Introduction
The somatic cell nuclear transfer technique combined with genome editing is a powerful tool for the development of transgenic cloned animals of great breeding value. In recent years, large numbers of transgenic cloned animals have been produced and, during this time, somatic cell cloning technology has demonstrated its potential in the production of genome-modified animals. Compared with other animals, the oocytes of a pig contain more lipid droplets and are particularly sensitive to temperature (Nagashima et al., Reference Nagashima, Kashiwazaki, Ashman, Grupen and Nottle1995; Li et al., Reference Li, Lai, Wax, Hao, Murphy, Rieke, Samuel, Linville, Korte, Evans, Turk, Kang, Witt, Dai and Prather2006). As a result, the generation of somatic cell cloned pigs involves a higher degree of difficulty. Furthermore, the frequent occurrence of miscarriage, postnatal death, and abnormal development and growth have also led to a low birth rate for cloned pigs (Yang et al., Reference Yang, Smith, Tian, Lewin, Renard and Wakayama2007). To rescue transgenic pig breeds with high economic value and important breeding characteristics, recloning technology has been used to preserve the genetic information from cloned transgenic breeding pigs that would otherwise not have survived to breed (Ahn et al., Reference Ahn, Kim, Kim, Lee, Heo, Kang, Kang, Lee, Lee, Kim, Nho, Hwang, Woo, Park, Park and Shim2011). Hence, the preservation of the breed resources of transgenic pigs will lay the foundation for expanding the successive transgenic swine population. Although researchers have produced transgenic pigs (Cho et al., Reference Cho, Kim, Park, Choi, Bang, Hwang, Cho, Sohn, Uhm, Koo, Lee, Kim and Kim2007; Park et al., Reference Park, Choi, Hong, Han, Yoo, Jin, Seol and Park2008), cattle (Kuroiwa et al., Reference Kuroiwa, Kasinathan, Sathiyaseelan, Jiao, Matsushita, Sathiyaseelan, Wu, Mellquist, Hammitt, Koster, Kamoda, Tachibana, Ishida and Robl2009; Wang et al., Reference Wang, Sun, Ding, Zhang, Zhao, Li, Li, Tang, Zhang, Liu, Li, Gao, Wang, Wang, Dai and Li2009) and cats (Cho et al., Reference Cho, Bang, Yu, Lee, Kim, Jeon, Yee and Kong2010) successfully using recloning technology, it has not been reported whether recloning technology affects the in vivo development of recloned transgenic pig embryos and the reproductive performance and germline (genetic) transmission capacity of recloned transgenic pigs. In the present study, we have successfully produced male and female recloned transgenic pigs using recloning technology and have investigated systematically the characteristics of the reproductive performance and the germline (genetic) transmission capacity of the recloned transgenic pigs to establish a theoretical foundation for the development of new breeds of transgenic cloned pigs.
Materials and methods
Except when otherwise noted, all chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), and all cell culture-related consumables were purchased from Corning Incorporated. Nunc brand consumables were used for oocyte in vitro maturation (IVM) and in vitro embryo culture.
