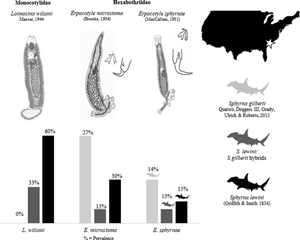
Introduction
Two cryptically similar and sister species of hammerhead sharks (Sphyrnidae), Sphyrna lewini (Griffith and Smith, Reference Griffith and Smith1834) and Sphyrna gilberti Quattro et al., Reference Quattro, Driggers, Grady, Ulrich and Roberts2013 occur along the coast of South Carolina (SC), USA (Quattro et al., Reference Quattro, Driggers, Grady, Ulrich and Roberts2013). These species are commonly known as the scalloped and Carolina hammerhead, respectively. They can be distinguished only by a difference in the number of precaudal vertebrae (S. gilberti has about 10 fewer than S. lewini) and genetic data. Their divergence was estimated to have occurred 4.5 million years ago (Pinhal et al., Reference Pinhal, Shivji, Vallinoto, Chapman, Gadig and Martins2012; Quattro et al., Reference Quattro, Driggers, Grady, Ulrich and Roberts2013). In addition, these species are capable of hybridization (Barker et al., Reference Barker, Adams, Driggers, Frazier and Portnoy2019). Because of the morphological similarity, S. gilberti was described only recently from the Carolina coasts (South and North Carolina states, USA) in the western North Atlantic Ocean (Quattro et al., Reference Quattro, Driggers, Grady, Ulrich and Roberts2013). Hence, knowledge about its biology and distribution is limited, and adults' range remains uncertain. However, specimens of S. gilberti have been found along the Atlantic coast of Florida (FL), prior to being officially recognized as a distinct species (Abercrombie et al., Reference Abercrombie, Clarke and Shivji2005; Duncan et al., Reference Duncan, Martin, Bowen and De Couet2006; Quattro et al., Reference Quattro, Stoner, Driggers, Anderson, Priede, Hoppmann, Campbell, Duncan and Grady2006). Barker et al. (Reference Barker, Frazier, Adams, Bedore, Belcher, Driggers, Galloway, Gelsleichter, Grubbs, Reyier and Portnoy2021) showed that neonates of S. gilberti are most common along coastal nurseries in SC, with abundances decreasing latitudinally to be at its lowest in southern FL, and were not found in the Gulf of Mexico. Furthermore, at least 3 adult specimens of S. gilberti were captured in southeast Brazil (Pinhal et al., Reference Pinhal, Shivji, Vallinoto, Chapman, Gadig and Martins2012). Knowledge of the parasite fauna of S. gilberti is sparse. Presently, the only parasite known for the Carolina hammerhead is a nematode in the spiral valve (Moravec et al., Reference Moravec, Dalrymple, Galloway, Barker and de Buron2020), although it is possible that specimens of S. gilberti have been unknowingly included in previous parasitic fauna studies of S. lewini in geographic locales where both species occur, and vice versa.
By contrast, the scalloped hammerhead, S. lewini, has been formally recognized as a species since the 19th century. It has a global range (Compagno, Reference Compagno1984). Five species of monogenoids were described from this shark. Two of these species are members of Hexabothriidae Price, 1942: Erpocotyle microstoma (Brooks, Reference Brooks1934), originally described in Sphyrna zygaena (Linnaeus, 1758) (type host) from the coast of Beaufort, North Carolina (NC), USA and reported in S. lewini from the western South Atlantic Ocean (Uruguay; Suriano and Labriola, Reference Suriano and Labriola1998); and Erpocotyle sphyrnae (MacCallum, Reference MacCallum1931), also described from S. zygaena off Woods Hole, Massachusetts (MA), USA and reported in S. lewini from the eastern North Atlantic Ocean (Senegal; Euzet and Maillard, Reference Euzet and Maillard1967) and the Pacific Ocean (Hawaii, USA; Yamaguti, Reference Yamaguti1968). The other 3 species are members of Monocotylidae Taschenberg, 1879: Cathariotrema selachii (MacCallum, 1916), originally described from S. zygaena (exact locale unknown, but is likely off Woods Hole, MA – see Bullard et al., Reference Bullard, Warren and Dutton2021) and reported in S. lewini from the Northern Gulf of Mexico off Mississippi, Alabama and Louisiana in Bullard et al. (Reference Bullard, Warren and Dutton2021); Loimosina wilsoni Manter, Reference Manter1944, originally described from S. zygaena from Montego Bay, Jamaica and reported in S. lewini from Alligator Harbor, FL, USA in Hargis (Reference Hargis1955); and Loimosina parawilsoni Bravo-Hollis, Reference Bravo-Hollis1970, described in S. lewini from the eastern tropical Pacific Ocean (Sinaloa, Mexico; Bravo-Hollis, Reference Bravo-Hollis1970) (the latter 2 were formerly considered Loimoidae – see Boeger et al., Reference Boeger, Kritsky, Domingues and Bueno-Silva2014).
In the present study, monogenoids infecting the gills of neonates of S. gilberti, S. lewini and their hybrids were identified, sequenced, illustrated and redescribed based on specimens collected from the western North Atlantic Ocean (SC, USA) and their types.
Materials and methods
Sampling and collection
A total of 87 neonates, all moribund upon capture, were collected. Because the shark species identification could not be determined at time of capture, the number of sharks sampled was necessary to gain a sufficient sample size for both species of hammerheads, S. lewini and S. gilberti, and their hybrids.
Sharks were captured using a 231 m long, 3 m deep gillnet with a stretched mesh of 10.3 cm in Bulls Bay, SC (Five Fathom Creek, 33.0095/-79.4853), a nursery area where both species of hammerhead and their hybrids are found in sympatry (Barker et al., Reference Barker, Adams, Driggers, Frazier and Portnoy2019). Fresh carcasses were kept on ice, individually labelled and fin clips from each specimen preserved in 20% salt-saturated DMSO and sent to the Marine Genomics Laboratory at Texas A&M University – Corpus Christi, USA, for molecular identification following the methods of Barker et al. (Reference Barker, Adams, Driggers, Frazier and Portnoy2019).
