Lactic Acid Bacteria (LAB) include a vast group of microorganisms, sharing several morphological, metabolic and physiological characteristics. LAB are Gram positive, non-spore forming, anaerobic, air and acid tolerant, fastidious with a strictly fermentative metabolism, and present lactic acid as the main final product of carbohydrate fermentation (Salminen et al. Reference Salminen, Von Wright and Ouwehand2004).
Several LAB species are used as starter cultures in the production of fermented milk and meats. During the manufacture of fermented milks, LAB starter cultures are added to heat-treated milk, followed by incubation in specific conditions to promote coagulation and characteristic flavour (Tamine & Robinson, Reference Tamine and Robinson2007). To control this transformation properly, the added LAB populations must be monitored during and after the fermentation process.
LAB enumeration in dairy products typically uses de Mann-Rogosa-Sharpe (MRS) agar incubated at anaerobic or microaerophilic conditions (Wehr & Frank, Reference Wehr and Frank2004). However, these specific conditions are not sufficiently selective for LAB enumeration (Kang & Fung, Reference Kang and Fung1998; Kim et al. Reference Kim, Fung and Kang2001; Giraffa, Reference Giraffa2004), which has led to modifications in the methodology, such as reducing pH and adding selective agents such as bile salts, sodium propionate, lithium chloride, cistein, gentamicin and dicloxacilin (Lapierre et al. Reference Lapierre, Undeland and Cox1992; Lim et al. Reference Lim, Huh and Baek1995; Ingham, Reference Ingham1999; Vinderola & Reinheimer, Reference Vinderola and Reinheimer1999). These substances inhibit non-LAB microorganisms, and even specific LAB genera or species. Specifically for this purpose, other culture media can also be used, such as M17 for Streptococcus (Tabasco et al. Reference Tabasco, Paarup, Janer, Peláez and Requena2007) and LC for Lactobacillus casei (Shah, Reference Shah2000).
Petrifilm™ system (3M Microbiology, St. Paul, MN, USA) has often been used as an alternative method for monitoring the microbiological quality of foods (Beuchat et al. Reference Beuchat, Copeland and Curiale1998; Ferrati et al. Reference Ferrati, Tavolaro, Destro, Landgraf and Franco2005; Tavolaro et al. Reference Tavolaro, Ferrati, Destro, Landgraf and Franco2005). Compared with conventional plating, the main advantages of Petrifilm™ are the simplicity of execution, shorter time for final results and fewer steps for culture media and glassware preparation. Petrifilm™ Aerobic Count (AC) is initially recommended for enumeration of mesophilic aerobes, but can also be used for LAB enumeration when associated with MRS both followed by incubation in anaerobic or microaerophilic conditions (Champagne et al. Reference Champagne, Gardner, Piette and St-Gelais1994; McGregor et al. Reference Mcgregor, Traylor, Gough, Hazlett and Bird1995; Nero et al. Reference Nero, Beloti, Barros, Ortolani, Tamanini and Franco2006; Ortolani et al. Reference Ortolani, Viçosa, Beloti and Nero2007).
This study aimed to evaluate the efficiency of Petrifilm™ AC plates associated with acidified MRS and M17 broths in specific conditions of incubation, as an alternative method for enumerating LAB starter cultures (Streptococcus salivarius subsp. thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) for yogurt production.
Material and Methods
Starter cultures
Strep. salivarius subsp. thermophilus (NCDO 1968) and Lb. delbrueckii subsp. bulgaricus (ATCC 11842) was stored at 4°C in MRS (Oxoid Ltd., Basingstoke, Hampshire, England) agar slants. At the moment of use, the cultures were streaked on MRS agar plates and incubated at 35°C for 24 h, when an isolated colony was transferred to MRS broth (Oxoid) and incubated at 35°C for 24 h. The obtained culture was then diluted in MRS broth to achieve turbidity similar to tube 1 from the MacFarland scale, which corresponds to approximately 3×108 CFU/ml, used as starter culture for yogurt production.
Lyophilized commercial culture for yogurt production (Danisco A/S, Copenhagen, Denmark) was aseptically added to 500 ml of nonfat milk (after heat treatment at 90°C for 10 mins and cooling), poured in sterile tubes and maintained at −20°C. At the moment of use, 1 ml aliquots were used for yogurt preparation.
Yogurt production
Nonfat pasteurized milk was submitted to heat treatment (90°C for 5 mins) cooled and 1000 ml aliquots were distributed in sterile flasks, completing 30 repetitions. Next, 20 repetitions were inoculated with the reference cultures of Strep. thermophilus and Lb. bulgaricus at the final concentration of approximately 106 CFU/ml. The remaining 10 repetitions were inoculated with the commercial starter culture previously prepared. All repetitions were submitted to the fermentation process at 42°C for 4 h.
Samples collection and microbiological analysis
Immediately after cultures inoculation and after the fermentation process, aliquots of each repetition were aseptically collected in sterile conditions and submitted to ten-fold serial dilution using MRS (pH adjusted to 5·4) and M17 (BD – Becton, Dickinson and Company, Franklin Lakes, NJ, USA) broths for microbiological analysis.