Preparation of solutions
The cell culture medium contained 1% (v/v) non-essential amino acids (NEAA), 75 μg/ml penicillin, 50 μg/ml streptomycin sulphate and 10% (v/v) fetal calf serum (FBS, HyClone) in high-glucose DMEM (HyClone). The cell digestion solution contained 0.25% (w/v) trypsin plus 0.02% (w/v) EDTA, and the oocyte-washing solution was Dulbecco's phosphate-buffered saline (DPBS, without Ca2+ and Mg2+, Gibco) that contained 0.1% (w/v) polyvinyl alcohol (PVA). The in vitro maturation medium used TCM199 (without HEPES) as the base medium and contained 10% (v/v) pig follicular fluid (pFF), 10% (v/v) fetal calf serum (FBS), 10 IU/ml human chorionic gonadotropin (hCG), 10 IU/ml pregnant mare serum gonadotrophin (eCG), 0.1 mg/ml l-cysteine and 10 ng/ml epidermal growth factor (EGF). For the pFF preparation, follicle fluid was aspirated from ovarian follicles approximately 4–8 mm in diameter and pipetted into a 15 ml centrifuge tube. After 20 min of centrifugation at 3000 g, the supernatant of the follicle fluid was filtered using 0.22–0.45 μm syringe filters, dispensed in aliquots into 1.5 ml centrifuge tubes and stored at –20°C. The in vitro operation medium consisted of TCM199 (buffered with HEPES) that contained 2% (v/v) FBS (namely T2). The micromanipulation medium consisted of T2 plus 5–7.5 μg/ml cytochalasin B (CB). The aqueous fusion/activation solution was composed of 0.25 M mannitol, 0.1 mM calcium chloride, 0.1 mM magnesium chloride, 0.5 mM HEPES, and 0.01% (w/v) PVA. The embryonic basal medium consisted of PZM (Yoshioka et al., Reference Yoshioka, Suzuki, Tanaka, Anas and Iwamura2002) and 2.7756 M inositol, and the embryo culture medium (PZM-3) consisted of PZM plus 3 mg/ml BSA.
In vitro maturation of swine oocytes
This experiment was performed according to the protocol of Zhang et al. (Reference Zhang, Li, Villemoes, Pedersen, Purup and Vajta2007). Briefly, ovaries were taken from prepubertal pigs and sows at the local slaughterhouse. These were placed at 28–35°C in a physiological saline solution that contained penicillin and streptomycin sulphate and were brought to the laboratory within 2 h and washed with saline. Immediately upon arrival, the ovarian follicles 3–6 mm in diameter were aspirated using a sterile 10 ml syringe with an 18 G needle. The aspirated follicular fluid was slowly injected into a 38°C-preincubated 15 ml centrifuge tube to sediment the cumulus–oocyte complexes (COCs). After approximately 15 min of sedimentation, a clear interface was visible. Following the removal of the supernatant, the cell pellets were diluted with oocyte-washing medium (DPBS + 0.01% PVA) and aspirated gently. Using a stereomicroscope, the cumulus–oocyte complexes having more than two layers of compact cumulus investment and a dense, homogeneous cytoplasm were selected rapidly. These cells were then washed three times with DPBS that contained 0.01% PVA and another three times with IVM medium. Subsequently, 50 of the washed COCs were matured in 400 μl IVM medium at 38.5°C with 5% CO2 and saturated humidity for 42–44 h. COCs were then treated with DPBS (without Ca2+ and Mg2+, Gibco) that contained 1 mg/ml hyaluronidase to remove the surrounding cumulus cells. Finally, the oocytes with clear perivitelline spaces, intact cell membranes and extruded first polar bodies (pb1) were selected for use.
Preparation of donor cells for recloned transgenic pigs
Somatic fibroblast cell lines were established from the ear tissue of primary transgenic cloned pigs (Park et al., Reference Park, Lai, Cheong, Cabot, Sun, Wu, Rucker, Durtschi, Bonk, Samuel, Rieke, Day, Murphy, Carter and Prather2002). The animal experiments in the current study have been approved by the Animal Care and Use Committee of China Agricultural University. Prior to nuclear transfer, the contact-inhibited fibroblasts were subjected to conventional digestion, centrifugation and washing. The cell pellets were then resuspended in cell culture medium and used as nuclear donors.