Monogenoids from each hammerhead specimen were identified before the host species identifications were determined. Gills were resected from each host within 10 h post-capture, flooded and shaken rapidly in hot water (68°C) to relax, kill and detach worms from the gill filaments. Some monogenoids were processed immediately – the haptor was fixed in 95% ethanol (EtOH) and the anterior end in 10% neutral buffer formalin (NBF) to generate hologenophores sensu Pleijel et al. (Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008); the remaining specimens were fixed with either 10% NBF or 100% EtOH to obtain final concentrations of 5% NBF and 70% EtOH, respectively. Other hologenophores were generated via some EtOH-fixed specimens by removing a small lateral part of their body.
Morphology
Parasites were stained with either acetocarmine or Gomori's trichrome (Humason, Reference Humason1979) and mounted using Canada balsam or Permount Mounting Medium (Fisher Chemical, Fairlawn, New Jersey, USA). The haptor of some specimens were mounted separately in Hoyer's medium to examine sclerite and anchor morphology. Drawings were made using an Olympus BX50 differential interference contrast compound microscope mounted with camera lucida. Measurements of the haptoral hook-like sclerites and anchors were obtained using ImageJ software (www.nih.org) following measurements schemes of MacCallum (Reference MacCallum1931), Euzet and Maillard (Reference Euzet and Maillard1967) and Bullard and Dippenaar (Reference Bullard and Dippenaar2003) (Fig. 1A–E). Anchors were measured as indicated in Fig. 2. Measurements are in micrometres; the range is presented followed by the average and the number of measured structures (n) in parentheses. Haptoral sucker pairs and their respective sclerites are numbered 1–3, with 1 being closest to the point of attachment for the haptoral appendix.

Fig. 1. Measurements of sclerite curvature were taken using ImageJ software (www.nih.org): (A) tip-to-tip (MacCallum, Reference MacCallum1931), (B) perimeter hook length (Euzet and Maillard, Reference Euzet and Maillard1967), (C) perimeter shaft length (Euzet and Maillard, Reference Euzet and Maillard1967), (D) shaft length (Bullard and Dippenaar, Reference Bullard and Dippenaar2003) and (E) max shaft width (Bullard and Dippenaar, Reference Bullard and Dippenaar2003). Scale bar 80 μm.

Fig. 2. Width (A) and length (B) measurements of anchors of the haptoral appendix. Scale bar 13 μm.
Vouchers and hologenophores are deposited at the National Museum of Natural History, Smithsonian Institution (USNM) in Washington DC, USA, and the Harold W. Manter Laboratory of Parasitology (HWML) in Nebraska, USA.
For comparison, micrographs of the following specimens from USNM, HWML and Nacional Collection of Helminths, Institute of Biology, National Autonomous University of Mexico (CNHE), Mexico (available at http://unibio.unam.mx) were examined: E. microstoma (syntypes: USNM 132155, HWML 1437), E. sphyrnae (syntypes: USNM 1320885 and USNM 1320884), L. wilsoni (syntypes: USNM 1337561, USNM 1337564, HWML 1425) and L. parawilsoni (holotype: CNHE 153, syntype CNHE 154).
Molecular analyses
DNA was extracted from parasite tissue using a DNeasyBlood and Tissue kit (Qiagen, Valencia, California, USA) and manufacturer's protocol. Sequences of DNA from portions of the nuclear-encoded 28S ribosomal RNA (rDNA; 28S) and the mitochondrially encoded cytochrome c oxidase I (COI) genes were amplified and sequenced for species comparisons and confirmations. Primers LSU5 (5′-TAGGTCGACCCGCTGAAYTTAAGCA-3′; Jensen and Bullard, Reference Jensen and Bullard2010) and 28S_ECD2 (5′-CTTGGTCCGTGTTTCAAGACGGG-3′; Tkach et al., Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003) were used to amplify a1100 base pair portion of the 28S gene: a 25 μL total volume reaction contained 1× polymerase chain reaction (PCR) buffer (Promega, Madison, Wisconsin, USA), 3 mm MgCl2, 0.4 mm deoxynucleotide triphosphates (dNTPs) (Promega), 0.4× Rediload gel loading buffer (Invitrogen, Waltham, MA, USA), 0.6 μ m of each primer, 1 U GoTaq® Flexi DNA Polymerase (Promega) and 3 μL template DNA. Cycling was as follows: 5 min initial denaturation at 94°C, followed by 40 cycles of denaturation at 94°C for 45 s, annealing at 62°C for 45 s, extension at 72°C for 45 s, then a final extension at 72°C for 5 min. Primers JB3 (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and JB4.5 (5′-TAAAGAAAGAACATAATGAAAATG-3′; Bowles et al., Reference Bowles, Blair and Mcmanus1995) were used to amplify a portion of COI: a 25 μL total volume reaction contained 1× PCR buffer (Promega), 3 mm MgCl2, 0.2 mm dNTPs (Promega), 0.4× Rediload gel loading buffer (Invitrogen), 0.2 μ m of each primer, 1 U GoTaq® Flexi DNA Polymerase (Promega) and 1 μL template DNA. Cycling was as follows: 4 min at 94°C, followed by 40 cycles at 94°C for 30 s, annealing at 48°C for 40 s, extension at 72°C for 50 s and final extension at 72°C for 7 min.
All products were electrophoresed through 1% agarose gels stained with GelRed (Biotium, Fremont, California, USA) and visualized under ultraviolet light. Samples that produced a faint band were subjected to another round of PCR, done as above, except the template was the product from the initial PCR. Products were purified using ExoSAP-IT (Affymetrix, Santa Clara, California, USA) or by gel extraction using a QIAquick Gel Extraction kit (Qiagen), following the manufacturer's protocol for both methods and then sent to Eurofins MWG Operon LLC (Louisville, Kentucky, USA) for bi-directional Sanger sequencing. Complementary sequences were assembled, and their chromatograms were assessed and edited by eye using Sequencher version 5.3 (Gene Codes Corp., Ann Arbor, Michigan, USA). Resulting sequences were compared with sequences available in the National Center for Biotechnology Information (NCBI) GenBank database using the Basic Local Alignment Search Tool (BLAST – Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990) and were deposited into GenBank (Table 1).
