Stone and DeLay1960). ELISA-detectable FMDV antibody can be detected in mammary secretions for up to 12 months post-vaccination in cattle, 24 weeks post-vaccination in pigs, and 83 days post-vaccination in sheep (Burrows, Reference Burrows1968; de Leeuw et al., Reference De Leeuw, Van Bekkum and Tiessink1978; Blackwell et al., Reference Blackwell, McKercher, Kosikowski, Carmichael and Gorewit1982; Francis and Black, Reference Francis and Black1983; Armstrong, Reference Armstrong1997; Kim et al., Reference Kim, Tark, Kim, Kim, Lee, Kwon, Bae, Kim and Ko2017).
Nasal and upper respiratory tract secretions
Respiratory tract mucosa is the initial site of FMDV replication and the virus is present in both upper and lower respiratory tract secretions during the acute phase of infection (Korn, Reference Korn1957; Donaldson and Ferris, Reference Donaldson and Ferris1980; Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a, Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b). The specimens can be used in preclinical diagnosis because FMDV RNA may be detected in nasal swabs from 1 day before clinical signs through 10–14 days after the appearance of serum antibodies (Marquardt et al., Reference Marquardt, Straub, Ahl and Haas1995; Callahan et al., Reference Callahan, Brown, Osorio, Sur, Kramer, Long, Lubroth, Ellis, Shoulars, Gaffney and Rock2002; Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a, Reference Alexandersen, Quan, Murphy, Knight and Zhang2003b). In pigs, FMDV RNA can be detected in nasal swabs from 6 h through 7 DPI, i.e., up to 2 days after the appearance of serum antibody (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a).
Aerosols
Airborne droplets or droplet nuclei containing infectious FMDV derived from secretions or excretions produced in respiratory, oral, and pedal epithelia present a significant challenge for prevention and control (Sutmoller and McVicar, Reference Sutmoller and McVicar1976; Burrows et al., Reference Burrows, Mann, Garland, Greig and Goodridge1981; Brown et al., Reference Brown, Meyer, Olander, House and Mebus1992; Sørensen et al., Reference Sørensen, Mackay, Jensen and Donaldson2000). Re-analysis of epidemiological and meteorological data collected during the 1982–1983 epidemic in Denmark suggested that FMDV was aerosolized and transmitted over a distance of 70 km (Christensen et al., Reference Christensen, Normann, Thykier-Nielsen, Sørensen, de Stricker and Rosenørn2005). Infectious FMDV can be detected in respiratory exhalations 1–6 days post-exposure in cattle (Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a). FMDV RNA can be detected in respiratory exhalations 6 h to 4 days post-exposure in pigs (Alexandersen et al., Reference Alexandersen, Oleksiewicz and Donaldson2001; Oleksiewicz et al., Reference Oleksiewicz, Donaldson and Alexandersen2001). Notably, pigs aerosolize more virus than ruminants, i.e., 1 × 106.1 median tissue culture infective dose (TCID50) per day in pigs (Sellers et al., Reference Sellers, Herniman and Mann1971) compared with 1 × 104.3 TCID50/day in cattle and sheep (McVicar and Sutmoller, Reference McVicar and Sutmoller1976), because the virus replicates more extensively in swine respiratory mucosa (Oleksiewicz et al., Reference Oleksiewicz, Donaldson and Alexandersen2001; Alexandersen and Donaldson, Reference Alexandersen and Donaldson2002; Alexandersen et al., Reference Alexandersen, Zhang and Donaldson2002a, Reference Alexandersen, Brotherhood and Donaldson2002b, Reference Alexandersen, Zhang, Reid, Hutchings and Donaldson2002c; Arzt et al., Reference Arzt, Baxt, Grubman, Jackson, Juleff, Rhyan, Rieder, Waters and Rodriguez2011a). In sheep, FMDV was detectable in respirations 17 h to 13 days post-exposure, i.e., FMDV is shed in aerosol 1–2 days before the appearance of clinical signs (Burrows, Reference Burrows1968; Sellers and Parker, Reference Sellers and Parker1969; Alexandersen et al., Reference Alexandersen, Brotherhood and Donaldson2002b). Experimentally, cattle and sheep can be infected by airborne exposure to as little as 1 × 101 TCID50, whereas pigs require more than 1 × 103 TCID50 (Alexandersen and Donaldson, Reference Alexandersen and Donaldson2002; Donaldson and Alexandersen, Reference Donaldson and Alexandersen2002; Alexandersen et al., Reference Alexandersen, Zhang and Donaldson2002a; Stenfeldt et al., Reference Stenfeldt, Pacheco, Smoliga, Bishop, Pauszek, Hartwig, Rodriguez and Arzt2016).