Construction, fusion and activation of somatic cell nuclear-transferred embryos
Construction of reconstructed oocytes
Mature oocytes and donor cells were simultaneously transferred into micromanipulation medium droplets. After equilibration at 38.5°C, 5% CO2 and saturated humidity for 10–15 min, the oocytes were held with a holding pipette (inner diameter of 25–35 μm, outer diameter of 100–120 μm) under an inverted microscope (Eclipse Ti, Nikon) equipped with a micromanipulator (Narishige, Japan) and a warmed stage (Tokihai, Japan). The first polar body was adjusted to the 1 o'clock position using an enucleation/microinjection needle with a 15–25 μm inner diameter. Subsequently, from the 3 o'clock position, the microinjection needle was inserted to draw the first polar body together with 10–20% of the adjacent cytoplasm, which potentially contains the oocyte nucleus. The ear tissue fibroblasts of the primary transgenic cloned pig, which were 15–20 μm in diameter, highly refractive and rounded to a smooth shape, were selected for transfer into the perivitelline space from the enucleating cut. Following this micromanipulation, the reconstructed oocytes composed of the donor cell and the oocyte cytoplasm were transferred into a T2 droplet and placed in an incubator for 30 min at 38.5°C, 5% CO2 in air and saturated humidity for a recovery period.
Fusion and activation of reconstructed oocytes
After the 30-min recovery period in the T2 droplet, the reconstructed oocytes were transferred into fusion medium in batches to equilibrate for 2 min. Following three washes with fusion/activation medium, ten oocytes per batch were placed in a cell fusion chamber and were spread with fusion medium. By moving the reconstructed oocytes with a pulled fine glass needle, the contact surfaces of the donor cell and the recipient oocyte was oriented parallel to the electrodes. With the CF-150B cell fusion device (BLS, Hungary), a pulse direct current (DC) of 1.56 kv/cm for 100 μs was administered to induce cell fusion and activation. Subsequently, couplets were washed three times with embryo culture medium and immediately transferred into chemically assisted activation medium (PZM-3 + 10 μg/ml CHX + 10 μg/ml CB) and covered with mineral oil. After 4 h of culture in an incubator at 38.5°C, 5% CO2 in air and saturated humidity, the results of the fusion were determined using a stereomicroscope.
In vitro culture and transplantation of recloned embryos
Following activation, the recloned embryos were washed three times with PZM-3. These cells were then transferred into a 4-well plate that contained 400 μL PZM-3, which had been buffered in the incubator 4 h in advance, and incubated at 38.5°C, 5% O2, 5% CO2, 90% N2 and saturated humidity. After 12–20 h of culture, the recloned embryos were surgically transplanted into the oviducts of surrogate pigs that were at a natural estrus of day 0 or day 3 (the occurrence of a back-reaction to pressure was considered a symbol of estrus day 0). Each surrogate pig was implanted with 160–300 embryos, and the full-term births of the recloned piglets (one mini-pig and three Landrace pigs) from four surrogate mother pigs were recorded. In addition, anesthetics, analgesics, tranquilizers, and care were taken to minimize pain and discomfort during preoperative, operative, and postoperative procedures.
Reproductive performance analysis for recloned transgenic pigs (female and male)
The status of the genital tracts and ovaries, puberty, estrous cycle, gestation period, the numbers of pregnant, farrowed pigs and piglets, and the litter sizes of the female recloned transgenic miniature pigs were recorded. Furthermore, the onset of puberty, semen volume, sperm concentration, sperm motility rate and semen colour and smell of the male recloned transgenic Landrace pigs were recorded. Subsequently, the four female recipient pigs were artificially inseminated, and the numbers of pregnant pigs, farrowed pigs, piglets born and the litter size for each pig were monitored.