Table 1. Sequences generated and used in the present study from families Monocotylidae and Hexabothriidae

COI sequences were used for distance analysis (species delimitation) and 28S sequences were used for phylogenetic analysis of the family Monocotylidae.
COI sequences were translated to confirm the absence of premature stop codons, which if present were corrected using Open Reading Frame Finder (RRID:SCR_016643) by NCBI and Geneious software version 4.8.5 (Kearse et al., Reference Kearse, Moir, Wilson, Stones-Havas, Cheung, Sturrock, Buxton, Cooper, Markowitz, Duran, Thierer, Ashton, Meintjes and Drummond2012). Sequences produced in this study and sequences of species of Hexabothriidae and Monocotylidae obtained from GenBank (Table 1) were aligned by hand in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018), and distance analyses were done using the same program. The maximum composite likelihood method (Tamura et al., Reference Tamura, Nei and Kumar2004) was used to calculate the number of base substitutions per site between pairs of sequences (p-distance), and phylograms were generated using the neighbour-joining (NJ) method (Saitou and Nei, Reference Saitou and Nei1987), implementing pairwise deletions and support values obtained by 1000 bootstrap replicates (Felsenstein, Reference Felsenstein1985). Resulting trees represent intraspecific and interspecific distances between sequences but not phylogenetic relationships (Figs 3 and 4).

Fig. 3. Neighbour-joining tree based on Tamura and Nei (Reference Tamura and Nei1993) distances for Hexabothriidae COI sequences from this study and from the GenBank database conducted in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018). Sum of branch length = 1.11 and bootstrap support is shown, for 15 nucleotide sequences representing 6 species with a total of 821 positions in the final dataset. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances (number of base substitutions per site, see Table 4). Codon positions included were 1st + 2nd + 3rd.

Fig. 4. Neighbour-joining tree based on Tamura and Nei (Reference Tamura and Nei1993) distances for Monocotylidae COI sequences from this study and from GenBank conducted in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018). Sum of branch length = 0.532 and bootstrap support is shown, for 14 nucleotide sequences representing 4 species with a total of 348 positions in the final dataset. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances (number of base substitutions per site, see Table 5). Codon positions included were 1st + 2nd + 3rd.
A maximum likelihood phylogenetic tree for Monocotylidae was constructed using 28S sequences obtained herein and from GenBank (see Table 1 for accession numbers) – the only fragment available for the group at the moment for species previously allocated in Loimoidae. Sequences were aligned using GUIDANCE 2 (Penn et al., Reference Penn, Privman, Ashkenazy, Landan, Graur and Pupko2010a, Reference Penn, Privman, Landan, Graur and Pupko2010b) using the multiple sequence alignment algorithm MAFFT (Katoh et al., Reference Katoh, Misawa, Kuma and Miyata2002) and set to 100 alternative guide trees. The phylogeny was generated using raxmlGUI 2.0 (version 2.0.6) (Silvestro and Michalak, Reference Silvestro and Michalak2012; Stamatakis, Reference Stamatakis2014) with the general time reversible model with gamma rates (GTR + G) for 1000 bootstrap repetitions. The final tree was opened and edited in MEGA X (Fig. 5).

Fig. 5. Maximum likelihood tree (GTR + G substitution model, 1000 bootstraps) for Monocotylidae 28S sequences from this study and GenBank conducted in raxmlGUI 2.0 (version 2.0.6) (Silvestro and Michalak, Reference Silvestro and Michalak2012; Stamatakis, Reference Stamatakis2014) using 92 sequences representing 54 species with a total of 4022 positions in the final dataset. The consensus tree is shown with bootstraps as support; values lower than 50% have been removed.
Genomic DNA was extracted from hosts using a Mag-Bind Blood and Tissue DNA Kit (Omega Bio-Tek, Norcross, Georgia, USA). Double-digest restriction site-associated DNA sequencing libraries were prepared following the methods of Barker et al. (Reference Barker, Adams, Driggers, Frazier and Portnoy2019) and sequenced on an Illumina HiSeq 4000 DNA sequencer. Subsequent data processing and species identification was performed following the methods of Barker et al. (Reference Barker, Frazier, Adams, Bedore, Belcher, Driggers, Galloway, Gelsleichter, Grubbs, Reyier and Portnoy2021). Briefly, the dDocent pipeline (Puritz et al., Reference Puritz, Hollenbeck and Gold2014) was used to trim reads, map reads and call single-nucleotide polymorphisms (SNPs). Hosts were identified as S. lewini or S. gilberti using a panel of 1491 diagnostic SNPs, and a match of at least 95% to 1 species was required for identification. The program NewHybrids (Anderson and Thompson, Reference Anderson and Thompson2002) was used to determine if ambiguous individuals could be assigned into a hybrid category [first-generation hybrid (F1), S. lewini backcross (BX), S. gilberti backcross].
Prevalence determination
Upon receipt of shark identifications, prevalence of infection per parasite species, including coinfections, as well as per family, was calculated. Coinfections included instances where specimens of multiple species were found to infect the same host individual. Monogenoid prevalence is the per cent of infected sharks, regardless of the parasite species; Hexabothriidae and Monocotylidae prevalence includes all species from their respective families. In some instances, only fragments of specimens were collected but could not be identified to species or even family level, in which case these were included within the category of highest taxonomic classification possible.
Results
Species determination and prevalence of infection
Of the 87 neonates examined, 44 were determined molecularly as S. gilberti, 20 as S. lewini and 15 as hybrids of the 2 species (6 F1 hybrids, 5 S. lewini BX and 4 S. gilberti BX). Eight sharks could not be determined at the species level (i.e. no DNA or morphology available could ascertain Sphyrna species, as only the head of these specimens was provided and attempts to sequence were unsuccessful). Thus, only the remaining 79 neonates with confirmed species identities were used to report prevalence (Table 2). In total, 3 species of monogenoids were determined as E. microstoma, E. sphyrnae, and L. wilsoni (see redescriptions below). Both species of Erpocotyle were found to infect both species and hybrids of the studied hammerhead sharks, while L. wilsoni was found only on specimens of S. lewini and hybrids.
