Introduction
The colorful and intricately patterned shells of cone snails (Fig. 1), which comprise the neogastropod family Conidae, have attracted the interest of naturalists and collectors for centuries. Besides having shells that are beautiful natural objects, cone snails are highly specialized venomous predators of worms, mollusks, or fish (e.g., Kohn, Reference Kohn1956; Duda et al., Reference Duda, Kohn and Palumbi2001; Olivera et al., Reference Olivera, Showers Corneli, Watkins and Fedosov2014), and are ecologically important in tropical and subtropical marine habitats (e.g., Kohn 1959, Reference Kohn2001). The complex venoms (conopeptides) that they use to paralyze their prey are noteworthy for sometimes being dangerous to people (see Kohn, Reference Kohn2016), though they also hold significant pharmacological potential for treating varied human ailments (e.g., Vetter and Lewis, Reference Vetter and Lewis2012; Gorson and Holford, Reference Gorson and Holford2016). Finally, with nearly 900 extant species (see below), cone snails are remarkably diverse and their relationships to each other are becoming better understood. Based on the branching topology of a new molecular phylogenetic hypothesis for cone snails (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014), Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) divided extant members of the family into four genera: (1) the basal genus Profundiconus Kuroda, Reference Kuroda1956, represented by 28 species (Marshall and Bouchet, Reference Marshall and Bouchet2016); (2) Californiconus Tucker and Tenorio, Reference Tucker and Tenorio2009, represented by one living eastern Pacific species (Bouchet, Reference Bouchet2011); (3) Conasprella Thiele, Reference Thiele1929, represented by 113 species (Marshall and Bouchet, Reference Marshall and Bouchet2017); and (4) the hyperdiverse genus Conus Linnaeus, Reference Linnaeus1758, represented by 755 species (Bouchet and Gofas, Reference Bouchet and Gofas2015). Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) further divided Conasprella and Conus into subgenera corresponding with individual subclades; many of these subgenera correspond with genus-level rankings proposed earlier by Tucker and Tenorio (Reference Tucker and Tenorio2009). Uribe et al. (Reference Uribe, Puillandre and Zardoya2017) very recently published a phylogenetic analysis of mitochondrial genomic data and recognized two additional genus-level clades within the Conidae: Lilliconus Raybaudi Massilia, Reference Raybaudi Massilia1994 and Pseudolilliconus Tucker and Tenorio, Reference Tucker and Tenorio2009.

Figure 1 A fossil specimen (UF 271043) of Conus spurius Gmelin from the Gatun Formation of Panama shown under (1) regular light, (2), ultraviolet light, and (3) as a inverse UV image, causing the brightly fluorescing regions in (2) to become darkened, as they would have appeared in life. Scale=1 cm.
Cone snails have a fossil record that extends back to the early Eocene (Kohn, Reference Kohn1990; Röckel et al., Reference Röckel, Korn and Kohn1995; Hendricks and Portell, Reference Hendricks and Portell2008), and their shells are common constituents of many Miocene, Pliocene, and Pleistocene marine fossil deposits, especially in tropical America. Several of these cone snail faunas have received recent attention by paleontologists, including those of the Miocene (Landau et al., Reference Landau, da Silva, Heitz and Janssen2016) and early Pliocene (Landau and da Silva, Reference Landau and da Silva2010) of Venezuela, the late Miocene and early Pliocene of the Dominican Republic (Hendricks, Reference Hendricks2015), and the Plio-Pleistocene of the southeastern United States (Hendricks, Reference Hendricks2009; Petuch et al., Reference Petuch, Drolshagen and Herndl2015). An exception is the cone snail fauna of the late Miocene Gatun Formation of Panama, which was last intensively studied by Woodring (Reference Woodring1970) (supplemental information about the fauna was later provided by Pitt and Pitt [Reference Pitt and Pitt1993]). This richly fossiliferous, siliciclastic unit, which is exposed on the Caribbean coast of Panama in the province of Colón, has attracted the attention of paleontologists for over a century (e.g., Toula, 1909, Reference Toula1911; Brown and Pilsbry, Reference Brown and Pilsbry1911; Cossmann, Reference Cossmann1913; Vaughan, Reference Vaughan1919; Olsson, Reference Olsson1922; Woodring, 1957, 1959, 1964, 1970, 1973, Reference Woodring1982; Hendy, Reference Hendy2013; Pimiento et al., Reference Pimiento, González-Barba, Ehret, Hendy, MacFadden and Jaramillo2013; Anderson et al., Reference Anderson, Hendy, Johnson and Allmon2017). Nearly 400 species of mollusks have been reported from the Gatun Formation (Paleobiology Database, accessed July 12, 2016) and modern work on the fauna continues to reveal additional diversity (e.g., Landau et al., Reference Landau, Petit and da Silva2012). As noted by Pimiento et al. (Reference Pimiento, González-Barba, Ehret, Hendy, MacFadden and Jaramillo2013), exposures of the Gatun Formation tend to depend upon recent construction activity because urban development and fast-growing vegetation cause many collecting localities to be short lived.
Building on earlier work by Coates (Reference Coates1999), Hendy (Reference Hendy2013) provided a detailed overview of the stratigraphy and paleoenvironmental context of the Gatun Formation (also see Pimiento et al., Reference Pimiento, González-Barba, Ehret, Hendy, MacFadden and Jaramillo2013; Anderson et al., Reference Anderson, Hendy, Johnson and Allmon2017). The total thickness of the Gatun Formation, which is divided into a lower, middle, and upper unit (see Woodring, Reference Woodring1957), is likely >600 m and accumulated over a four-million-year interval between ca. 12–8 Ma (Hendy, Reference Hendy2013). Based on quantitative paleoecological analyses of molluscan assemblages, Hendy (Reference Hendy2013, p. 223) found that “different parts of the formation accumulated in a range of depositional environments, despite only subtle variations in their sedimentology” and that these marine environments ranged in depth from 0–100 m. This contrasts somewhat with earlier estimates that suggested that the Gatun Formation was not formed in depths exceeding ~50 m; for example, Collins (1999) used benthic foraminifera to estimate that the lower, middle, and upper Gatun Formation were each deposited at a depth of ~25 m.
Twenty-five species-group names (species and subspecies, including conferred records) have been applied to fossil Conidae from the Gatun Formation of the Panama Canal Zone (Table 1). Toula (Reference Toula1909) published the first report of a cone snail fossil from the Gatun Formation (not identified to species); this was followed two years later by his descriptions of three taxa (Toula, Reference Toula1911). Cossmann (Reference Cossmann1913) reported four species from the Gatun Formation, one of which was described as new. Brown and Pilsbry (Reference Brown and Pilsbry1911) described five new species from the Gatun Formation and reported the occurrence of four additional taxa that were originally described from elsewhere. Olsson (Reference Olsson1922) focused on the Neogene molluscan fauna of Costa Rica, reporting that several cone snail species co-occurred in the Gatun Formation of the Canal Zone, including one newly described species. Woodring (Reference Woodring1970) provided the most thorough systematic treatment of Conidae from the Gatun Formation and included detailed taxonomic summaries of the work described above. Further, Woodring (Reference Woodring1970) described two additional cone snail species from the Gatun Formation and reported on taxa found in the Gatun Formation that were originally described from other tropical American Neogene localities. His work on the cone snails from the Gatun Formation was based on ~350 specimens from existing collections at the Smithsonian Institution and material that he collected himself in 1947 (Woodring, Reference Woodring1957, p. 46). He recognized 16 species and subspecies of Conidae from the Gatun Formation: “eight in the lower part, 14 in the middle part, seven in the upper part in the eastern area, and six in the upper part in the western area” (Woodring, Reference Woodring1970, p. 346). Woodring (Reference Woodring1970) further noted that two of the 54 localities he studied had a maximum of eight co-occurring species; one of these localities (Woodring’s locality 138c) is from the lower Gatun Formation, while the other (Woodring’s locality 155) is from the middle Gatun Formation. A maximum of five co-occurring species was reported from a locality positioned in the upper Gatun Formation (Woodring’s locality 175). Pitt and Pitt (Reference Pitt and Pitt1993) published the most recent work on fossil Conidae from the Gatun Formation; these authors illustrated three specimens that they could not confidently assign to known taxa, but otherwise did not add to the total diversity of Conidae from the Gatun Formation.
Table 1 Species of Conidae reported from the Gatun Formation of Panama, including literature sources for those reports, information about type specimens and localities, and the current taxonomic status of these species as treated herein. (H)=holotype; (L)=lectotype; and (S)=syntype.

In contrast to Woodring’s (Reference Woodring1970) broad treatment, the focus of this paper is characterization of the diversity of fossil Conidae from a single well-studied locality positioned in the lower Gatun Formation. Cone snail specimens were intensively sampled from this locality in July and October 2015 by the author and others, which resulted in the collection of nearly 900 specimens belonging to at least nine species, allowing intraspecific variability to be characterized for many of these taxa. Special attention is given to description of the preserved coloration patterns of many of the species, which are often revealed by exposure to ultraviolet (UV) light (see Fig. 1), an approach that Hendricks (Reference Hendricks2015) recently discussed and applied to fossil Conidae from the Dominican Republic. This technique, pioneered by Olsson (Reference Olsson1967), has been previously applied to other molluscan fossils (e.g., Vokes and Vokes, Reference Vokes and Vokes1968; Krueger, Reference Krueger1974; Hoerle, Reference Hoerle1976; Kase et al., Reference Kase, Kitao, Aguilar, Kurihara and Pandita2008; Hendricks, Reference Hendricks2009; Caze et al., 2010, 2011a, Reference Caze, Merle, Saint Martin and Pacaudb; Landau et al., Reference Landau, Harzhauser, Islamoglu and da Silva2013; Harzhauser and Landau, Reference Harzhauser and Landau2016), including by Pitt and Pitt (Reference Pitt and Pitt1993) who were the first to publish photographs of Conidae (as well as other taxa) from the Gatun Formation under UV light. This paper builds upon the work of Pitt and Pitt (Reference Pitt and Pitt1993) by fully characterizing the variability in coloration patterns in cone snail fossils from the Gatun Formation, including those of taxa that they did not study. These preserved coloration patterns are of special interest because they—in conjunction with other shell characteristics—are useful for understanding the relationships of members of this Miocene fauna to extant tropical American cone snail species. Where possible, the Conidae treated here are assigned to the modern clades recognized by Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) and the implications of these assignments for understanding the evolution of the extant tropical American fauna are discussed.
Geological setting
Locality information
With the exception of previously published material, all of the study specimens were collected from a quarry adjacent to a housing development called “San Judas Tadeo” in Cativa, Colón Province, Panama. The Florida Museum of Natural History (FLMNH) Division of Invertebrate Paleontology at the University of Florida (UF) recognizes this locality by the name “San Judas 01” and by the locality code YN020. (Equivalent museum locality codes: FLMNH Division of Vertebrate Paleontology YPA032; Smithsonian Tropical Research Institute [STRI] 290,307 [Anderson et al., Reference Anderson, Hendy, Johnson and Allmon2017]; Los Angeles County Museum Invertebrate Paleontology 41,707 [Anderson et al., Reference Anderson, Hendy, Johnson and Allmon2017]). It is positioned at ~9.3521170°N, 79.8368540°W (WGS84) and was included on a map recently published by Anderson et al. (Reference Anderson, Hendy, Johnson and Allmon2017, fig. 2).
Stratigraphic and paleoenvironmental information
Hendy (Reference Hendy2013), Pimiento et al. (Reference Pimiento, González-Barba, Ehret, Hendy, MacFadden and Jaramillo2013), and Anderson et al. (Reference Anderson, Hendy, Johnson and Allmon2017) recently published stratigraphic columns for the Gatun Formation that include locality YN020, referred to as “San Judas” in all three studies. Hendy (Reference Hendy2013) and Pimiento et al. (Reference Pimiento, González-Barba, Ehret, Hendy, MacFadden and Jaramillo2013) show YN020 located at ~200 m in the section of Coates (Reference Coates1999), with an estimated age of ca. 11–10 Ma. Presuming that this is correct (absolute age dates are not available for this locality), YN020 is Tortonian in age (i.e., late Miocene). Using faunal occurrence records, Hendy (Reference Hendy2013) also estimated changes in paleobathymetry across the section exposed at YN020, suggesting that water depth was usually ~20–60 m as this portion of the lower Gatun Formation was deposited (also see Anderson et al., Reference Anderson, Hendy, Johnson and Allmon2017).
Materials and methods
Specimens from locality YN020
Most of the studied specimens were collected from YN020 on either July 24, 2015 or October 8, 2015 by the author, with the generous assistance of others (see acknowledgments). Effort was made to collect all discovered specimens of Conidae, regardless of preservational quality, resulting in nearly 900 newly collected specimens. A modest number of Conidae specimens collected by teacher participants during the July 24, 2015 trip were not included in this study. A small number of additional cone snail fossils collected from YN020 prior to July 2015 are also included in this study. All studied specimen lots from YN020 are listed in Supplementary data set 1.
Specimen preparation
Prior to study, most specimens were scrubbed clean in water and, in most cases, soaked overnight in diluted (~50%) Clorox® bleach, which sometimes favors the process by which ultraviolet light reveals original coloration patterns (see detailed overview in Krueger, Reference Krueger1974). Following the bleach treatment, shells were again rinsed in water and allowed to dry overnight.
Digital photography and photo processing
Digital photography of specimens under ultraviolet light followed the approach described recently in detail by Hendricks (Reference Hendricks2015). Most images were captured using a Nikon D7100 camera and two Raytech LS-7CB lamps were used for longwave UV illumination. Because brightly fluorescing regions of the shell (e.g., Fig. 1.2) correspond to regions that would have been pigmented during life, Adobe Photoshop was used to create inversed images (e.g., Fig. 1.3) in order to reconstruct the appearance of the original coloration pattern; thus, fluorescing regions become darkened. Importantly, this approach does not reconstruct the actual pigment color of the shell of the once-living animal. These inversed color images are useful, however, for recognizing different elements of coloration patterning. Besides being used to create inverse images, Adobe Photoshop was utilized for uniformly adjusting the white balance of images (often applying the default “shade” setting to photographs taken under UV light and the “auto” setting to photographs taken under regular light), as well as their levels (which were adjusted manually). Some small shell features were digitally photographed at multiple focal levels using a Nikon SMZ1500 stereoscopic zoom microscope in concert with a Nikon Digital Sight DS-Fi2 Camera Head and DS-U3 Digital Camera Control Unit. The resulting image series were then merged into single composite focus stacked images using the software Helicon Focus (v. 6.2.2; Kozub et al., Reference Kozub, Khmelik, Shapoval, Chentsov, Yatsenko, Litovchenko and Starikh2014).
Morphological terminology
Most of the terminology used here for cone snail shell morphology follows that of Röckel et al. (Reference Röckel, Korn and Kohn1995), Hendricks (2009, Reference Hendricks2015), and Kohn (Reference Kohn2014). Consistent with these past studies, four simple measurements were collected in most cases using digital calipers from well-preserved specimens in order to quantify shell form (in some cases, however, measurements were captured from digital images). These are illustrated on Figure 2.1 and include maximum shell length (SL), maximum diameter (MD), aperture height (AH), and height of maximum diameter (HMD). Three ratios were derived from these linear measurements and were used to assign qualitative descriptors to different aspects cone snail shell shape following Röckel et al. (Reference Röckel, Korn and Kohn1995). These ratios include relative diameter (RD), position of maximum diameter (PMD), and the relative height of the spire (RSH).

