Introduction
Bovine viral diarrhea virus (BVDV) is one of the most important infectious disease agents of cattle worldwide (Lanyon et al., Reference Lanyon, Hill, Reichel and Brownlie2014; Givens and Newcomer, Reference Givens and Newcomer2015; Richter et al., Reference Richter, Lebl, Baumgartner, Obritzhauser, Kasbohrer and Pinior2017). It was identified in 1957 as the causative agent for bovine viral diarrhea or BVD (Lee and Gillespie, Reference Lee and Gillespie1957). BVDV is a single-stranded positive sense RNA virus classified in the genus Pestivirus of the family Flaviviridae. BVDV strains of each distinct genotype (BVDV1 and BVDV2) are further classified as one of two biotypes: cytopathic (cp) and non-cytopathic (ncp) as defined by the lytic activity of the virus in cell culture (Gamlen et al., Reference Gamlen, Richards, Mankouri, Hudson, McCauley, Harris and Macdonald2010; Ridpath, Reference Ridpath2010b). Cp BVDV strains are not common and are mainly involved in outbreaks of mucosal disease whereas ncp BVDV strains are more common in nature and are often associated with the most clinically severe form of acute infection (Ridpath, Reference Ridpath2010b).
Cattle are the natural host for BVDV (Walz et al., Reference Walz, Grooms, Passler, Ridpath, Tremblay, Step, Callan and Givens2010) and infections with the virus are endemic in cattle populations in many different parts of the world (Chernick and van der Meer, Reference Chernick and van der Meer2017; Velasova et al., Reference Velasova, Damaso, Prakashbabu, Gibbons, Wheelhouse, Longbottom, Van Winden, Green and Guitian2017; Yesilbag et al., Reference Yesilbag, Alpay and Becher2017; Aragaw et al., Reference Aragaw, Sibhat, Ayelet, Skjerve, Gebremedhin and Asmare2018; Han et al., Reference Han, Ryu, Park and Choi2018; Scharnbock et al., Reference Scharnbock, Roch, Richter, Funke, Firth, Obritzhauser, Baumgartner, Kasbohrer and Pinior2018). The prevalence of BVDV infection based on serological surveys in different geographic regions range from 40 to 90% in individual cattle and 28–66% in cattle herds, while 0.5–2.5% of cattle were persistently infected (PI) with the virus (Walz et al., Reference Walz, Grooms, Passler, Ridpath, Tremblay, Step, Callan and Givens2010; Velasova et al., Reference Velasova, Damaso, Prakashbabu, Gibbons, Wheelhouse, Longbottom, Van Winden, Green and Guitian2017; Scharnbock et al., Reference Scharnbock, Roch, Richter, Funke, Firth, Obritzhauser, Baumgartner, Kasbohrer and Pinior2018).
Although cattle with transient BVDV infection are important sources of virus, PI cattle play a substantially larger role in the infection of susceptible cattle and maintenance of BVDV in cattle populations (Lindberg and Houe, Reference Lindberg and Houe2005). The most common route of BVDV transmission is direct contact between animals (Laureyns et al., Reference Laureyns, Ribbens and de Kruif2010). Infected cattle shed BVDV in body fluids and excretions including nasal discharge, saliva, semen, urine, feces, tears, milk, and uterine flushing (Thurmond, Reference Thurmond, Goyal and Ridpath2005; Lanyon et al., Reference Lanyon, Hill, Reichel and Brownlie2014). BVDV can also be transmitted during rectal examination (Lang-Ree et al., Reference Lang-Ree, Vatn, Kommisrud and Loken1994), as well as during natural breeding or artificial insemination (AI) of cows with semen from infected bulls (Rikula et al., Reference Rikula, Nuotio, Laamanen and Sihvonen2008; Newcomer et al., Reference Newcomer, Toohey-Kurth, Zhang, Brodersen, Marley, Joiner, Galik, Riddell and Givens2014).
The outcome of BVDV infection depends on viral characteristics such as biotype, genotype or strain (antigenic diversity), and virulence, and host factors such as species of host, immune status, pregnancy status, and concurrent infections with other pathogens (Brownlie, Reference Brownlie1991; Liebler-Tenorio et al., Reference Liebler-Tenorio, Ridpath and Neill2003; Walz et al., Reference Walz, Grooms, Passler, Ridpath, Tremblay, Step, Callan and Givens2010). Transient or acute infection is said to occur when postnatal immunocompetent cattle are infected with BVDV. Cattle with acute infection usually recover and eliminate the virus within 2 weeks post-infection although the clinical manifestations with acute BVDV infection may range from subclinical infection, clinical disease to fatal disease (Baker, Reference Baker1995; Hansen et al., Reference Hansen, Smirnova, Van Campen, Shoemaker, Ptitsyn and Bielefeldt-Ohmann2010). Vertical transmission of BVDV occurs when the virus is transmitted from the infected dam to her offspring (Kennedy, Reference Kennedy2005). Infection of susceptible pregnant cows with the ncp virus before the development of fetal immunocompetence results in the birth of PI cattle (Lanyon et al., Reference Lanyon, Hill, Reichel and Brownlie2014). Apart from BVDV presence in PI cattle, there is evidence that following apparent recovery from transient infection, BVDV may maintain prolonged or chronic infections within immunoprivileged sites such as in tissues of the ovary, testes, central nervous system, and in circulating white blood cells (Givens and Marley, Reference Givens and Marley2013). These apparently recovered animals can remain infectious for BVDV-naïve cattle for months post-infection (Collins et al., Reference Collins, Heaney, Thomas and Brownlie2009) although it is not clear if such chronic infections may reactivate future outbreaks of BVDV infections or predispose the reproductive organs to invasion by other pathogens including bacteria.
The focus of the review is on the impact of BVDV infection on reproduction and fertility in cattle. This review seeks to highlight possible mechanisms through which BVDV may interfere with reproductive processes. The reference to biotypes offered a broader means of discussing the effects of BVDV while maintaining focus on the topic. Different strains of BVDV had various effects as reported in numerous in vitro and in vivo studies. This also depended on the viral biotype, virulence, and host-related factors. Although outcomes of infection will undoubtedly be influenced by viral strains and virulence, it is largely unknown how these relate to herd fertility data. Information is lacking on the influence of tissue types in determining factors likely to influence virulence, survival, and pathogenicity for differing strains of BVDV within the female reproductive tract.
Reproductive and economic losses associated with BVDV infection in cattle
Reproductive losses in cattle due to BVDV infection were first described in 1946 (Olafson et al., Reference Olafson, Mac and Fox1946). Although BVDV is recognized as a major component of respiratory disease, particularly in calves, it is the invasion of reproductive tissues by the virus that have pronounced delayed effects (Brownlie et al., Reference Brownlie, Hooper, Thompson and Collins1998). BVDV can utilize the reproductive system to maintain and spread itself in cattle populations (Grooms, Reference Grooms2004).
Infection with BVDV has been associated with a decline in the fertility of affected cattle (McGowan and Kirkland, Reference McGowan and Kirkland1995; Fray et al., Reference Fray, Paton and Alenius2000; Robert et al., Reference Robert, Beaudeau, Seegers, Joly and Philipot2004; Burgstaller et al., Reference Burgstaller, Obritzhauser, Kuchling, Kopacka, Pinior and Kofer2016). BVDV infection was associated with increased incidence of embryonic and fetal losses, calf losses, and retained placenta postpartum (Larsson et al., Reference Larsson, Niskanen and Alenius1994). Other observations include decreased conception and pregnancy rates (Virakul et al., Reference Virakul, Fahning, Joo and Zemjanis1988; Houe et al., Reference Houe, Pedersen and Meyling1993; McGowan et al., Reference McGowan, Kirkland, Richards and Littlejohns1993a, Reference McGowan, Kirkland, Rodwell, Kerr and Carroll1993b; Burgstaller et al., Reference Burgstaller, Obritzhauser, Kuchling, Kopacka, Pinior and Kofer2016), prolonged calving interval (Niskanen et al., Reference Niskanen, Emanuelson, Sundberg, Larsson and Alenius1995; Burgstaller et al., Reference Burgstaller, Obritzhauser, Kuchling, Kopacka, Pinior and Kofer2016), prolonged time to first calving (Valle et al., Reference Valle, Martin and Skjerve2001), and increased risk of late return to service (Robert et al., Reference Robert, Beaudeau, Seegers, Joly and Philipot2004). Munoz-Zanzi et al. (Reference Munoz-Zanzi, Thurmond and Hietala2004) considered the overall impact of endemic BVDV infection on fertility of dairy heifers to depend on the type and timing of infection relative to reproductive development. Infection with BVDV during the first 45 days of gestation had no effect on the rate of return to estrus but was associated with increased mid-gestation abortion rates (7%) in dairy cows (Rufenacht et al., Reference Rufenacht, Schaller, Audige, Knutti, Kupfer and Peterhans2001). A decrease in calving rate and fertility was also reported in cows PI with BVDV (Kale et al., Reference Kale, Ata, Yavru, Yapkic, Bulut and Gulay2006). Moreover, fertility was lowered in apparently healthy heifers that had detectable BVDV antibodies, BVDV antigen, or both (Kale et al., Reference Kale, Yavru, Ata, Kocamuftuoglu, Yaplcl and Haslrcloglu2011). The presence of BVDV antigen but not BVDV antibody in the blood of cows was associated with a decrease from 71 to 28% in the first service conception rate (Yavru et al., Reference Yavru, Kale, Gulay, Yapici, Bulut and Ata2013). A meta-analysis of 41 studies from different geographic regions revealed that, compared with unvaccinated cattle, BVDV vaccination was associated with a 45% decrease in the abortion rate, 85% decrease in the fetal-infection rate, and a 5% increase in pregnancy risk (Newcomer et al., Reference Newcomer, Walz, Givens and Wilson2015).