Table 2. Summary of prevalence of infection and coinfection (%) per monogenoid species (L. wilsoni, E. microstoma and E. sphyrnae) and per monogenoid family (Hexabothriidae is represented by the combined prevalence of both Erpocotyle species; L. wilsoni was the only monocotylid found, thus its prevalence is representative of the family in the present study) successfully identified shark individuals (n = 79)

BX, backcross.
The shaded boxes represent the numbers of each species of host sampled.
Molecular species identity
In total, 22 COI and 4 28S sequences were generated (accession numbers in Table 1; sequence composition in Table 3). The 28S sequences from the Monocotylidae specimens (n = 4) were 100% identical to that of a Loimosina sp. (Table 1; 99% query coverage).
Table 3. Sequence compositions, with no gaps, per monogenoid species collected from gills of Sphyrna spp.: length in base pairs (bp), reported as a range; variable sites (V); parsimony-informative sites (PI); per cent composition of each nucleotide

Variable and PI sites are compared between both species of Erpocotyle. Loimosina wilsoni was the only monocotylid identified in the present study, and was not compared to the other species of Hexabothriidae.
There is no GenBank sequence available for Erpocotyle; however, NJ analysis using COI sequences obtained herein supported the finding of 2 distinct species of Erpocotyle in the sharks examined (Fig. 3). Erpocotyle sphyrnae and E. microstoma sequences formed 2 distinct groups each with 100% bootstrap support. Likewise, the 2 available sequences of species of Loimosina formed a clade (100% bootstrap support) distinct from all available Monocotylidae sequences from GenBank (Fig. 4). Intraspecific variation for E. sphrynae was 0.00–0.02 and 0.01–0.04 for E. microstoma; for L. wilsoni, these values were 0.01–0.02 (Tables 4 and 5, respectively).
Table 4. Tamura and Nei (Reference Tamura and Nei1993) distances between Hexabothriidae COI sequences from this study (shaded) and from GenBank based on an 821 base pair alignment

The number of base substitutions per site between sequences was calculated in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018) using the maximum composite likelihood method (Tamura et al., Reference Tamura, Nei and Kumar2004). Standard error estimates are above the diagonal. This analysis involved 6 species with a total of 15 sequences. Codon positions included were 1st + 2nd + 3rd.
Table 5. Tamura and Nei (Reference Tamura and Nei1993) distances between Monocotylidae COI sequences from this study (shaded) and from the GenBank database based on a 348 base pair alignment
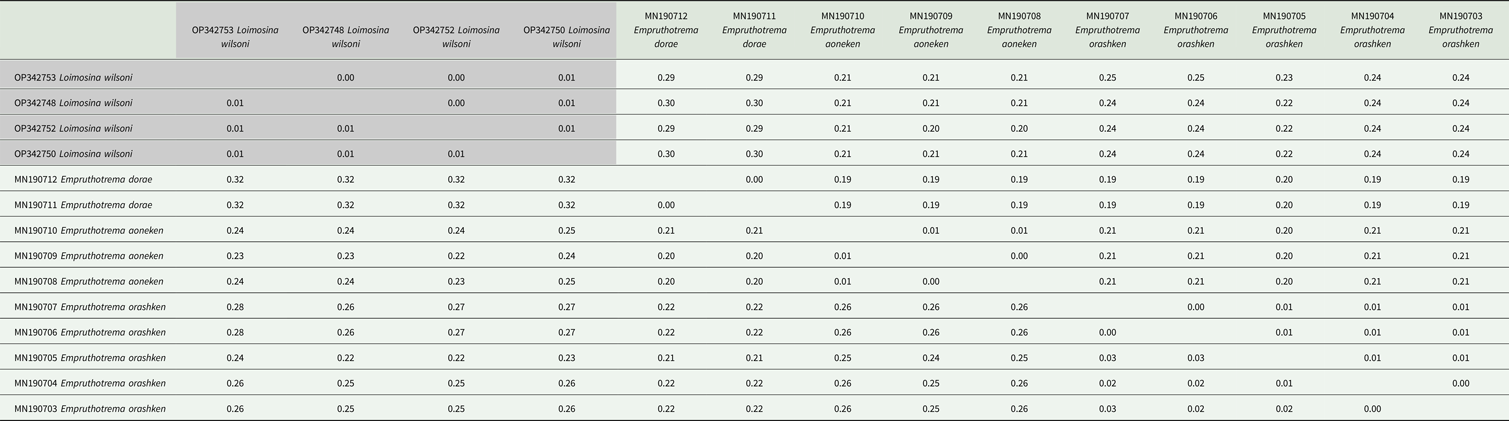
The number of base substitutions per site between sequences is shown and was calculated in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018) using the maximum composite likelihood method (Tamura et al., Reference Tamura, Nei and Kumar2004). Standard error estimates are above the diagonal. This analysis involved 4 species with a total of 14 sequences. Codon positions included were 1st + 2nd + 3rd.
The maximum likelihood phylogeny using 28S sequences of Monocotylidae supports the proximity of L. wilsoni and Loimosina sp. (of Boeger et al., Reference Boeger, Kritsky, Domingues and Bueno-Silva2014), which comprise a monophyletic clade to the paraphyletic Neoheterocotyle and its sister Troglocephalus (Fig. 5). Cathariotrema (another monocotylid from hammerheads) is much further distanced in the tree, forming a sister clade to Triloculotrema.