Figure 2 Specimen measurements. (1) Conus spurius Gmelin, Reference Gmelin1791 (UF 256537), illustrating measurements of maximum shell length (SL), maximum diameter (MD), aperture height (AH), and height of maximum diameter (HMD). (2) Spire whorls of Conus woodringi n. sp. (UF 259874) showing trace of the subsutural flexure (SSF) along a growth line; arrow indicates the position where the growth line intersects the abaxial margin of the sutural ramp. (3) Enlarged version of the trace shown in (2); measurements include depth of subsutural flexure (SSFD), width of the SSF from the adaxial margin of sutural ramp to position of maximum curvature of the SSF (SSFW1), and width of the SSF from the position of its maximum curvature to the abaxial margin of the sutural ramp (SSFW2).
While landmark- (e.g., Cunha et al., Reference Cunha, Tenorio, Afonso, Castilho and Zardoya2008; Cruz et al., Reference Cruz, Pante and Rohlf2012; Tenorio et al., Reference Tenorio, Tucker and Chaney2012) and outline-based (e.g., Smith and Hendricks, Reference Smith and Hendricks2013) geometric morphometric techniques have previously been applied to the shells of Conidae, these three simple ratios and their descriptors have been widely applied to many modern and fossil species (e.g., Röckel et al., Reference Röckel, Korn and Kohn1995; Hendricks, 2009, Reference Hendricks2015; Kohn, Reference Kohn2014; Harzhauser and Landau, Reference Harzhauser and Landau2016; Helwerda, Reference Helwerda2017) and allow for straightforward comparisons to be made between species. It is important to recognize, however, that measurements of RD and PMD (and related ratios) are not necessarily homologous across species (also see Smith and Hendricks, Reference Smith and Hendricks2013; Harzhauser and Landau, Reference Harzhauser and Landau2016). The shell shoulder (defined here as the intersection of the abaxial margin of the sutural ramp with the last whorl) is sometimes positioned at the HMD, especially in species with sharply angled shoulders. In other species, the HMD may be beneath (i.e., anterior to) the shoulder. Even so, these simple metrics are useful for characterizing shell shape, assessing intraspecific variation, and have the advantage—unlike outline-based geometric morphometric approaches—of being collectable from imperfectly preserved fossil specimens. Shell measurement data for individual specimens are presented in Supplementary data set 2. Additionally, reports of typical shell size follow the approach of Kohn (Reference Kohn2014, p. 45), who reported “the median length of shells larger than one-half the maximum size, in order to minimize the effect of varying proportions of juvenile shells in the samples.”
Smith (Reference Smith1930) recognized the importance of the shape of the subsutural flexure (SSF) for differentiating cone snail species (also see Hendricks, Reference Hendricks2009). The SSF traces the growing edge of the shell across the sutural ramp and may be shallow to deep in depth, and symmetrical to asymmetrical in shape. While the final interval of growth is often damaged on fossil cone snail shells, earlier growth lines very often preserve the shape of the SSF, especially where shell production temporarily ceased, leaving high-relief traces of past growth (e.g., Fig. 2.2).
For this study, three simple measurements were collected from digital images to quantify the shape of the SSF for individual species. Prior to photography, shells were positioned with the apex pointed towards the camera lens, with the axis of coiling oriented perpendicular to it. Following photography, the resulting images were digitally rotated such that the adaxial region of the SSF (i.e., where it intersects the previous whorl) was positioned directly beneath the apex of the shell (see dashed line on Fig. 2.2). Measurements were then collected as indicated in Figure 2.3 and include: (1) the depth of the subsutural flexure (SSFD); (2) the width from the origin point to the position of maximum SSF depth, which is usually the point of maximum curvature (SSFW1); and (3) the width from the position of maximum depth to the abaxial margin of the sutural ramp (SSFW2).
From these measurements, two ratios were used to quantify the general morphology of the SSF. First, the depth-to-width ratio of the SSF (DWSSF) was calculated as follows.
Second, the asymmetry of the SSF (ASSF) was calculated as follows.
Species with ASSF values <1 have asymmetrical SSFs, while those with ASSF values of ~1 have symmetrical SSFs. Note that Harzhauser and Landau (Reference Harzhauser and Landau2016) very recently pioneered an alternative approach to quantifying the shape of the SSF, wherein measurements were collected from images taken at an oblique angle relative to the SSF. Subsutural flexure data for individual specimens are presented in Supplementary data set 3.
The terminology used here to characterize and describe preserved shell coloration patterns revealed by UV light follows the recently developed approach of Hendricks (Reference Hendricks2015), which in turn was built upon earlier descriptive terminology developed by Röckel et al. (Reference Röckel, Korn and Kohn1995), Hendricks (Reference Hendricks2009), and Kohn (Reference Kohn2014). Briefly, some cone snail shells show elements of coloration patterning that seem to occupy a single layer (e.g., rows of spiral blotches covering a shell’s last whorl). Others seemingly show two layers of patterning, with a basal (or, primary) layer that appears to be overprinted by a secondary layer (e.g., axial streaks overprinted by spiral bands). In some cases, these two layers of patterning show no evidence of interactions (i.e., a noninteracting pattern, where one pattern simply appears to cover another). In other cases, however, the two layers may interact with one another where they intersect (i.e., an interacting pattern); these interactions are described on a case-by-case basis. See Hendricks (Reference Hendricks2015) for additional discussion.
Some of the Conidae species from YN020 have also been reported from other tropical American localities and strata. The new morphological descriptions below pertain only to material from YN020 and studied type material. Future work will more broadly consider intraspecific differences across the Neogene of tropical America.
Repositories and institutional abbreviations
All newly collected specimens from YN020 are reposited in the Florida Museum of Natural History Division of Invertebrate Paleontology collections at the University of Florida (UF). Previously published fossils, including type and figured specimens, are from the following museum collections: the Academy of Natural Sciences of Drexel University, Philadelphia, Pennsylvania (ANSP); the California Academy of Sciences Department of Invertebrate Zoology and Geology, San Francisco (CASG); the Colección Nacional de Paleontología, Instituto de Geología, Universidad Nacional Autónoma de México, Mexico City (IGM); the Natural History Museum, London (NHMUK); the Naturhistorisches Museum Wien, Austria (NHMW); the Paleontological Research Institution, Ithaca, New York (PRI); the University of California Museum of Paleontology, Berkeley (UCMP); and the Smithsonian Institution National Museum of Natural History, Washington D.C. (USNM).
Systematic paleontology
Family Conidae Fleming, Reference Fleming1822
Remarks
While repeated tests have confirmed the monophyly of the Conidae (e.g., most recently by Uribe et al., Reference Uribe, Puillandre and Zardoya2017), the internal classification of this hyperdiverse clade has been in a state of continuous upheaval in recent years, with significant disagreement about how to subdivide the clade into manageable Linnaean categories consistent with modern hypotheses of phylogeny (e.g., see varied views in Röckel et al., Reference Röckel, Korn and Kohn1995; Tucker and Tenorio, Reference Tucker and Tenorio2009; Hendricks et al., Reference Hendricks, Saupe, Myers, Hermsen and Allmon2014; Hendricks, Reference Hendricks2015; Petuch et al., Reference Petuch, Drolshagen and Herndl2015; Puillandre et al., Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015; Harzhauser and Landau, Reference Harzhauser and Landau2016; Landau et al., Reference Landau, da Silva, Heitz and Janssen2016). While a suitable classification for extant cone snails is now available (Puillandre et al., 2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015; Uribe et al., Reference Uribe, Puillandre and Zardoya2017), morphological features of their shells have yet to be coded and mapped upon the existing molecular trees. Because of this, detailed morphological diagnoses of individual clades supported by molecular sequence data remain largely lacking. For example, while molecular data suggest a great genetic divide between the two largest genera, Conasprella and Conus, morphological features of the shell present no obvious features that separate the two clades. Thus, detailed analyses of the phylogenetic distribution of cone snail shell characters in light of molecular sequence data are badly needed, most especially to diagnose the shell features of the different genera and subgenera of Conidae. The assignment below of fossil species of Conidae to individual clades, therefore, is based largely on comparison with the shells—and especially the shell coloration patterns—of similar modern taxa of known phylogenetic position. Species with no obvious relationship to modern taxa are assigned for now to Conus, reflecting the traditional classification of cone snails (see Röckel et al., Reference Röckel, Korn and Kohn1995; Hendricks, Reference Hendricks2009; Kohn, Reference Kohn2014).
Genus Conasprella Thiele, Reference Thiele1929
Type species
Conasprella pagoda (Kiener, Reference Kiener1847) by subsequent designation (Tucker and Tenorio, Reference Tucker and Tenorio2009). Species is extant and occurs in the Indo-Pacific.
Remarks
Based on molecular phylogenetic results, Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) divided the extant clade Conasprella into seven subgenera, three of which (Kohniconus Tucker and Tenorio, Reference Tucker and Tenorio2009; Dalliconus Tucker and Tenorio, Reference Tucker and Tenorio2009; Ximeniconus Emerson and Old, Reference Emerson and Old1962) include tropical American members. All extant species of Conasprella are vermivorous (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014), so this feeding ecology can also be assumed for fossil taxa assigned to this clade.
Conasprella imitator (Brown and Pilsbry, Reference Brown and Pilsbry1911)
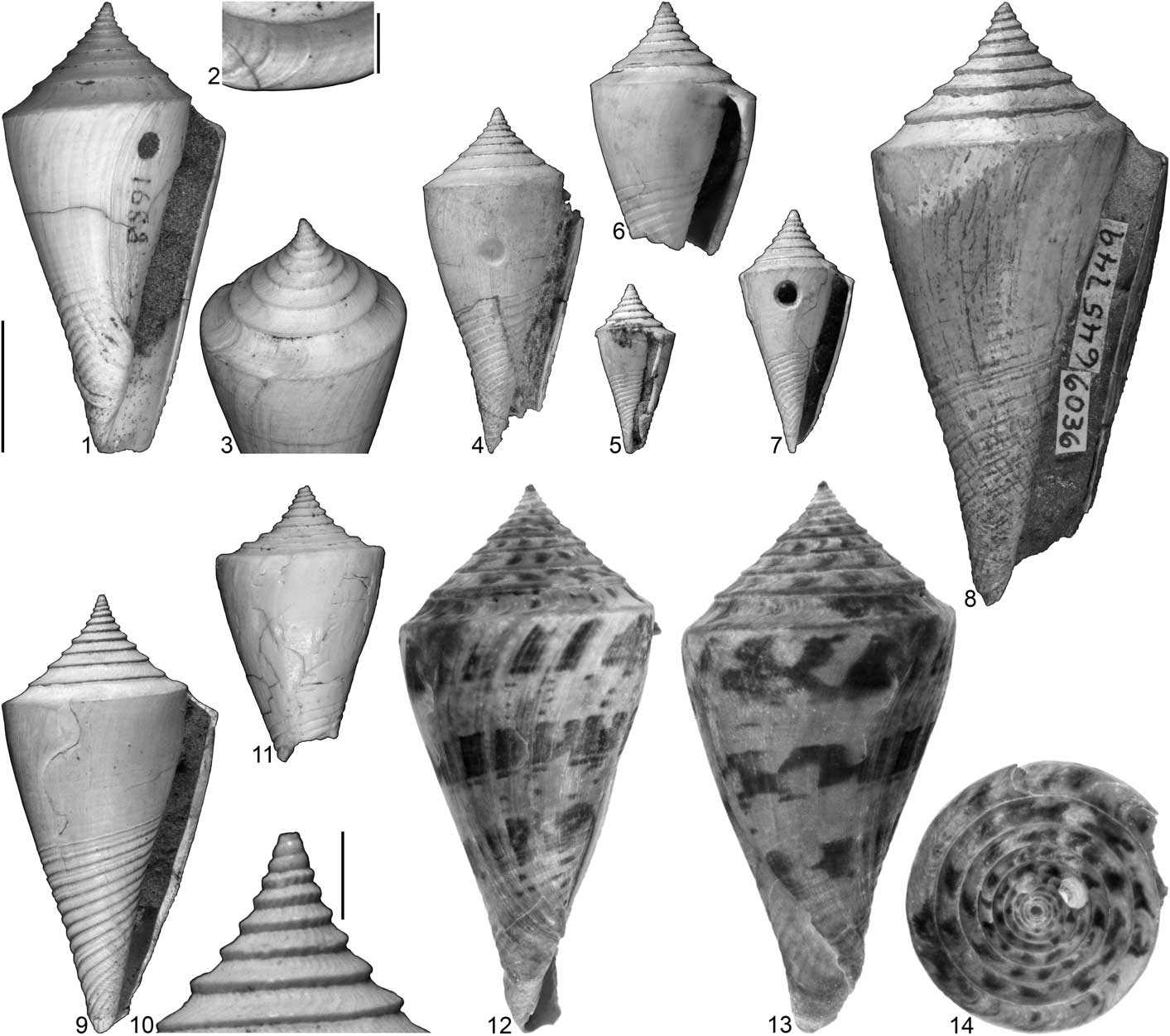
Figure 3 Conasprella imitator (Brown and Pilsbry, Reference Brown and Pilsbry1911): specimens photographed under regular light unless otherwise noted. (1–3) ANSP 1688, lectotype of Conus imitator, “from the excavations for the locks at Gatun” (Brown and Pilsbry, Reference Brown and Pilsbry1911, p. 336), SL 34.3 mm (measured from a digital image); (4) NHMW 1933/0018/0225-1, lectotype of Conus dalli, from Gatun, SL 26.3 mm (measured from a digital image); (5) NHMW 1933/0018/0225-3, paralectotype of Conus dalli, from Gatun, SL 12.8 mm (measured from a digital image); (6) NHMW 1933/0018/0226-2, paralectotype of Conus dalli, from Gatun, SL 19.2 mm (measured from a digital image; anterior portion missing); (7) NHMW 1933/0018/0226-1, paralectotype of Conus dalli, from Gatun, SL 18.4 mm (measured from a digital image); (8) USNM 645749, specimen figured by Woodring (Reference Woodring1970, pl. 55, fig. 1), Panama Canal Zone, Woodring locality 177d, upper Gatun Formation, SL 45.4 mm; (9, 10) USNM 645750, specimen figured by Woodring (Reference Woodring1970, pl. 55, fig. 2), Panama Canal Zone, Woodring locality 138c, lower Gatun Formation, SL 33.0 mm; (11) UF 271035, UF locality YN020, lower Gatun Formation, SL 20.6 mm; (12–14) UF 259873, UF locality YN020, lower Gatun Formation, SL 41.4 mm, photographed under UV light. Scale bar to left of (1) is 10 mm and pertains to (1), (3–9), and (11–14); scale bars associated with (2) and (10) are 2 mm.
1911 Conus dalli Reference ToulaToula, p. 509, pl. 31, fig. 23a–d (not Conus dalli Stearns, Reference Stearns1873, an extant eastern Pacific species).
1911 Conus imitator Reference Brown and PilsbryBrown and Pilsbry, p. 342, pl. 23, fig. 4.
1917 ?Conus dalli; Reference MauryMaury, p. 212, pl. 7, fig. 15.
1921 Conus imitator; Reference PilsbryPilsbry, p. 327.
1928 Conus imitator lius Reference WoodringWoodring, p. 209, pl. 10, figs. 5, 6.
1970 Conus imitator imitator; Reference WoodringWoodring, p. 354, pl. 55, figs. 1, 2.
2009 Gradiconus imitator (Brown and Pilsbry); Reference Tucker and TenorioTucker and Tenorio, p. 97.
2010 Conus imitator; Reference Landau and da SilvaLandau and da Silva, p. 101, pl. 20, fig. 9a–c, pl. 21, fig. 1a, b.
Lectotype
ANSP 1688 (Fig. 3.1–3.3), from the lower locks at Gatun, Panama, presumably Gatun Formation.
Occurrence
Based on the material examined, Conasprella imitator is confirmed to span the lower to upper Gatun Formation, northern Canal Zone, Panama. Woodring (Reference Woodring1970) reported numerous additional Neogene occurrences for this species from throughout tropical America, including from elsewhere in Panama, as well as Costa Rica, Mexico, Colombia, Jamaica, and the Dominican Republic, but these await confirmation. More recently, Landau and da Silva (Reference Landau and da Silva2010) recognized the species as occurring in the lower Pliocene Araya Formation of Cubagua Island, Venezuela (also see Landau et al., Reference Landau, Vermeij and da Silva2008).
Description
Maximum shell size: medium. Largest observed specimen (USNM 645749) has SL 45.4 mm. Woodring (Reference Woodring1970, p. 355) reported a specimen with SL 55 mm.
Last whorl
Shape conical (RD 0.62–0.67,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 64}$$
; PMD 0.91–0.94,
$$\bar{x}\, {\equals}\, 0.{\rm 64}$$
; PMD 0.91–0.94,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 93}$$
; N=4); outline slightly convex on posterior half, slightly concave on anterior half, resulting in sigmoidal profile. Shoulder carinate to angulate; smooth. Widest part of shell at or just below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Spiral grooves present on anterior third to half of last whorl, with cords between; axial growth lines sometimes evident in grooves.
$$\bar{x}\, {\equals}\, 0.{\rm 93}$$
; N=4); outline slightly convex on posterior half, slightly concave on anterior half, resulting in sigmoidal profile. Shoulder carinate to angulate; smooth. Widest part of shell at or just below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Spiral grooves present on anterior third to half of last whorl, with cords between; axial growth lines sometimes evident in grooves.
Spire whorls
Spire moderate to high (RSH 0.20–0.24;
![]() $$\bar{x}\, {\equals}\, 0.{\rm 22}$$
; N=4); outline slightly concave. Protoconch multispiral. First several postnuclear whorls tuberculate. Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp typically concave; sometimes flat or sigmoidal. Spiral ornamentation on ramp is variable: several strong spiral threads are present on ANSP 1688, while these are reduced to fine spiral threads on USNM 645749 and USNM 645750; on UF 259873, a single spiral cord and one to two fine spiral grooves are present. Subsutural flexure asymmetrical (ASSF 0.6–0.8,
$$\bar{x}\, {\equals}\, 0.{\rm 22}$$
; N=4); outline slightly concave. Protoconch multispiral. First several postnuclear whorls tuberculate. Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp typically concave; sometimes flat or sigmoidal. Spiral ornamentation on ramp is variable: several strong spiral threads are present on ANSP 1688, while these are reduced to fine spiral threads on USNM 645749 and USNM 645750; on UF 259873, a single spiral cord and one to two fine spiral grooves are present. Subsutural flexure asymmetrical (ASSF 0.6–0.8,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 7}$$
, N=3), depth approximately equal to width (DWSSF 0.9–1.1,
$$\bar{x}\, {\equals}\, 0.{\rm 7}$$
, N=3), depth approximately equal to width (DWSSF 0.9–1.1,
![]() $$\bar{x}\, {\equals}\, {\rm 1}.0$$
, N=3) (Fig. 3.2).
$$\bar{x}\, {\equals}\, {\rm 1}.0$$
, N=3) (Fig. 3.2).
Coloration pattern
The coloration pattern of this species is characterized from a single specimen, UF 259873 (Fig. 3.12–3.14). It is not possible to determine if only a single pattern is present, or if there are two noninteracting patterns. The stronger component of the pattern consists of three well-defined, discontinuous spiral bands that result in irregularly shaped blotches. The weaker component consists of spiral rows of irregularly spaced dots and dashes between these bands. The two components do not differ in the color of emitted light under exposure to UV, nor does one component appear to overlay the other. Sutural ramp with irregular blotches.
Materials
ANSP 1688 (lectotype of Conus imitator Brown and Pilsbry, Reference Brown and Pilsbry1911; Fig. 3.1–3.3); ANSP 78910 (paralectotype of C. imitator); NHMW 1933/0018/0225 (lectotype, Fig. 3.4, and two paralectotypes, one of which is shown in Fig. 3.5, of Conus dalli Toula, Reference Toula1911); NHMW 1933/0018/0226 (two paralectotypes of C. dalli Toula; Fig. 3.6, 3.7); USNM 645749 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 3.8); USNM 645750 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 3.9, 3.10); UF 259770 (one specimen; Fig. 3.11); and UF 259873 (one specimen; Fig. 3.12–3.14).
Remarks
Toula’s (Reference Toula1911) name for this species, Conus dalli, is occupied by the extant eastern Pacific species Conus dalli Stearns, Reference Stearns1873. The type material for this species resides at the NHMW and is represented by five specimens, four of which are figured here (Fig. 3.4–3.7), including the lectotype designated by Woodring (Reference Woodring1970, p. 355), NHMW 1933/0018/0225-1 (Fig. 3.4). These correspond closely with the lectotype of Conus imitator Brown and Pilsbry, Reference Brown and Pilsbry1911 (ANSP 1688; Fig. 3.1–3.3), as do the two specimens figured by Woodring (Reference Woodring1970; Fig. 3.8–3.10). Additional work is needed to determine if Maury’s (Reference Maury1917) specimen (PRI 28624) from the Dominican Republic is Conasprella imitator. Woodring (Reference Woodring1970) synonymized his subspecies Conus imitator lius Woodring, Reference Woodring1928 from the Bowden Formation of Jamaica as C. imitator imitator based on the Jamaican specimens apparently lacking tubercles on the early postnuclear whorls, a trait that Woodring (Reference Woodring1970) considered variable in the species based on his material from the Gatun Formation: he reported that some lower Gatun Formation specimens lack tubercles on the early postnuclear whorls, while other specimens of similar age possess the feature. The presence or absence of tubercles on early postnuclear whorls is not known to be an intraspecifically variable feature of cone snail shells, so additional work is needed to determine whether the early whorls of the Jamaican and lower Gatun Formation specimens in question are eroded or not. In any case, Woodring’s decision to synonymize his earlier Jamaican subspecies is tentatively followed here pending future study.
The coloration pattern of Conasprella imitator revealed under UV light, in addition to other aspects of its shell morphology, support a close relationship with the extant eastern Pacific species Conasprella (Kohniconus) arcuata (Broderip and Sowerby I, 1829), an association also recognized by Woodring (Reference Woodring1966), and the extant western Atlantic species Conasprella delessertii (Récluz, Reference Récluz1843). Given their similar shells and radular tooth morphologies, Tucker and Tenorio (Reference Tucker and Tenorio2009) assigned both extant taxa to their new genus Kohniconus. More recent molecular phylogenetic work (Puillandre et al., 2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015), however, suggests that while they both belong to the clade Conasprella sensu Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015), they are otherwise not closely related; other species assigned to Kohniconus by Tucker and Tenorio (Reference Tucker and Tenorio2009) were not included in the molecular phylogenetic analysis of Puillandre et al. (Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014). Thus, additional work will need to be done to test the degree of relationship between C. arcuata and C. delessertii, as well as other similar species. In any case, C. imitator shares much in common with both extant species and can be confidently assigned to the genus Conasprella sensu Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015), if not the subgenus Kohniconus sensu Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015). In terms of coloration pattern, C. imitator shares more in common with C. arcuata, which has only one layer of pigmentation; C. delessertii has two interacting layers of pigmentation. Tucker and Tenorio (Reference Tucker and Tenorio2009) assigned C. imitator to the genus Gradiconus (genus Conus, subgenus Dauciconus sensu Puillandre et al., 2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015), but for the reasons stated above, an assignment to Conasprella is better supported. Among co-occurring fossil species, C. imitator might be confused with a species that is assigned here to the subgenus Dauciconus: Conus taphrus Woodring, Reference Woodring1970. Both species have somewhat similar overall shell shapes, but their coloration patterns are very different (see remarks under Conus taphrus for additional discussion).
Even though Brown and Pilsbry (Reference Brown and Pilsbry1911, p. 342) reported Conasprella imitator as “rather abundant at Gatun,” only one specimen (UF 259873) is confirmed from UF locality YN020. Brown and Pilsbry’s (Reference Brown and Pilsbry1911) specimens, which were collected from exposures associated with the construction of the locks at Gatun, are from the middle Gatun Formation, while the two records from YN020 (UF 259873, UF 271035) are from the lower Gatun Formation. Woodring (Reference Woodring1970, p. 355) reported C. imitator as spanning the lower (six localities) to middle (six localities) to upper (10 localities) Gatun Formation. Of the 70 specimens available to him, the greatest abundance of C. imitator (20 specimens) came from his locality 177b, which is positioned in the upper Gatun Formation. At the very least, these reports suggest that, owing to differences in paleoenvironment and/or geological age, C. imitator may have been less common in the lower Gatun Formation relative to the upper Gatun Formation.
Subgenus Ximeniconus Emerson and Old, Reference Emerson and Old1962
Type species
Conus ximenes Gray, Reference Gray1839.
Remarks
Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) assigned 29 extant species to the subgenus Ximeniconus. Eight of these species were included in the phylogenetic analysis of Puillandre et al. (Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014); four occur in the eastern Pacific and four occur in the western Atlantic.
Conasprella (Ximeniconus) burckhardti (Böse, Reference Böse1906)