Results
Preparation of somatic fibroblast cell lines stably expressing GFP for recloned embryos
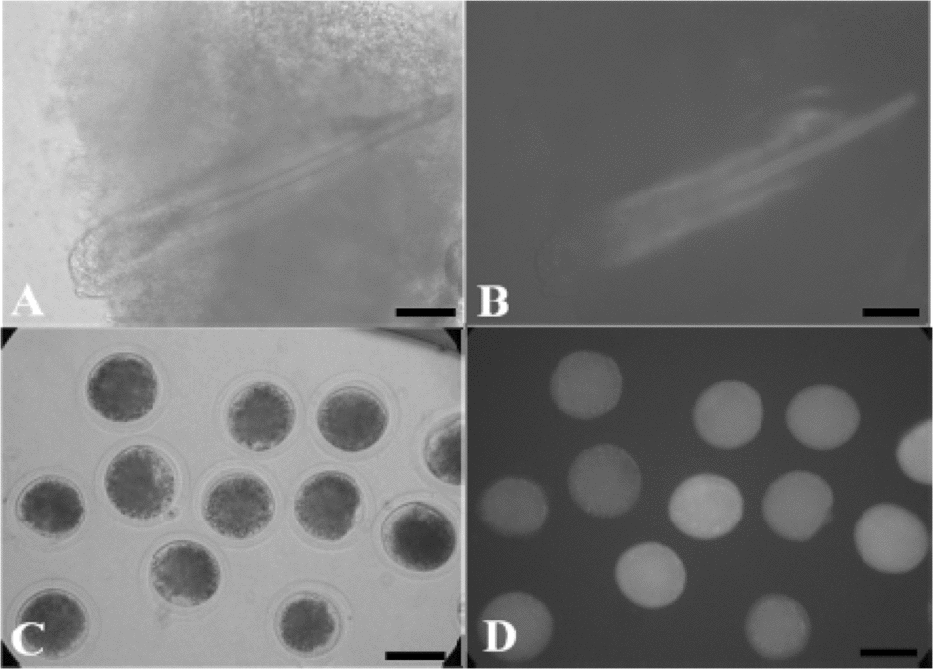
As shown in Figure 1, ear tissues were taken from female Chinese experimental primary transgenic cloned miniature pigs and male primary transgenic cloned Landrace pigs at postnatal day 7 to establish primary somatic fibroblast cell lines that stably expressed GFP (Fig. 1A,B). These cells showed normal shape and growth, and moreover, the recloned embryos produced by somatic cell nuclear transfer were GFP positive (Fig. 1C,D).

Figure 1 (A) An ear tissue sample from the primary transgenic cloned pig visualized using bright field microscopy. (B) The same ear tissue sample as in (A) visualized under ultraviolet light. (C) Recloned transgenic pig embryos at 2 h after fusion and activation under bright field. (D) The same recloned transgenic pig embryos as in C under ultraviolet light. Scale bar = 100 μm.
In vivo viability of recloned transgenic embryos
The numbers of surrogate pigs, pregnant pigs, postpartum pigs, piglets born and the GFP+ offspring are shown in Table 1. These values were calculated after the recloned embryos derived from the female primary Chinese experimental transgenic cloned miniature pigs and male primary transgenic cloned Landrace pigs were transplanted (Figs. 2, 3 and Table 1).
Table 1 In vivo viabilities of recloned transgenic embryos


Figure 2 (A) The recloned transgenic Chinese experimental miniature pig (CEMP) at 5 days of age (black). (B) The recloned transgenic Landrace pigs at 5 days of age (white).

Figure 3 Lane 1: The PCR product amplified using genomic DNA from the recloned female mini-pig. Lane 2–4: The PCR products amplified using genomic DNA from the recloned male Landrace pigs. Lane 5: The PCR product amplified using genomic DNA from the primary GFP+ cloned pig. Lane 6: The negative control. M: DNA markers ranged from 100–1500 bp.
Reproductive characteristics of recloned transgenic pigs
As shown in Tables 2 and 3, the status of the genital tracts and ovaries, puberty, estrous cycle, gestation period, the numbers of pregnant pigs, postpartum pigs, piglets born and the litter sizes of the female recloned Chinese experimental transgenic miniature pigs were recorded (Fig. 4A and Table 2). In addition, the puberty onset, semen volume, sperm concentration, sperm motility rate, semen colour and smell, the numbers of artificially inseminated pigs, pregnant pigs, postpartum pigs, and piglets born and litter size from the male recloned transgenic Landrace pigs were also recorded (Fig. 4B and Table 3).