Taxonomy
Subclass Heteronchoinea Boeger and Kritsky, Reference Boeger and Kritksy2001
Hexabothriidae Price, 1942
Erpocotyle microstoma (Brooks, Reference Brooks1934)

Fig. 6. Erpocotyle microstoma (A) whole body, scale bar 1000 μm; (B) male copulatory organ (MCO) and parallel vaginae, scale bar 50 μm; (C) midsection of specimen containing the Ce (caeca), GI (gastrointestinal) canal, reproductive organs Ger (germarium), MGl (Mehlis' gland), Oo (ootype), SR (seminal receptacle) and Vag (vaginae), and VC (vitelline canal), scale bar 100 μm; (D) anchors of the haptoral appendix, scale bar 60 μm; (E) curvature of the terminal hook of a sucker sclerite, scale bar 60 μm; (F–H) sucker sclerites 1–3, respectively, scale bar 60 μm.
Redescription (based on 9 whole- and 9 partially mounted specimens, including hologenophores, 2 syntypes; measurements based on specimens collected in present study): Body 3623–7425 (5562; n = 7) long, 800–1575 (1172; n = 10) at greatest width, tapering anteriorly (Fig. 5A). Oral sucker 160–283 (224; n = 9) long, 200–375 (278; n = 10) wide, multiple papillae present within mouth. Pharynx bulbous, 73–150 (98; n = 7) long, 63–150 (88; n = 7) wide. Oesophagus short. Caeca double, diverticulated, fusing just anterior to haptor, extends as non-diverticulated branches into haptor and haptoral appendix. Haptor symmetrical, squared-oval shaped 950–1550 (1257; n = 7) long, 800–1075 (938; n = 6) wide, armed with 2 parallel symmetrical rows of bell-shaped suckers, and haptoral appendix; haptoral suckers in 3 pairs; pairs 1–3 measuring 380–550 (464; n = 7), 425–550 (493; n = 6) and 375–500 (451; n = 6) in diameters, respectively; hook-shaped sclerites embedded within haptoral suckers (see Fig. 6E–H), composed by long slightly curved shaft, short recurved hook, measurements are included in Table 6; haptoral appendix 1013–1875 (1515; n = 8) long, 255–500 (407; n = 10) wide, bearing 2 distal bell-shaped suckers composed of a small proximal and distal bulb and pair of anchors (Fig. 5D): proximal bulb 36–65 (51; n = 8) long, 65–85 (73; n = 8) wide; distal bulb 95–225 (164; n = 8) long, 125–185 (151; n = 8) wide; anchors 50–75 (62; n = 9) in total length, 25–40 (31; n = 3) wide. Testes numerous (40–55; n = 3), irregular-shaped, located in posterior third of the body; vas deferens winding from testes to base of male copulatory organ (MCO). Germarium (ovary) J-shaped (Fig. 6C), 554–670 (612; n = 2) long, 268–430 (349; n = 2) wide, pre-testicular, proximally poorly lobate, descending branch straight, ascending branch straight. Ootype smooth (see Fig. 6C); seminal receptacle present, 225–340 (278; n = 3) long, 105–125 (117; n = 3) wide; uterus ventral to vas deferens, containing few eggs; eggs 138–190 (168; n = 4) long, 40–65 (53; n = 4) wide, with polar filaments 70–120 (98; n = 3). Common genital pore posterior to caeca bifurcation. MCO unarmed (Fig. 6B), distally bulbous, ovate, 18–63 (35; n = 5) in diameter; prostatic region 33–120 (77; n = 5) long, 13–65 (36; n = 5) wide; distal portion of vas deferens with thick walls. Two latero-ventral vaginal apertures; vagina parallel, proximally connected to vitelline commissure; distal vaginal duct expanded with glands along entire length.
Table 6. Erpocotyle microstoma sclerite measurements in micrometres

Sclerites are numbered 1 through 3, with 1 being closest to the point of attachment of the haptoral appendix. Ranges are given, with averages in parentheses and number of specimens measured. See Fig. 1 for images of measurements: (A) tip-to-tip (MacCallum, Reference MacCallum1931), (B) perimeter hook length (Euzet and Maillard, Reference Euzet and Maillard1967), (C) perimeter shaft length (Euzet and Maillard, Reference Euzet and Maillard1967), (D) shaft length (Bullard and Dippenaar, Reference Bullard and Dippenaar2003) and (E) max shaft width (Bullard and Dippenaar, Reference Bullard and Dippenaar2003).
Taxonomic summary:
Type host and locality: Sphyrna zygaena Beaufort, NC, USA (western North Atlantic Ocean)
Present hosts and localities: Sphyrna lewini, S. gilberti, hybrid (S. lewini/S. gilberti) Bulls Bay, Awendaw, SC, USA (western North Atlantic Ocean)
Other reported localities: Sphyrna mokarran Panama Canal, Panama (Pacific Ocean); Sphyrna tudes Punta del Este, Uruguay (western South Atlantic Ocean)
Site of infection: gill filaments
Specimens studied: syntypes USNM 132155, HWML 1437; vouchers: USNM 1666647, USNM 1666648, USNM 1666649, USNM 1666651, USNM 1666652, USNM 1666653, USNM 1666654, HWML 216856, HWML 216857, HWML 216858, HWML 216859, HWML 216860, HWML 216861, HWML 216863, HWML 216864; hologenophores: USNM 1666642, USNM 1666643, USNM 1666644, USNM 1666645, USNM 1666646, USNM 1666650, HWML 216851, HWML 216852, HWML 216853, HWML 216854, HWML 216855, HWML 216861, HWML 216862, HWML 216863, HWML 216864, HWML 216865, HWML 216866
Representative sequences: COI – Genbank OP342755, OP342756, OP342757, OP342758, OP342759, OP342760, OP342761, OP342762, OP342763, OP342764, OP342765, OP342766, OP342767, OP342768, OP342769, OP342770, OP342771
Remarks: Brooks (Reference Brooks1934) indicated the absence of the MCO in his original description of this species. It thus far has been either considered missed or assumed absent in all other redescriptions (Price, Reference Price1942; Caballero et al., Reference Caballero, Hidalgo and Grocott1956; Suriano and Labriola, Reference Suriano and Labriola1998). However, the MCO is clearly present in available syntypes and specimens collected in the present study, and it corresponds to the morphology of MCO of Erpocotyle species as diagnosed by Boeger and Kritsky (Reference Boeger and Kritsky1989), except for the relatively expanded prostatic region, which can represent either an artefact or an autapomorphic feature of the species. The vaginae, which were never previously described in detail (see Price, Reference Price1942; Caballero et al., Reference Caballero, Hidalgo and Grocott1956; Suriano and Labriola, Reference Suriano and Labriola1998), also conform with the general morphology for species of the genus as diagnosed by Boeger and Kritsky (Reference Boeger and Kritsky1989) – the vaginae are parallel, non-muscular, differentiated into 2 segments and distally expanded with basal glands. The generic diagnosis for Erpocotyle spp. includes glando-muscular terminal vaginae, a homologous character. However, compared to those from USNM, our specimens displayed a more dilated distal vagina in E. microstoma than in E. sphyrnae. This feature, along with the shape and size of the sucker sclerites, may potentially be used to distinguish E. microstoma from the remaining species of the genus.