Reproductive losses contribute to the significant economic damage associated with BVDV infection. These reproductive losses vary from insidious reduction in reproductive performance at the herd level to devastating abortion storms (Grooms, Reference Grooms2004). BVDV infection may cause no obvious clinical signs or a broad range of signs in association with other disease complexes, thereby making assessment of its economic impact difficult and likely to be underestimated (Laureyns et al., Reference Laureyns, Ribbens and de Kruif2010). A review of the studies carried out in different countries showed estimated losses in individual dairy herd outbreaks varied from a few thousands up to a hundred thousand US dollars while losses at the national level ranged between $10 and 40 million per million calving (Houe, Reference Houe2003). Losses in Scottish beef suckler herds were estimated at £37 mean loss per cow per annum (Gunn et al., Reference Gunn, Stott and Humphry2004). In addition, a 10-year BVD eradication program increased milk yield per cow for all herd sizes, and generated around £47 million in discounted economic gain in Scotland (Weldegebriel et al., Reference Weldegebriel, Gunn and Stott2009). In New Zealand, the rate of financial return when BVDV was controlled compared with the cost of uncontrolled BVDV infection was as high as 123% over a 10-year term (Reichel et al., Reference Reichel, Hill and Voges2008). The analysis of a 6-year eradication program revealed that the annualized benefits of BVDV eradication in Ireland exceeded the costs by a factor of five in the beef suckler sector and a factor of 14 in the dairy sector (Stott et al., Reference Stott, Humphry, Gunn, Higgins, Hennessy, O'Flaherty and Graham2012). In Switzerland, the estimated annual financial losses in BVDV-infected herds ranged CHF 85–89 per dairy cow and CHF 1337–2535 for an average farm (Thomann et al., Reference Thomann, Tschopp, Magouras, Meylan, Schupbach-Regula and Hasler2017). A recent global review revealed that direct financial losses due to BVDV infection in cattle in 15 countries were dependent on several factors but ranged from US$0.50 to 688 per animal, with naïve dairy cows having US$25 more direct losses per animal than beef cows (Richter et al., Reference Richter, Lebl, Baumgartner, Obritzhauser, Kasbohrer and Pinior2017).
Mechanisms linking BVDV infection with infertility in cattle
BVDV is known to invade most organs of the reproductive tract in infected cattle. BVDV or the viral-specific antigen was present in testicular tissue (Givens et al., Reference Givens, Heath, Brock, Brodersen, Carson and Stringfellow2003), oviductal cells (Booth et al., Reference Booth, Stevens, Collins and Brownlie1995), in macrophage-like cells in the endometrial stroma (Firat et al., Reference Firat, Ak, Bozkurt, Ak, Turan and Bagcigil2002), in vaginal mucus and uterine flush fluid (Brock et al., Reference Brock, Redman, Vickers and Irvine1991), and in both epithelial and non-epithelial cells of the endometrium, myometrium, and placenta (Fredriksen et al., Reference Fredriksen, Press, Loken and Odegaard1999a). BVDV has also been demonstrated in the epithelial, luteal, granulosa, and macrophage-like cells of the ovary and in follicular fluid (Bielanski et al., Reference Bielanski, Loewen, Del Campo, Sirard and Willadsen1993; Booth et al., Reference Booth, Stevens, Collins and Brownlie1995; Grooms et al., Reference Grooms, Ward and Brock1996; Fredriksen et al., Reference Fredriksen, Press, Loken and Odegaard1999a; Firat et al., Reference Firat, Ak, Bozkurt, Ak, Turan and Bagcigil2002; Gonzalez Altamiranda et al., Reference Gonzalez Altamiranda, Kaiser, Mucci, Verna, Campero and Odeon2013). Moreover, viral antigens have been detected in the oocytes of infected cows (Brownlie et al., Reference Brownlie, Booth, Stevens and Collins1997; Fray et al., Reference Fray, Prentice, Clarke and Charleston1998), in embryos (Gonzalez Altamiranda et al., Reference Gonzalez Altamiranda, Kaiser, Mucci, Verna, Campero and Odeon2013), and in fetuses (Harding et al., Reference Harding, Cao, Shams, Johnson, Vassilev, Gil, Wheeler, Haines, Sibert, Nelson, Campos and Donis2002; Morarie-Kane et al., Reference Morarie-Kane, Smirnova, Hansen, Mediger, Braun and Chase2018).
Previous studies have suggested various mechanisms through which BVDV infection can impact fertility. These include viral effects on reproductive organs, gametes, embryo, and the fetus. Many of the underlying mechanisms, particularly those associated with early pregnancy losses have however not yet been described clearly. Viral infection is also thought to predispose cattle to other diseases. More recent studies have also provided evidence of viral interference with endometrial functions during exposure to infection and also in the period of early pregnancy. These observations are discussed further below.
Viral disruption of reproductive function in bulls
There is evidence that BVDV can infect tissues of the male reproductive tract although there are varying reports on the consequence of viral infection on testicular function and male fertility. BVDV replicates in the seminal vesicles and the prostate gland and can be shed in the semen of bulls following both acute and persistent infection (Meyling and Mikél Jensen, Reference Meyling and Mikél Jensen1988; Kirkland et al., Reference Kirkland, Richards, Rothwell and Stanley1991; Kommisrud et al., Reference Kommisrud, Vatn, Lang-Ree and Loken1996; Rikula et al., Reference Rikula, Nuotio, Laamanen and Sihvonen2008). BVDV can also localize in the testes of infected bulls to cause a persistent testicular infection for several weeks, forming potential sources of infection via semen (Voges et al., Reference Voges, Horner, Rowe and Wellenberg1998; Givens et al., Reference Givens, Heath, Brock, Brodersen, Carson and Stringfellow2003; Newcomer et al., Reference Newcomer, Toohey-Kurth, Zhang, Brodersen, Marley, Joiner, Galik, Riddell and Givens2014). Some studies reported that neither acutely infected nor PI bulls showed any obvious abnormalities in semen or sperm quality (Kirkland et al., Reference Kirkland, Richards, Rothwell and Stanley1991, Reference Kirkland, Mackintosh and Moyle1994). In contrast, other studies reported abnormalities including poor semen volume, decreased sperm concentration and motility, and increased sperm abnormalities (Revell et al., Reference Revell, Chasey, Drew and Edwards1988; Kommisrud et al., Reference Kommisrud, Vatn, Lang-Ree and Loken1996). A lower conception rate of 38% was also recorded in cows bred with semen from a PI bull when compared with a rate of 66% in those bred with semen from an uninfected bull (Kirkland et al., Reference Kirkland, Mackintosh and Moyle1994). Therefore, BVDV infection has the potential to disrupt testicular function to cause abnormalities of spermatozoa. Semen from these infected bulls may also constitute a potential source of infection to susceptible cows, in addition to impacting negatively the conception rates and fertility in cows following natural breeding or AI.