Figure 4 Conasprella burckhardti (Böse, Reference Böse1906): (1, 2, 4–8) photographed under regular light; (3, 9–23) photographed under UV light; specimens are from UF locality YN020 unless otherwise indicated. (1, 2) Artificial cast of IGM 170 (PRI 70566), holotype of Conus burckhardti Böse, Reference Böse1906, Tuxtepec, Oaxaca, Mexico, portion of SL preserved 21.6 mm (measured from a digital image); (3) USNM 645752, specimen figured by Woodring (Reference Woodring1970, pl. 57, fig. 19), SL 42.3 mm, Panama Canal Zone, Woodring locality 155, middle Gatun Formation; (4) USNM 645754, specimen figured by Woodring (Reference Woodring1970, pl. 57, fig. 17), SL 37.8 mm, Panama Canal Zone, Woodring locality 138a, lower Gatun Formation; (5) PRI 20899, holotype of Conus harrisi Olsson, Reference Olsson1922, Banana River, Panama, portion of SL preserved 53.0 mm; (6, 12) UF 270984, SL 17.2 mm, showing features of the protoconch and early postnuclear whorls; (7, 19) UF 270985, SL 26.0 mm, showing growth lines on the sutural ramp, indicating the shape of the subsutural flexure; (8) UF 259819, showing ornamentation features of the last whorl; (9, 10) UF 270986, SL 34.9 mm; (11) UF 259811, SL 30.5 mm; (13) UF 270987, SL 25.9 mm; (14) UF 270988, SL 31.0 mm; (15) UF 270989, SL 24.5 mm; (16) UF 270990, SL 33.3 mm; (17) UF 259831, SL 26.6 mm; (18) UF 270991, SL 25.1 mm; (20) UF 259813, SL 23.3 mm; (21) UF 270992, SL 21.2 mm; (22) UF 270993, SL 26.7 mm; (23) UF 270994, SL 30.3 mm. Scale bar to left of (1) is 1 cm and pertains to all but (6–8), which are focus-stacked composite images; (6) scale bar equals 0.5 mm and (7, 8) scale bars equal 1 mm.
1906 Conus burckhardti Reference BöseBöse, p. 49, pl. 5, figs. 39, 40.
1922 Conus burckhardti; Reference OlssonOlsson, p. 52, pl. 39, figs. 4, 5.
1922 Conus harrisi Reference OlssonOlsson, p. 53, pl. 3, fig. 1.
1925 Conus burckhardti; Reference MauryMaury, p. 187, pl. 34, fig. 5.
1970 Conus burckhardti burckhardti; Reference WoodringWoodring, p. 357, pl. 57, figs. 19, 20.
1970 Conus burckhardti harrisi; Reference WoodringWoodring, p. 358, pl. 57, fig. 17.
1993 Conus burckhardti harrisi; Reference Pitt and PittPitt and Pitt, pl. 3, fig. 9.
2009 Ximeniconus burckhardti (Böse); Reference Tucker and TenorioTucker and Tenorio, p. 153.
Occurrence
The exact stratigraphic position of the type locality at Tuxtepec, Mexico is unknown, but Woodring (Reference Woodring1970) presented it as middle Miocene. Based on the species’ occurrence at UF locality YN020 and reports in Woodring (Reference Woodring1970) for both of the subspecies that he recognized, Conasprella burckhardti spans the lower to upper Gatun Formation. Woodring (Reference Woodring1970) also reported C. burckhardti from Miocene deposits in Ecuador, the Bocas del Toro area of Panama, Costa Rica, Venezuela, and Trinidad, but these require confirmation. There is no indication that the species persisted past the Miocene.
Description
Maximum shell size: medium. Typical shell size of specimens from UF locality YN020: moderately small (24.7 mm; N=136). Largest observed specimen from YN020 (UF 270995) has SL 36.9 mm, but has a slightly damaged apex and was thus slightly longer than this. The specimen (USNM 645752; Fig. 4.3) figured by Woodring (Reference Woodring1970) from his locality 155 (middle Gatun Formation) has SL 42.3 mm and the holotype (PRI 20899; Fig. 4.5) of C. harrisi, an incomplete specimen, has SL 53.0 mm.
Last whorl
Shape conical (RD 0.50–0.60,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 54}$$
; PMD 0.86–0.96,
$$\bar{x}\, {\equals}\, 0.{\rm 54}$$
; PMD 0.86–0.96,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 91}$$
; N=31); outline slightly convex on posterior half, slightly concave on anterior half, resulting in sigmoidal profile. Shoulder carinate; smooth. Widest part of shell at or below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Spiral ribs, which are often beaded, especially on anterior half, extending from base to shoulder; grooves between ribs exhibit closely spaced axial threads (Fig. 4.8).
$$\bar{x}\, {\equals}\, 0.{\rm 91}$$
; N=31); outline slightly convex on posterior half, slightly concave on anterior half, resulting in sigmoidal profile. Shoulder carinate; smooth. Widest part of shell at or below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Spiral ribs, which are often beaded, especially on anterior half, extending from base to shoulder; grooves between ribs exhibit closely spaced axial threads (Fig. 4.8).
Spire whorls
Spire moderate to high (RSH 0.20–0.31,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 25}$$
; N=31); outline nearly straight to slightly concave. Protoconch multispiral, with at least three whorls; diameter 0.8–0.9 mm (
$$\bar{x}\, {\equals}\, 0.{\rm 25}$$
; N=31); outline nearly straight to slightly concave. Protoconch multispiral, with at least three whorls; diameter 0.8–0.9 mm (
![]() $$\bar{x}\, {\equals}\, 0.{\rm 9}$$
; N=12) (Fig. 4.6). Tubercles present on first 0.25–1.0 whorls; these often become diminishing undulations before terminating. Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp slightly concave; spiral ornamentation absent. Subsutural flexure asymmetrical (ASSF 0.5–0.6,
$$\bar{x}\, {\equals}\, 0.{\rm 9}$$
; N=12) (Fig. 4.6). Tubercles present on first 0.25–1.0 whorls; these often become diminishing undulations before terminating. Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp slightly concave; spiral ornamentation absent. Subsutural flexure asymmetrical (ASSF 0.5–0.6,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 6}$$
, N=3), depth approximately equal to width (DWSSF 0.8–1.3,
$$\bar{x}\, {\equals}\, 0.{\rm 6}$$
, N=3), depth approximately equal to width (DWSSF 0.8–1.3,
![]() $$\bar{x}\, {\equals}\, {\rm 1}.{\rm 1}$$
, N=3) (Fig. 4.7).
$$\bar{x}\, {\equals}\, {\rm 1}.{\rm 1}$$
, N=3) (Fig. 4.7).
Coloration pattern
Two patterns present, which are noninteracting in most cases and differ slightly in the color of emitted light. The primary (base) pattern is highly variable, ranging from irregularly shaped, poorly organized axial blotches to continuous or discontinuous axial streaks to nearly continuous spiral bands. The secondary pattern, which is nearly obsolete in some specimens, consists of spiral dots or dashes on rib surfaces. The secondary dash pattern on UF 259813 (Fig. 4.20) shows weak interaction with the primary pattern in some areas, resulting in unpigmented spaces between the spiral dashes.
The coloration pattern of UF 270994 (Fig. 4.23) is unlike any of the other observed specimens. On this specimen, the primary pattern continuously covers the entire last whorl, with the exception of several irregularly shaped blotches that are devoid of pigmentation; the secondary pattern of this specimen consists of continuous lines that cover the rib surfaces, but do not cross the unpigmented blotches of the primary pattern, indicating interactions between the two patterns. This specimen is otherwise morphologically consistent with other specimens of C. burckhardti. Sutural ramp with irregular blotches that sometimes extend over the shoulder onto the last whorl, indicating an association with the primary pattern.
Materials
An artificial cast of IGM 170 (holotype of Conus burckhardti Böse, Reference Böse1906; Fig. 4.1, 4.2); USNM 645752 (one specimen, figured by Woodring; Fig. 4.3); USNM 645753 (one specimen, figured by Woodring, Reference Woodring1970); USNM 645754 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 4.4); PRI 20899 (holotype of Conus harrisi Olsson, Reference Olsson1922); and an additional 258 observed specimens, all from UF locality YN020.
Remarks
Conasprella burckhardti was described by Böse (Reference Böse1906) from Tuxtepec in the Tehuantepec region of Oaxaca, México from deposits that both Woodring (1966, Reference Woodring1970) and Beu (2009) reported as middle Miocene in age. An artificial cast (PRI 70566) of the type specimen (IGM 170; Fig. 4.1, 4.2) was kindly provided by Dr. Perrilliat at the Colección Nacional de Paleontología, Instituto de Geología. Woodring (Reference Woodring1970) was the first to apply the name C. burckhardti to material from the Gatun Formation, and his circumscription of the material he examined from the Gatun Formation is consistent with the features of IGM 170. Conasprella burckhardti was the second most commonly collected species at UF locality YN020.
Woodring (Reference Woodring1970) treated Conus harrisi Olsson, Reference Olsson1922 (Fig. 4.5) as a subspecies of Conus burckhardti. The specimen (USNM 645754; Fig. 4.4) figured by Woodring (Reference Woodring1970) differs from typical C. burckhardti in the very narrow width of its shell (RD 0.44), but it is otherwise consistent in shell characteristics with other C. burckhardti. Conasprella burckhardti harrisi is thus treated here simply as C. burckhardti. While Woodring (Reference Woodring1970) only reported C. burckhardti burckhardti from the middle and upper Gatun Formation, most of the shells found at UF locality YN020 (lower Gatun Formation) are more consistent with this wider form than they are with the narrower Conasprella burckhardti harrisi morphology. Both morphologies span the Gatun Formation.
Among extant taxa, Conasprella burckhardti is most similar to the eastern Pacific species Conasprella (Ximeniconus) tornata (Sowerby I, 1833), which ranges from Baja California, Mexico to Peru (for a detailed overview of this species, see Tenorio et al., Reference Tenorio, Tucker and Chaney2012). Both taxa have similar shell shapes, multispiral protoconchs, sutural ramps lacking spiral ornamentation, and moderately deep subsutural flexures. The coloration patterns of both taxa are also consistent: a primary pattern of axial blotches overlain by spiral rows of dots or dashes. A notable difference is that tubercles are present on the first postnuclear whorl of C. burckhardti, but are reportedly absent from C. tornata (Tenorio et al., Reference Tenorio, Tucker and Chaney2012). Among fossil species, C. burckhardti is similar to the recently described species C. ageri Hendricks, Reference Hendricks2015 from the lower Pliocene Gurabo Formation of the Dominican Republic. Like C. tornata, C. ageri also lacks tubercles on its early postnuclear whorls. Shells of C. ageri also typically have lower values of PMD (0.83–0.89,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 86}$$
; Hendricks, Reference Hendricks2015) than C. burckhardti (0.86–0.96,
$$\bar{x}\, {\equals}\, 0.{\rm 86}$$
; Hendricks, Reference Hendricks2015) than C. burckhardti (0.86–0.96,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 91}$$
), corresponding with the fact that they are usually widest below the shoulder, while specimens of C. burckhardti are usually widest at the shoulder. Finally, shells of C. burckhardti usually have higher spires (RSH 0.20–0.31,
$$\bar{x}\, {\equals}\, 0.{\rm 91}$$
), corresponding with the fact that they are usually widest below the shoulder, while specimens of C. burckhardti are usually widest at the shoulder. Finally, shells of C. burckhardti usually have higher spires (RSH 0.20–0.31,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 25}$$
) than shells of C. ageri (RSH 0.18–0.23,
$$\bar{x}\, {\equals}\, 0.{\rm 25}$$
) than shells of C. ageri (RSH 0.18–0.23,
![]() $$\bar{x}$$
0.20; Hendricks, Reference Hendricks2015). Presuming, as suggested here, that C. burckhardti is closely related to extant C. tornata, the occurrence of the fossil taxon in the lower Gatun Formation provides a useful minimum age of origination for the subgenus Ximeniconus at ca. 10 Ma.
$$\bar{x}$$
0.20; Hendricks, Reference Hendricks2015). Presuming, as suggested here, that C. burckhardti is closely related to extant C. tornata, the occurrence of the fossil taxon in the lower Gatun Formation provides a useful minimum age of origination for the subgenus Ximeniconus at ca. 10 Ma.
Genus Conus Linnaeus, Reference Linnaeus1758
Type species
Conus marmoreus Linnaeus, Reference Linnaeus1758 by subsequent designation (Children, Reference Children1823). Species is extant and occurs in the Indo-Pacific.
Remarks
Conus was recently subdivided by Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) into 44 extant subgenera. Species of modern western Atlantic and eastern Pacific species belong, respectively, to 11 and 12 of these clades. The genus includes vermivores, molluscivores, and piscivores, though only two tropical American species, C. purpurascens Sowerby I, 1833 (eastern Pacific) and C. ermineus Born, Reference Born1778 (western Atlantic), eat fish (e.g., Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014).
Conus symmetricus? Sowerby I, 1850

Figure 5 Specimens from the Gatun Formation questionably assigned to Conus symmetricus Sowerby I, 1850: (1, 3) photographed under regular light; (2) photographed under UV light. (1, 2) USNM 645747, specimen figured by Woodring (Reference Woodring1970, pl. 57, figs. 13, 14), Panama Canal Zone, Woodring locality 155a, middle Gatun Formation, SL 20.3 mm; (3) UF 271037, UF locality YN020 (lower Gatun Formation), SL 21.0 mm. Scale bar to left of (1) is 1 cm and pertains to all specimens.
1850 Conus symmetricus Reference SowerbySowerby I, p. 44, pl. 9, fig. 1.
1917 Conus symmetricus; Reference MauryMaury, pl. 7, fig. 7, 7a.
1921 Conus symmetricus; Reference PilsbryPilsbry, pl. 20, fig. 2, 2a, 2b.
1961 Conus (Leptoconus) symmetricus; Reference PflugPflug, p. 63, pl. 18, figs. 1–11.
2009 Purpuriconus symmetricus (Sowerby I); Reference Tucker and TenorioTucker and Tenorio, p. 116.
2015 Conus symmetricus; Reference HendricksHendricks, p. 22, fig. 9a–g.
Lectotype
NHMUK PI BM G 83969 (designated by Pflug, Reference Pflug1961); label reports specimen from the Miocene of the Yaque River, St. Domingo.
Occurrence
Conus symmetricus is a very common taxon in some Neogene assemblages in the Dominican Republic (see Pflug, Reference Pflug1961; Hendricks, Reference Hendricks2015). Its occurrence in the Gatun Formation of Panama is regarded as tentative (see below) and its occurrence at UF locality YN020 is considered questionable.
Materials
USNM 645747 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 5.1, 5.2); UF 271037 (one specimen, Fig. 5.3).
Remarks
On the basis of two specimens from the middle Gatun Formation (one of which is USNM 645747; Fig. 5.1, 5.2), Woodring (Reference Woodring1970, p. 353) declared a “first unequivocal record for” Conus symmetricus “beyond the Dominican Republic, where it is abundant in the Gurabo Formation.” While USNM 645747 appears consistent in shell form with C. symmetricus from the Dominican Republic, its coloration pattern is different. Hendricks (Reference Hendricks2015, p. 25) noted that specimens of C. symmetricus from the Dominican Republic “show a wide range of variability in coloration pattern,” which consistently show the presence of two non-interacting patterns. UV light reveals a very different pattern on the dorsal surface of USNM 645757: three rows of nearly circular blotches, with no secondary pattern (Fig. 5.2). If this different pattern were found to be consistent in the older, Gatunian specimens of C. symmetricus, it could be reasonably argued that the specimens from the Gatun Formation constitute a different species.
A single poorly preserved specimen (UF 271037, Fig. 5.3) found at UF locality YN020 is questionably assigned here to C. symmetricus. Unfortunately, it does not show any evidence of a preserved coloration pattern under UV light, so does not provide any clarification with respect to this question. Pending discovery of additional specimens bearing preserved coloration patterns, C. symmetricus is tentatively accepted as occurring in the Gatun Formation. See discussion in Hendricks (Reference Hendricks2015) for the reasons why C. symmetricus cannot currently be assigned to a subclade within Conus.
Conus aemulator Brown and Pilsbry, Reference Brown and Pilsbry1911

Figure 6 Conus aemulator Brown and Pilsbry, Reference Brown and Pilsbry1911 from the Gatun Formation of Panama: (1–6) photographed under regular light; (7–16) photographed under UV light; all specimens are from UF locality YN020 (lower Gatun Formation) unless otherwise indicated. (1–3) ANSP 1691, holotype, “excavations for the locks at Gatun” (Brown and Pilsbry, Reference Brown and Pilsbry1911, p. 336), middle Gatun Formation (Woodring, Reference Woodring1970, p. 351), Panama Canal Zone, SL 22.7 mm, MD 12.8 mm; (4, 5) USNM 645744, specimen figured by Woodring (Reference Woodring1970, pl. 56, figs. 4, 8), Panama Canal Zone, Woodring locality 155a, middle Gatun Formation, SL 45.2 mm, MD 28.7 mm; (6) UF 259747, showing the shape of the subsutural flexure and features of the sutural ramp; (7) UF 270975, SL 41.0 mm; (8) UF 270977, SL 32.3 mm; (9) UF 259757, SL 18.6 mm; (10) UF 270972, SL 22.2 mm; (11) UF 270973, SL 43.7 mm; (12) UF 270976, SL 33.7 mm; (13) UF 270974, SL 29.0 mm; (14) UF 259741, SL 28.0 mm; (15) UF 270979, SL 28.2 mm; (16) UF 270978, SL 35.4 mm. Scale bar to the left of (1) equals 10 mm and pertains to (1, 4, 5, 7–16); scale bar to the left of (3) equals 5 mm and pertains to (2, 3); and scale bar to the right of (6) equals 5 mm and pertains to that specimen.