Available sequences for hexabothriids are limited (only 9 COI, 14 Cytb, 13 28S and 7 18S). The 5 COI sequences of E. microstoma used in this analysis form a group in the NJ tree, with high bootstrap support (Fig. 3; Table 5). Conspecific distances among sequences of E. microstoma did not exceed 0.04 while interspecific distances varied between 0.29 and 0.40. Although sequences generated were short (~340 bp), such short sequences have been demonstrated to still be useful in species delimitation of other organisms (Hajibabaei et al., Reference Hajibabaei, Smith, Janzen, Rodriguez, Whitfield and Hebert2006). The distances (0.23–0.25; Table 4) between sequences of the 2 morphologically distinguishable species observed in this study support the delimitation between E. microstoma and E. sphyrnae.
Erpocotyle sphyrnae (MacCallum, Reference MacCallum1931)
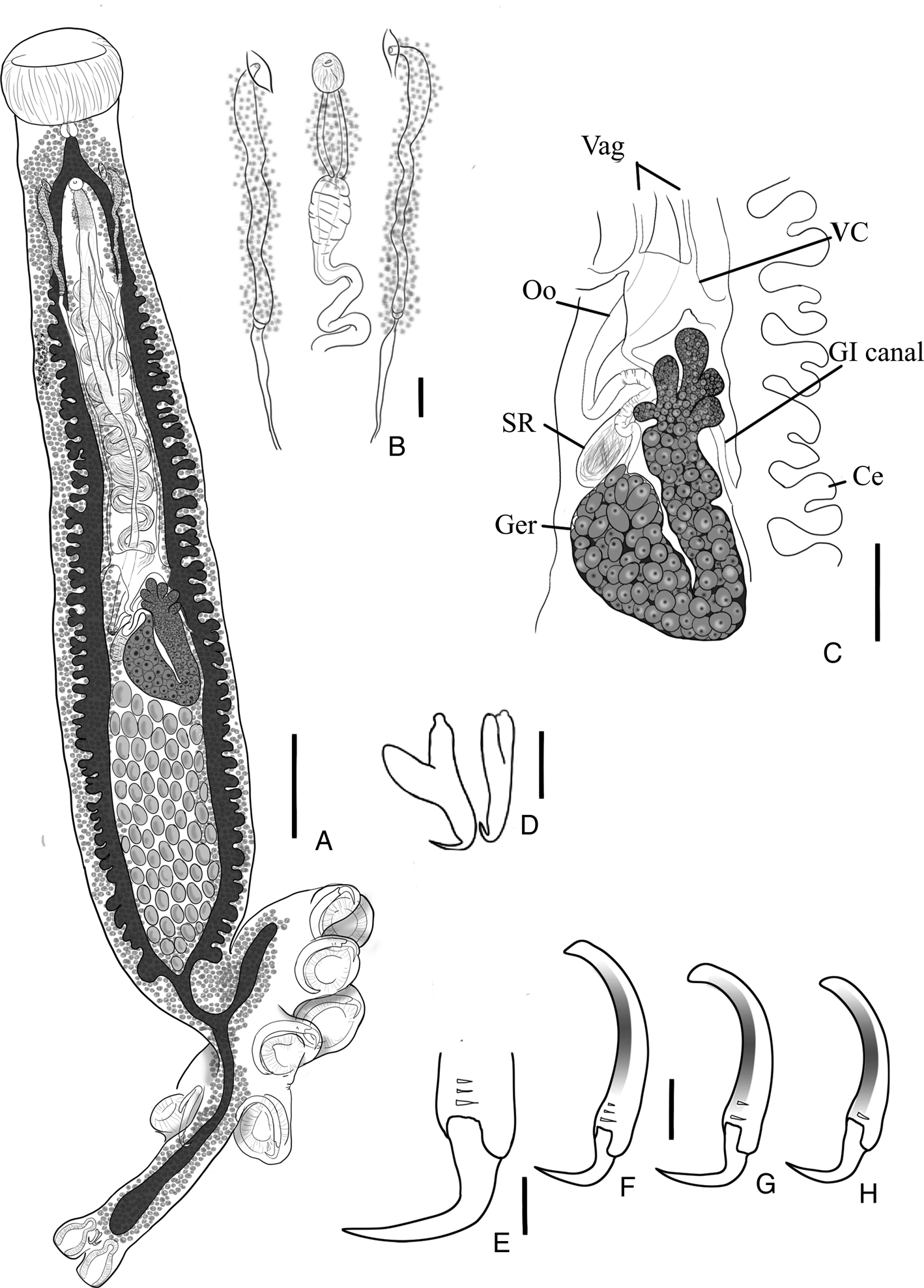
Fig. 7. Erpocotyle sphyrnae (A) whole body, scale bar 500 μm; (B) male copulatory organ (MCO) and parallel vaginae, scale bar 60 μm; (C) midsection of specimen (abbreviations as in Fig. 6), scale bar 200 μm; (D) anchors of the haptoral appendix, scale bar 25 μm; (E) curvature of the terminal hook of a sucker sclerite, scale bar 30 μm; (F–H) sucker sclerites 1–3, respectively, scale bar 60 μm.
