Introduction
The coffee berry borer (CBB), Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytidae), is a major insect pest of coffee crops worldwide (Le Pelley, Reference Le Pelley1968; Barrera, Reference Barrera1984; Waterhouse & Norris, Reference Waterhouse and Norris1989). It is a polyvoltin insect that remains most of its life inside the fruit (Baker, Reference Baker1984). Upon adult emergence, which occurs when they are about 15–16 days old (Mathieu et al., Reference Mathieu, Gaudichon, Brun and Frérot2001; da Silva et al., Reference da Silva, Moreira and Bento2014), females mate with their siblings, leaving their natal berries, and start flying to search for new berries (Baker et al., Reference Baker, Ley, Balbuena and Barrera1992; López-Guillén et al., Reference López-Guillén, Valdez-Carrasco, Cruz-López, Barrera, Malo and Rojas2011). Once CBB females find suitable berries, they begin to bore into them, making irregular galleries in the endosperm to deposit their eggs, and in which their progeny will eventually develop (Mansingh, Reference Mansingh1991; Damon, Reference Damon2000). In general, they bore one hole per berry, except in cases of artificially heavy infestations or during periods of intense infestation, where more than one female may bore into a single berry (Wrigley, Reference Wrigley1988; Vega et al., Reference Vega, Infante, Johnson, Vega and Hofstetter2015).
In wet tropical climates with two strongly marked seasons (i.e., dry season and wet season), mass emergence followed by the greatest dispersal of CBB occurs from residual infested berries on the ground before or during the harvest, and dry berries abandoned on the branches of the coffee trees after harvest (Dufour et al., Reference Dufour, González and Frérot2000). This phenomenon occurs during the inter-harvest period, after the first rains at the end of the dry season. The mass emergence of CBB from residual infested berries is due to an abrupt increase in humidity combined with high temperatures (Barrera, Reference Barrera1984; Baker et al., Reference Baker, Ley, Balbuena and Barrera1992; Giordanengo et al., Reference Giordanengo, Brun and Frérot1993; Mathieu et al., Reference Mathieu, Brun and Frérot1997; Dufour et al., Reference Dufour, González and Frérot2000; López-Guillén et al., Reference López-Guillén, Valdez-Carrasco, Cruz-López, Barrera, Malo and Rojas2011). The olfactory capacities of CBB females guide them towards suitable coffee berries, thus ensuring a food source and a breeding site (Ticheler, Reference Ticheler1961; Giordanengo et al., Reference Giordanengo, Brun and Frérot1993; Mathieu et al., Reference Mathieu, Gaudichon, Brun and Frérot2001).
During the fruiting period, from June to November, little migration movement is observed in the field and very few CBB females are caught by semiochemical-baited traps (Barrera et al., Reference Barrera, Herrera, Villacorta, García, Cruz-López, Barrera and Montoya2006), though CBB populations develop rapidly up to the harvest, and cause serious coffee crop losses. For instance, Guzmán et al. (Reference Guzmán, Castillo and López1997) reported that the infestation rate can rise from 27 to 67% in 4 months. However, it is unknown how the dispersal process occurs during infestations in the berry ripening period.
This study was carried out at this much understudied stage of the infestation process, therefore raised two main questions: (a) is walking the most frequent way of CBB displacement because evidence of flight activity is low? and (b) do CBB females move to the nearest available berries or disperse systematically to other nodes or other branches? It was expected that the results of this study might help in understanding the H. hampei dispersal process when its populations have plenty of available coffee berries, and provide a clearer picture of CBB ecology and biology, for its effective management.
Materials and methods
Study area
The study was conducted on the ‘La Alianza’ farm (15.046667N and −92.182778W, 670 m a.s.l.), Cacahoatán, Chiapas, Mexico, in the fruiting period from the end of June to the end of August 2015. The study area was characterized by an average annual temperature of 26°C and an annual rainfall of 4600 mm. A 1 ha plot planted with Coffea canephora var. robusta Pierre ex A. Froehner was selected in a plantation including other species such as Cajanus cajan (L.) Mill sp., Yucca elephantipes Baker in Regel, Musa × paradisiaca (L.), Cecropia peltata L. and Nectandra sp., as well as forest species such as Tabebuia donnell-smithii Rose and Cedrela odorata L. The coffee trees were about 9 years old and planted 5 m apart (400 plants ha−1), and were 3–4 m tall. The single flowering occurred at the end of January. No chemical pesticides were used to control insects and diseases.