Figure 7 Type specimens of Conus veatchi Olsson, Reference Olsson1922 and a calcitic cast questionably assigned to Conus aemulator Brown and Pilsbry, Reference Brown and Pilsbry1911: (1–4) photographed under regular light; (5) photographed under UV light. (1, 2) PRI 20897, holotype, Conus veatchi, SL 45.2 mm, Water Cay, Panama (stratum unknown); (3) PRI 20894, paratype, Conus veatchi, SL 39.9 mm, Water Cay, Panama (stratum unknown); (4, 5) UF 271027, Conus aemulator?, SL 27.0 mm, UF locality YN020 (lower Gatun Formation). Scale bar to the left of (1) equals 10 mm and pertains to all specimens.
1911 Conus aemulator Reference Brown and PilsbryBrown and Pilsbry, p. 342, pl. 23, fig. 9.
1922 Conus veatchi Reference OlssonOlsson, p. 44, pl. 2, figs. 5, 8.
1970 Conus aemulator aemulator; Reference WoodringWoodring, p. 351, pl. 55, figs. 5, 6, pl. 56, figs. 4, 8.
1993 Not Conus aemulator aemulator; Reference Pitt and PittPitt and Pitt, p. 10, pl. 3, fig. 6.
2009 Dauciconus aemulator (Brown and Pilsbry); Reference Tucker and TenorioTucker and Tenorio, p. 88.
2016 Dauciconus aemulator (Brown and Pilsbry); Reference Landau, da Silva, Heitz and JanssenLandau et al., p. 204, pl. 44, fig. 5, pl. 45, figs. 1, 2, pl. 46, fig. 8, pl. 47, fig. 8.
Holotype
ANSP 1691, “excavations for the locks at Gatun” (Brown and Pilsbry, Reference Brown and Pilsbry1911, p. 336), Panama Canal Zone.
Occurrence
Conus aemulator was described from excavations for the Gatun locks, which Woodring (Reference Woodring1970, p. 351) characterized as middle Gatun Formation. Woodring (Reference Woodring1970) reported the species as spanning the Gatun Formation. Additional records presented by Woodring (Reference Woodring1970) for C. aemulator come from Neogene localities throughout tropical America, including the Darién and Bocas del Toro areas of Panama, Ecuador, the Dominican Republic, Haiti, the Grenadine Islands, and Colombia; these all require confirmation. Very recently, Landau et al. (Reference Landau, da Silva, Heitz and Janssen2016) reported the species from the lower–middle Miocene Cantaure Formation of Venezuela.
Description
Maximum shell size: medium. Largest observed specimen (UF 270973) has SL 43.7 mm. Typical shell size of specimens from UF locality YN020: medium (32.3 mm; N=29).
Last whorl
Shape conical to broadly conical (RD 0.64–0.73,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 68}$$
; PMD 0.86–0.91,
$$\bar{x}\, {\equals}\, 0.{\rm 68}$$
; PMD 0.86–0.91,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 88}$$
; N=17); outline convex on posterior half, nearly straight on anterior half, resulting in a slightly convex profile. Shoulder carinate and forming a posterior-pointing ridge. Widest part of shell below shoulder. Aperture slightly wider at base than shoulder. Siphonal notch absent. Slightly wavy spiral threads or cords present, usually restricted to the anterior half, but in rare cases extend past the midline or are absent altogether. Some specimens (e.g., UF 259739, UF 270980) show evidence of weakly beaded spiral threads.
$$\bar{x}\, {\equals}\, 0.{\rm 88}$$
; N=17); outline convex on posterior half, nearly straight on anterior half, resulting in a slightly convex profile. Shoulder carinate and forming a posterior-pointing ridge. Widest part of shell below shoulder. Aperture slightly wider at base than shoulder. Siphonal notch absent. Slightly wavy spiral threads or cords present, usually restricted to the anterior half, but in rare cases extend past the midline or are absent altogether. Some specimens (e.g., UF 259739, UF 270980) show evidence of weakly beaded spiral threads.
Spire whorls
Spire low to moderate (RSH 0.05–0.19,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 11}$$
; N=17); outline concave to slightly sigmoidal. Early spire whorls stepped. Protoconch unknown. Ornamentation of early postnuclear whorls unknown. Sutural ramp slightly convex to flat on early whorls, concave or sigmoidal in later whorls, with 3–6 (typically four) spiral grooves that separate threads. Subsutural flexure asymmetrical to strongly asymmetrical (ASSF 0.2–0.7,
$$\bar{x}\, {\equals}\, 0.{\rm 11}$$
; N=17); outline concave to slightly sigmoidal. Early spire whorls stepped. Protoconch unknown. Ornamentation of early postnuclear whorls unknown. Sutural ramp slightly convex to flat on early whorls, concave or sigmoidal in later whorls, with 3–6 (typically four) spiral grooves that separate threads. Subsutural flexure asymmetrical to strongly asymmetrical (ASSF 0.2–0.7,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 4}$$
, N=4), depth typically slightly greater than width (DWSSF 0.9–1.7,
$$\bar{x}\, {\equals}\, 0.{\rm 4}$$
, N=4), depth typically slightly greater than width (DWSSF 0.9–1.7,
![]() $$\bar{x}\, {\equals}\, {\rm 1}.{\rm 3}$$
, N=4) (Fig. 6.6).
$$\bar{x}\, {\equals}\, {\rm 1}.{\rm 3}$$
, N=4) (Fig. 6.6).
Coloration pattern
One pattern present. Pattern usually consists of three rows of discontinuous spiral bands. The elements within the bands are complex, including sub-rectangular blotches (e.g., Fig. 6.7), diagonal streaks (e.g., Fig. 6.8), sub-triangular markings (e.g., Fig. 6.11, 6.13), irregular blotches (Fig. 6.14), or closely spaced axial streaks (Fig. 6.15); in one observed specimen (Fig. 6.16), the spiral elements form nearly continuous bands. Sutural ramp with occasional blotches.
Materials
ANSP 1691 (holotype, Fig. 6.1–6.3); USNM 645744 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 6.4, 6.5); 56 additional observed specimens, all from UF locality YN020, which are listed in Supplementary Data Set 1.
Remarks
This species was described from the Gatun Formation on the basis of a single calcitic cast (holotype, ANSP 1691; Fig. 6.1–6.3). This poorly preserved specimen has a conical last whorl (RD 0.66, PMD 0.86) and a moderately high spire (RSH 0.14); there is evidence of several spiral threads on the sutural ramp and on the anterior half of the last whorl. Despite the poor preservation of the holotype, the characteristics preserved allow it to be recognized as a valid taxon.
Shells of Conus aemulator are very similar to those of C. molis and the possibility that specimens of C. aemulator are in fact juvenile C. molis (see below) was carefully considered. Both taxa have shells that are similar in overall shape, are widest below the shoulder, have raised spiral ornamentation on the anterior half of the last whorl, and possess spiral ornamentation on the sutural ramp. One key difference is that mature specimens of C. molis are much larger than mature C. aemulator, but this does not assist with differentiating immature C. molis from mature C. aemulator. The very different coloration patterns (revealed by UV light) of the two species, however, do help to resolve this problem: C. aemulator has only one layer of pigmentation (three discontinuous spiral bands), while C. molis has two layers that vary in the color of emitted light (two or three regions of axial streaks covered by two or three nearly continuous spiral bands). Two features provide some additional assistance for differentiating the two species. The sutural ramp of C. aemulator features three to six (but usually four) grooves that separate spiral threads, while more such grooves (often five or more) are commonly present on the ramps of specimens of C. molis. Finally, relative to C. molis, the subsutural flexure of C. aemulator is much more asymmetrical across the ramp (mean ASSF in C. aemulator=0.4; mean ASSF in C. molis=0.7).
Olsson (Reference Olsson1922) described Conus veatchi from two specimens (PRI 20897, holotype, Fig. 7.1, 7.2; PRI 20894, paratype, Fig. 7.3) collected at “Water Cay” in Panama (stratigraphic context unknown). Conus veatchi was also reported by Olsson from the Gatun Formation of the Canal Zone, but associated specimens are lacking. Olsson (Reference Olsson1922, p. 44) stated that C. veatchi “is very unlike any of the associated Gatun species in its low, nearly flat spire.” Woodring (Reference Woodring1970) placed C. veatchi in synonymy with C. aemulator, a conclusion also reached recently by Landau et al. (Reference Landau, da Silva, Heitz and Janssen2016). Olsson’s type specimens are generally consistent in form with C. aemulator, although neither type specimen shows evidence of a preserved coloration pattern when exposed to UV light. While the stratigraphic position of the Water Cay locality relative to the Gatun Formation in the area of Colón is uncertain, the conclusion that C. veatchi is synonymous with C. aemulator is reasonable given the information available.
Landau et al. (Reference Landau, da Silva, Heitz and Janssen2016) reported C. aemulator (as Dauciconus aemulator; see below) from the lower–middle Miocene Cantaure Formation of Venezuela. Even though these Venezuelan specimens of C. aemulator do not reveal preserved coloration pattern under UV light (Landau et al., Reference Landau, da Silva, Heitz and Janssen2016, p. 204), they appear otherwise consistent in form with C. aemulator as circumscribed here.
Tucker and Tenorio (Reference Tucker and Tenorio2009, p. 88) assigned C. aemulator to the Dauciconus clade, a conclusion followed by Landau et al. (Reference Landau, da Silva, Heitz and Janssen2016). While C. aemulator has a general shell shape consistent with some, but certainly not all, species in this subgenus, its coloration pattern does not allow obvious comparison with extant species of Dauciconus. Pending detailed diagnosis of the shell characteristics of extant Dauciconus, C. aemulator is not assigned to a subgenus of Conus.
Finally, UF 259771, which is a calcitic cast questionably assigned to C. aemulator, is highlighted here for its potential importance for understanding the taphonomic process of mineral replacement in mollusk shells. A coloration pattern that is generally consistent with that of C. aemulator (three rows of discontinuous spiral bands) is revealed when this specimen is exposed to UV light (Fig. 7.4, 7.5). This suggests that the process of replacement of aragonite with calcite is highly localized, preserving in place the fluorescing material once associated with regions of pigmentation.
Subgenus Stephanoconus Mörch, Reference Mörch1852
Type species
Conus leucostictus Gmelin, Reference Gmelin1791 (=Conus regius Gmelin, Reference Gmelin1791).
Remarks
Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) assigned 21 extant cone snail species to the subgenus Stephanoconus. The phylogenetic positions of 10 of these have been determined (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014), and three occur in the Indo-Pacific, three in the eastern Pacific, and four in the western Atlantic. All Stephanoconus are vermivorous (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014) and this feeding ecology is assumed for fossil taxa assigned to this clade.
Conus (Stephanoconus) woodringi new species