Viral disruption of reproductive physiology and endocrine functions in cows
Regulation of the reproductive cycle and ovarian activity in cows is mainly under the control of hormones secreted by the hypothalamic–pituitary–ovarian axis via important negative and positive feedback control mechanisms (Noakes, Reference Noakes, Noakes, Parkinson and England2001a). A previous review (Fray et al., Reference Fray, Paton and Alenius2000) highlighted that BVDV may persist in the ovary of cows for several weeks following infection and was likely to impede ovarian function and fertility by disrupting the physiologic and endocrine functions of reproductive organs. BVDV infection has been associated with oophoritis (Ssentongo et al., Reference Ssentongo, Johnson and Smith1980; Grooms et al., Reference Grooms, Brock and Ward1998b), ovarian cyclic inactivity (Grooms et al., Reference Grooms, Ward and Brock1996), retarded follicular growth (Grooms et al., Reference Grooms, Brock, Pate and Day1998a; Gonzalez Altamiranda et al., Reference Gonzalez Altamiranda, Kaiser, Mucci, Verna, Campero and Odeon2013), and reduced ovulation rate in response to superovulation (Kafi et al., Reference Kafi, McGowan, Kirkland and Jillella1997). It is not clear how BVDV infection affects fertility by influencing ovarian function. BVDV infection caused necrosis of ovarian granulosa cells (McGowan et al., Reference McGowan, Kafi, Kirkland, Kelly, Bielefeldt-Ohmann, Occhio and Jillella2003) that may lead to a reduction in ovarian estradiol secretion in infected cows (Fray et al., Reference Fray, Mann, Clarke and Charleston1999; McGowan et al., Reference McGowan, Kafi, Kirkland, Kelly, Bielefeldt-Ohmann, Occhio and Jillella2003). Suppression of estradiol secretion may impair estrus and ovulation by negatively affecting the magnitude or timing of the pre-ovulatory luteinizing hormone (LH) surge (McGowan et al., Reference McGowan, Kafi, Kirkland, Kelly, Bielefeldt-Ohmann, Occhio and Jillella2003). Whereas acute infection with ncp BVDV was not found to alter serum concentrations of progesterone or estradiol (Grooms et al., Reference Grooms, Brock, Pate and Day1998a; Fray et al., Reference Fray, Mann, Clarke and Charleston1999), another study reported a decrease in the post-ovulatory plasma progesterone concentration in infected cows (Fray et al., Reference Fray, Mann, Bleach, Knight, Clarke and Charleston2002). Although a previous study observed decreased thyroid hormone levels associated with pituitary gland infection with the related border disease virus (Anderson et al., Reference Anderson, Higgins, Smith and Osburn1987), it is unknown if BVDV can invade the hypothalamus and pituitary gland to induce alterations in the secretion of gonadotrophin-releasing hormones or the gonadotrophins.
Leukocytes including macrophages are present in the ovary, and their distribution varied with the stage of the cycle suggesting important roles in ovarian activities (Wu et al., Reference Wu, Van der Hoek, Ryan, Norman and Robker2004). Leukocytes are known to secrete cytokines and other inflammatory mediators in a tightly regulated manner to regulate critical ovarian processes such as follicular growth, ovulation, luteinization, and luteolysis (Wu et al., Reference Wu, Van der Hoek, Ryan, Norman and Robker2004; Richards et al., Reference Richards, Liu and Shimada2008; Jabbour et al., Reference Jabbour, Sales, Catalano and Norman2009). It is likely that a massive depletion of leukocytes during acute BVDV infections may impede the deployment of leukocytes to the ovary thereby compromising these reproductive processes (Kelling et al., Reference Kelling, Steffen, Topliff, Eskridge, Donis and Higuchi2002).
The oviducts have important functions in bovine fertility including the transport, storage, and capacitation of spermatozoa, the pick-up of the newly ovulated oocyte by the infundibulum and the transport, maturation and fertilization of the oocyte. The secretory products of the oviducts should also provide an optimum environment for the sustenance of the spermatozoa, oocytes, and the early embryo that is undergoing cleavage (Senger, Reference Senger2003; Rodriguez-Martinez, Reference Rodriguez-Martinez2007). BVDV infection was associated with salpingitis in infected non-pregnant cows (Archbald et al., Reference Archbald, Gibson, Schultz, Fahning and Zemjanis1973). Inflammation of the oviducts can interfere with the secretive and other physiologic functions of the oviducts, thereby compromising the ideal environment required for oocyte and sperm transport, and for fertilization.
Viral degradation of the oocyte, embryo, and fetus
BVDV infection may cause infertility by adversely affecting the viability of the oocyte or the conceptus at the embryonic or fetal stages, although this depends on several factors including the viral genotype, biotype (ncp versus cp), and the stage of reproductive events during which infection occurred. Unlike ncp BVDV, cp biotypes express non-structural protein 3 (NS3) which induces apoptosis in infected cells (Gamlen et al., Reference Gamlen, Richards, Mankouri, Hudson, McCauley, Harris and Macdonald2010).
Infection prior to the time of breeding or conception is followed by viral invasion of the ovary, cumulus cell population, and the oocytes maturing in primordial, primary, and secondary follicles (Fray et al., Reference Fray, Prentice, Clarke and Charleston1998). There was evidence of necrosis of oocytes in the follicles of cows infected with ncp BVDV (McGowan et al., Reference McGowan, Kafi, Kirkland, Kelly, Bielefeldt-Ohmann, Occhio and Jillella2003). One study also reported that oocytes from PI heifers in an in vitro fertilization (IVF) procedure showed a decrease in both the cleavage and embryo production rates (Gonzalez Altamiranda et al., Reference Gonzalez Altamiranda, Kaiser, Mucci, Verna, Campero and Odeon2013). Infection with BVDV may also have harmful effects on sperm–oocyte integrity and interaction at the time of fertilization. An in vitro study observed that infection with cp and ncp BVDV induced detrimental effects on sperm attachment to the zona pellucida (ZP) of bovine oocytes and on the fertilization rate during bovine IVF (Garoussi and Mehrzad, Reference Garoussi and Mehrzad2011).
Following fertilization, BVDV infection can also affect the developing embryo, although viral invasiveness and the effects on viability and quality of embryos at different stages of development have been controversial. The ZP is an extracellular glycoprotein matrix surrounding the oocyte and the early embryo that exerts several important functions during fertilization and early embryonic development (Sinowatz et al., Reference Sinowatz, Topfer-Petersen, Kolle and Palma2001). BVDV-like particles were detected in the ZP of embryos from BVDV-infected uterine horns (Archbald et al., Reference Archbald, Fulton, Seger, Al-Bagdadi and Godke1979) or in association with un-hatched in vitro-infected embryos (Givens et al., Reference Givens, Galik, Riddell, Brock and Stringfellow2000). Virus released from washed embryos can also be infective to cell culture in vitro (Givens et al., Reference Givens, Galik, Riddell, Brock and Stringfellow2000). Over 50% of recipient cows that received embryos exposed to BVDV type 2 became infected after embryo transfer, and a large proportion of the pregnancies in these cows were lost (Bielanski et al., Reference Bielanski, Algire, Lalonde and Nadin-Davis2009). On the other hand, embryos recovered from PI donor cows (Brock et al., Reference Brock, Lapin and Skrade1997) or from cows inseminated with BVDV-infected semen (Bielanski et al., Reference Bielanski, Algire, Lalonde and Garceac2013) when washed remained un-infective to the recipient cow or the produced calf. The failure of infection was attributed to the washing process, which significantly reduced virus copies associated with the embryos (Gard et al., Reference Gard, Givens, Marley, Galik, Riddell, Stringfellow, Zhang and Edmondson2009). In addition, both cp and ncp BVDV infection did not affect in vitro oocyte fertilization or embryo development in the presence of the complete ZP (Tsuboi and Imada, Reference Tsuboi and Imada1996; Stringfellow et al., Reference Stringfellow, Riddell, Brock, Riddell, Galik, Wright and Hasler1997). In contrast, both cp and ncp BVDV invade and replicate in ZP-free embryos or in hatched blastocysts but not in ZP-intact embryos, in vitro (Vanroose et al., Reference Vanroose, Nauwynck, Van Soom, Vanopdenbosch and de Kruif1998). Therefore, the consensus is that the ZP protects the oocyte and the unhatched embryo from infection by BVDV.