Table 2 Reproductive and developmental capacities of the female recloned transgenic Chinese experimental miniature pigs (CEMPs)

Table 3 Reproductive and developmental capacities of the male recloned transgenic landrace pigs


Figure 4 (A) The mature female recloned transgenic Chinese experimental miniature pig (CEMP) and her F1 piglets. (B) A mature male recloned transgenic Landrace pig.
Discussion
In the present study, somatic fibroblast cell lines derived from ear tissue of primary GFP-transgenic cloned pigs were successfully established. Furthermore, these cells were used as donor cells to produce recloned transgenic embryos, which were capable of in vivo development to full term. In addition, each of the recloned transgenic pigs (male and female) possessed normal reproductive performance. Moreover, after genotype identification by polymerase chain reaction (PCR), the female and the three male recloned transgenic pigs were shown to be GFP positive. Among their F1 offspring, 53% (16/30) of the piglets had successfully integrated the GFP gene (data not shown), which demonstrated that the recloned transgenic pigs had stable genetic transmission capacities. Previous studies have shown that pigs (Park et al., Reference Park, Choi, Hong, Han, Yoo, Jin, Seol and Park2008 Cho et al., Reference Cho, Kim, Park, Choi, Bang, Hwang, Cho, Sohn, Uhm, Koo, Lee, Kim and Kim2007), cattle (Kuroiwa et al., Reference Kuroiwa, Kasinathan, Sathiyaseelan, Jiao, Matsushita, Sathiyaseelan, Wu, Mellquist, Hammitt, Koster, Kamoda, Tachibana, Ishida and Robl2009; Wang et al., Reference Wang, Sun, Ding, Zhang, Zhao, Li, Li, Tang, Zhang, Liu, Li, Gao, Wang, Wang, Dai and Li2009) and cats (Cho et al., Reference Cho, Bang, Yu, Lee, Kim, Jeon, Yee and Kong2010) had been cloned successfully using recloning technology, which demonstrates that recloning technology has become a routine and effective animal breeding technique. However, this research had focused on the early development of the recloned embryos (Hill et al., Reference Hill, Winger, Long, Looney, Thompson and Westhusin2000, Reference Hill, Winger, Burghardt and Westhusin2001; Uhm et al., Reference Uhm, Gupta, Das, Kim, Park, Kim and Lee2009), the birth rate of the recloned animals (Fujimura et al., Reference Fujimura, Murakami, Kurome, Takahagi, Shigehisa and Nagashima2008) and the length of life of the recloned animals (Fujimura et al., Reference Fujimura, Murakami, Kurome, Takahagi, Shigehisa and Nagashima2008). However, the reproductive performance and the genetic transmission capacities of the recloned animals have not yet been reported.
In this study, somatic fibroblasts derived from primary cloned pigs of two different genetic backgrounds were used to produce recloned transgenic pigs. The birth rates of the recloned pigs from each genetic background were 0.6% and 0.4%. Due to limitations in the sample size of our experiment, accurate statistical analyses were not carried out. However, based on the data recorded, the production efficiencies of the recloned transgenic pigs from each genetic background were similar. In addition, the pregnancy rates, the parturition rates and the birth rates of the recloned transgenic pigs in this study were very close to the experimental results for recloned transgenic pigs from other studies (Kurome et al., Reference Kurome, Hilsatomi, Matsumoto, Tomii, Ueno, Hiruma, Saito, Nakamura, Okumura, Matsumoto, Kaji, Endo and Nagashima2008; Park et al., Reference Park, Choi, Hong, Han, Yoo, Jin, Seol and Park2008). However, the cloning efficiency in this study was significantly greater than that from Cho's study (0.04%) (Cho et al., Reference Cho, Kim, Park, Choi, Bang, Hwang, Cho, Sohn, Uhm, Koo, Lee, Kim and Kim2007), but it was lower than that from the study of Kurome et al. (3.3%) (Fujimura et al., Reference Fujimura, Murakami, Kurome, Takahagi, Shigehisa and Nagashima2008). These differences may be associated with the genetic backgrounds of the donors used in the experiments, the different exogenous genes used or the computing methods of the cloning efficiency.