Theoretically, on-farm air sampling could be used for pre-clinical non-invasive FMDV surveillance. For example, Pacheco et al. (Reference Pacheco, Brito, Hartwig, Smoliga, Perez, Arzt and Rodriguez2017) reported that FMDV RNA could be detected by passing air through filters, then disrupting the filters, extracting FMDV RNA, and performing RT-PCR. Similarly, Oem et al. (Reference Oem, Kye, Lee, Kim, Park, Park, Joo and Song2005) detected FMDV RNA in exhaled air from infected cattle using a microchip-based hand-held air sampling device (Ilochip A/S, Denmark). FMDV RNA was harvested by washing the chip chamber with 25 µl of 0.1% (v/v) TritonX-100 solution (Sigma-Aldrich) followed by QIAamp Viral RNA Mini Kit (Qiagen, Germany) (Oem et al., Reference Oem, Kye, Lee, Kim, Park, Park, Joo and Song2005). However, routine FMDV surveillance based on air sampling would need to account for the fact that viral aerosols are highly dynamic, non-uniform, and subject to atmospheric and climatic conditions (Verreault et al., Reference Verreault, Moineau and Duchaine2008). Furthermore, air sampling devices differ in recovery efficiency (Tseng and Li, Reference Tseng and Li2005; Verreault et al., Reference Verreault, Moineau and Duchaine2008). Comparing all air sampling methods reported from 1960 to 2008, Verreault et al. (Reference Verreault, Moineau and Duchaine2008) concluded that no single sampling method was optimal for all climatic conditions. Perhaps for these reasons, aerosol sampling has primarily been a research tool for understanding and modeling the transmission of FMDV over distances.
Other sample types
Information concerning the shedding and detection of FMDV in urine or feces from FMDV-susceptible species is sparse, but shedding of FMDV in cattle urine and feces between 2 and 6 DPI has been reported (Bachrach, Reference Bachrach1968; Garland, Reference Garland1974). FMDV may be resistant in the environment, depending on the virus strain and the ambient conditions, and has been detected by virus isolation for up to 39 days in cattle urine and 14 days in feces (Bachrach, Reference Bachrach1968; Cottral, Reference Cottral1969; Donaldson et al., Reference Donaldson, Gibson, Oliver, Hamblin and Kitching1987; McColl et al., Reference McColl, Westbury, Kitching and Lewis1995; Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a). In general, urine and feces have not been considered suitable diagnostic specimens because they contain little virus and are likely to be mixed with environmental contaminants and other body fluids (Parker, Reference Parker1971; Alexandersen et al., Reference Alexandersen, Zhang, Donaldson and Garland2003a). However, in the context of molecular diagnostics, these sample types may deserve further evaluation in terms of their suitability for environmental surveillance and monitoring.
Conclusions
FMDV remains an important pathogen of livestock more than 120 years after it was first identified because it is highly contagious, genetically and antigenically diverse, infectious for a wide variety of species, able to establish subclinically infected carriers in some species, and widely geographically distributed (Brito et al., Reference Brito, Rodriguez, Hammond, Pinto and Perez2017). The ‘burden of disease’ imposed by FMDV is economically astonishing. Globally, Knight-Jones et al. (Reference Knight-Jones, McLaws and Rushton2017) estimated the annual costs from production losses and vaccination at €5.3–€17 billion (US$6.5–US$21 billion) in FMDV-endemic areas. In FMDV-free areas, they estimated the annual costs of FMDV outbreaks at ≥€1.2 billion (US$1.5 billion).