Experimental design and experimental conditions
In the study area, 15 coffee trees were randomly selected. In the central part of each one, six branches with five nodes and at least four fruits per node were chosen. The selected coffee trees were bearing the first production of suitable berries, which were at the green stage with at least 20% dry content (Baker, Reference Baker1984, Reference Baker and Baker1999; Bustillo et al., Reference Bustillo, Bernal, Benavides and Chaves1998; Barrera et al., Reference Barrera, Herrera, Villacorta, García, Cruz-López, Barrera and Montoya2006; Vega et al., Reference Vega, Infante, Johnson, Vega and Hofstetter2015). The experimental design included three treatments assigned to branches infested by H. hampei and three identical treatments assigned to branches free of infestation. Thus, the treatments involved were: (1) without glue and protection + first node infested (control), (2) application of glue (Pelton Glu®, France) between nodes + first node infested, (3) application of glue between nodes and complete protection of the branch with a tulle net + first node infested, (4) without glue and protection + initially free of infestation, (5) application of glue between nodes + initially free of infestation, and (6) application of glue between nodes and complete protection of the branch with a tulle net + initially free of infestation (fig. 1). The last treatment without initial infestation and with tulle net was used as an absolute control and consequently, was not included in the analysis of dynamics. The treatments were positioned in each tree at random. On infested branches, only the first node berries infested by H. hampei were used (fig. 1). If the nodes had more than three infested berries, the excess was removed. In each coffee tree, the berries and nodes on the selected branches were marked (fig. 1). Preliminary trials showed that the glue effectively trapped CBB. The pieces of tulle surrounding the branches like sleeves were sealed at the ends of nodes one and five with rigid plastic links.
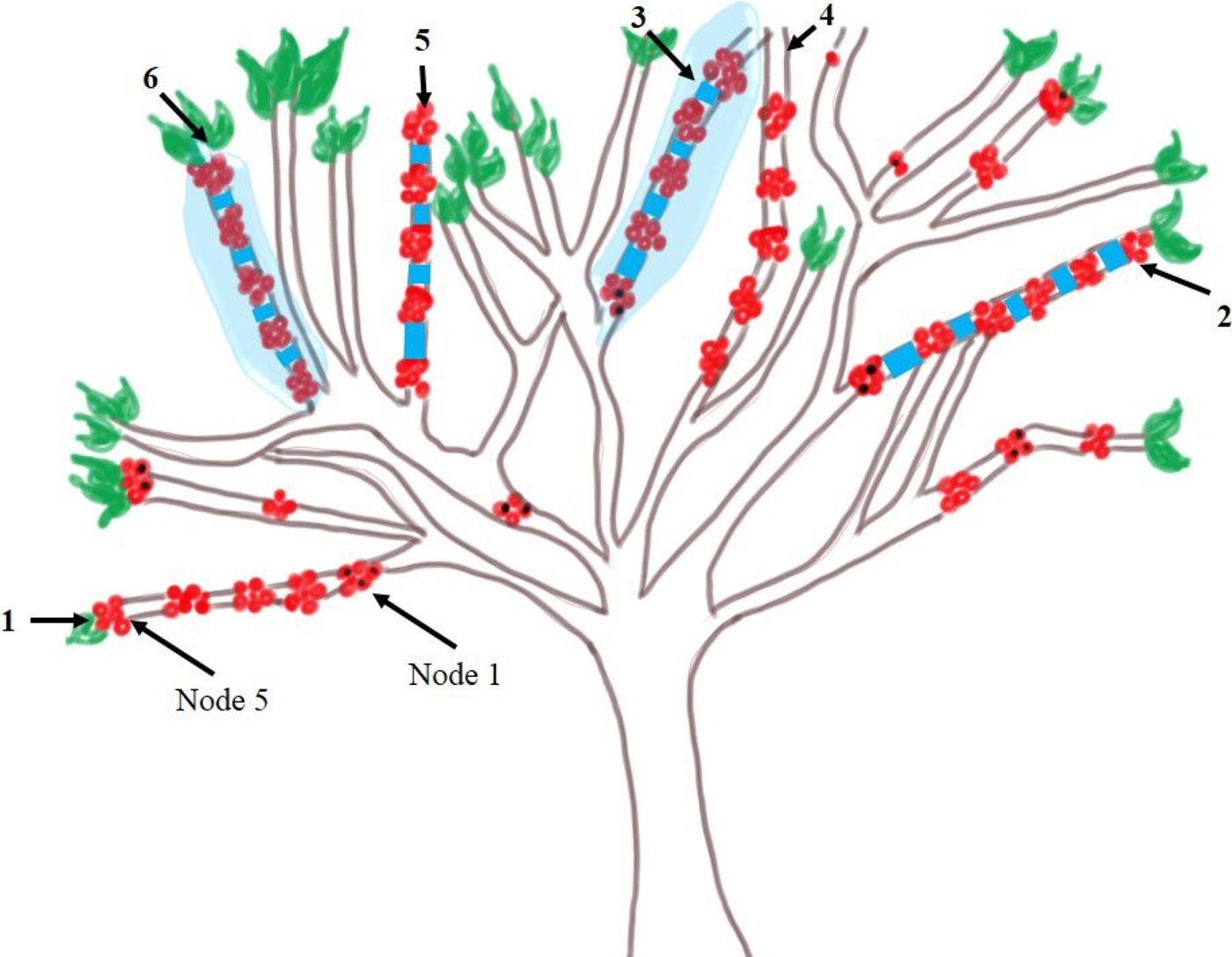
Fig. 1. Design of applied treatments. (1) Without glue and protection + first node infested (control); (2) application of glue between nodes + first node infested; (3) application of glue between nodes and complete protection of the branch with a tulle net + first node infested; (4) without glue and protection + initially free of infestation (control); (5) application of glue between nodes + initially free of infestation; (6) application of glue between nodes and complete protection of the branch with a tulle net + initially free of infestation (absolute control).