Redescription (based on 7 whole- and 5 partially mounted specimens, including hologenophores, 5 syntypes; measurements are from specimens collected in the present study): Body fusiform, 1425–4550 (2719; n = 7) long, 263–890 (521; n = 9) wide at posterior third of body (see Fig. 7A). Oral sucker 140–445 (258; n = 9) long, 190–550 (350; n = 8) wide, multiple papillae present within the mouth. Pharynx bulbous, 48–80 (62; n = 6) in diameter. Oesophagus short. Caeca double, diverticulated, fusing just anterior to haptor, extend as non-diverticulated branches into haptor and haptoral appendix. Vitellaria extending posteriolaterally, branching into haptor and haptoral appendix. Haptor symmetrical, in the shape of a squared-oval 480–970 (704; n = 8) long and 375–562 (478; n = 4) wide, armed with 2 parallel symmetrical rows of bell-shaped suckers. Haptoral appendix projecting marginally between first sucker–sclerite pair; hook-shaped sclerites embedded within haptoral suckers, comprises long shaft and long recurved hook (see Fig. 7E–H). Haptoral suckers 1–3 measure 180–325 (252; n = 7), 180–330 (258; n = 7) and 180–325 (250; n = 7) in diameter measurements of sucker sclerites in Table 7. Haptoral appendix 410–1030 (740; n = 8) long, 140–320 (222; n = 8) wide with 2 terminal bell-shaped suckers, each containing a muscular proximal bulb 13–53 (31; n = 7) long, 25–63 (46; n = 7) wide, and a distal bulb, 48−145 (107; n = 8) long, 58–148 (107; n = 8) wide and a pair of anchors between suckers (Fig. 7D), 45–58 (52; n = 10) long and 13–33 (22; n = 5) wide. Genital pore common. Testes multiple (60–98; n = 3), post-germarium, each subovate. Vas deferens distally wide with thick walls, winding from anterior testes to base of MCO; MCO composed by a distal unarmed, ovate muscular bulb 25–50 (40; n = 4) in diameter and a proximal elongate prostatic region 36–100 (79; n = 4) long and 29–45 (37; n = 4) wide (Fig. 7B). Two parallel vaginae (see Fig. 7B), located on either side of the MCO, differentiated into 2 segments: distal segment expanded with glands along entire length and a thin muscular layer on the most distal segment. Vaginal pores opening at level of genital pore, with each vaginal duct connecting proximally to the vitelline reservoir/commissure. Ootype smooth (see Fig. 7C); uterus with up to 3 eggs; eggs fusiform, 125–235 (197; n = 3) long, 35–50 (42; n = 3) wide, with 2 polar filaments 25–95 (53; n = 3) long. Germarium J-shaped, 180–648 (357; n = 5) long by 95–348 (185; n = 5) wide, adjacent to testes to the left of the medial line; proximal germarium lobate, straight descending and ascending germarium branches. Seminal receptacle ovate, 85–188 (139; n = 4) long and 40–90 (70; n = 4) wide.
Table 7. Erpocotyle sphyrnae sclerite measurements in micrometres

Sclerites are numbered 1 through 3, with 1 being closest to the point of attachment of the haptoral appendix. Ranges are given with averages in parentheses and number of measured specimens. See Fig. 1 for images of measurements: (A) tip-to-tip (MacCallum, Reference MacCallum1931), (B) perimeter hook length (Euzet and Maillard, Reference Euzet and Maillard1967), (C) perimeter shaft length (Euzet and Maillard, Reference Euzet and Maillard1967), (D) shaft length (Bullard and Dippenaar, Reference Bullard and Dippenaar2003) and (E) max shaft width (Bullard and Dippenaar, Reference Bullard and Dippenaar2003).
Taxonomic summary:
Type host and locality: Sphyrna zygaena Woods Hole, MA, USA (western North Atlantic Ocean)
Present host and localities: Sphyrna lewini, S. gilberti, hybrid (S. lewini/S. gilberti) Bulls Bay, Awendaw, SC, USA (western North Atlantic Ocean)
Other hosts and localities: Sphyrna zygaena, S. lewini Dakar, Senegal (eastern North Atlantic Ocean); S. lewini Hawaii, USA (Pacific Ocean); S. mokarran Nueweiba, Egypt (Gulf of Aqaba)
Site of infection: gill filaments
Specimens studied: syntypes USNM 1320884, USNM 1320885; vouchers: USNM 1666656, USNM 1666657, USNM 1666659, USNM 1666660, USNM 1666661, HWML 216867, HWML 216868, HWML 216869; hologenophores: USNM 1666655, USNM 1666658, HWML 216870, HWML 216871
Representative sequences: COI_Genbank OP342754, OP342755
Remarks: Yamaguti (Reference Yamaguti1968) presented the latest redescription of this species, which provides its morphology in detail, but indicated the presence of 6 irregular muscular pits, 2 median and 4 submedian, within the anterior sucker. These structures were not present in any of the specimens examined herein nor were they noted in any of the previous descriptions/redescriptions (MacCallum, Reference MacCallum1931; Price, Reference Price1942; Euzet and Maillard, Reference Euzet and Maillard1967).
Compared to the syntype specimens, our specimens depicted a protruding bulge on one of the proximal ends of the anchors (Fig. 7D). However, this feature was not visible in the museum specimens likely because their anchors were not properly flattened. Anchors have been shown to have high morphological variation among hexabothriids (Teo et al., Reference Teo, Dhillon and Lim2013; Khang et al., Reference Khang, Soo, Tan and Lim2016); thus, the presence of this bulge may be a possible differential diagnostic between E. sphrynae and E. microstoma.
The 2 COI sequences generated for E. sphyrnae formed a group in the NJ tree (Fig. 3). This group is adjacent to the group composed by E. microstoma sequences, presenting a distance of 0.23–0.25 among these species, compared to distances between 0.35 and 0.41 with other, non-Erpocotyle Hexabothriidae sequences (Table 4).