From each sample, two dilutions from each broth were selected and plated in Petrifilm™ AC (1 plate for dilution). The same selected dilutions in MRS broth were also pour plated in MRS agar (pH 5·4), and the same selected dilutions in M17 broth were pour plated in M17 agar, both in duplicate as recommended by the conventional plating procedure (Wehr & Frank, Reference Wehr and Frank2004). All MRS plates were incubated at 35°C for 48 h under anaerobiosis (Anaerobac, Probac do Brasil, São Paulo, SP, Brazil) and all M17 plates were incubated at 42°C for 48 h. After incubation, the formed colonies were enumerated and the results were expressed as CFU/ml or CFU/g. The described conventional plating methodology is recommended by the International Standardization Organization and International Dairy Federation for starter culture enumeration in yogurt (ISO, 2003).
Morphological characterization of colonies from MRS (pH 5·4) and M17 plates
The selectivity of MRS (pH 5·4) and M17 was verified by morphological study of the colonies formed in the plates after incubation conditions and counting. Twenty-six samples prepared with reference and commercial starter cultures were randomly selected during the analysis and 10% of the formed colonies in each plate (Petrifilm™ AC and MRS and M17 Petri dishes) were classified according to Gram staining in cocci (Strep. thermophilus) or rods (Lb. bulgaricus).
Data analysis
The obtained counts were converted in log10 and compared by correlation analysis. The mean values obtained from each culture media and methodology were also calculated and compared by Analysis of Variance to verify significant differences (P<0·05). Finally, the frequencies of cocci and rods were calculated considering each culture media and plating procedure. All analyses were conducted using the software Statistica 7.0 (StatSoft Inc., Tulsa, OK, USA).
Results and Discussion
As seen in Table 1, Petrifilm™ AC plates presented high correlation indices with the conventional plating procedure when all samples were analysed as one group (milk after inoculation and after fermentation), independent of the type of added starter culture (reference or commercial) or culture media (MRS at pH 5·4 or M17). The distribution of these results, considering distinct periods of fermentation and culture media, are presented in Fig. 1, and a good performance of Petrifilm™ AC plates can be observed associated with M17 just after the inoculation, and associated with MRS (pH 5·4) after fermentation. Despite some inefficiency observed at specific conditions (M17 after fermentation and MRS pH 5·4 just after inoculation), no significant differences were observed between the mean counts from each culture media obtained from each methodology, starter culture and period of fermentation (Table 2), suggesting equivalency between Petrifilm™ AC and conventional plating.

Fig. 1. Dispersion of microbiological counts obtained from MRS (pH 5·4) and M17 by conventional plating and Petrifilm™ AC of nonfat milk inoculated with starter cultures (reference and commercial) (A and B) and after fermentation at 42°C for 4 h (C and D). In each graph: n: number of repetitions, r: correlation, r2: coefficient of determination, p: level of significance, a: slope, b: intercept, mv: mean variance.
Table 1. Statistical parameters of correlation between microbiological counts obtained for MRS (pH 5·4) and M17 by conventional plating and Petrifilm™ AC of nonfat milk inoculated with starter cultures (reference and commercial) and submitted to fermentation at 42°C for 4 h

n: number of repetitions, r: correlation, r2: coefficient of determination, p: level of significance, a: slope, b: intercept, mv: mean variance
Table 2. Mean values (±standard device) of microbiological counts obtained from MRS (pH 5·4) and M17 by conventional plating and Petrifilm™ AC plates of nonfat milk inoculated with LAB starter cultures and submitted to fermentation at 42°C for 4 hours
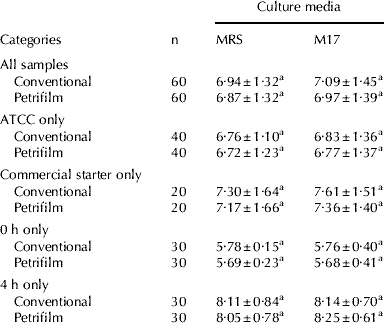
Obs. Mean values with same uppercase letters are not significantly different in each column in each category (P<0·05)
Considering the obtained results, Petrifilm™ AC plates presented appropriate performance for LAB enumeration in the samples, as previously observed in other studies (Champagne et al. Reference Champagne, Gardner, Piette and St-Gelais1994; McGregor et al. Reference Mcgregor, Traylor, Gough, Hazlett and Bird1995; Nero et al. Reference Nero, Beloti, Barros, Ortolani, Tamanini and Franco2006; Ortolani et al. Reference Ortolani, Viçosa, Beloti and Nero2007). However, in specific situations, this performance was clearly hindered by the low capacity for reducing 2,3,5-triphenyltetrazolium chlorine (TTC) by the LAB starter cultures. TTC is the growth indicator used in Petrifilm™ AC plates, providing a red color in the formed colonies when reduced (Dawkins et al. Reference Dawkins, Hollingsworth and Hamilton2005). Beloti et al. (Reference Beloti, Barros, Freitas, Nero, Souza, Santana and Franco1999) verified a high frequency of non-reducing TCC microorganisms in pasteurized milk classified mainly as Gram positive cocci and rods, characteristics directly associated with the cultures used in this study. The low capability of reducing TTC by Streptococcus and Lactobacillus has been previously observed by Nero et al. (Reference Nero, Beloti, Barros, Ortolani, Tamanini and Franco2006) and Ortolani et al. (Reference Ortolani, Viçosa, Beloti and Nero2007), suggesting some difficulty in properly identifying the colonies from these genera in Petrifilm™ AC plates, especially in specific stress situations.