The CBB caught with glue, as well as infested berries, were recorded and removed weekly between 11 am and 2 pm over the duration of the experiment. Also, the total number of infested and un-infested berries was recorded according to the node location, at the beginning and the end of the experiment. During the experiment, the temperature ranged from 26 to 28°C and the relative humidity from 80 to 100%.
Statistical analysis
For each date and treatment, we calculated: (1) the number of infested nodes per branch (from 0 to 5), and (2) the average number of infested berries per infested node and per branch. The differences between the initial and final number of berries per branch and per treatment and the differences between infested nodes per branch and infested berries per infested node were analysed by the non-parametric Friedman test. To compare treatments, coffee trees were considered as blocks and treatments as groups. To compare initial and final dates, coffee trees per treatment were considered as blocks and date as groups. The curves of the trajectories (infestation as a function of time) which reflected the number of infested nodes per branch and the number of infested berries per infested node were classed. While the development of infestation was more important than the time when it occurred, we chose a method of clustering based on the shape-respecting ‘generalized distances of Fréchet’ between trajectories rather than on classic distances. We used the kmIShape method based on k-means (Genolini et al., Reference Genolini, Ecochard, Benghezal, Driss, Andrieu and Subtil2016) with a timescale parameter of 0.1 giving five times more importance to shape than to the number of nodes infested. We obtained a partitioning where each class contained branches whose trajectories had similar shapes whatever the time lag between them. The classification was repeated 100 times with the random choice of initial cluster centres, and within this framework, the partition most frequently met was selected. The independence of classes for the number of berries in the branch was analysed with the Kruskal–Wallis non-parametric test. The description of the composition of the classes, and the mean trajectories led to a hypothesis about the development of the infestations over time: influence of tulle net, influence of initial state (infested or not) and influence of the glue. The hypothesis was tested using the Friedman test. All analyses were done using R version 2.5 (R Core Team, 2016) and R ‘Agricolae’ package (de Mendiburu, Reference de Mendiburu2016). The classification method was implemented using the R ‘kmIShape’ package (Genolini, Reference Genolini2016).