Viral-induced damage to embryos was previously linked to infection with the cp biotype. Infection with cp but not ncp BVDV was observed to cause embryonic cell death (Brock and Stringfellow, Reference Brock and Stringfellow1993) or inhibited embryonic development (Vanroose et al., Reference Vanroose, Nauwynck, Van Soom, Vanopdenbosch and de Kruif1998). In other studies on ncp BVDV, Booth et al. (Reference Booth, Collins, Jenner, Prentice, Ross, Badsberg and Brownlie1998) observed a reduction in the initial cleavage of zygotes but an increased blastocyst yield whereas Stringfellow et al. (Reference Stringfellow, Riddell, Galik, Damiani, Bishop and Wright2000) reported reduced cleavage in zygotes, embryos beyond the 4-cell stage and blastocyst yield. There was variation in cleavage, blastocyst development, and hatching among cultures contaminated with different strains of ncp virus but none of these effects was considered prominent (Givens et al., Reference Givens, Galik, Riddell, Brock and Stringfellow2000). Recently, infection with ncp BVDV was also observed to cause early embryonic death and marked decline in serum progesterone levels in experimentally infected cows (Tsuboi et al., Reference Tsuboi, Osawa, Hirata, Kawashima, Kimura and Haritani2013). Both BVDV1 and 2 were present in 100% of degenerate embryos produced in vitro from infected oocytes while 20–100% of viable embryos carried the virus but appeared to develop normally (da Silva Cardoso Pinto et al., Reference da Silva Cardoso Pinto, Alves, de Souza Nunes Martins, Basso, Tannura, Pontes, Lima, Garcia da Silva, Okuda, Stefano, Romaldini, Arnold and Pituco2017).
BVDV infection of the fetus via the placenta depends on the fetal age at the time of infection, the immunocompetence of the developing fetus, and the biotype, strain, and virulence of the infecting BVDV (Brownlie et al., Reference Brownlie, Hooper, Thompson and Collins1998; Grooms, Reference Grooms2004; Lanyon et al., Reference Lanyon, Hill, Reichel and Brownlie2014). BVDV can invade the placentome and access the fetus following acute (Fredriksen et al., Reference Fredriksen, Press, Sandvik, Odegaard and Loken1999b) and persistent infections (Fredriksen et al., Reference Fredriksen, Press, Loken and Odegaard1999a). Infection of fetuses of seropositive cows is rare due to the presence of maternal antibodies that can prevent viral invasion of the placentome (Brownlie et al., Reference Brownlie, Hooper, Thompson and Collins1998). Infection with BVDV can result in fetal death (Done et al., Reference Done, Terlecki, Richardson, Harkness, Sands, Patterson, Sweasey, Shaw, Winkler and Duffell1980; Sprecher et al., Reference Sprecher, Baker, Holland and Yamini1991; Lanyon et al., Reference Lanyon, Hill, Reichel and Brownlie2014). Depending on the time of infection, fetal death is followed by fetal reabsorption, mummification, or expulsion usually within the first trimester of pregnancy (Sprecher et al., Reference Sprecher, Baker, Holland and Yamini1991; Grooms, Reference Grooms2004). The mechanisms of viral-induced fetal death and abortion are not clear but may be due to cp effects in fetal and placental tissues, degeneration, and separation of the feto-maternal unit or a viral-induced inflammatory environment that is unfavorable for fetal survival and development. Some of the lesions observed were considered to be non-specific as the primary cause of abortion and included inflammatory cell infiltration of the fetal eyelid, lung, myocardium, and peribronchiolar and inter-alveolar tissues and placental vasculitis, degeneration, and necrosis (Murray, Reference Murray1991).
Apart from causing fetal death, BVDV infection can also lead to persistent fetal infection if dams are infected during the period of development of fetal immunocompetence. Infection of susceptible pregnant cows within days 18–125 of pregnancy with the ncp virus biotype has been associated with transplacental and persistent fetal infection (Brownlie et al., Reference Brownlie, Hooper, Thompson and Collins1998; Harding et al., Reference Harding, Cao, Shams, Johnson, Vassilev, Gil, Wheeler, Haines, Sibert, Nelson, Campos and Donis2002; Grooms, Reference Grooms2004; Lanyon et al., Reference Lanyon, Hill, Reichel and Brownlie2014). The mechanism of persistent infection is not clear. There was evidence of hepatic immune response to fetal infection by 14 days post-infection of the dam (Morarie-Kane et al., Reference Morarie-Kane, Smirnova, Hansen, Mediger, Braun and Chase2018). Moreover, persistent infection has been related to the ability of the ncp virus biotype to inhibit fetal induction of type-I interferon (IFN) response to the virus (Charleston et al., Reference Charleston, Fray, Baigent, Carr and Morrison2001; Peterhans and Schweizer, Reference Peterhans and Schweizer2013) thereby permitting fetal immunotolerance to BVDV and the birth of PI calves. Although some PI cattle may appear clinically normal, there are reports of poor growth, poor milk production, poor survivability, and increased susceptibility to other diseases as well as mucosal disease in PI cattle (Houe, Reference Houe1993; Baker, Reference Baker1995; Voges et al., Reference Voges, Young and Nash2006). Moreover, BVDV infection can also result in fetal malformations in dams infected during the period of fetal organ formation, most probably due to viral-induced lesions, and disruption of embryogenesis. As previously described, transplacental BVDV infection of the fetus within 80–150 days of pregnancy can lead to the development of congenital defects of several organ systems including cerebellar hypoplasia, hydrocephalus, ocular degeneration, thymic hypoplasia, pulmonary hypoplasia, brachygnathism, arthrogryposis, and growth retardation (Baker, Reference Baker1995; Blanchard et al., Reference Blanchard, Ridpath, Walker and Hietala2010; Lanyon et al., Reference Lanyon, Hill, Reichel and Brownlie2014). These congenital deformities invariably lead to significant reproductive losses in the form of fetal losses, decreased calf yield, decreased availability of replacement heifers, dystocia that may be associated with increased maternal mortality, and cows culled for reproductive problems.
Viral-induced immune dysfunction and susceptibility to diseases
There is no doubt that both male and female cattle that readily succumb to prevalent diseases will have compromised reproductive efficiency. Males with clinical or subclinical disease will have poor libido and mating capacity. Moreover, the reproductive process imposes significant biological demands on the female; therefore, it should not be surprising that the reproductive activities are often the first to be arrested when the health of the female is compromised (Pineda, Reference Pineda and Pineda2003).
There is evidence that infection with BVDV can render host cattle more susceptible to secondary infection with other pathogens. The presence of BVDV infection is known to increase the severity of respiratory disease in calves infected with bovine herpes virus 1, bovine respiratory syncytial virus, and the bacteria Mannheimia haemolytica and Histophilus somni (Edwards et al., Reference Edwards, Wood, Hewitt-Taylor and Drew1986; Potgieter, Reference Potgieter1997; Brodersen and Kelling, Reference Brodersen and Kelling1998; Ridpath, Reference Ridpath2010a).
Infection with BVDV also increased the severity of enteric diseases in cattle infected with bovine rotavirus (de Verdier Klingenberg, Reference de Verdier Klingenberg2000) and Salmonella typhimurium (Wray and Roeder, Reference Wray and Roeder1987; Penny et al., Reference Penny, Low, Nettleton, Scott, Sargison, Strachan and Honeyman1996). Calves predisposed to other systemic diseases are prone to be unthrifty with poor reproductive development and delayed onset of puberty.
Immune dysfunction following BVDV infection in heifers and cows may also increase the severity of reproductive tract disease by facilitating placental invasion by specific and opportunistic pathogens or by exacerbating fetal lesions. Intercurrent infections of BVDV with some bacteria such as Trueperella pyogenes and Bacillus spp., or other fungi have been demonstrated in some aborted fetuses (Kirkbride, Reference Kirkbride1992). Other studies also reported increased severity of abortions or fetal lesions when BVDV infection coexisted with other bacteria such as Leptospira hardjo and Coxiella burnetii (Pritchard et al., Reference Pritchard, Borland, Wood and Pritchard1989) or Campylobacter fetus (Jeffrey and Hogg, Reference Jeffrey and Hogg1988). Co-infections of BVDV with the protozoan parasite Neospora caninum (Bjorkman et al., Reference Bjorkman, Alenius, Manuelsson and Uggla2000) or the bacteria H. somni (Headley et al., Reference Headley, Voltarelli, de Oliveira, Bronkhorst, Alfieri, Filho, Okano and Alfieri2015) were also associated with abortions in dairy cows. Increased susceptibility of the dam to specific and opportunistic pathogens of the reproductive tract can result in reproductive abnormalities such as puerperal metritis, endometritis, pyometra, embryonic and fetal death, abortion and retained fetal membranes.