Figure 8 Conus (Stephanoconus) woodringi new species: (1, 2, 11, 12) photographed under regular light; (3–10) photographed under UV light; all specimens are from UF locality YN020 (lower Gatun Formation) unless otherwise indicated. (1–5) UF 259874, holotype, SL 50.2 mm, MD 28.0 mm; (6) UF 271017, SL 44.0 mm; (7) UF 271018, preserved portion of SL 33.7 mm; (8) UF 259753, SL 38.7 mm; (9) UF 256535, SL 41.7 mm; (10) USNM 645745, specimen figured by Woodring (Reference Woodring1970, pl. 56, figs. 3, 7), Panama Canal Zone, Woodring locality 138c, lower Gatun Formation, SL 47.7 mm; (11, 12) USNM 645746, specimen figured by Woodring (Reference Woodring1970, pl. 56, fig. 9), Panama Canal Zone, Woodring locality 161b, middle Gatun Formation, SL 61.7 mm. Scale bar to left of (1) is 10 mm and pertains to all specimens.
1970 Conus consobrinus consobrinus; Reference WoodringWoodring, p. 352, pl. 56, figs. 3, 7, 9 (not Conus consobrinus Sowerby I, 1850).
1993 Conus consobrinus consobrinus; Reference Pitt and PittPitt and Pitt, p. 10, pl. 4, fig. 1 (not Conus consobrinus Sowerby I, 1850).
2010 ?Conus spurius; Reference Landau and da SilvaLandau and da Silva, pl. 21, fig. 6a–c (not Conus spurius Gmelin, Reference Gmelin1791).
Holotype
UF 259874, UF locality YN020 (“San Judas 01”), lower Gatun Formation, Cativa, Colón Province, Panama (latitude and longitude: 9.3521170° N, 79.8368540° W (WGS84); determined using Google Earth Pro) (Fig. 8.1–8.5).
Paratypes
UF 271017 (Fig. 8.6), UF 271018 (Fig. 8.7), UF 259753 (Fig. 8.8), UF256535 (Fig. 8.9), and UF 271019 (all from UF locality YN020, same as holotype).
Diagnosis
Shell moderately large; spire low to moderate; spire tuberculate; subsutural flexure asymmetrical and deep; two sometimes interacting coloration patterns present, one often consisting of zig-zagging axial streaks.
Occurrence
Based on records here and in Woodring (Reference Woodring1970), the species spans the lower to upper Gatun Formation. It may also occur in the lower Pliocene Araya Formation of Cubagua Island, Venezuela (see remarks).
Description
Shell size: moderately large. The holotype specimen (UF 259874) from UF locality YN020 has SL 50.2 mm, while a middle Gatun Formation specimen (USNM 645746; Fig. 8.11, 8.12) figured by Woodring (Reference Woodring1970) has SL 61.7 mm.
Last whorl
Shape conical (RD 0.65–0.70,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 68}$$
; PMD 0.88–0.95,
$$\bar{x}\, {\equals}\, 0.{\rm 68}$$
; PMD 0.88–0.95,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 91}$$
; N=4); outline convex on posterior half, nearly straight on posterior half, resulting in a slightly convex profile. Shoulder sharply angulate to angulate and forming a posterior-pointing ridge; smooth in mature individuals. Widest part of shell below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Fine to strong spiral threads on anterior half, diminishing towards shoulder; threads are frequently beaded.
$$\bar{x}\, {\equals}\, 0.{\rm 91}$$
; N=4); outline convex on posterior half, nearly straight on posterior half, resulting in a slightly convex profile. Shoulder sharply angulate to angulate and forming a posterior-pointing ridge; smooth in mature individuals. Widest part of shell below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Fine to strong spiral threads on anterior half, diminishing towards shoulder; threads are frequently beaded.
Spire whorls
Spire low to moderate (RSH 0.10–0.20,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 15}$$
; N=4); outline concave to slightly concave. Protoconch unknown. Early postnuclear whorls unknown, but at least seven of the teleoconch whorls bear large, elongate tubercles that diminish thereafter. Sutural ramp of early whorls convex, sigmoidal on later whorls; several spiral grooves present, with threads in between. Subsutural flexure asymmetrical (ASSF 0.4–0.7,
$$\bar{x}\, {\equals}\, 0.{\rm 15}$$
; N=4); outline concave to slightly concave. Protoconch unknown. Early postnuclear whorls unknown, but at least seven of the teleoconch whorls bear large, elongate tubercles that diminish thereafter. Sutural ramp of early whorls convex, sigmoidal on later whorls; several spiral grooves present, with threads in between. Subsutural flexure asymmetrical (ASSF 0.4–0.7,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 6}$$
, N=3), depth often nearly twice width (DWSSF 1.2–2.1,
$$\bar{x}\, {\equals}\, 0.{\rm 6}$$
, N=3), depth often nearly twice width (DWSSF 1.2–2.1,
![]() $$\bar{x}\, {\equals}\, {\rm 1}.{\rm 8}$$
, N=3) (Fig. 8.2, 8.12).
$$\bar{x}\, {\equals}\, {\rm 1}.{\rm 8}$$
, N=3) (Fig. 8.2, 8.12).
Coloration pattern
Two sometimes interacting patterns present that vary in the color of emitted light. The primary pattern usually consists of two discontinuous bands made up of bold, zig-zagging axial streaks; the bands are usually divided at the midline by a narrow, unpigmented spiral band. In one specimen (Fig. 8.9), the primary bands are continuous, though an unpigmented region at the midline remains. The secondary pattern consists of numerous spiral rows of dots, dashes, or chevron-shape spots that extend from the base to the spire. Interactions between the two patterns are sometimes evident in cases where spaces between the elements of the secondary pattern overlap the primary pattern and result in small, unpigmented dots or spots. Sutural ramp with irregular blotches.
Etymology
Named for Dr. Wendell P. Woodring (1891–1983) in honor of his important contributions to Cenozoic tropical American paleontology (see Moore, Reference Moore1992).
Materials
In addition to type specimens listed above, the following specimens were studied: NHMUK PI G 83962 (lectotype of Conus consobrinus); USNM 645745 (one specimen, figured by Woodring, Reference Woodring1970; see Fig. 8.10); USNM 645746 (one specimen, figured by Woodring, Reference Woodring1970; see Fig. 8.11, 8.12); and CASG 66695.09 (one specimen from the Gatun Formation of Panama figured by Pitt and Pitt, Reference Pitt and Pitt1993, pl. 4, fig. 1).
Remarks
Conus consobrinus Sowerby I, 1850 was described from the Neogene of the Dominican Republic (the most recent formal treatment of this material was by Pflug, Reference Pflug1961). Occurrences of C. consobrinus have been widely reported from throughout tropical America (see Woodring, Reference Woodring1970). Based on ANSP 1682, Brown and Pilsbry (Reference Brown and Pilsbry1911) were the first to report C. consobrinus from the Gatun Formation of Panama, but Woodring (Reference Woodring1970) considered this specimen to instead represent C. tortuosostriatus Toula, Reference Toula1911 and included it in his circumscription of that species; ANSP 1682 was viewed and Woodring’s assignment is accepted here (see below). Woodring (Reference Woodring1970) nevertheless recognized C. consobrinus consobrinus as occurring in the Gatun Formation on the basis of other material: “two specimens from the lower part, two from the middle part, and one from the upper part” (Woodring, Reference Woodring1970, p. 353). Following Woodring (Reference Woodring1970), Pitt and Pitt (Reference Pitt and Pitt1993) applied the name C. consobrinus consobrinus to a specimen (CASG 66695.09) that shows a fluorescing coloration pattern under UV light (Pitt and Pitt, Reference Pitt and Pitt1993, pl. 4, fig. 1). Thus, the reported occurrence of C. consobrinus in the Gatun Formation is based on a total of six specimens.
While some aspects of shell form (perhaps most notably the presence of large tubercles on most spire whorls and commonly beaded spiral threads on the last whorl) support an association between specimens of C. consobrinus from the Dominican Republic and somewhat similar, older material from the Gatun Formation, coloration patterns revealed under UV light and comparison of shell shape parameters instead better support the hypothesis that the Gatun material represents a species—Conus woodringi n. sp.—distinct from younger C. consobrinus from the early Pliocene of the Dominican Republic. While the coloration pattern of C. consobrinus from its type region remains unpublished, it differs markedly from that of the new species (Hendricks, 2017, personal observation of specimens in the collections of the PRI from Tulane University locality TU 1219, lower Pliocene Gurabo Formation of the Dominican Republic). Most notably, the coloration pattern of C. consobrinus seems to lack the distinctive, zig-zagging axial streaks that are present on most of the specimens of C. woodringi n. sp. from UF locality YN020. The shell morphology of C. consobrinus from the Dominican Republic also differs substantially from the specimens assigned here to C. woodringi n. sp. In particular, measurements collected from 13 specimens of C. consobrinus in lot PRI 65989 from TU locality 1219 show that shells of C. consobrinus are narrower (RD 0.60–0.64,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 62}$$
) and have higher spires (RSH 0.22–0.28,
$$\bar{x}\, {\equals}\, 0.{\rm 62}$$
) and have higher spires (RSH 0.22–0.28,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 25}$$
) than shells of C. woodringi n. sp. from the Gatun Formation (compare with values above, which do not overlap with these simple metrics). It is therefore concluded that C. consobrinus does not occur in the Gatun Formation of Panama and that reports of this species by Woodring (Reference Woodring1970) and Pitt and Pitt (Reference Pitt and Pitt1993) should instead be referred to C. woodringi n. sp.
$$\bar{x}\, {\equals}\, 0.{\rm 25}$$
) than shells of C. woodringi n. sp. from the Gatun Formation (compare with values above, which do not overlap with these simple metrics). It is therefore concluded that C. consobrinus does not occur in the Gatun Formation of Panama and that reports of this species by Woodring (Reference Woodring1970) and Pitt and Pitt (Reference Pitt and Pitt1993) should instead be referred to C. woodringi n. sp.
Woodring (Reference Woodring1970) considered Conus lavillei Cossmann, Reference Cossmann1913 to be a junior synonym of C. consobrinus. The type specimen figured by Cossmann (Reference Cossmann1913) has a much narrower shell and higher spire than specimens assigned here to C. woodringi n. sp., and it is therefore considered to be a different species. Conus lavillei is much more similar in shell form to Conus tortuosostriatus Toula, Reference Toula1911, a species known from the Gatun Formation (Woodring, Reference Woodring1970), but not found at UF locality YN020.
A specimen (NHMW 2010/0038/0214) from the lower Pliocene Araya Formation of Cubagua Island, Venezuela that was assigned by Landau and Silva (2010) to Conus spurius instead appears to be consistent in several respects with Conus woodringi n. sp. In particular, this specimen (see Landau and da Silva, Reference Landau and da Silva2010, pl. 21, fig. 6a–c) exhibits a similar overall shell shape, large tubercles on its early postnuclear whorls, and a visible coloration pattern that shows some evidence of the zig-zagging axial streaks of C. woodringi n. sp. Importantly, specimens of C. spurius never have large tubercles on early postnuclear whorls, negating the possibility that NHMW 2010/0038/0214 belongs to that species. The specimen from the Araya Formation, however, is somewhat younger (early Pliocene) than the new species from the Gatun Formation (late Miocene). Because of this, the assignment of NHMW 2010/0038/0214 to Conus woodringi n. sp. is considered tentative at the present time, pending study of additional material from the Araya Formation.
The assignment of C. woodringi n. sp. to the subgenus Stephanoconus is supported in large part by its overall shell shape, presence of large tubercles on most spire whorls, deep subsutural flexure, and especially its complex, two-element coloration pattern. Indeed, C. woodringi n. sp. is very similar in shell morphology to the extant eastern Pacific species Conus (Stephanoconus) archon Broderip, Reference Broderip1833, which has a known phylogenetic position (Puillandre et al., 2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015). In addition to similarities shared with C. consobrinus, C. woodringi n. sp. is also somewhat similar to two additional Neogene tropical American species of Stephanoconus, both from the late Miocene and early Pliocene of the Dominican Republic: Conus sewalli Maury, Reference Maury1917 and C. bellacoensis Hendricks, Reference Hendricks2015. Notable differences, however, include the lack of pigmented zig-zagging axial streaks on C. sewalli and that shells of C. bellacoensis are narrower (mean RD 0.57) and have higher spires (mean RSH=0.28) (Hendricks, Reference Hendricks2015) than specimens of C. woodringi. It is anticipated that future study of C. consobrinus will also support its assignment to the Stephanoconus clade.
Subgenus Dauciconus Cotton, Reference Cotton1945
Remarks
Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) assigned a total of 105 extant species to the subgenus Dauciconus Cotton, Reference Cotton1945, making it one of the most diverse subgenera of Conus. Some of these species may be synonymous (see Kohn, Reference Kohn2014), and only 17 have been subjected to molecular phylogenetic analysis (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014). Even so, Dauciconus is one of the most important cone snail clades in tropical America and is restricted to the eastern Pacific and western Atlantic. The clade also has a substantial Neogene fossil record in tropical America. For example, Hendricks (Reference Hendricks2015) assigned six species (one questionably) from the late Miocene and early Pliocene of the Dominican Republic to this subgenus. Additionally, Petuch et al. (Reference Petuch, Drolshagen and Herndl2015) assigned 23 Plio-Pleistocene species from southern Florida to the genera Gradiconus da Motta, Reference da Motta1991, Magelliconus da Motta, Reference da Motta1991, Purpuriconus da Motta, Reference da Motta1991, Cariboconus Petuch, Reference Petuch2003, and Dauciconus Cotton, Reference Cotton1945, although Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) considered all of these genus-level groupings to be synonymous with Dauciconus and Hendricks (Reference Hendricks2009) synonymized many of the taxa that Petuch et al. (Reference Petuch, Drolshagen and Herndl2015) accepted as valid. Given that species of Dauciconus are important constituents of other Neogene tropical American deposits, it is somewhat surprising that only one species from the Gatun Formation of Panama can be confidently assigned to this clade: Conus taphrus Woodring, Reference Woodring1970. All Dauciconus are vermivorous (Puillandre et al., 2014) and this feeding ecology is similarly assumed for fossil taxa assigned to this clade.
Conus (Dauciconus) taphrus Woodring, Reference Woodring1970

Figure 9 Conus (Dauciconus) taphrus Woodring, Reference Woodring1970: (1–5) photographed under regular light; (6–9) photographed under UV light; all specimens are from UF locality YN020 (lower Gatun Formation) unless otherwise indicated. (1–5) USNM 645748, holotype of Conus taphrus, Panama Canal Zone, Woodring locality 161d, middle Gatun Formation, SL 28.1 mm, MD 14.9 mm; (6, 7) UF 256543, from UF locality 5299 (see text), MD 21.5 mm (measured from digital image); (8) UF 271020, MD 17.4 mm; (9) UF 224823, SL 26.5 mm. Scale bar to the left of (1) equals 10 mm and pertains to (1–3, 6–9); scale bars to the right of (4) and (5) equal 10 mm and 3 mm, respectively, and pertain to those specimens.
1970 Conus taphrus Reference WoodringWoodring, p. 354, pl. 57, figs. 1, 7.
2009 Gradiconus taphrus; Reference Tucker and TenorioTucker and Tenorio, p. 97.
Type
Holotype USNM 645748, Woodring (Reference Woodring1970) locality 161d (= USGS locality 8366), Canal Zone, Panama, middle Gatun Formation.
Occurrence
Previously, Conus taphrus was only known from the holotype specimen, which is from the middle Gatun Formation. Based on its presence at UF locality YN020 and UF locality 5299 (9.35711° N, 79.8387° W (WGS84), Colón Province, Panama), the species also occurs in the lower Gatun Formation. Conus taphrus is not known from beyond the Canal Zone of Panama.
Description
Note that characteristics and values below are derived from the holotype, specimens from UF locality YN020, and a single specimen from UF locality 5299.
Maximum shell size
Moderately small. The largest nearly complete specimen (holotype, USNM 645748) has SL 28.1 mm, but has a damaged base and was thus slightly longer than this.
Last whorl
Shape conical (RD 0.66–0.69,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 68}$$
; PMD 0.93,
$$\bar{x}\, {\equals}\, 0.{\rm 68}$$
; PMD 0.93,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 93}$$
; N=2); outline slightly convex on posterior half, slightly concave on anterior half, resulting in sigmoidal profile. Shoulder angulate or subangulate; smooth. Widest part of shell at or just below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Spiral grooves present on anterior third of last whorl.
$$\bar{x}\, {\equals}\, 0.{\rm 93}$$
; N=2); outline slightly convex on posterior half, slightly concave on anterior half, resulting in sigmoidal profile. Shoulder angulate or subangulate; smooth. Widest part of shell at or just below shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Spiral grooves present on anterior third of last whorl.
Spire whorls
Spire moderate (RSH 0.20–0.21;
![]() $$\bar{x}\, {\equals}\, 0.{\rm 21}$$
; N=2); outline concave. Protoconch unknown. The apex of the holotype is broken, but may record evidence of a single weakly tuberculate postnuclear whorl; similarly, the eroded apex of UF 224823 appears to show evidence of several weakly tuberculate early postnuclear whorls. Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp sigmoidal to nearly convex; weak spiral striae present. Sutural groove present. Subsutural flexure asymmetrical (ASSF 0.4–0.6,
$$\bar{x}\, {\equals}\, 0.{\rm 21}$$
; N=2); outline concave. Protoconch unknown. The apex of the holotype is broken, but may record evidence of a single weakly tuberculate postnuclear whorl; similarly, the eroded apex of UF 224823 appears to show evidence of several weakly tuberculate early postnuclear whorls. Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp sigmoidal to nearly convex; weak spiral striae present. Sutural groove present. Subsutural flexure asymmetrical (ASSF 0.4–0.6,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 5}$$
, N=3), depth less than width (DWSSF 0.5–0.7,
$$\bar{x}\, {\equals}\, 0.{\rm 5}$$
, N=3), depth less than width (DWSSF 0.5–0.7,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 7}$$
, N=3) (Fig. 9.5).
$$\bar{x}\, {\equals}\, 0.{\rm 7}$$
, N=3) (Fig. 9.5).
Coloration pattern
Two noninteracting patterns present; these differ slightly in the color of emitted light. The primary (base) pattern consists of irregularly shaped axial blotches in UF 256543 (Fig. 9.6, 9.7; specimen from UF locality 5299), but in UF 271020 (Fig. 9.8) and UF 224823 (Fig. 9.9) consists of spirally arranged dots and spots, which are sometimes sub-triangular in shape. The secondary pattern consists of a continuous (UF 224823 and UF 256543) or nearly continuous (UF 271020) spiral band at the midline of the last whorl; UF 224823 shows evidence of a second continuous spiral band that covers the anterior third of the shell. The coloration pattern of the anterior half of the last whorl is unknown. Sutural ramp with irregular blotches.
Materials
Holotype: USNM 645748 (Fig. 9.1–9.5). Other material: two specimens from UF locality YN020 (UF 224823, UF 271020) and one from UF locality 5299 (UF 256543).
Remarks
Prior to this study, the only known specimen of Conus taphrus was Woodring’s (Reference Woodring1970) holotype. Woodring (Reference Woodring1970, p. 354) considered the holotype’s “sutural channel” to be “a distinctive feature.” While none of the three newly recognized specimens of C. taphrus possesses a sutural channel that is as well developed as that of the holotype, they are otherwise consistent with it in other aspects of shell form. The holotype reveals no evidence of its coloration pattern under UV light, but the new specimens fluoresce strongly, showing some variability in their primary and secondary patterns. Pending discovery of additional specimens, this is assumed to represent intraspecific variability.
Among co-occurring species, C. taphrus can only be mistaken for Conasprella imitator (Brown and Pilsbry, Reference Brown and Pilsbry1911). While coloration patterns revealed by UV light readily separate the two species, other differences in shell morphology are less distinctive. Both species have sigmoidal last whorl profiles, but the curves are more pronounced in Conasprella imitator, which also has a more angulate shoulder. The subsutural flexure of Conasprella imitator (
![]() $${\rm DW}_{{{\rm SSF}}} \,\bar{x}\, {\equals}\, {\rm 1}.0$$
) is also deeper than that of Conus taphrus (
$${\rm DW}_{{{\rm SSF}}} \,\bar{x}\, {\equals}\, {\rm 1}.0$$
) is also deeper than that of Conus taphrus (
![]() $${\rm DW}_{{{\rm SSF}}} \,\bar{x}\, {\equals}\, 0.{\rm 7}$$
).
$${\rm DW}_{{{\rm SSF}}} \,\bar{x}\, {\equals}\, 0.{\rm 7}$$
).
Conus taphrus is similar to the extant western Atlantic species C. daucus Hwass in Bruguière, 1792 and C. amphiurgus Dall, Reference Dall1889, which, while not closely related to one another, are both members of the Dauciconus clade (Puillandre et al., 2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015). Kohn (Reference Kohn2014) recently revised both of these extant taxa. His figured specimens of each species exhibit a large amount of variability in coloration pattern, but the basic elements of their patterns (e.g., a primary pattern consisting of axial and/or spiral elements overlain by a secondary pattern of bands) are broadly consistent with the pattern described here for C. taphrus. Other features of the shell form of C. taphrus are also generally consistent with C. daucus and C. amphiurgus. Perhaps most notably, Kohn (Reference Kohn2014, p. 228) stated that the early whorls of C. daucus exhibit a “prominent suture,” which is one of the most diagnostic features of C. taphrus.
While knowledge of C. taphrus will improve with discovery of additional, better-preserved specimens, the known shell features of this species are similar enough to extant taxa such as C. daucus and C. amphiurgus to assign the species to the subgenus Dauciconus. Tucker and Tenorio (Reference Tucker and Tenorio2009) included C. taphrus in a listing of fossil species that they considered to belong to the genus Gradiconus da Motta, Reference da Motta1991. Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) synonymized Gradiconus with the diverse subgenus Dauciconus Cotton, Reference Cotton1945. This adds further support to the opinion here that C. taphrus belongs in the Dauciconus clade, even if determination of its position within Dauciconus requires additional specimens and study.
Conus? (Dauciconus?) multiliratus Böse, Reference Böse1906