Results
Average values of infestation-free berries, infested berries and nodes per branch and treatment, at the beginning and the end of the experiment
At the beginning, there were 15.4 berries per node, on average. The number of berries per branch was not significantly different between treatments at the beginning and at the end of the experiment (table 1). The treatments varied from 77 to 74 berries per branch, on average, between weeks 1 and 10 (table 1). The number of berries was not significantly different between the beginning and the end of the experiment (Friedman, P = 0.06). Regarding the level of infestation, the number of infested berries per branch varied from 0 to 1.5 at the beginning and was not significantly different between treatments with the initial infestation (table 1). The number of infested berries then increased significantly up to the end of the trial (P < 0.01), with the exception of the treatment with tulle net free of initial infestation. No infestation was observed in this treatment used as an absolute control, and consequently, it was not included in the analysis of dynamics.
Table 1. Average values of infestation-free berries, infested berries and nodes per branch and treatment, at the beginning and the end of the experiment.

* NS = not significant at 5%; means with the same letter are not significantly different.
(number) data with the same numbers were analysed in the same test.
At the end of the experiment, the number of infested berries, the final number of infested nodes and the average percentage of infested berries were higher in the Net-Glue-Infested treatment than in the treatments initially free of infestation (table 1). We did not find any females trapped in the inter-node spaces of branches previously coated with glue (i.e., Glue-Infested, Glue-Initially free of infestation and Net-Glue-Infested treatments). The percentage of infested berries on Glue-Infested and Glue-Initially free of infestation treatments was not significantly different at the end of the experiment. In addition, it is important to note that the infestation rates were from 1.4 to 7.6% at the end of the experiment, which were considered moderate because they were located at the start of new fruiting development.
Mean trajectories for classes obtained by the kmIShape method for infested nodes per branch over 9 weeks with individual branch trajectories