Pathogenic organisms can invade the reproductive tract of the cow during breeding (Newcomer et al., Reference Newcomer, Toohey-Kurth, Zhang, Brodersen, Marley, Joiner, Galik, Riddell and Givens2014), during parturition or the postpartum period (Bondurant, Reference Bondurant1999; Bicalho et al., Reference Bicalho, Santin, Rodrigues, Marques, Lima and Bicalho2017b) or through the blood circulation following a systemic microbial infection (Jeon et al., Reference Jeon, Cunha, Vieira-Neto, Bicalho, Lima, Bicalho and Galvão2017). For instance, the uteri of almost all cows are contaminated within a few days postpartum with a variety of both specific and non-specific bacteria including Escherichia coli, T. pyogenes, and other anaerobes such as Fusobacterium, Prevotella, and Bacteroides species (Huszenicza et al., Reference Huszenicza, Fodor, Gacs, Kulcsar, Dohmen, Vamos, Porkolab, Kegl, Bartyik, Lohuis, Janosi and Szita1999; Williams et al., Reference Williams, Fischer, Pfeiffer, England, Noakes, Dobson and Sheldon2005; Bicalho et al., Reference Bicalho, Machado, Higgins, Lima and Bicalho2017a). In most normal cows, the reproductive tract is protected by the innate immune system which acts immediately and within hours to prevent infection. Much later, usually after a few days, the adaptive immune response sets in for weeks or months to provide a sustained protection.
Innate immune response involves the recognition of microbial patterns by resident cells and migrant immune cells of the reproductive tract which leads to increased expression of inflammatory products and innate immune mediators such as antimicrobial peptides (AMPs), mucins, pro-inflammatory cytokines, acute phase proteins (APPs), type-I IFNs, and prostaglandins (Oguejiofor et al., Reference Oguejiofor, Cheng and Wathes2017b). This activation of an early inflammatory cascade is critical in mobilizing specialized innate immune cells such as granulocytes and macrophages from the blood circulation toward the endometrium to phagocytize and eliminate the pathogens (Butt et al., Reference Butt, Senger and Widders1991; Singh et al., Reference Singh, Murray, Mshelia and Woldehiwet2008; Oguejiofor et al., Reference Oguejiofor, Cheng and Wathes2017b). Subsequently, the innate immune response stimulates the adaptive immunity resulting in the generation of pathogen-specific B and T lymphocytes that drive the antibody and cell-mediated immune response (Turvey and Broide, Reference Turvey and Broide2010; Hickey et al., Reference Hickey, Patel, Fahey and Wira2011). When innate immune response fails, reproductive tract infection occurs and may persist until cleared by the adaptive immunity often resulting in subsequent decrease or absence of fertility in affected cows. However, uterine immune function may become compromised resulting in bacterial persistence and uterine diseases such as metritis, endometritis, or cervicitis in up to 50% of postpartum cows (Sheldon et al., Reference Sheldon, Cronin, Goetze, Donofrio and Schuberth2009; LeBlanc, Reference LeBlanc2014).
The mechanisms via which BVDV-induced immune dysfunction may predispose the cow's reproductive tract to infection and infertility are not clearly understood, but may include viral-induced leukocyte depletion (leukopenia), viral interference with the functions of immune cells in affected animals, or viral interference with innate functions of endometrial cells.
Viral-induced leukocyte depletion (leukopenia)
A massive depletion of leukocytes occurs in the systemic circulation in cattle acutely infected (Kelling et al., Reference Kelling, Steffen, Topliff, Eskridge, Donis and Higuchi2002) or PI (Piccinini et al., Reference Piccinini, Luzzago, Frigerio, Dapra, Liandris and Zecconi2006) with BVDV. Immune dysfunction associated with BVDV infection may be a consequence of the marked tropism of the virus for antigen-presenting cells (APCs) (Brackenbury et al., Reference Brackenbury, Carr and Charleston2003). BVDV is lymphotropic, with acute infection resulting in lymphoid depletion in the thymus, spleen, lymph nodes, and Peyer's patches depending on the virus strain (Walz et al., Reference Walz, Bell, Wells, Grooms, Kaiser, Maes and Baker2001). The leukopenia is mainly due to lymphopenia and neutropenia as a result of removal of BVDV-infected leukocytes by the immune system, destruction of immune cells by BVDV, and increased trafficking of immune cells into tissue sites of viral replication (Walz et al., Reference Walz, Grooms, Passler, Ridpath, Tremblay, Step, Callan and Givens2010). It is possible that a significant depletion of circulating leukocytes may decrease the number of leukocytes mobilized to the cow's reproductive tract during infection. This can compromise immune response to infection thereby leading to the development of reproductive- tract disease and infertility.
Viral interference with the functions of immune cells
There is abundant evidence that BVDV infects immune cells and significantly alters their immune mechanisms and functions that have critical roles in both innate and adaptive immune response to infection. The reader is referred to previous reviews on the subject (Brackenbury et al., Reference Brackenbury, Carr and Charleston2003; Chase et al., Reference Chase, Elmowalid and Yousif2004; Peterhans and Schweizer, Reference Peterhans and Schweizer2010; Chase, Reference Chase2013; Chase et al., Reference Chase, Thakur, Darweesh, Morarie-Kane and Rajput2015).
Immune cells possess pattern recognition receptors including Toll-like receptors (TLRs) 3, 7, and 8 that recognize viral RNA in endolysosomal compartments and retinoic acid inducible gene I (RIG-I)-like receptors (RLRs), RIG-I and melanoma differentiation-associated protein 5 (MDA5) that recognize viral RNA in the cytoplasm (Berke et al., Reference Berke, Li and Modis2013). Viral recognition induces host immune response by activating signaling pathways that lead to the expression of pro-inflammatory cytokines, type-I IFNs, and antimicrobial proteins to eliminate the virus (Kumar et al., Reference Kumar, Kawai and Akira2009). However, BVDV has evolved different means of evading the host immune response to survive either by avoiding detection by host cells or by disabling the antiviral response of the host. Autophagy is a critical cellular process during innate and adaptive immune response to pathogens including viruses and bacteria (Deretic and Levine, Reference Deretic and Levine2009). Both cp and ncp BVDV infection induces autophagy, which may impair the innate immune response in bovine cells and facilitate BVDV replication (Zhou et al., Reference Zhou, Ren, Cong, Mu, Yin and Ding2017). Depending on the virus biotype and strain, infection with BVDV can interfere with several innate and adaptive immune mechanisms including IFN response, phagocytic activity, antigen-presenting functions, and humoral and cell-mediated functions of immune cells.
Type-I IFNs are important cytokines secreted by innate immune cells to protect uninfected cells and prevent viral replication by activating macrophages, dendritic cells (DCs), and other cells involved in the innate and adaptive immune interphase (Randall and Goodbourn, Reference Randall and Goodbourn2008). These cytokines also serve as a key link to the adaptive immune response by enhancing the differentiation of virus-specific cytotoxic T cells (Stetson and Medzhitov, Reference Stetson and Medzhitov2006). Type-I IFNs induce the expression of a large number of IFN-stimulated genes (ISGs), which are responsible for the antiviral and immunomodulatory properties of IFNs (Hertzog and Williams, Reference Hertzog and Williams2013). Infection with ncp BVDV is known to inhibit the synthesis of IFN suggesting an important mechanism by which ncp BVDV establishes a persistent infection (Charleston et al., Reference Charleston, Fray, Baigent, Carr and Morrison2001; Schweizer and Peterhans, Reference Schweizer and Peterhans2001; Baigent et al., Reference Baigent, Zhang, Fray, Flick-Smith, Goodbourn and McCauley2002). This virus survival strategy involves the production of the viral protein Npro which degrades the transcription factor IFN regulatory factor (IRF) 3, thereby preventing downstream signaling and the activation of an IFN response (Chen et al., Reference Chen, Rijnbrand, Jangra, Devaraj, Qu, Ma, Lemon and Li2007; Peterhans and Schweizer, Reference Peterhans and Schweizer2010). A recent study also provided evidence that BVDV Npro may suppress the activity of S100 calcium binding protein A9 (S100A9, a cell protein that stimulates innate immunity), resulting in reduced type-I IFN production (Darweesh et al., Reference Darweesh, Rajput, Braun, Rohila and Chase2018). Although the type-I IFNs are typically considered to be most important in the host antiviral immune response, they are also induced by almost all bacterial pathogens (Perry et al., Reference Perry, Chen, Zheng, Tang and Cheng2005; Monroe et al., Reference Monroe, McWhirter and Vance2010). These suggest mechanisms through which ncp BVDV inhibition of IFN response can escalate other viral and bacterial infections in affected cows.