Figure 10 Conus? (Dauciconus?) multiliratus Böse, Reference Böse1906: (1–7) photographed under regular light; (8–18) photographed under UV light; (3–7) are focus-stacked composite images; specimens are from UF locality YN020 unless otherwise indicated. (1) USNM 645751, specimen figured by Woodring (Reference Woodring1970, pl. 57, figs. 3, 4), Panama Canal Zone, Woodring locality 175, upper Gatun Formation, SL 27.1 mm; (2) ANSP 2554, syntype of Conus gaza Johnson and Pilsbry in Brown and Pilsbry, Reference Brown and Pilsbry1911, from the Dominican Republic, SL 23.3 mm (measured from a digital image); (3, 4, 8) UF 270996, SL 34.6 mm, (3, 4) showing features of the protoconch and early postnuclear whorls; (5, 6) UF 270997, showing features of the subsutural flexure and sutural ramp; (7) UF 270998, showing features of the last whorl; (9) UF 259868, SL 21.6 mm; (10, 11) UF 259855, SL 23.7 mm; (12) UF 270999, SL 22.9 mm; (13) UF 259860, SL 18.9 mm; (14) UF 271000, SL 18.5 mm (measured from a digital image); (15) UF 271001, SL 27.2 mm; (16) UF 259864, SL 23.7 mm; (17) UF 259862, SL 26.5 mm; (18) UF 271002, SL 29.0 mm. Scale bar to left of (1) is 10 mm and pertains to (1, 2) and (8–18); scale bars associated with (3–7) are 1 mm.
1906 Conus agassizi var. multiliratus Reference BöseBöse, p. 49, pl. 5, figs. 34–38.
1911 Conus gaza Johnson and Pilsbry in Reference Brown and PilsbryBrown and Pilsbry, p. 342, pl. 23, figs. 2, 3.
1917 Conus gaza; Reference MauryMaury, p. 210, pl. 7, fig. 12.
1921 Conus gaza; Reference PilsbryPilsbry, p. 330.
1970 Conus multiliratus multiliratus; Reference WoodringWoodring, p. 356, pl. 57, figs. 3, 4.
1993 Conus multiliratus multiliratus; Reference Pitt and PittPitt and Pitt, p. 12, pl. 4, fig. 3.
2009 Conasprelloides multiliratus (Böse); Reference Tucker and TenorioTucker and Tenorio, p. 88.
2015 Conus (Dauciconus) multiliratus; Reference HendricksHendricks, p. 46, fig. 22a–e.
Holotype
IGM (no known catalog number), Tuxtepec, Oaxaca Province, Mexico (stratum unknown); Woodring (Reference Woodring1970) reported the specimen as missing and this remained the case as of 2010 (M.d.C. Perrilliat, personal communication, September 14, 2010).
Occurrence
Woodring (Reference Woodring1970) presented Böse’s (Reference Böse1906) type locality at Tuxtepec as middle Miocene, but its exact stratigraphic position is not known. In the Panama Canal Zone, Conus multiliratus spans the lower to upper Gatun Formation (Woodring, Reference Woodring1970). Recently, Hendricks (Reference Hendricks2015) reported the species from the early Pliocene Gurabo Formation of the Dominican Republic. Additional occurrences come from the Bocas del Toro area of Panama (Limón Formation), Colombia, Jamaica (Bowden Formation), and the Dominican Republic (Cercado Formation) (Woodring, Reference Woodring1970), but these require confirmation.
Description
Maximum shell size: moderately small. Largest observed specimen from YN020 (UF 270996; Fig. 10.8) has SL 34.7 mm. Typical shell size of specimens from UF locality YN020: moderately small (23.4 mm; N=87).
Last whorl
Shape typically broadly conical, sometimes conical (RD 0.69–0.80,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 75}$$
; PMD 0.89–0.94,
$$\bar{x}\, {\equals}\, 0.{\rm 75}$$
; PMD 0.89–0.94,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 92}$$
; N=24); outline slightly sigmoidal (posterior half convex, anterior half concave). Shoulder carinate or sharply angulate, smooth. Widest point of shell at shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Prominent ribs extend from base to shoulder; ribs on the anterior half are sometimes beaded. Grooves between ribs exhibit prominent axial threads that result in a cancellate pattern (Fig. 10.7); grooves often decrease in width towards the shoulder.
$$\bar{x}\, {\equals}\, 0.{\rm 92}$$
; N=24); outline slightly sigmoidal (posterior half convex, anterior half concave). Shoulder carinate or sharply angulate, smooth. Widest point of shell at shoulder. Aperture uniform in width from base to shoulder. Siphonal notch absent. Prominent ribs extend from base to shoulder; ribs on the anterior half are sometimes beaded. Grooves between ribs exhibit prominent axial threads that result in a cancellate pattern (Fig. 10.7); grooves often decrease in width towards the shoulder.
Spire whorls
Spire moderate to high (RSH 0.21–0.30,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 25}$$
; N=24); outline often slightly sigmoidal (concave near apex; convex near later whorls). Protoconch multispiral, with at least 3.5 whorls; diameter 0.8–0.9 mm (
$$\bar{x}\, {\equals}\, 0.{\rm 25}$$
; N=24); outline often slightly sigmoidal (concave near apex; convex near later whorls). Protoconch multispiral, with at least 3.5 whorls; diameter 0.8–0.9 mm (
![]() $$\bar{x}\, {\equals}\, 0.{\rm 9}$$
; N=14). Tubercles present on first 0.5–1.75 whorls; these become diminishing undulations before terminating (Fig. 10.4). Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp usually concave, but may be nearly flat or slightly sigmoidal; very fine spiral grooves sometimes present. Subsutural flexure asymmetrical (ASSF 0.3–0.5,
$$\bar{x}\, {\equals}\, 0.{\rm 9}$$
; N=14). Tubercles present on first 0.5–1.75 whorls; these become diminishing undulations before terminating (Fig. 10.4). Early whorls strongly stepped; later whorls weakly stepped. Sutural ramp usually concave, but may be nearly flat or slightly sigmoidal; very fine spiral grooves sometimes present. Subsutural flexure asymmetrical (ASSF 0.3–0.5,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 4}$$
, N=5), depth usually slightly less than width (DWSSF 0.7–1.3,
$$\bar{x}\, {\equals}\, 0.{\rm 4}$$
, N=5), depth usually slightly less than width (DWSSF 0.7–1.3,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 9}$$
, N=5) (Fig. 10.5, 10.6).
$$\bar{x}\, {\equals}\, 0.{\rm 9}$$
, N=5) (Fig. 10.5, 10.6).
Coloration pattern
One pattern present. Pattern consists of pigmented dashes or blotches on rib surfaces that are often organized into axial streaks or blotches; an unpigmented or weakly pigmented region is often present just anterior to the midline of the last whorl, dividing the regions of pigmentation into two broad, discontinuous bands. In one observed specimen (UF 259862; Fig. 10.17), pigmentation on the rib surfaces nearly form two solid bands just above and below the midline. In another specimen (UF 271002, Fig. 10.18), very well organized axial streaks are present. Sutural ramp with irregularly shaped pigmented radial blotches.
Materials
USNM 645751 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 10.1); ANSP 2554, the syntype series (nine specimens, all from the Gatun Formation) of Conus gaza, including the specimen illustrated by Brown and Pilsbry (Fig. 10.2), and an additional 156 observed specimens, all from UF locality YN020, which are listed in Supplementary Data Set 1.
Remarks
Woodring (Reference Woodring1970) reported that the type specimen of Conus multiliratus is lost and communication with the staff of the Instituto de Geología at the Universidad Nacional de México in 2010 confirms that this remains the case. Nevertheless, the specimens figured by Böse from Tuxtepec, Mexico appear consistent in form with the specimens considered here from the Gatun Formation, allowing them to be confidently assigned to the same species. Inspection of the syntype series in ANSP 2554 supports the conclusion of Woodring (Reference Woodring1970) that Conus gaza is a junior synonym of Conus multiliratus.
Hendricks (Reference Hendricks2015) assigned the fossil species to the genus Conus and the subgenus Dauciconus sensu Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) because the overall shell morphology of Conus multiliratus is very similar to that of the extant western Atlantic species Conus (Dauciconus) cancellatus Hwass in Bruguière, 1792 (see Kohn, Reference Kohn2014 for a recent circumscription). This assignment is considered more tentative here, however, because shells of C. multiliratus are also broadly similar to extant taxa assigned to the subgenus Conasprella (Kohniconus) sensu Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015). For example, specimens of the eastern Pacific species Conasprella (Kohniconus) arcuata (Broderip and Sowerby I, 1829) figured by Tenorio et al. (Reference Tenorio, Tucker and Chaney2012, pl. 228, figs. 1a–11b) have shell shapes, coloration patterns, and ornamentation features that are also generally consistent with those of C. multiliratus; perhaps importantly, however, weak spiral ornamentation is sometimes present on the sutural ramp of C. multiliratus, but is apparently absent from Conasprella arcuata (Tenorio et al., Reference Tenorio, Tucker and Chaney2012). Tucker and Tenorio (Reference Tucker and Tenorio2009, p. 146) noted this apparent convergence of form between the Conasprella (Kohniconus) and Conus (Dauciconus) clades: “[s]ome species of Kohniconus … have been confused … with species of Dauciconus” and this “underscores the utility of radular morphology in confirming identifications for such conchologically similar but distantly related species.” Tucker and Tenorio (Reference Tucker and Tenorio2009), however, assigned C. cancellatus as the type species for the genus Conasprelloides, not Dauciconus. Puillandre et al. (Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014) showed Conasprelloides sensu Tucker and Tenorio (Reference Tucker and Tenorio2009) to be polyphyletic within Conus (Dauciconus) sensu Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015). While information about the radula of C. multiliratus will likely never be recovered, construction of detailed morphological diagnoses of modern cone snail clades informed by molecular phylogenetic results (e.g., Puillandre et al., 2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) may help to clarify the phylogenetic position of this fossil species in the future. Pending completion of such work, C. multiliratus remains tentatively assigned to Conus (Dauciconus) sensu Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015).
Hendricks (Reference Hendricks2015) reported on the coloration pattern of Conus multiliratus from the lower Pliocene Gurabo Formation of the Dominican Republic. He demonstrated the presence of two continuous spiral bands on the last whorl that might be overprinted by spiral dashes. This varies significantly from the coloration pattern described here for specimens from UF locality YN020. Additional work is needed to determine if this amounts to a species-level difference, or instead an example of coloration pattern variation (or, potentially evolution) within a single lineage. While some specimens of extant Conus cancellatus have patterns that are consistent with C. multiliratus from the Dominican Republic (e.g., Kohn, Reference Kohn2014, pl. 69, figs. 16, 17), others have patterns that are generally consistent with the specimens documented here from the Gatun Formation (e.g., Kohn, Reference Kohn2014, pl. 69, fig. 12).
Among co-occurring fossil species at UF locality YN020, Conus multiliratus might be confused with Conasprella burckhardti, which also has strong ribs (also with intervening grooves showing prominent axial threads) that extend from the base of the last whorl to the shoulder. Shells of Conus multiliratus, however, are much wider than shells of Conasprella burckhardti and the two species do not overlap in RD. Conus multiliratus also bears some similarity in shape to Conasprella imitator, but the latter species has a very different coloration pattern, a greater number of tuberculate postnuclear whorls, and the spiral ornamentation on its last whorl is restricted to the anterior half. Conus multiliratus was the third most commonly collected species of Conidae at UF locality YN020.
Subgenus Pyruconus Olsson, Reference Olsson1967
Type species
Conus patricius Hinds, Reference Hinds1843 from the Recent of the eastern Pacific, designated by Olsson (Reference Olsson1967).
Remarks
Tucker and Tenorio (Reference Tucker and Tenorio2009) recognized Pyruconus as a genus of Conidae comprising two extant species from the eastern Pacific, C. patricius Hinds, Reference Hinds1843 and C. fergusoni Sowerby II, 1873. While the two species share large shells, large opercula, thick periostraca, and similar radulae, the molecular phylogenetic analysis of Puillandre et al. (Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014) did not support the close relationship between the two species that is suggested by morphological data. Despite this, the classification of Puillandre et al. (Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) retained both species in Pyruconus, which was recognized as a subgenus of Conus (see further discussion in Hendricks, Reference Hendricks2015). If future phylogenetic work confirms that C. patricius and C. fergusoni belong to different clades, a new subgeneric name for the clade that includes C. fergusoni will be necessary. This issue has bearing on the present study because one species from the Gatun Formation, Conus recognitus Guppy, Reference Guppy1867, bears close resemblance to C. patricius, while another Gatun species, Conus molis Brown and Pilsbry, Reference Brown and Pilsbry1911, is very similar to C. fergusoni. For the sake of recognizing and communicating the present taxonomic uncertainty concerning the membership of Pyruconus, fossil species that are more similar to C. recognitus are referred informally to “Pyruconus I,” while those that are more similar to C. fergusoni are referred to “Pyruconus II.”
Conus patricius is vermivorous (Nybakken, Reference Nybakken1988; Tenorio et al., Reference Tenorio, Tucker and Chaney2012) and the similar radular tooth structure of C. fergusoni, as well as its overall phylogenetic position (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014), strongly suggests that it shares this feeding ecology. Thus, a vermivorous feeding ecology may also be assumed for fossil species assigned to Pyruconus I and II.
Conus (Pyruconus I) recognitus Guppy, Reference Guppy1867

Figure 11 Conus (Pyruconus I) recognitus Guppy, Reference Guppy1867; all specimens photographed under regular light and are from UF locality YN020 unless otherwise indicated. (1) USNM 645736, specimen figured by Woodring (Reference Woodring1970, pl. 55, fig. 4), Panama Canal Zone, Woodring locality 182, upper Gatun Formation, SL 39.2 mm; (2–4) UF 190572, SL 33.8 mm, (4) showing features of the early postnuclear whorls; (5) UF 259879, SL 36.9 mm (measured from digital image); (6) UF 259876, calcitic cast, SL 56.0 mm (measured from digital image); (7) UF 259875, calcitic cast, MD 37.4 mm (measured from digital image). Scale bar to left of (1) is 10 mm and pertains to (1–3) and (5–7); scale bar associated with (4) is 5 mm.
1850 Conus solidus Reference SowerbySowerby I, p. 45 (not Conus solidus Gmelin, Reference Gmelin1791, which, according to Kohn [Reference Kohn2014], is a junior synonym of Conus mappa [Lightfoot, Reference Lightfoot1786], an extant western Atlantic species).
1866 Conus solidus; Guppy, p. 287, pl. 16, fig. 1.
1867 Conus recognitus Reference GuppyGuppy, p. 171.
1876 Conus recognitus; Reference GuppyGuppy, p. 527.
1917 Conus recognitus; Reference MauryMaury, pl. 7, fig. 9.
1921 Conus recognitus; Reference PilsbryPilsbry, pl. 19, fig. 2.
1961 Conus (Lithoconus) recognitus; Reference PflugPflug, p. 59, pl. 18, figs. 12–15.
1970 Conus recognitus; Reference WoodringWoodring, p. 346, pl. 55, fig. 4.
2009 Pyruconus recognitus (Guppy); Reference Tucker and TenorioTucker and Tenorio, p. 117.
2015 Conus (Pyruconus) recognitus; Reference HendricksHendricks, p. 42, fig. 17a–d.
Lectotype
NHMUK BM G83971, Yaque River, Dominican Republic, stratum unknown; designated by Pflug (Reference Pflug1961).
Occurrence
The exact type locality for the species is not known, but it is located somewhere in the Cibao Valley region of the northern Dominican Republic. In the Dominican Republic, the species is known to occur in both the upper Miocene Cercado Formation (personal observation) and lower Pliocene Gurabo Formation (Hendricks, Reference Hendricks2015), so the type stratum could potentially be either formation (Woodring, Reference Woodring1970, also reported the species from the Baitoa Formation of the Dominican Republic, presenting another possibility). Guppy (Reference Guppy1866) reported the species (as Conus solidus) from Jamaica (presumably from the Pliocene Bowden Formation) and later (Guppy, Reference Guppy1876) from Haiti (stratum unknown). Woodring (Reference Woodring1970) was the first to report the species from Panama based on a single specimen (USNM 645736; Fig. 11.1) from the upper Gatun Formation. Specimens from the lower Gatun Formation at UF locality YN020 confirm that the species ranges through the formation. Additional occurrences from Costa Rica and Colombia reported by Woodring (Reference Woodring1970) require confirmation.
Description
Maximum shell size: moderately large. While the largest complete specimen (UF 259876) from UF locality YN020 has SL 52.1 mm and MD 28.4 mm, an incomplete specimen (UF 259875) has MD 36.8 mm, suggesting that it had an estimated SL 65–70 mm. The lectotype has SL 65 mm (Pflug, Reference Pflug1961).
Last whorl
Shape slightly pyriform (RD 0.61,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 61}$$
; PMD 0.85–0.86,
$$\bar{x}\, {\equals}\, 0.{\rm 61}$$
; PMD 0.85–0.86,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 86}$$
; N=2); outline convex on posterior half, slightly concave on anterior third, resulting in a sigmoidal profile. Shoulder round; smooth. Widest part of shell below shoulder. Aperture uniform in width from base to shoulder. A slight siphonal notch may be present (Fig. 11.6), but confirmation of this requires more completely preserved specimens to be discovered. Spiral threads on anterior half, becoming obsolete towards shoulder.
$$\bar{x}\, {\equals}\, 0.{\rm 86}$$
; N=2); outline convex on posterior half, slightly concave on anterior third, resulting in a sigmoidal profile. Shoulder round; smooth. Widest part of shell below shoulder. Aperture uniform in width from base to shoulder. A slight siphonal notch may be present (Fig. 11.6), but confirmation of this requires more completely preserved specimens to be discovered. Spiral threads on anterior half, becoming obsolete towards shoulder.
Spire whorls
Spire low (RSH 0.09–0.10;
![]() $$\bar{x}\, {\equals}\, 0.{\rm 1}0$$
; N=2); outline nearly flat to slightly sigmoidal. Protoconch unknown. Early postnuclear whorls unknown, but at least five of the teleoconch whorls of UF 190572 bear tubercles, which diminish thereafter (Fig. 11.4). Sutural ramp convex, with fine spiral treads. Subsutural flexure asymmetrical (ASSF 0.2–0.7,
$$\bar{x}\, {\equals}\, 0.{\rm 1}0$$
; N=2); outline nearly flat to slightly sigmoidal. Protoconch unknown. Early postnuclear whorls unknown, but at least five of the teleoconch whorls of UF 190572 bear tubercles, which diminish thereafter (Fig. 11.4). Sutural ramp convex, with fine spiral treads. Subsutural flexure asymmetrical (ASSF 0.2–0.7,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 4}$$
, N=5), depth usually slightly greater than width (DWSSF 0.9–1.4,
$$\bar{x}\, {\equals}\, 0.{\rm 4}$$
, N=5), depth usually slightly greater than width (DWSSF 0.9–1.4,
![]() $$\bar{x}\, {\equals}\, {\rm 1}.{\rm 2}$$
, N=5) (Fig. 11.3, 11.4).
$$\bar{x}\, {\equals}\, {\rm 1}.{\rm 2}$$
, N=5) (Fig. 11.3, 11.4).
Coloration pattern
No observed specimens from UF locality YN020 show evidence of a preserved coloration pattern.
Materials
NHMUK BM G83971 (lectotype of Conus recognitus Guppy, Reference Guppy1867); USNM 645736 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 11.1); and an additional 18 observed specimens, all from UF locality YN020.
Remarks
A thorough review of this species and its complicated nomenclatural history were provided by Woodring (Reference Woodring1970; also see Woodring, 1928), who reported only a single specimen (USNM 645736; Fig. 11.1) from the Gatun Formation of Panama (Woodring locality 182, which is located in the upper Gatun Formation). Intensive searching at UF locality YN020 resulted in the discovery of 18 additional specimens from the Gatun Formation, though all but two of these were fragmentary or otherwise poorly preserved. Interestingly, seven of the 18 specimens that were found at YN020 are preserved as calcitic casts (e.g., Fig. 11.6, 11.7), a much higher proportion than observed in other species from this locality. Why this mode of preservation was so much more common in C. recognitus than the other species found is unknown.
The unaltered specimens found do not show any clear evidence of a coloration pattern under ultraviolet light. It could be the case that the pattern is simply not preserved in the specimens found, or that the living snails had a solid pattern of pigmentation that covered the entire shell, resulting in an undifferentiated pattern of fluorescence under UV light. Hendricks (Reference Hendricks2015) suggested the latter for specimens of C. recognitus from the lower Pliocene Gurabo Formation of the Dominican Republic, and this conclusion seems likely for this Panamanian material as well.
As also recognized by Woodring (1966, Reference Woodring1970) and Hendricks (Reference Hendricks2015), C. recognitus shares numerous shell similarities with the extant eastern Pacific species C. patricius Hinds, Reference Hinds1843—a taxon of known phylogenetic position (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014)—justifying assignment of this taxon to the same subgenus, Pyruconus Olsson, Reference Olsson1967. The pyriform shells of members of the Pyruconus I clade are distinctive and the establishment here of C. recognitus in the lower Gatun Formation provides a potentially useful calibration date for future molecular phylogenetic studies. Given its unique shell shape, especially its rounded shoulder, C. recognitus cannot be easily confused with co-occurring fossil species in the Gatun Formation.
Conus (Pyruconus II) molis Brown and Pilsbry, Reference Brown and Pilsbry1911