The curves of the trajectories that reflected the number of infested nodes per branch enabled four classes to be defined (fig. 2). This classification was achieved with 35% of the replications. The first class (red) had almost flat trajectories, containing branches with few infestations, principally Control-Initially free of infestation and Glue-Initially free of infestation branches, initially free of CBB infestations (14/21), along with some branches of the Glue-Infested treatment (4/21). The second class (green) contained the branches whose number of infested nodes increased rapidly in two growth steps around weeks 3 and 6. Half of the Net-Glue-Infested treatment was in this class (8/15). The third class (dark blue) had a similar shape to class two (green) with two steps of node infestation but reaching a lower level of infestation. This class included the Control-Infested, Glue-Infested (12/18) and Control-Initially free of infestation treatments (5/18). For the second and third classes, a decrease was observed after each increase. The fourth class (light blue) presented only one peak that included some branches of all the treatments.
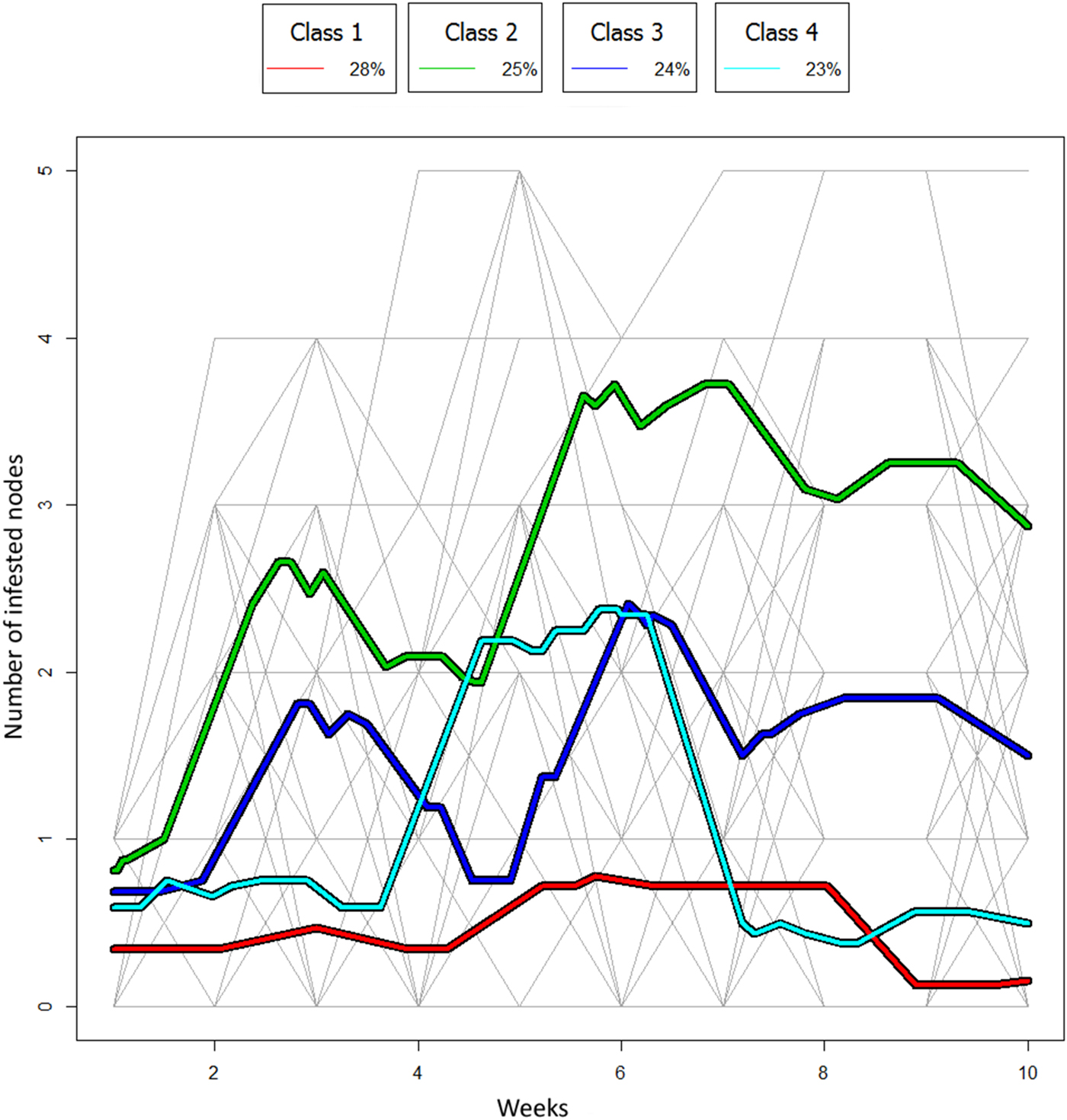
Fig. 2. Mean trajectories for classes obtained by the kmIShape method for infested nodes per branch over 9 weeks with individual branch trajectories. Trajectories are grouped giving more importance to shape than to the number of nodes infested on the same date. For each class, the number of branches in each class is given as a percentage of total branches.
Mean trajectories for classes obtained by the kmIShape method for infested berries per infested node per branch over 9 weeks with individual branch trajectories
The trajectories per branch of the infested berries per infested node were grouped into three classes (fig. 3). This classification was achieved with 18% of the replications. The first class (green) had a mean trajectory that was almost flat, grouping the branches for which the number of infested berries per infested node grew slowly, including the Control-Initially free of infestation and Glue-Initially free of infestation (20/29) treatments. The second class (dark blue) represented trajectories with a peak, which clustered the branches for which the number of infested berries per infested node grew rapidly, including a majority of infested treatments (12/16). The third class (red) had an intermediate trajectory that grouped the branches for which the number of infested berries per infested node increased before weeks 3 and 6 and then stabilized. These branches belonged to the Control-Infested, Glue-Infested and Net-Glue-Infested treatments (24/30).