Professional phagocytes are effector cells that have important roles in the innate immune clearance of intracellular and extracellular pathogens. Macrophages and neutrophils produce several enzymes and reactive oxygen species such as superoxide anion, hydrogen peroxide, and nitric oxide that have critical roles in the killing of invading pathogens (Dale et al., Reference Dale, Boxer and Liles2008). A suppression of these crucial functions can therefore predispose affected cows to other diseases. There are several reports of various forms of viral interference with the phagocytic and inflammatory functions of phagocytes following infection with BVDV. Neutrophils from cattle PI with BVDV were characterized by a significant decrease in random migration, bacterial ingestion, oxidant production, and antibody independent cell-mediated cytotoxicity (Brown et al., Reference Brown, Bolin, Frank and Roth1991). There was also a significant decrease in polymorphonuclear leukocytes respiratory burst and cellular enzymes NAGase and lysozyme in PI heifers (Piccinini et al., Reference Piccinini, Luzzago, Frigerio, Dapra, Liandris and Zecconi2006). Both ncp and cp BVDV decreased CD18 and CD62L (L-selectin) expression in bovine neutrophils. Because these two receptors are important for endothelial adhesion and extravasation, this suggested a mechanism through which BVDV could inhibit neutrophil migration (Chase et al., Reference Chase, Thakur, Darweesh, Morarie-Kane and Rajput2015). In macrophages infected with BVDV in vitro, there was reduced production of superoxide anion (Adler et al., Reference Adler, Frech, Meier, Jungi and Peterhans1994) and the pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α) (Adler et al., Reference Adler, Jungi, Pfister, Strasser, Sileghem and Peterhans1996) following lipopolysaccharide (LPS) treatment. Fc receptor (FcR) and complement factors have important roles in the opsonization and cytotoxic killing of bacteria by effector cells (Ravetch and Clynes, Reference Ravetch and Clynes1998). FcR and complement receptor (C3R) expression, phagocytosis and microbicidal activity and the production of neutrophil chemotactic factors were all reduced in macrophages recovered from BVDV-infected calves (Welsh et al., Reference Welsh, Adair and Foster1995). In bovine monocytes, ncp BVDV infection suppressed gene expression of pro-inflammatory cytokines TNF-α, interleukin (IL)1-β, and IL6 and co-stimulatory molecules CD80 and CD86 (Lee et al., Reference Lee, Pharr, Boyd and Pinchuk2008). BVDV type-2 infections modulated mRNA responses and induced a suppression of pro-inflammatory cytokine protein responses to TLR ligation in monocyte derived macrophages with the exception of TLR7 ligation (Schaut et al., Reference Schaut, Ridpath and Sacco2016). Prostaglandins and leukotrienes are lipid mediators that can regulate immunity. Leukotrienes have immune modulatory and pro-inflammatory properties (Di Gennaro and Haeggstrom, Reference Di Gennaro and Haeggstrom2012). In general, PGE2 suppresses acute inflammatory mediators and is predominant at the late or chronic stages of immunity (Kalinski, Reference Kalinski2012), although its immunomodulatory effect may vary during other physiologic processes such as in the uterus. Infection with BVDV stimulates the production of PGE2 in bovine macrophages (Van Reeth and Adair, Reference Van Reeth and Adair1997) and inhibits the synthesis of leukotriene B4 in bovine mononuclear cells (Atluru et al., Reference Atluru, Gudapaty, Xue, Gurria, Chengappa, McVey, Minocha and Atluru1992). The alteration of these lipid mediators suggests another mechanism through which BVDV may disrupt immune response in infected cattle.
Classical APCs include macrophages, dendritic cells, and B cells that process antigens and present them together with major histocompatibility complex II (MHC II) to T cells thereby facilitating antibody mediated and cell-mediated immune response. In addition, cytokines produced by APCs serve as an important link between the innate and adaptive immune response (Parkin and Cohen, Reference Parkin and Cohen2001). Infection with both cp and ncp BVDV compromised antigen uptake in bovine monocytes (Boyd et al., Reference Boyd, Lee, Kruger and Pinchuk2004). Monocytes infected with ncp BVDV were compromised in their ability to stimulate T cell responses (Glew et al., Reference Glew, Carr, Brackenbury, Hope, Charleston and Howard2003). Ncp BVDV infection diminished the expression of CD80/CD86 and MHC II antigen presentation molecules on the surface of peripheral blood mononuclear cells (Archambault et al., Reference Archambault, Beliveau, Couture and Carman2000). Infection of monocytes with cp BVDV altered the expression of multiple proteins involved in immune function of APCs including cell adhesion, apoptosis, antigen uptake processing and presentation, APPs, and MHC molecules (Lee et al., Reference Lee, Nanduri, Pharr, Stokes and Pinchuk2009). The depression of T and B lymphocytes in lymphatic tissues and in peripheral circulation (Ellis et al., Reference Ellis, Davis, Belden and Pratt1988; Brodersen and Kelling, Reference Brodersen and Kelling1999) can inhibit cell-mediated and humoral immune response in affected cows.
Taken together, BVDV alters the different aspects of innate immunity including IFN and inflammatory pathways, and phagocytosis. BVDV also influences adaptive immunity by altering the earliest phase of innate response involving pattern recognition, antigen presentation, co-stimulatory signaling and lymphocyte recruitment, and by inducing apoptosis of lymphoid tissues and altered B and T cellular response (Chase, Reference Chase2013). BVDV has evolved this interference with the host's immune mechanisms as a means of survival by evading immune elimination by the host. However, viral suppression of immune response can predispose affected cows to other systemic secondary infections that can inhibit fertility. Compromised migrant immune cells may also fail to protect the cow from other secondary infections of the reproductive tract following coitus, parturition or postpartum thereby leading to reproductive tract infection and infertility.
Viral interference with innate immune functions of endometrial cells
The epithelial cells and the underlying stromal cells are the majority cell types that constitute the endometrium. The epithelial cells comprise the first line of cells in contact with microbes that contaminate the uterine lumen, but erosion of the maternal caruncles following placental separation postpartum can also expose both cell types to the contents of the uterine lumen (Noakes, Reference Noakes, Noakes, Parkinson and England2001b). The innate immune response of the endometrium constitutes an important barrier to infection by pathogens that contaminate the uterus following breeding, at parturition and during the postpartum period (Singh et al., Reference Singh, Murray, Mshelia and Woldehiwet2008; Oguejiofor et al., Reference Oguejiofor, Cheng and Wathes2017b). During early pregnancy, genes of the innate immune response may function to protect the uterus against infection (Walker et al., Reference Walker, Meier, Littlejohn, Lehnert, Roche and Mitchell2010). Endometrial epithelial cells and stromal cells express the extra-cytosolic receptors, TLRs 1–10 (Davies et al., Reference Davies, Meade, Herath, Eckersall, Gonzalez, White, Conlan, O'Farrelly and Sheldon2008; Swangchan-Uthai et al., Reference Swangchan-Uthai, Lavender, Cheng, Fouladi-Nashta and Wathes2012; Oguejiofor et al., Reference Oguejiofor, Cheng, Abudureyimu, Fouladi-Nashta and Wathes2015b, Reference Oguejiofor, Cheng, Fouladi-Nashta and Wathes2017a), as well as the cytosolic receptors: IFN-induced with helicase C domain 1 (IFIH1 also known as MDA5), DExD/H-box helicase 58 (DDX58, also known as RIG-I), and leucine-rich repeat (in FLII) interacting protein 1, LRRFIP1 (Cheng et al., Reference Cheng, Chauhan, Barry, Abudureyimu, Oguejiofor, Chen and Wathes2017; Oguejiofor et al., Reference Oguejiofor, Cheng, Abudureyimu, Fouladi-Nashta and Wathes2015b, Reference Oguejiofor, Cheng, Fouladi-Nashta and Wathes2017a). These receptors are known to detect extracellular and intracellular pathogen-associated molecular patterns (PAMPs) during innate immunity (Kumar et al., Reference Kumar, Kawai and Akira2011).
Bovine endometrial cells respond to either E. coli or LPS stimulation by increased expression of gene transcripts and proteins of pro-inflammatory cytokines, type-I IFNs, AMPs, mucins, APPs, and the prostaglandins PGF2α and PGE2 (Davies et al., Reference Davies, Meade, Herath, Eckersall, Gonzalez, White, Conlan, O'Farrelly and Sheldon2008; Swangchan-Uthai et al., Reference Swangchan-Uthai, Lavender, Cheng, Fouladi-Nashta and Wathes2012; Chapwanya et al., Reference Chapwanya, Meade, Doherty, Callanan and O'Farrelly2013; Fu et al., Reference Fu, Liu, Feng, Liu, Liang, Li, Li, Cao, Feng, Zhang, Zhang and Yang2013; Oguejiofor et al., Reference Oguejiofor, Cheng, Abudureyimu, Fouladi-Nashta and Wathes2015b). Bacterial LPS also induced increased expression of many genes that may be involved in innate defence against uterine bacterial infection including several ISGs, IRFs, type-I IFN receptors, immunoproteasomes, complement factors, guanylate-binding proteins, cell adhesion molecules, matrix metalloproteinases, growth factors, and genes involved in the intracellular recognition of pathogens (Oguejiofor et al., Reference Oguejiofor, Cheng, Abudureyimu, Fouladi-Nashta and Wathes2015b).