With good reason, the OIE and the Food and Agriculture Organization (FAO) have proposed the global eradication of FMD by the year 2030 (Rodriguez and Gay, Reference Rodriguez and Gay2011). This objective creates the need for alternative control methods, i.e., vaccines that provide broad-range protective immunity and diagnostic methods that can differentiate vaccinated from infected animals. Nevertheless, eradication is not feasible without the inclusion of accurate, cost-effective surveillance.
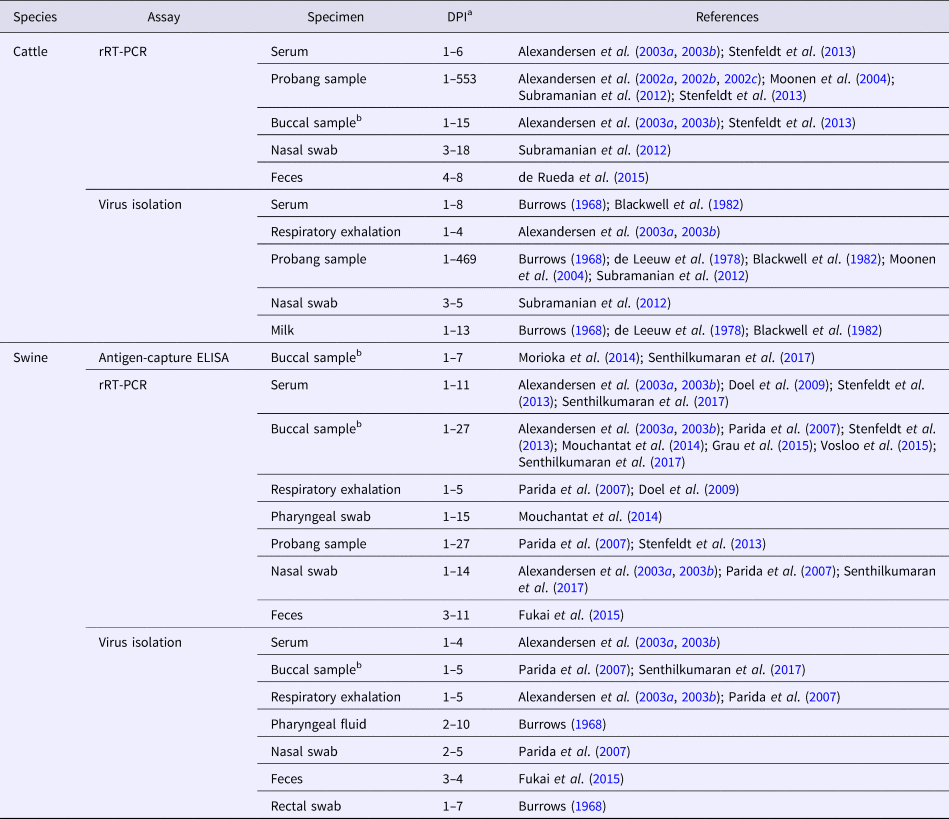
Historically, FMDV surveillance has typically been based on individual animal serum, vesicular fluid, or epithelial samples. Although current methods are still necessary for FMDV diagnoses, individual animal sampling and testing is impractical and expensive for surveillance in countries endemic with the disease. In an outbreak scenario, it would be feasible for individual sampling to occur. However, FMDV or antibody are also present in other body secretions, e.g., buccal and nasal secretions, respiratory exhalations (aerosols), mammary secretions, urine, feces, and environmental samples (Table 2). Alternative specimens can be used to support control and elimination programs by enabling herd-level sampling for FMDV surveillance at a lower cost and with less effort. Future research should focus on the development of diagnostic assays able to exploit the detection opportunities offered by alternative specimens, because, without these tools, the goal of FMDV eradication is unlikely to succeed.
Table 2. Temporal range for the detection of FMDV or viral components in alternative specimens

a Days post-inoculation (DPI) represent the minimum and maximum detection points reported.
b Buccal samples including samples collected with cotton swabs, cotton rope, or rope-in-a-bait collection devices.