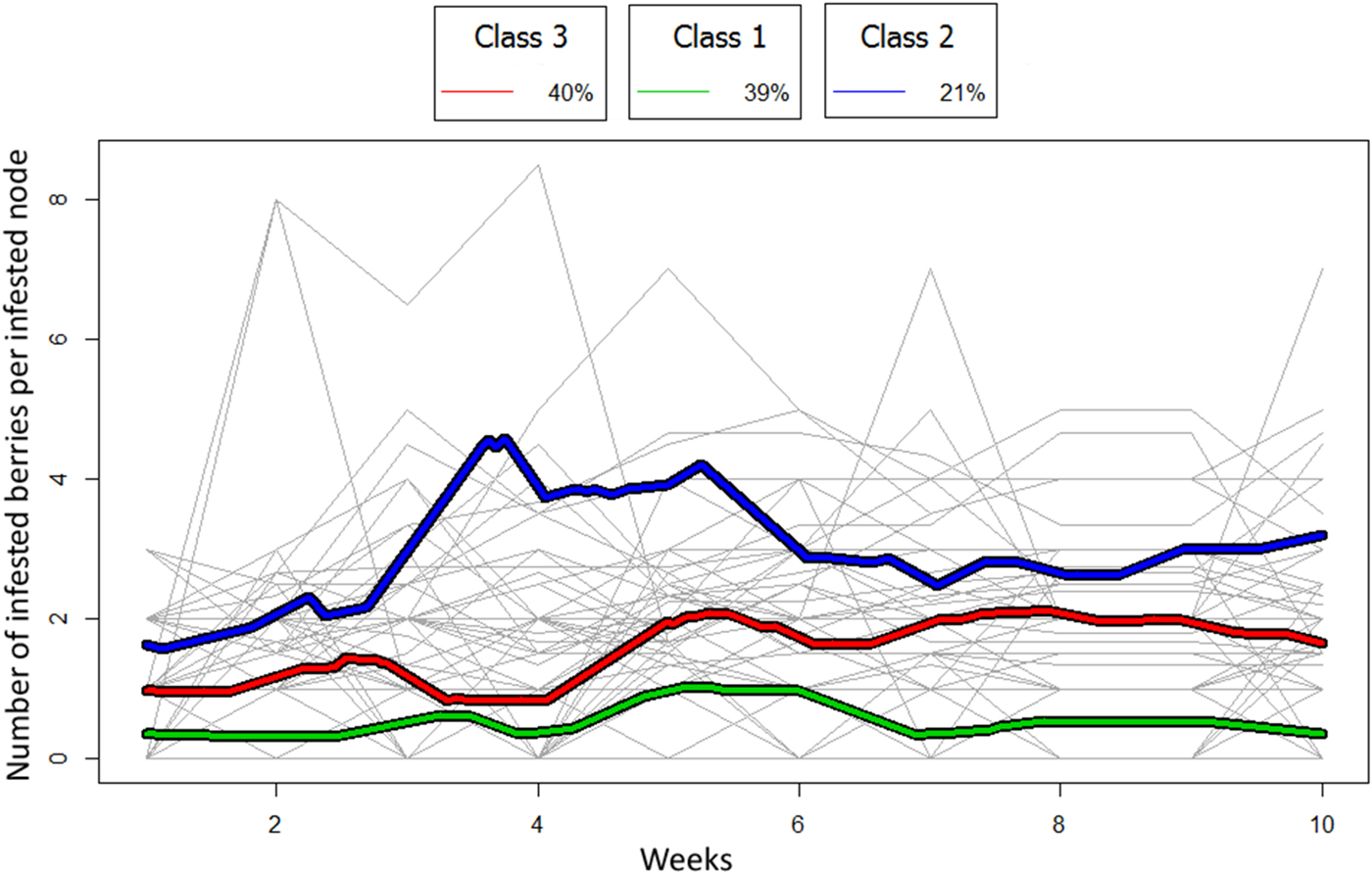
Fig. 3. Mean trajectories for classes obtained by the kmIShape method for infested berries per node per branch over 9 weeks with individual branch trajectories. Trajectories are grouped giving more importance to shape than to the number of nodes infested on the same date. The number of branches in each class is given as a percentage of total branches.
Differences between classes 1 and classes 2 and 3 for nodes and berries suggested that the initial state (infested or not) could have a great influence on nodes and berries infestation. In the same way, differences between classes 2 and 3 for nodes suggested that the tulle net could have a great influence on nodes infestation. Furthermore, treatments with glue or control were often grouped in the same class (classes 1 and 3 for nodes and all classes for berries). This assumed that glue did not have any influence on berries or nodes infestation.
Average values for infested nodes per branch and infested berries per infested node on each date
Differences existed in infestation dynamics between treatments. Graphic representation of the classes of infestation dynamics for nodes and berries (table 2)
Table 2. Average values for infested nodes per branch and infested berries per infested node on each date and significance of the Friedman test for the three hypotheses built from the classification of infestation dynamics.