Figure 12 Conus (Pyruconus II) molis Brown and Pilsbry, Reference Brown and Pilsbry1911 from the Gatun Formation of Panama: (1–4) photographed under regular light; (5–12) photographed under UV light; all specimens are from UF locality YN020 (lower Gatun Formation) unless otherwise indicated. (1) USNM 645743, specimen figured by Woodring (Reference Woodring1970, pl. 55, figs. 9, 10), Panama Canal Zone, Woodring locality 155b, middle Gatun Formation, SL 54.7 mm; (2) ANSP 1684, a syntype of Conus concavitectum Brown and Pilsbry, Reference Brown and Pilsbry1911, Panama Canal Zone, Gatun Formation, SL 37.0 mm (measured from digital image); (3) UF 259785, showing the shape of the subsutural flexure and features of the sutural ramp; (4, 5) UF 259776, MD 40.0 mm (damaged), with (4) showing features of the early postnuclear whorls; (6) UF 259787, MD 28.0 mm (damaged); (7) UF 270981, SL 26.5 mm; (8, 9) UF 259780, MD 38.1 mm (damaged); (10) UF 270982, MD 51.0 mm (damaged); (11) UF 190574, SL 79.0 mm; (12) UF 270983, SL 83.1 mm. Scale bar to the left of (1) equals 10 mm and pertains to (1, 2, 5–12); scale bar to the right of (3) equals 5 mm and pertains to that specimen; and scale bar to the right of (4), which is a focus-stacked composite image, equals 1 mm and pertains to that specimen.
1911 Conus molis Reference Brown and PilsbryBrown and Pilsbry, p. 343, pl. 23, fig. 1.
1911 Conus concavitectum Reference Brown and PilsbryBrown and Pilsbry, p. 341, pl. 23, figs. 5, 6.
1911 ?Conus haytensis; Reference Brown and PilsbryBrown and Pilsbry, p. 341 (not Sowerby I, 1850).
1917 Not Conus molis; Reference MauryMaury, p. 200 (likely=Conus haytensis Sowerby I, 1850).
1970 Conus molis; Woodring, Reference Woodring1970, p. 350, pl. 55, figs. 8–10.
1993 Not Conus cf. molis Brown and Pilsbry; Reference Pitt and PittPitt and Pitt, p. 10, pl. 4, fig. 2 (= Conus spurius Gmelin, Reference Gmelin1791).
Holotype
USNM 636043, “excavations for the locks at Gatun” (Brown and Pilsbry, Reference Brown and Pilsbry1911, p. 336), Panama Canal Zone. Woodring (Reference Woodring1970, p. 350) stated that the type locality is positioned in the “middle part of the Gatun [F]ormation.”
Occurrence
Woodring (Reference Woodring1970) reported that Conus molis spans the lower to upper Gatun Formation. He also reported that the species occurs in the Limón Formation of Panama, the Tubará Formation of Colombia, and unnamed deposits in Costa Rica; these records require confirmation. Maury’s (Reference Maury1917) report of the species from the Dominican Republic is considered incorrect (see below).
Description
Maximum shell size: large. The largest nearly complete observed specimen (UF 256531) from UF locality YN020 has SL 98.2 mm. Woodring (Reference Woodring1970, pl. 55, fig. 8) figured a specimen (USNM 645742) from the upper Gatun Formation (Woodring locality 185) that he reported as having an SL of 160 mm.
Last whorl.—Shape broadly and ventricosely conical, broadly conical, or conical (RD 0.68–0.74,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 71}$$
; PMD 0.84–0.94,
$$\bar{x}\, {\equals}\, 0.{\rm 71}$$
; PMD 0.84–0.94,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 89}$$
; values based on three specimens, two of which are slightly damaged); outline convex on posterior half, slightly concave to nearly straight on anterior half, resulting in sigmoidal to slightly convex profile. Shoulder sharply angulate or angulate, rarely forming an adaxially slanting carinate ridge; smooth. Widest part of shell below shoulder. Aperture nearly uniform in width from base to shoulder. Siphonal notch absent. Spiral threads present on anterior half of last whorl of immature specimens, sometimes diminishing towards shoulder and occasionally forming beads (e.g., UF 270981); weak and slightly wavy spiral threads on anterior quarter of mature specimens, with very fine spiral threads sometimes extending to the shoulder.
$$\bar{x}\, {\equals}\, 0.{\rm 89}$$
; values based on three specimens, two of which are slightly damaged); outline convex on posterior half, slightly concave to nearly straight on anterior half, resulting in sigmoidal to slightly convex profile. Shoulder sharply angulate or angulate, rarely forming an adaxially slanting carinate ridge; smooth. Widest part of shell below shoulder. Aperture nearly uniform in width from base to shoulder. Siphonal notch absent. Spiral threads present on anterior half of last whorl of immature specimens, sometimes diminishing towards shoulder and occasionally forming beads (e.g., UF 270981); weak and slightly wavy spiral threads on anterior quarter of mature specimens, with very fine spiral threads sometimes extending to the shoulder.
Spire whorls
Spire low to moderate (RSH 0.10–0.13;
![]() $$\bar{x}\, {\equals}\, 0.{\rm 12}$$
; values based on three specimens, two of which are slightly damaged); outline concave to nearly straight. Protoconch unknown. Most specimens have poorly preserved apices, but two observed specimens (UF 259776 and UF 271034) show evidence of weakly tuberculate early postnuclear whorls. Early whorls are stepped. Sutural ramp concave to nearly flat. Four or five spiral grooves separate threads on the ramps of immature specimens; mature individuals may have several more grooves and threads, though spiral ornamentation sometimes becomes obsolete on the final intervals of growth. Subsutural flexure nearly symmetrical (ASSF 0.7–0.9;
$$\bar{x}\, {\equals}\, 0.{\rm 12}$$
; values based on three specimens, two of which are slightly damaged); outline concave to nearly straight. Protoconch unknown. Most specimens have poorly preserved apices, but two observed specimens (UF 259776 and UF 271034) show evidence of weakly tuberculate early postnuclear whorls. Early whorls are stepped. Sutural ramp concave to nearly flat. Four or five spiral grooves separate threads on the ramps of immature specimens; mature individuals may have several more grooves and threads, though spiral ornamentation sometimes becomes obsolete on the final intervals of growth. Subsutural flexure nearly symmetrical (ASSF 0.7–0.9;
![]() $$\bar{x}\, {\equals}\, 0.{\rm 7}$$
; N=4), depth typically slightly greater than width (DWSSF 0.9–1.7;
$$\bar{x}\, {\equals}\, 0.{\rm 7}$$
; N=4), depth typically slightly greater than width (DWSSF 0.9–1.7;
![]() $$\bar{x}\, {\equals}\, 1.4$$
; n=4).
$$\bar{x}\, {\equals}\, 1.4$$
; n=4).
Coloration pattern
Two occasionally interacting patterns present that vary in the color of emitted light. The primary pattern consists of fine to wide axial streaks that are often separated into two (e.g., Fig. 12.7, 12.8, 12.12) or three (e.g., Fig. 12.6) regions along the length of the last whorl. The streaks range from nearly straight (e.g., 12.7, 12.11) to irregular (e.g., Fig. 12.8), sometimes forming diagonal streaks composed of sub-triangular elements (e.g., Fig. 12.10). Several rows of spiral dots and dashes are visible on the anterior half of one large specimen (Fig. 12.11) and the primary pattern is nearly absent from another large specimen (Fig. 12.12). The secondary pattern usually consists of at least two nearly continuous spiral bands; a third band is present just below the shoulder in some specimens, though it tends to be weaker than the bands positioned at the midline and on the anterior third of the last whorl. The immature specimen shown in Figure 12.7 does not show evidence of the secondary pattern. In one specimen (Fig. 12.10), the secondary pattern has the appearance of smearing the primary pattern, suggesting a degree of interaction between the two layers. Sutural ramp with diffuse radial blotches (Fig. 12.9).
Materials
USNM 645743 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 12.1); ANSP 1684, syntype of Conus concavitectum Brown and Pilsbry, Reference Brown and Pilsbry1911 (Fig. 12.2); and 81 specimens from UF locality YN020.
Remarks
The holotype of Conus molis was part of the Princeton University invertebrate paleontology collection (formerly PU 5502), but now resides in the Smithsonian collection (USNM 636043). Woodring (Reference Woodring1970) considered Conus concavitectum Brown and Pilsbry (Reference Brown and Pilsbry1911) to represent a juvenile C. molis; on this basis, he conferred taxonomic precedence to C. molis over C. concavitectum. The syntypes of C. concavitectum, one of which is figured here (Fig. 12.2), were observed and Woodring’s conclusion that this species is synonymous with C. molis is accepted here. Brown and Pilsbry (Reference Brown and Pilsbry1911, p. 341) reported, but did not figure, a “perfect but small specimen” of Conus haytensis Sowerby I, 1850 from the Gatun Formation; this unknown specimen is presumed to instead be C. molis, which is very similar (see below). Conversely, Maury (Reference Maury1917) reported C. molis from the Neogene of the Dominican Republic, but Woodring (Reference Woodring1970, p. 351) noted, “[i]t is likely that Maury’s C. molis is an immature C. haytensis.” Pending future study, Maury’s reported occurrence of this taxon in the Dominican Republic is considered incorrect. The specimen of Conus cf. molis figured by Pitt and Pitt (Reference Pitt and Pitt1993, pl. 4, fig. 2) has a coloration pattern that is consistent with C. spurius Gmelin, Reference Gmelin1791 as circumscribed herein (see below). See Woodring (Reference Woodring1970) for a complete synonymy list.
The most notable feature of Conus molis is its remarkably large size, which readily dwarfs co-occurring species from the Gatun Formation, as well as most other fossil and extant tropical American cone snail species. Complete and nearly complete specimens of mature C. molis are very rare at UF locality YN020, and most of the specimens that can be unequivocally assigned to C. molis consist of large spire fragments. While these mature specimens from YN020 were easily assigned to C. molis, mature individuals of Conus aemulator are very similar to immature specimens of C. molis and can be readily confused; see the remarks for C. aemulator for differences between the two species. Woodring’s (Reference Woodring1970, p. 351) description of C. molis noted that “immature shells” sometimes preserve a coloration pattern “consisting of spiral rows of brownish crude rectangles, much like the pattern of C. spurius.” Such a pattern, however, is different from that described here for both immature and mature specimens of C. molis (e.g., Fig. 12.5–12.12). Instead, these “immature shells” of C. molis mentioned by Woodring (Reference Woodring1970) are considered here to be specimens of C. spurius (see description of that species below). While specimens of C. spurius from UF locality YN020 have overall shell shapes that are somewhat similar to those of C. molis and C. aemulator, they lack the prominent spiral threads that cover the sutural ramps of the latter two species.
Beyond the Gatun Formation, C. molis shares many morphological similarities with Conus haytensis Sowerby I, 1850 from the Pliocene of the Dominican Republic and Florida, which has a somewhat different coloration pattern (see Hendricks, 2009, Reference Hendricks2015; a more detailed comparison will require re-description of the Dominican material). Conus molis is also similar in shell morphology to the extant eastern Pacific taxon Conus fergusoni Sowerby II, 1873 (also recognized by Woodring, Reference Woodring1966), which has a known phylogenetic position (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014), allowing both fossil taxa to be assigned with confidence to the clade (subgenus “Pyruconus II”) represented today only by C. fergusoni.
Subgenus Spuriconus Petuch, Reference Petuch2003
Type species
Conus spurius Gmelin, Reference Gmelin1791, from the Recent of the western Atlantic, designated by Petuch (Reference Petuch2003).
Remarks
Conus spurius was included in the molecular phylogenetic analysis of Puillandre et al. (Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014) and was found to occupy a derived position within Conus. Puillandre et al. (2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) assigned C. spurius to the Conus subgenus Lindaconus Petuch, Reference Petuch2002. As recently noted by Hendricks (Reference Hendricks2015), however, the type species for Lindaconus—Conus lindae Petuch, Reference Petuch1987—is a synonym of Conus flavescens Sowerby I, 1834 (see Kohn, Reference Kohn2014), which belongs in the Conus subgenus Dauciconus (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014). Therefore, the name Spuriconus Petuch, Reference Petuch2003 is favored for this subgenus over Lindaconus. Conus spurius is vermivorous (Kohn, Reference Kohn2014) and possibly also molluscivorous (Leal et al., Reference Leal, Kohn and Mensch2017); the feeding ecologies of fossil species assigned to Spuriconus are therefore uncertain, though vermivory is likely.
Conus (Spuriconus) spurius Gmelin, Reference Gmelin1791