The Chinese experimental miniature pig (CEMP) originates from the Chinese Bama miniature pig. After reaching sexual maturity, the female recloned transgenic miniature pigs obtained in the experiment were examined using b-mode ultrasonography, and the results showed the genital tract and ovaries to be normal. In this study, the recloned transgenic pig's acceptance of a normal miniature boar's mounting was considered the start of her estrus. Furthermore, the age (puberty) when the female recloned transgenic pigs began estrus was within the normal range (120–130 days) and was essentially identical to the puberty onset of conventionally bred CEMPs and other miniature pig species (Shibata et al., Reference Shibata, Otake, Tsuchiya, Chikyu, Horiuchi and Kawarasaki2006; Wakai et al., Reference Wakai, Tanaka, Yamanaka, Sugimura, Sasada, Kawahara, Kobayashi and Sato2008). Additionally, the oestrous cycles of the recloned transgenic pigs were not significantly different from those of ordinary miniature pigs and other pig species (Shibata et al., Reference Shibata, Otake, Tsuchiya, Chikyu, Horiuchi and Kawarasaki2006). Following insemination with semen of normal male miniature pigs, the recloned transgenic miniature pigs showed very similar gestation periods, pregnancy rates, parturition rates and litter sizes compared with ordinary female miniature pigs (Liu et al., Reference Liu, Lv, He, Yang, Qin, Pan, Huang, Huang, Lu, Lu, Li and Lu2010). Moreover, of the seven F1 piglets, four were GFP positive. Hence, the female recloned transgenic miniature pigs had normal reproductive performance and genetic transmission capacities.
In this experiment, the first ejaculations of the male recloned transgenic Landrace pigs were considered to represent the onset of puberty. Our research found that the ages when the three male recloned transgenic pigs began ejaculating were within the reported range (Martin et al., Reference Martin, Adams and Wiseman2004). Upon analysis, the average semen volume, sperm concentration and sperm motility rate of the three experimental pigs were similar to the results of other studies (Martin et al., Reference Martin, Adams and Wiseman2004; Yeste et al., Reference Yeste, Barrera, Coll and Bonet2011). In addition, the semen of the recloned Landrace pigs was ivory-coloured and smelled normal and was similar to that of ordinary Landrace pigs (pictures not provided). To investigate whether the male recloned Landrace pigs had normal reproductive performance, the semen was collected to artificially inseminate four normal Landrace pigs. It was found that the pregnancy rates, parturition rates and litter sizes of the recipient Landrace mother pigs were within normal range. Furthermore, among the 23 natal F1 piglets, 12 had integrated the GFP gene. Thus, the male recloned transgenic Landrace pigs had normal reproductive performance and genetic transmission capacities.
Therefore, the recloned transgenic pigs possessed normal reproductive performance and stable germline (genetic) transmission capacities, and they can be used as breeding animals for the development of new swine breeds in the future.
Conclusion
Ear tissue fibroblasts derived from primary transgenic cloned pigs stably expressing green fluorescent protein (GFP) could efficiently sustain the full-term development of recloned transgenic embryos. Furthermore, these recloned transgenic pigs had normal reproductive performance and stable germline (genetic) transmission capacities.
Acknowledgements
We thank Mr Gang Wang of the Beijing Jiajie Kongning Bioengineering Technology Co. Ltd. for his constructive support, and we would also like to thank Ms Jing Fei of the State Key Laboratories of Agrobiotechnology for her kind help with the experimental materials. Funding was provided by National Transgenic Breeding Program (2008ZX08006-001).