* NS = not significant at level P = 0.05; S, significant.
† On this date, there was high variability between branches of the same treatment due to the small number of infested nodes.
The results of table 2 indicated that (1) the number of infested nodes on protected branches (Net-Glue-Infested) was significantly higher than the number of nodes on unprotected branches (Glue-Infested) in weeks 5 and 6. However, the number of infested berries per node was not different in both treatments; (2) the number of infested nodes was significantly larger on infested branches than on branches free of Infestation up to the 8th week. However, the number of infested berries per infested node was not significantly different with or without initial infestation: (3) the final number of infested berries per node was not different on glue or control type branches except on one date for node infestation; and (4) the final number of infested berries per node was not correlated with the initial number of berries (R 2 = −0.09). The initial number of berries per branch was not related to the classes of infestation dynamics for nodes (χ2 = 3.0; df = 3; P = 0.4) or berries (χ 2 = 2.9; df = 2; P = 0.2).
Discussion
In this study, infestation dynamics were studied for 9 weeks of the fruiting period on a branch scale on several coffee trees with suitable berries for CBB and some already infested by H. hampei. Under these conditions, we demonstrated that the number of berries was uniform on the branches for all the treatments and that the number of infested berries per branch increased over time, except on branches of the absolute control that were initially free of infestation and protected with a tulle net (table 1). If the rate of infestation appeared moderate at the end of the study, it is because the infestation of new berries by H. hampei was beginning (table 1). As is already known, the levels of CBB infestation can continue to rise until the harvest (Guzmán et al., Reference Guzmán, Castillo and López1997).
Several combined classes were defined for CBB infestation dynamics: classes for infested nodes per branch and classes for infested berries per infested node. The first classes showed one or two expansion phases or peaks separated by approximately 4 weeks (fig. 2). The top of the two peaks could be interpreted as the beginning of two periods of abundant fall of infested berries (Borbón-Martínez, Reference Borbón-Martínez1989; Teixeira et al., Reference Teixeira, de Souza and Costa2006; Contreras & Camilo, Reference Contreras, Camilo, Barrera, García, Domínguez and Luna2007). One class presented a clear expansion phase. It grouped some of the initially infested branches. By contrast, the dynamics of berry infestation per node was not so clear. Only one class presented a distinct expansion phase. It grouped some of the initially infested branches. The average period of the peak was between weeks 3 and 4. Although the number of berries per node was high (15.4, on average), the final number of infested berries per infested node was low (2.2, on average). This level could be explained by the falling of many infested berries during the expansion phase (fig. 3) (Teixeira et al., Reference Teixeira, de Souza and Costa2006). It should also be noted that berry fall might explain the decrease in node infestation after the peaks (fig. 2). The fall was due to the physiological weakening of berries under the effect of more or less deep perforations (Borbón-Martínez, Reference Borbón-Martínez1989). Consequently, such berries which will eventually deteriorate on the ground are part of the crop losses associated with H. hampei infestations. We know that the emergence of females leads to a reinforcing of infestations on healthy berries during ripening and to an increase in the infestation level up to the harvest (Teixeira et al., Reference Teixeira, de Souza and Costa2006). This study of infestation dynamics at the branch level during the fruiting season was totally different from what is observed in the inter-harvest period when females emerge from the residual berries searching for scarce news berries, which leads to further dispersal (Mathieu et al., Reference Mathieu, Brun and Frérot1997; Dufour et al., Reference Dufour, González and Frérot2000; Dufour & Frérot, Reference Dufour and Frérot2008). Indeed, during the fruiting period up to the harvest, step-by-step dispersal is facilitated by the abundance of suitable berries on the branches.