Recently, ncp BVDV has been established to readily infect both epithelial and stromal cells of the bovine endometrium in vitro, and to suppress the ability of these cells to mount an innate immune response to bacterial LPS (Oguejiofor et al., Reference Oguejiofor, Cheng, Abudureyimu, Anstaett, Brownlie, Fouladi-Nashta and Wathes2015a). Viral infection inhibited many genes that are typically up-regulated in response to bacterial presence including genes involved in pathogen recognition, IFN response, inflammatory response, chemokine activity, transcription regulation, tissue remodeling and cell migration, and cell death/survival (Oguejiofor et al., Reference Oguejiofor, Cheng, Abudureyimu, Anstaett, Brownlie, Fouladi-Nashta and Wathes2015a). In the bovine endometrial cells, type-I IFN stimulated expression of many ISGs which play important roles in various immune, especially antiviral pathways. However, in the cells infected with ncp BVDV, the stimulatory effect was significantly inhibited or neutralized (Cheng et al., Reference Cheng, Chauhan, Barry, Abudureyimu, Oguejiofor, Chen and Wathes2017). Viral proteins produced by BVDV are thought to interfere with TLR4 and myeloid differentiation primary response 88 (MyD88) signaling pathways thereby subverting cellular response to bacterial LPS (Schaut et al., Reference Schaut, McGill, Neill, Ridpath and Sacco2015). Consequently, viral suppression of endometrial innate immune response may be another mechanism through which ncp BVDV infection can compromise endometrial signaling, cytokine activity, and the mobilization of leukocytes toward the uterine lumen to clear microbial contaminants.
Moreover, infection of endometrial cells with ncp BVDV increased the mRNA expression of prostaglandin-endoperoxide synthase 1 (PTGS1) and microsomal prostaglandin E synthase-1 (mPGES1), and attenuated aldo–keto reductase family 1, member B1 (AKR1B1) expression, leading to increased PGE2 and decreased PGF2α concentrations and an increase in PGE2:PGF2α ratios in bovine uterine endometrium (Cheng et al., Reference Cheng, Abudureyimu, Oguejiofor, Ellis, Barry, Chen, Anstaett, Brownlie and Wathes2016). Prostaglandins are known to modulate the immune response in the endometrium. PGF2α enhances immune response whereas PGE2 is an immune suppressor (Lewis, Reference Lewis2003; Herath et al., Reference Herath, Lilly, Fischer, Williams, Dobson, Bryant and Sheldon2009). In addition, PGE2 also inhibits luteal regression due to its luteotropic effect on the corpus luteum (Arosh et al., Reference Arosh, Banu, Kimmins, Chapdelaine, Maclaren and Fortier2004), and persistent corpora lutea and over production of progesterone in cases of uterine disease, can disrupt the reproductive cycle and inhibit uterine immunity to cause subfertility (Opsomer et al., Reference Opsomer, Grohn, Hertl, Coryn, Deluyker and de Kruif2000). Hence this switch in prostaglandin secretion may comprise another mechanism whereby BVDV infection can predispose affected cows to uterine infection. Inadequate endometrial innate immune response leads to microbial persistence and endometritis (LeBlanc, Reference LeBlanc2014). In addition, direct effects of bacterial LPS or indirect effects of inflammatory mediators such as cytokines, prostaglandins, and oxidative stress can disrupt sperm, ovarian, uterine, and embryonic function leading to decreased fertility (Gilbert, Reference Gilbert2012).
Potential viral effects on maternal early pregnancy recognition
Infection of susceptible heifers and cows with BVDV a few days before or after breeding was observed to cause significant decline in conception rates. In BVDV-infected cows, animals bred before they seroconverted had a 22% first-service conception rate compared with a 79% rate in cows seropositive at the time of breeding (Virakul et al., Reference Virakul, Fahning, Joo and Zemjanis1988). Moreover, the conception rates of 60% in naturally infected cows and 44% in experimentally infected cows were both lower than 79% observed in non-infected cows at 21 days following insemination (McGowan et al., Reference McGowan, Kirkland, Richards and Littlejohns1993a). Hence, ncp BVDV infection of susceptible cows has been associated with failure of early pregnancy (McGowan and Kirkland, Reference McGowan and Kirkland1995; Tsuboi et al., Reference Tsuboi, Osawa, Hirata, Kawashima, Kimura and Haritani2013) but the mechanisms have remained largely undefined. Interestingly, recent in vitro studies have provided new evidence that may link BVDV infection with early pregnancy losses in cows.
Following conception, the bovine embryo enters the uterus on days 4–6 after breeding and must signal its presence for effective maternal recognition and hence maintenance of pregnancy prior to implantation. Interferon-τ (IFNT) is a member of the type-I IFNs that have the same functional receptors in bovine endometrium (Li and Roberts, Reference Li and Roberts1994; Roberts et al., Reference Roberts, Ezashi, Rosenfeld, Ealy and Kubisch2003). The bovine conceptus trophectoderm begins IFNT secretion into the uterine lumen on around day 8 of gestation, with secretion increasing significantly during the period of trophectoderm elongation (Kimura et al., Reference Kimura, Spate, Green, Murphy, Seidel and Roberts2004; Robinson et al., Reference Robinson, Fray, Wathes, Lamming and Mann2006). Following sufficient IFNT stimulation of the endometrium (around day 16 of gestation), there is inhibition of the development of oxytocin receptors which prevents luteolysis and ensures the continued production of progesterone needed for maintenance of pregnancy (Mann et al., Reference Mann, Lamming, Robinson and Wathes1999; Forde et al., Reference Forde, Carter, Spencer, Bazer, Sandra, Mansouri-Attia, Okumu, McGettigan, Mehta, McBride, O'Gaora, Roche and Lonergan2011; Lonergan and Forde, Reference Lonergan and Forde2014). Failure of pregnancy recognition results in luteolysis and loss of progesterone, a significant risk factor for embryonic death (Diskin et al., Reference Diskin, Parr and Morris2011). In addition to inhibition of luteolysis, IFNT is thought to stimulate a receptive endometrium for implantation by modulating maternal endometrial activity of hormones and their receptors, type-I IFNs, cytokines, prostaglandins, and nutrient transporters (Forde et al., Reference Forde, Carter, Spencer, Bazer, Sandra, Mansouri-Attia, Okumu, McGettigan, Mehta, McBride, O'Gaora, Roche and Lonergan2011; Bazer, Reference Bazer2013; Lonergan and Forde, Reference Lonergan and Forde2014).
One mechanism through which ncp BVDV infection may disrupt early pregnancy is by alteration of endometrial prostaglandin production and signaling during pregnancy recognition in cows. In previous reports from bovine studies, IFNT stimulated increased expression of prostaglandin-endoperoxide synthase 2 (PTGS2), the rate-limiting enzyme in PG synthesis, in the endometrium during the peri-implantation period (Arosh et al., Reference Arosh, Banu, Kimmins, Chapdelaine, Maclaren and Fortier2004; Emond et al., Reference Emond, MacLaren, Kimmins, Arosh, Fortier and Lambert2004). Increased biosynthesis of PGE2 was cell specific and temporal in endometrium, myometrium, and corpus luteum, suggesting important roles of PGE2 in endometrial receptivity, myometrial quiescence, and luteal maintenance during maternal recognition of pregnancy (MRP) (Arosh et al., Reference Arosh, Banu, Kimmins, Chapdelaine, Maclaren and Fortier2004). Evidence from studies in small ruminants (sheep) showed the importance of interaction between prostaglandins produced by the conceptus and endometrial epithelial and stromal cells and IFNT in the regulation of endometrial gene expression and functions that promote conceptus elongation, development and implantation (Simmons et al., Reference Simmons, Satterfield, Welsh, Bazer and Spencer2010; Dorniak et al., Reference Dorniak, Bazer, Wu and Spencer2012; Bazer, Reference Bazer2013). Therefore, PGs play crucial roles in early pregnancy in ruminants. The intra-uterine inhibition of PTGS2 suppressed uterine PG production and led to failure of elongation of ovine conceptuses (Dorniak et al., Reference Dorniak, Bazer and Spencer2011) and decreased pregnancy rate (Erdem and Guzeloglu, Reference Erdem and Guzeloglu2010). IFNT stimulates PGE2 production by ovine endometrial cells (Dorniak et al., Reference Dorniak, Bazer and Spencer2011). In a recent in vitro study, IFNT treatment also increased PGE2 secretion, and in addition up-regulated the expression of PTGS1 and the PGE2 receptor PTGER3 in bovine endometrial epithelial and stromal cells, suggesting that IFNT activates the PGE2 signaling pathway (Cheng et al., Reference Cheng, Abudureyimu, Oguejiofor, Ellis, Barry, Chen, Anstaett, Brownlie and Wathes2016). However, ncp BVDV infection suppressed the IFNT-induced production of PGE2 and the expression of its receptor PTGER3 in infected endometrial cells (Cheng et al., Reference Cheng, Abudureyimu, Oguejiofor, Ellis, Barry, Chen, Anstaett, Brownlie and Wathes2016). Furthermore, whereas IFNT inhibits the oxytocin-stimulated pulsatile release of PGF2α by the ruminant endometrium, the basal secretion of PGE2 and PGF2α is known to increase during early pregnancy (Ulbrich et al., Reference Ulbrich, Schulke, Groebner, Reichenbach, Angioni, Geisslinger and Meyer2009; Dorniak et al., Reference Dorniak, Bazer and Spencer2011). However, ncp BVDV infection also suppressed basal PGF2α secretion and the expression of AKR1B1, the predominant isoform for PGF2α production (Cheng et al., Reference Cheng, Abudureyimu, Oguejiofor, Ellis, Barry, Chen, Anstaett, Brownlie and Wathes2016). These new observations therefore suggest that ncp BVDV infection may disrupt the recognition or maintenance of pregnancy by suppressing IFNT-induced PG production and signaling in the endometrium during early pregnancy.