The acidity of certain foods has also been indicated as a possible cause for poorer performance of the Petrifilm™ system compared with conventional plating procedures. Ferrati et al. (Reference Ferrati, Tavolaro, Destro, Landgraf and Franco2005) observed that the low pH of certain foods can interfere with the development of certain microorganisms in Petrifilm™ AC plates, affecting their efficiency in the enumeration of hygiene indicator microorganisms. The results obtained in the present study may indicate that the acidity interfered with the performance of Petrifilm™ AC plates in two specific situations: directly in the Petrifilm™ AC plates, when associated with MRS broth (pH 5·4) as previously observed by Ortolani et al. (Reference Ortolani, Viçosa, Beloti and Nero2007), and indirectly in yogurt production, when after 4 h fermentation, the acid production may have stressed the added LAB cultures, as suggested by Caplice & Fitzgerald (Reference Caplice and Fitzgerald1999). The direct interference of acidity on Petrifilm™ AC plates can be observed when MRS at pH 5·4 is employed for LAB enumeration just after the inoculation in milk repetitions, showing low correlation (r=0·35, Fig. 1A). However, after the fermentation process, the results suggest a possible adaption of LAB cultures to the low pH of the yogurt, resulting in the excellent performance of Petrifilm™ AC associated with MRS (pH 5·4) for LAB enumeration (Fig. 1C). Considering M17, which is not acidified, the performance of Petrifilm™ AC plates improved just after inoculating LAB cultures (Fig. 1B). After fermentation, the acidity of the yogurt may have stressed LAB cultures, hindering the appropriate reduction of TTC in Petrifilm™ AC plates and explaining the low correlation indices obtained in this situation (Fig. 1D).
Considering the methodology described by ISO 9232 (ISO, 2003), the counts obtained from MRS (pH 5·4) plates correspond to Lb. delbrueckii, and from M17 plates correspond to Strep. thermophilus. According to Charteris et al. (Reference Charteris, Kelly, Morelli and Collins1997), this protocol is selective enough to properly enumerate these two LAB cultures in yogurt. The obtained frequencies of cocci and rods observed in each culture media, before (h 0) and after (h 4) fermentation of the repetitions are presented in Table 3. Considering these findings, it can be concluded that the low pH of MRS and the anaerobic environment were not sufficiently selective for Lb. delbrueckii, neither in conventional plating nor Petrifilm™ AC plates. Despite the absence of selectivity in MRS (pH 5·4), Lb. delbrueckii predominate just after the inoculation, and then were reduced after 4 h of fermentation. The low selectivity of MRS has been described as a limiting factor in the enumeration of LAB genera and species, even when adding inhibitory substances such as acids (Kang & Fung, Reference Kang and Fung1998; Kim et al. Reference Kim, Fung and Kang2001). However, M17 presented adequate selectivity in both enumerating systems for Strep. thermophilus, with predominance of cocci just after the inoculation and fermentation process. The incubation temperature of the plates (42°C) was the probable cause for this selectivity, since Strep. thermophilus are known to be tolerant of higher temperatures (Delorme, Reference Delorme2008).
Table 3. Morphological characteristics of colonies randomly selected from MRS (pH 5·4) and M17 plated by conventional procedure and Petrifilm™ AC with nonfat milk inoculated with LAB starter cultures and submitted to fermentation at 42°C for 4 hours
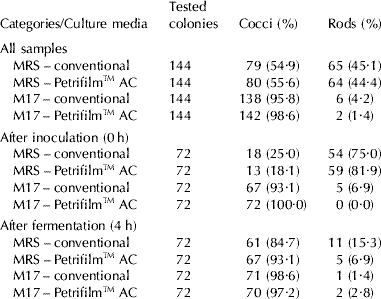
Our results indicate that MRS and M17 broths can be associated with Petrifilm™ AC plates to enumerate LAB starter cultures in yogurt production. However, in specific situations the Petrifilm™ system differed from conventional plating, primarily due to the adjusted pH of MRS broth and the final acidity of yogurt production. The standardized protocol for enumeration of Lb. delbrueckii in yogurt by both tested methodologies (MRS pH 5·4) was not sufficiently selective, whereas the enumeration of Strep. thermophilus was adequate using M17 at 42°C.
Luís A Nero acknowledges the support of FAPEMIG (PPM – CVZ APQ-5946-5.04/07).