In this study, we examined the displacement mode associated with colonization dynamics. Walking is an activity that CBB performs naturally; for instance, this behaviour is observed in CBB breeding installations (Mathieu et al., Reference Mathieu, Brun and Frérot1997), when recently emerged females try to get out, or in olfactometers where females move towards the attractant source (Giordanengo et al., Reference Giordanengo, Brun and Frérot1993). It is, therefore, possible that a CBB female could walk from one node to another. However, no CBB was trapped on the glue-coated branches protected with tulle net and glue-coated branches exposed to colonizing females from different origins, while numerous nodes were infested over time (table 2). This suggests an absence of displacement by walking. In addition, we showed that the presence or absence of glue did not modify the result of the node infestation process. Differences were only significant on one date (table 2). Thus, the absence of displacement by walking was not the consequence of a repulsive effect of the glue on the CBB, which confirms the safety of glue and the predisposition of H. hampei to use flight as a mechanism of dispersing from one node to another. Therefore, in the fruiting season, CBB does not colonize by walking but by flying, although it is quite capable of moving in that way. The origin of this behaviour was uncertain at the time when coffee trees were not cultivated, and plants grew naturally with low productivity (Tisserant, Reference Tisserant1929). It is possible that the colonization of more or less isolated nodes made CBB displacement by flight compulsory.
The average number of infested nodes per branch and infested berries per infested node, with or without tulle net protection, showed no significant difference between Glue-Infested and Net-Glue-Infested treatments, from weeks 1 to 4 (table 2). A difference appeared in weeks 5 and 6 for nodes. Despite the opportunity for CBB to fly out of the Glue-Infested branches or to colonize them, the infestation level remained similar to that with a net for the first 4 weeks. So, in the first 4 weeks for Control-Infested and Glue-Infested treatments, it seemed that H. hampei mainly dispersed to colonize nodes and berries on the same branch (table 2). CBB stayed close to the berry from which it emerged or to the nodes in the immediate vicinity. After that, there was a phase of dispersal away from the branch. For instance, in weeks 5 and 6, the nodes of Net-Glue-Infested branches were more infested than Glue-Infested branches (table 2), because the tulle protection prevented CBB from flying away from the branch. On that date, only a small share of berries and nodes were infested, suggesting that wider dispersal of CBB can occur despite fruit availability nearby. A previous study showed that there is a correlation between the percentage of infested berries and the total number of berries at a given time (Ticheler, Reference Ticheler1961).
The results showed that the number of infested nodes per branch without tulle net protection (control and glue) was significantly larger up to the 9th week when the first node was previously infested (table 2). This means that early infestation was conducive to node infestation dynamics. By contrast, the number of infested berries per infested branch did not depend on the initial status (table 2), but it seemed to be subject to strong fluctuations caused by infested berry fall.
Several studies on the dynamics of CBB populations in the tropics have shown how groups of colonizing females emerge and fly from the residual coffee berries during the inter-harvest period. They can produce substantial migration peaks before dispersing around the place of emergence up to several metres or tens of metres away (Leplae, Reference Leplae1928; Dufour et al., Reference Dufour, Barrera, Decazy, Bertrand and Rapidel1999, Reference Dufour, González and Frérot2000; Pereira et al., Reference Pereira, Vilela, Tinoco, de Lima, Fantine, Morais and França2011). On the other hand, we observed that in the fruiting period, the emergence of females was prolonged over time that migrations were of small amplitude and that dispersal took place over short distances within each coffee tree. We demonstrated that CBB does not walk but flies, even over short distances and that dispersal occurs in two steps at the beginning of the fruiting season: (1) intra-branch dispersal during which CBB colonize nodes and berries step by step, (2) inter-branch dispersal which extends the operational range within the coffee trees.
The increase in coffee berry production during the fruiting season elicits more volatile compounds emission that causes a strong attraction and directs CBB to the berries. Thus, there is a real problem for CBB control, especially for trapping with semiochemicals, whose attractiveness seems insufficient to divert CBB from its natural host. This explains why trapping has not been effective at this period and has not been performed under these climatic conditions. However, our results prompt us to explore new strategies for CBB control during fruiting. Lastly, it is possible to study the influence of coffee architecture, densities and planting systems on infestation dynamics in order to identify new integrated protection strategies against CBB. Consequently, in an environment conducive to the development of Beauveria bassiana that entomopathogen should be used during intra-branch colonization to limit the expansion of the pest. In addition, the attractant potential of traps should also be improved and the trapping system should be optimized in order to attract CBB more efficiently during inter-branch colonization.
Acknowledgements
We would like to thank Enrique López of El Colegio de la Frontera Sur (ECOSUR) for his technical contribution. We also thank Peter Biggins for his valuable help in revising the English of this manuscript.