Figure 13 Conus (Spuriconus) spurius Gmelin, Reference Gmelin1791 from the Gatun Formation of Panama: (1, 2) photographed under regular light; (3–20) photographed under UV light; all specimens are from UF locality YN020 unless otherwise indicated. (1, 2) USNM 645738, specimen figured by Woodring (Reference Woodring1970, pl. 55, fig. 7), Panama Canal Zone, Woodring locality 155, middle Gatun Formation, SL 45.8 mm, MD 28.9 mm; (3, 4) UF 256537, SL 43.2 mm, MD 23.7 mm; (5) UF 271003, SL 42.6 mm; (6) UF 271004, SL 33.9 mm; (7) UF 259760, SL 35.6 mm; (8) UF 259745, SL 36.5 mm; (9) UF 259766, SL 19.9 mm; (10) UF 271005, SL 39.3 mm; (11) UF 271006, SL 40.0 mm; (12) UF 271007, SL 36.5 mm; (13) UF 271008, SL 31.7 mm; (14) UF 271009, SL 37.2 mm; (15) UF 271010, SL 27.7 mm; (16) UF 271011, SL 35.7 mm; (17) UF 271012, SL 43.8 mm; (18) UF 271013, SL 42.5 mm; (19) UF 271014, SL 46.4 mm; (20) UF 271015, SL 46.6 mm. Scale bar to the left of (1) equals 10 mm and pertains to all specimens.
1791 Conus spurius Gmelin, Reference Gmelin1791, p. 3396, no. 67.
1970 ?Conus spurius; Reference WoodringWoodring, p. 348, pl. 55, fig. 7.
1993 Conus cf. molis Brown and Pilsbry; Reference Pitt and PittPitt and Pitt, p. 10, pl. 4, fig. 2 (not C. molis Brown and Pilsbry, Reference Brown and Pilsbry1911).
2008 Conus spurius s.l.; Reference Landau, Vermeij and da SilvaLandau, Vermeij, and da Silva, p. 458.
2009 Conus spurius; Reference HendricksHendricks, p. 19, pl. 3, figs. 1–14, pl. 4, figs. 1–15 (includes complete synonym list for Plio-Pleistocene records for the southeastern U.S.).
2009 Spuriconus spurius (Gmelin, Reference Gmelin1791); Reference Tucker and TenorioTucker and Tenorio, p. 121, pl. 7, fig. 7.
2013 Lindaconus spurius spurius (Gmelin, Reference Gmelin1791); Reference TuckerTucker, p. 125, figs. 44–46.
2014 Conus spurius; Reference KohnKohn, p. 345, pls. 97–100 (includes complete synonym list for modern records).
2015 Conus (Spuriconus) spurius; Reference HendricksHendricks, p. 49, fig. 26a–f, k.
2017 Conus sp.; Reference WilliamsWilliams, fig. 6b, f.
Lectotype
Illustration in Gualtieri (Reference Gualtieri1742, pl. 21, figs. d, f) designated by Clench (Reference Clench1942) and recently reproduced in Kohn (Reference Kohn2014, pl. 97, figs. 1, 2), Virgin Gorda, Virgin Islands (see Vink, Reference Vink1985; Kohn, Reference Kohn2014).
Occurrence
Kohn (Reference Kohn2014) reviewed the distribution of extant Conus spurius and published an occurrence map (Kohn, Reference Kohn2014, map 5.29) showing the species spanning most of the Caribbean, the east and west coasts of Florida, and the northern Yucatán Peninsula. The species also has an extensive Neogene tropical American fossil record, including the Plio-Pleistocene of Florida (Hendricks, Reference Hendricks2009) and the early Pliocene Gurabo Formation of the Dominican Republic (Hendricks, Reference Hendricks2015). Landau and da Silva (Reference Landau and da Silva2010) assigned 24 specimens from the lower Pliocene Araya Formation of Cubagua Island, Venezuela to C. spurius. One of their two figured specimens (NHMW 2010/0038/0150; pl. 21, fig. 5a–c), however, appears to be Conus humerosus Pilsbry, Reference Pilsbry1921 (see Hendricks, Reference Hendricks2015, figs. 3, 26g–j), while the other (NHMW 2010/0038/0214, pl. 21, fig. 6a, b) is assigned to C. woodringi n. sp. (see remarks associated with that species). It is nevertheless possible that some (or all) of the remaining 22 specimens belong to C. spurius, although these have not been observed by the author. Woodring (Reference Woodring1970) reported C. spurius from the middle Gatun Formation of Panama on the basis of a single specimen (USNM 645738, Fig. 13.1, 13.2), but its identification is in question (see below). Numerous specimens found at UF locality YN020 confirm the presence of the species in the lower Gatun Formation. Other occurrence records reported by Woodring (Reference Woodring1970) from the Neogene of Panama, Costa Rica, Colombia, and Jamaica (Bowden Formation) require confirmation.
Description
Note that this description pertains only to specimens from UF locality YN020, not recent or fossil specimens of C. spurius from elsewhere. Maximum shell size: medium. Typical shell size of specimens from UF locality YN020: medium (36.7 mm; N=120). Largest observed specimen from YN020 (UF 271016) has SL 53.5 mm.
Last whorl
Shape usually conical, sometimes broadly conical, rarely ventricosely conical or broadly and ventricosely conical (RD 0.60–0.74,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 67}$$
; PMD 0.84–0.93,
$$\bar{x}\, {\equals}\, 0.{\rm 67}$$
; PMD 0.84–0.93,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 89}$$
; N=47); outline convex on posterior half, slightly concave to straight on anterior half, resulting in a slightly sigmoidal to slightly convex profile. Shoulder carinate and forming a posterior-pointing ridge. Widest part of shell below shoulder. Aperture slightly wider at base than shoulder. Siphonal notch absent. Spiral threads on anterior half, sometimes extending past midline, but rarely extending to shoulder.
$$\bar{x}\, {\equals}\, 0.{\rm 89}$$
; N=47); outline convex on posterior half, slightly concave to straight on anterior half, resulting in a slightly sigmoidal to slightly convex profile. Shoulder carinate and forming a posterior-pointing ridge. Widest part of shell below shoulder. Aperture slightly wider at base than shoulder. Siphonal notch absent. Spiral threads on anterior half, sometimes extending past midline, but rarely extending to shoulder.
Spire whorls
Spire low to high (RSH 0.06–0.25;
![]() $$\bar{x}\, {\equals}\, 0.{\rm 14}$$
; N=47); outline concave to slightly sigmoidal. Early spire whorls sometimes stepped, with angulate to subangulate shoulders. In some low-spired specimens (Fig. 13.8, 13.19), the later whorls rise slightly above those that formed earlier, giving the spire a sunken appearance. Quality of preservation prevents the number of protoconch whorls from being determined with confidence, but UF 259766 (Fig. 13.9) appears to show more than two whorls. Similarly, quality of preservation prevents definitive characterization of the features of the earliest whorls, but UF 259756 and UF 259766 suggest that at least one postnuclear whorl may bear fine tubercles. Sutural ramp often convex on early whorls, often concave on later whorls. Spiral ornamentation on the sutural ramp is highly variable: specimens often have 2–4 fine spiral grooves and/or threads on earlier whorls, but these usually become obsolete later in growth and most mature specimens lack spiral ornamentation on the terminal portion of the ramp (e.g., UF 259746). Subsutural flexure asymmetrical (ASSF 0.2–0.7,
$$\bar{x}\, {\equals}\, 0.{\rm 14}$$
; N=47); outline concave to slightly sigmoidal. Early spire whorls sometimes stepped, with angulate to subangulate shoulders. In some low-spired specimens (Fig. 13.8, 13.19), the later whorls rise slightly above those that formed earlier, giving the spire a sunken appearance. Quality of preservation prevents the number of protoconch whorls from being determined with confidence, but UF 259766 (Fig. 13.9) appears to show more than two whorls. Similarly, quality of preservation prevents definitive characterization of the features of the earliest whorls, but UF 259756 and UF 259766 suggest that at least one postnuclear whorl may bear fine tubercles. Sutural ramp often convex on early whorls, often concave on later whorls. Spiral ornamentation on the sutural ramp is highly variable: specimens often have 2–4 fine spiral grooves and/or threads on earlier whorls, but these usually become obsolete later in growth and most mature specimens lack spiral ornamentation on the terminal portion of the ramp (e.g., UF 259746). Subsutural flexure asymmetrical (ASSF 0.2–0.7,
![]() $$\bar{x}\, {\equals}\, 0.{\rm 5}$$
, N=4), depth approximately twice width (DWSSF 1.7–2.2,
$$\bar{x}\, {\equals}\, 0.{\rm 5}$$
, N=4), depth approximately twice width (DWSSF 1.7–2.2,
![]() $$\bar{x}\, {\equals}\, {\rm 1}.{\rm 8}$$
, N=4).
$$\bar{x}\, {\equals}\, {\rm 1}.{\rm 8}$$
, N=4).
Coloration pattern
One pattern present. Pattern consists of 9–18 rows of spiral elements. The most conspicuous elements on most specimens are large rectangular or sub-rectangular blotches, although these are sometimes irregular in shape, including on the same specimen (Fig. 13.11); these rows are sometimes separated by one or more rows of small dots or dashes. In some specimens, the spiral elements merge at their margins (Fig. 13.14), sometimes producing axial streaks (Fig. 13.15, 13.16). In other specimens, the rectangular blotches are closely spaced (Fig. 13.17) and sometimes connect to form nearly continuous (Fig. 13.18) or continuous (Fig. 13.19) spiral bands. In one observed specimen (Fig. 13.20), spiral elements on the posterior third of the last whorl form markings that are nearly triangular in shape. Sutural ramp with irregularly shaped radial botches that sometimes extend over the shoulder onto the last whorl.
Materials
USNM 645738 (one specimen, figured by Woodring, Reference Woodring1970; Fig. 13.1, 13.2) and an additional 304 observed specimens, all from UF locality YN020.
Remarks
Woodring’s (Reference Woodring1970, p. 348) assertion that the extant western Atlantic species Conus spurius occurs in the Gatun Formation was based on a single specimen (USNM 645738; Fig. 13.1, 13.2) found at his locality 155, “Middle part of Gatun formation, eastern area.” The >300 specimens found at UF locality YN020—the most of any species—provide substantial additional support for this occurrence in the Gatun Formation of Panama, although USNM 645738 presents additional questions (see below).
Extant C. spurius have highly variable, but also distinctive, shell coloration patterns (e.g., Tucker, Reference Tucker2013, figs. 41–43; Kohn, Reference Kohn2014, pls. 97–100), which is also observed in the specimens from UF locality YN020. Some specimens (e.g., Fig. 13.3–13.8) from YN020 show patterns that are typical of many extant C. spurius, featuring irregularly shaped spiral blotches. More commonly, however, specimens from YN020 have spiral blotches that are nearly rectangular in shape (e.g., Fig. 13.13), although some specimens (e.g., Fig. 13.11) exhibit both irregular and rectangular blotches. In some specimens (e.g., Fig. 13.15, 13.16), spiral rows of blotches coalesce to form axial streaks, while in others (e.g., Fig. 13.17–13.19) they merge to form continuous or nearly continuous spiral bands; these variations of pigmentation patterning are also observed in some extant C. spurius (see Kohn, Reference Kohn2014, pls. 97–100).
In his description of Conus molis Brown and Pilsbry, Reference Brown and Pilsbry1911, Woodring (Reference Woodring1970, p. 350) mentioned “immature shells” with a coloration pattern “consisting of spiral rows of brownish crude rectangles, much like the pattern of Conus spurius.” As shown above, the coloration pattern of C. molis is very different from that of C. spurius and the shells to which Woodring referred are assumed to represent specimens of C. spurius. Indeed, Pitt and Pitt (Reference Pitt and Pitt1993) used UV photography to characterize the coloration pattern of a specimen (apparently one of many in their collection) of Conus cf. molis that has a “pattern of spiral rows of crude rectangles, much like the color pattern of C. spurius” (Pitt and Pitt, Reference Pitt and Pitt1993, p. 10, 12, pl. 4, fig. 2). Williams (Reference Williams2017, fig. 6f) recently figured a specimen of “Conus sp.” from the Gatun Formation that has a coloration pattern consistent with most specimens of C. spurius from YN020.
Apart from variability in coloration pattern, the shells from YN020 assigned here to C. spurius are otherwise consistent in shell form with one another and overlap in shell shape with extant C. spurius as circumscribed by Kohn (Reference Kohn2014), who provided the following shell shape data for the species: RD 0.56–0.75, mean=0.67; PMD 0.81–0.95, mean=0.89; RSH 0.05–0.26, mean=0.17. The specimens from YN020 are on average smaller than modern specimens (typical SL 58 mm, maximum SL 105 mm; Kohn, Reference Kohn2014, p. 346). Further, the distinctive carinate shoulder of specimens from YN020 is “subangulate to rounded” (Kohn, Reference Kohn2014, p. 346) in modern C. spurius, which is also observed in Woodring’s specimen from the Gatun Formation (Fig. 13.1).
The specimens found at UF locality YN020 add additional support to Kohn’s (Reference Kohn2014, p. 356) suggestion that Conus spurius may have the longest fossil record of any known extant species of Conidae (also see Hendricks, Reference Hendricks2015). Its occurrence in the lower Gatun Formation puts a minimum age for the taxon at 10 Ma, offering a potentially useful calibration date for the clade that includes C. spurius (Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014). Landau et al. (Reference Landau, Vermeij and da Silva2008) listed C. spurius as occurring in the lower Miocene of Cubagua, Venezuela so, pending additional study, the duration of the species may extend even further back in time.
Without the aid of UV light to reveal their very different shell coloration patterns, specimens of C. spurius from the Gatun Formation could be most easily confused with immature specimens of Conus molis as circumscribed here. A key difference is that C. molis has strong spiral threads on its sutural ramps, while such ornamentation is either very fine or absent in C. spurius. Notably, the absence of this spiral ornamentation is what led Pitt and Pitt (Reference Pitt and Pitt1993) to assign their figured specimen Conus cf. molis, rather than to assign it to C. molis outright. For comparisons between C. spurius and other species with which it co-occurs in other deposits, see Hendricks (2009, Reference Hendricks2015).
Dr. Judy Smith of the Smithsonian Institution recently recognized (J. Smith, personal communication, October 24, 2016) that Woodring’s (Reference Woodring1970) sole specimen (USNM 645738; Fig. 13.1, 13.2) of C. spurius from the Gatun Formation bears great similarity to Conus bramkampi Hanna and Strong, Reference Hanna and Strong1949, a species described from the Pliocene (Redman et al., Reference Redman, Leighton, Schellenberg, Gale, Nielsen, Dressler and Klinger2007) Imperial Formation of Alverson Canyon, Imperial County, California (holotype UCMP 34199). Conus bramkampi has a coloration pattern that is very similar to that of C. spurius, but has a much more rounded shoulder, which is evident on USNM 645738. Comparison of C. spurius with C. bramkampi requires additional study, especially because of the potential biogeographic importance of C. bramkampi for understanding the history of the Spuriconus clade in tropical America prior to the closure of the Central American Seaway.
Results
Of the 25 species-group Conidae taxa previously reported from the Gatun Formation of Panama (Table 1), nine (including a questionable record for Conus symmetricus) are recognized as occurring in the lower Gatun Formation at UF locality YN020 and one additional species was newly recognized as Conus (Stephanoconus) woodringi (Table 2). An additional five of the 25 taxa are considered valid, but were not found at YN020; type and previously figured specimens of four of the latter group were examined and photographed as part of this study and are shown in Figure 14 (Conus tortuosostriatus Toula, Reference Toula1911, Conus musaensis Olsson, Reference Olsson1922, Conus acolus Woodring, Reference Woodring1970, and Conus bravoi Spieker, Reference Spieker1922). Finally, 11 of the 25 species are considered synonymous with other species, or are otherwise dubious. For example, Conus tortuosopunctatus Toula, Reference Toula1911 (NHMW 1933/0018/0223), which is known from a single shell (Fig. 14.1), is almost certainly not from the Gatun Formation. Woodring (Reference Woodring1970) noted that sediment preserved in its aperture is not consistent with that of the Gatun Formation. The relatively well-preserved coloration pattern of this shell suggests that it may be a worn specimen of a modern species, possibly Conasprella (Ximeniconus) mahogani (Reeve, Reference Reeve1843).

Figure 14 Type and other specimens of taxa previously reported from the Gatun Formation of Panama, but not found at UF locality YN020: (1–6, 8, 10, 11) photographed under regular light; (7, 9) photographed under UV light. (1) NHMW 1933/0018/0223, holotype, Conus tortuosopunctatus Toula, Reference Toula1911, Gatun, Panama Canal, SL 19.5 mm (measured from digital image); (2) NHMW 1933/0018/0224, holotype, Conus tortuosostriatus Toula, Reference Toula1911, Gatun, Panama Canal, SL 34.3 mm (measured from digital image); (3) USNM 645756, specimen of Conus tortuosostriatus Toula, Reference Toula1911 figured by Woodring (Reference Woodring1970, pl. 57, fig. 5), Panama Canal Zone (eastern area), Woodring locality 175, upper Gatun Formation, SL 26.2 mm; (4) USNM 645755, specimen of Conus tortuosostriatus Toula, Reference Toula1911 figured by Woodring (Reference Woodring1970, pl. 57, fig. 6), Panama Canal Zone (eastern area), Woodring locality 175, upper Gatun Formation, SL 28.3 mm; (5) PRI 20887, lectotype, Conus musaensis Olsson, Reference Olsson1922, Banana River, Costa Rica, SL 17.7 mm; (6) USNM 645737, specimen of Conus musaensis Olsson, Reference Olsson1922 figured by Woodring (Reference Woodring1970, pl. 57, fig. 2), Panama Canal Zone, Woodring locality 170, middle Gatun Formation, SL 20.6 mm; (7) USNM 645741, holotype, Conus acolus Woodring, Reference Woodring1970, Panama Canal Zone, Woodring locality 136a, lower Gatun Formation, SL 35.1 mm; (8, 9) USNM 645739, specimen of Conus bravoi Spieker, Reference Spieker1922 figured by Woodring (Reference Woodring1970, pl. 56, fig. 10), Panama Canal Zone, Woodring locality 138c, lower Gatun Formation, SL 58.5 mm; (10) USNM 645740, specimen of Conus bravoi Spieker, Reference Spieker1922 figured by Woodring (Reference Woodring1970, pl. 56, fig. 11), Panama Canal Zone, Woodring locality 137a, lower Gatun Formation, SL 63.9 mm; (11) YPM 520, syntype (one of four), Conus molis var. bravoi Spieker, Reference Spieker1922, near mouth of Quebrada Tusillal, Zorritos Formation, Peru, SL 76.8 mm (measured from digital image). Scale bar to the left of (1) equals 10 mm and pertains to (1–7); scale bar to the left of (8) equals 10 mm and pertains to (8–11).
Table 2 Numbers of specimens of each species found at UF locality YN020.

Discussion
The 10 species recognized here from UF locality YN020 are represented by a total of 884 specimens (Table 2). This collection highlights the importance of attaining large sample sizes for documenting species-level and phylogenetic diversity at local scales. Indeed, the four rarest species were represented by a total of only 11 specimens and likely would not have been collected without intensive, taxonomically focused sampling conducted by multiple individuals. Five additional specimens found at YN020, all of which are fragmentary or poorly preserved, may represent additional, and possibly undescribed, biodiversity. Following the argument in Hendricks (Reference Hendricks2015), which suggested that at least three specimens are necessary to adequately characterize a new species of fossil cone snail, these are left undescribed pending discovery of additional, better-preserved specimens. They are illustrated in Figure 15, however, to facilitate future comparative study should additional material becomes available.

Figure 15 Fragmentary or poorly preserved Conidae of indeterminate taxonomic assignment; all specimens are from UF locality YN020 and were photographed under regular light. (1, 2) UF 256536, SL 42.8 mm; (3) UF 271042, SL 25.6 mm; (4) UF 271040, SL 27.4 mm; (5) UF 259805, SL 26.7 mm; (6) UF 271036, SL 13.5 mm. Scale bar to left of (1) is 10 mm and pertains to all specimens.
Many thousands of years likely separate the geologically oldest cone snail specimen found at UF locality YN020 from the youngest and, as noted above, water depth likely fluctuated on the scale of tens of meters during the interval in which the study strata were deposited (see Hendy, Reference Hendy2013; Anderson et al., Reference Anderson, Hendy, Johnson and Allmon2017). For these reasons, and because of the non-standardized sampling protocol employed, it is not possible to determine which of the 10 species documented here from YN020 were members of the same ecological communities during particular intervals of time. It is possible, for instance, that the total study sample is derived from multiple depth gradients, which captured different components of the total Conidae fauna as water depth shifted over time. This might also explain why some of the fossil Conidae documented from the Gatun Formation by Woodring (Reference Woodring1970) were not found at YN020.
Considered as a whole, however, the total biodiversity of the UF locality YN020 Conidae fauna can be considered moderate when compared to other recently documented tropical American assemblages. For example, Landau et al. (Reference Landau, da Silva, Heitz and Janssen2016) recently documented eight species from the Miocene Cantaure Formation of Venezuela (including Conus aemulator; see above), and Hendricks (Reference Hendricks2015) demonstrated that three late Miocene and early Pliocene coral reef-associated assemblages from the Dominican Republic were represented by 14–16 species, though collectively the three assemblages represent at least 28 different species. Today, ~19 species of Conidae are found off the Atlantic coast of Panama (data compiled from Kohn, Reference Kohn2014) and at least 32 off the Pacific coast of the country (Tenorio et al., Reference Tenorio, Tucker and Chaney2012). While the modern fauna appears more diverse than that of YN020, suggesting an increase in diversification since the late Miocene, the modern counts are sampled across a wider geographical range and presumably a greater range of sampling depths, so direct comparisons are somewhat problematic.
While the biodiversity of the Conidae fauna documented here from UF locality YN020 is moderate, it is phylogenetically diverse. The quality of preservation—particularly ancient coloration patterns revealed by UV light—allows six of the 10 species found at this locality to be assigned with confidence to six different clades (subgenera; see Puillandre et al., 2014, Reference Puillandre, Duda, Meyer, Olivera and Bouchet2015) of extant Conidae: Conasprella (Ximeniconus), Conus (Stephanoconus), Conus (Dauciconus), Conus (Pyruconus I), Conus (Pyruconus II), and Conus (Spuriconus). The lower Gatun Formation strata from which these taxa were collected were deposited ca. 10–11 Ma (late Miocene). All six of these clades, therefore, had minimum ages of origination of 10 Ma and as such may provide useful calibration points for future phylogenetic studies. The phylogenetic positions of the other four species cannot currently be estimated beyond the genus level and it is possible that some of them may represent extinct clades of Conidae. Combined with the similar pattern of substantial phylogenetic diversity documented recently in Neogene Dominican Conidae (Hendricks, Reference Hendricks2015), the Conidae fauna of YN020 adds to the view that the biogeographic history of the modern tropical American Conidae fauna is complex and is, at least in part, built from many different, long-lived clades. To date, reconstruction of the phylogenetic biogeographic history of tropical American Conidae has relied predominantly on analysis of molecular sequence data (e.g., Duda and Kohn, Reference Duda and Kohn2005; Puillandre et al., Reference Puillandre, Bouchet, Duda, Kauferstein, Kohn, Olivera, Watkins and Meyer2014), with limited reference to the rich cone snail fossil record of this region. Combining these sequence data with shell character data derived from extant and fossil species (i.e., a total evidence framework; see Hermsen and Hendricks, Reference Hendricks2008) has the potential to not only to directly test the phylogenetic assignments posited above, but also to more broadly explore the evolutionary history of this remarkable clade of dazzling, deadly, and diverse marine gastropods.
Acknowledgments
Fieldwork related to this study was facilitated by the National Science Foundation-supported project Great American Biotic Interchange Research Experiences for Teachers (GABI-RET; EAR 1358919 to B. MacFadden) and Partnerships for International Research and Education Panama Canal Project (PIRE PCP; OISE 0966884 to B. MacFadden), as well as the staff of the Smithsonian Tropical Research Institute (STRI). Thank you to B. MacFadden for the invitation to participate in the 2015 GABI-RET, C.A. Grant for coordinating many aspects of the project, and especially to the enthusiastic participating teachers who helped collect cone snail specimens in the field. Additional thanks for assistance in the field go to R. Portell, A. Klompmaker, C. Robbins, and J.W. Morena Bernal. Access to additional material, including type specimens, was provided by M. del Carmen Perrilliat (Instituo de Geología, Universidad Nacional Autónoma de México, Colección Nacional de Paleontología), K. Hollis and D. Levin (National Museum of Natural History, Washington D.C.), S. Butts (Yale Peabody Museum), J. Todd (Natural History Museum, London), P. Calloman (Academy of Natural Sciences of Drexel University), J. DeMouthe (California Academy of Sciences), and G. Dietl and L. Skibinski (Paleontological Research Institution). A. Schumacher and O. Mandic (Naturhistorisches Museum Wien) kindly provided the images of NHMW specimens that are shown in this paper. A special thank you is extended to R. Portell and the staff of the Division of Invertebrate Paleontology at the Florida Museum of Natural History for facilitating large loans of specimens for this study. At Ohio University, E. Hermsen (Dept. of Environmental & Plant Biology) and D. López (Dept. of Geological Sciences) are gratefully acknowledged for providing research space that supported this project. Finally, I thank A.J. Kohn and B. Landau for constructive reviews of an earlier version of this manuscript, and J. Kastigar, D. Davis, and B. Hunda for editorial assistance.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: doi:10.5061/dryad.2q2s4



