Interestingly, another mechanism through which ncp BVDV infection may disrupt early pregnancy is by alteration of the activities of ISGs in the endometrium during pregnancy recognition in cows. During the period of MRP, IFNT is known to differentially regulate the endometrial expression of many genes of which the most upregulated genes were ISGs. These include MX dynamin like GTPase 2 (MX2), bone marrow stromal cell antigen 2 (BST2), radical S-adenosyl methionine domain containing 2 (RSAD2), ISG15 ubiquitin-like modifier (ISG15), 2′,5′-oligoadenylate synthetase 1 (OAS1), ubiquitin specific peptidase 18 (USP18), IFN-induced protein 44 (IFI44), IFN-stimulated exonuclease gene 20 (ISG20), sterile alpha motif domain containing 9 (SAMD9), eukaryotic translation initiation factor 4E (EIF4E), and IFN-induced protein with tetratricopeptide repeats 2 (IFIT2) (Mansouri-Attia et al., Reference Mansouri-Attia, Aubert, Reinaud, Giraud-Delville, Taghouti, Galio, Everts, Degrelle, Richard, Hue, Yang, Tian, Lewin, Renard and Sandra2009; Forde et al., Reference Forde, Carter, Spencer, Bazer, Sandra, Mansouri-Attia, Okumu, McGettigan, Mehta, McBride, O'Gaora, Roche and Lonergan2011; Lonergan and Forde, Reference Lonergan and Forde2014). ISGylation and the up-regulation of ISG15 is an important maternal response to the developing conceptus that is conserved across mammalian pregnancy (Hansen and Pru, Reference Hansen and Pru2014). These ISGs are thought to have important roles in ruminants during early pregnancy in the regulation of uterine immunity, endometrial stromal remodeling, and the development of endometrial glands and uterine vasculature (Hansen, Reference Hansen2011; Bazer, Reference Bazer2013). Infection of endometrial cells with ncp BVDV significantly inhibited IFNT-stimulated expression of many tested ISGs including ISG15, USP18, DDX58, IFIH1, IFIT1, IFIT3, BST2, MX1, MX2, RSAD2, OAS1Y, and SAMD9, in addition to ISG15-secreted protein (Cheng et al., Reference Cheng, Chauhan, Barry, Abudureyimu, Oguejiofor, Chen and Wathes2017). Our recent studies demonstrated that BVDV interfered with the ISG regulatory pathway of IRF-STAT1 and IRF-STAT2 to inhibit IFNT-induced ISG expression in the bovine endometrium. In the bovine endometrial cells, IFNT treatment significantly stimulated the expression of many important genes in this pathway, including STAT1, STAT2, IRF9, TYK2, etc. However, in the cells infected with ncp BVDV, the IFNT-induced expression of those genes was significantly suppressed (Cheng et al., Reference Cheng, Brown and Wathes2018). This suggests yet another mechanism through which ncp BVDV infection may disrupt MRP and early pregnancy by suppressing the functions of ISGs in endometrial immunity and development in early pregnancy.
Summary
Reproductive diseases can have damaging consequences on fertility in both dairy and beef cattle. Reproductive losses associated with BVDV infection contribute to significant economic damage. Although infection with BVDV is known to cause poor fertility in cattle, a greater part of the underlying mechanisms, and differences in effect due to strain variations are still being investigated. Several mechanisms have been suggested through which BVDV infection may cause decreased fertility in cattle (Fig. 1). BVDV infections induce immune dysfunction, and predispose cows to other diseases that cause poor health and reduced fertility. Viral infection may also kill the oocyte, embryo, or fetus directly, or induce lesions that result in fetal abortion, mummification, teratogenesis, and the birth of malformed calves. BVDV infection is also thought to disrupt the reproductive endocrine system and leukocyte and cytokine functions in the reproductive organs. Recent studies provided evidence of viral-induced suppression of endometrial innate immunity that may predispose to uterine disease. Furthermore, there is new evidence that BVDV may potentially disrupt the MRP or the immune protection of the conceptus. To better describe how BVDV infection causes losses in early pregnancy, it is recommended that more investigation be carried out to further understand the interaction between BVDV and the bovine conceptus and endometrium during MRP and early pregnancy. Nevertheless, progress has been made in some regions of the world toward to control of BVDV for instance through elimination of PI animals in cattle herds (Wernike et al., Reference Wernike, Gethmann, Schirrmeier, Schroder, Conraths and Beer2017). However, even in countries where BVDV has been intensively controlled there is significant risk of reintroduction of BVDV (Santman-Berends et al., Reference Santman-Berends, Mars, Van Duijn, Van den Broek and Van Schaik2017) to a large number of naïve and susceptible cattle, underscoring the importance of continual testing, and vigilance of cattle movement and trade.
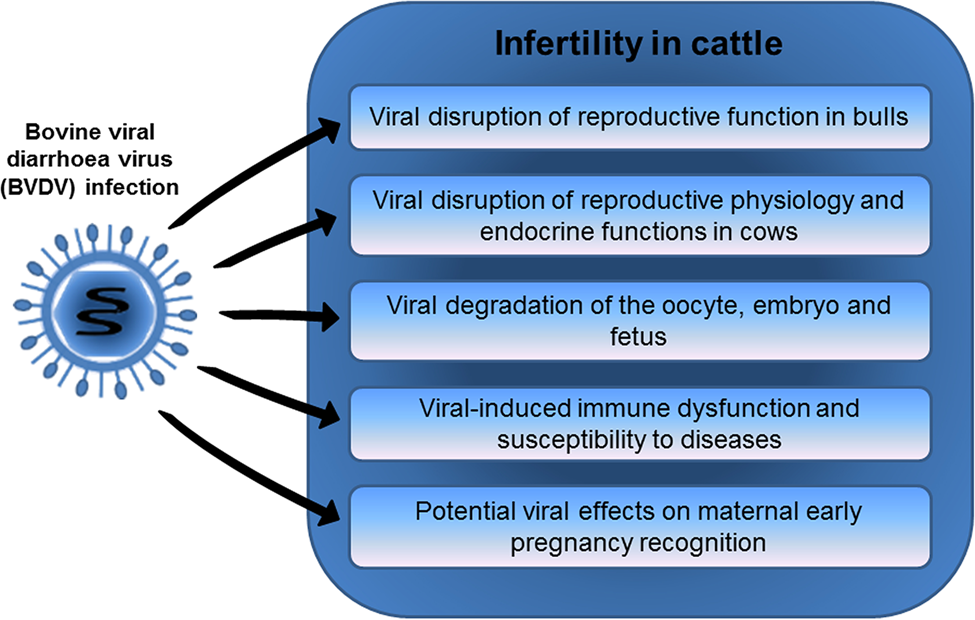
Fig. 1. Mechanisms linking BVDV infection with infertility in cattle.
Acknowledgments
The series of research in the authors' laboratory were funded by contributions from the Royal Veterinary College, the China Scholarship Commission and the Commonwealth Scholarship Commission. The authors thank Professor Joe Brownlie and Dr Olivia Anstaett for their generous provision of BVDV for use in the experiments. RVC manuscript approval number PPS_01908.