Subclass Polyonchoinea Bychowsky, 1937
Monocotylidae Taschenberg, 1879
Loimosina wilsoni (Manter, Reference Manter1944)
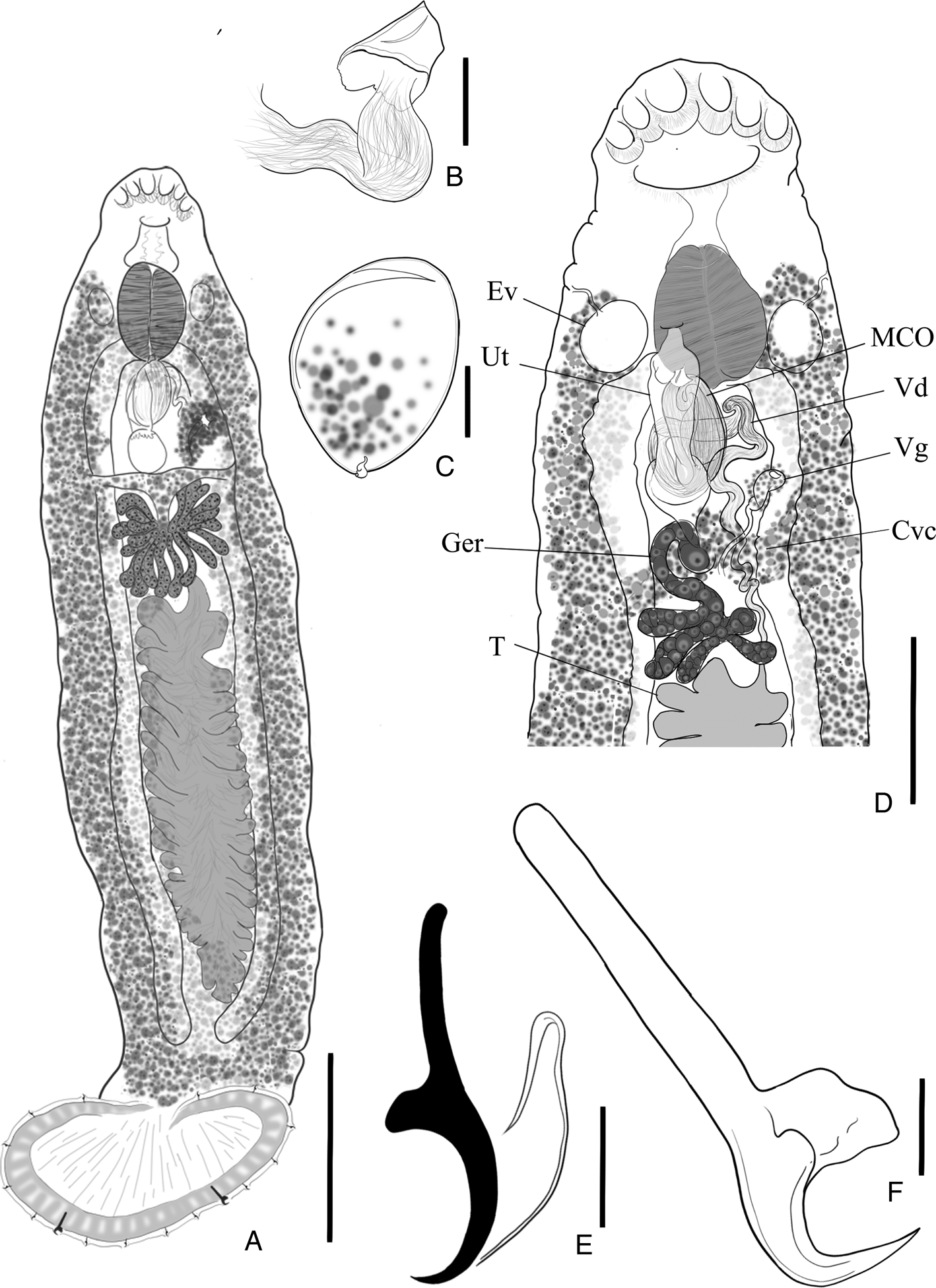
Fig. 8. Loimosina wilsoni (A) whole body, scale bar 500 μm; (B) sclerotized, cone-shaped male copulatory organ (MCO), scale bar 16 μm; (C) egg, scale bar 25 μm; (D) mid- to terminal portion of specimen containing the Cvc (common vitelline commissure), Ev (excretory vesicle), Ger (germarium), MCO, T (teste), Ut (uterus), Vd (vas deferens) and Vg (vagina), scale bar 150 μm; (E) haptoral hook, scale bar 10 μm; (F) haptoral anchor, scale bar 10 μm.
Redescription (based on 9 whole- and 2 partially mounted specimens, including hologenephores and 3 syntypes; measurements are from specimens collected in present study): |Body 1415–3226 (2089; n = 4) long by 229–506 (371; n = 5) wide at mid-length (see Fig. 8A). Head tapering anteriorly bearing 3 pairs of symmetrical pits. Mouth subterminal, ventral with prepharynx; large bulbous pharynx, 104–221 (166; n = 8) long, 103–255 (155; n = 8) wide, large excretory vesicle on each side (see Fig. 8D); oesophagus inconspicuous; caeca lacking diverticulae, extending posterolaterally, terminating blind just anterior to haptor. Haptor subcircular, 184–393 (281; n = 8) long, 378–673 (504; n = 8) wide, with an external row of small and often inconspicuous loculi, a single latero-posterior pair of anchors (Fig. 8F), 7 pairs of smaller hooks organized evenly and symmetrically around the rim of the haptor (Fig. 8E). Anchors 27–60 (44; n = 10) long, 9–16 (12; n = 5) wide, evenly curved point and shaft, truncated superficial root, elongate deep root 16–37 (28; n = 7). Hooks similar in shape and size, 8 (n = 2) long, with short, recurved point, slightly curved shaft, erected truncate thumb, shank about as long as shaft; filamentous hook loop reaching ½ of shank. Common reproductive pore overlaps pharynx, common genital atrium expanded. Single, large, heavily lobed testis, 55–105 (85; n = 3) long, 21–28 (24; n = 3) wide, post-germarium, occupying middle third of body; vas deferens intercaecal, convoluted, several distal loops expanding into seminal vesicle; ejaculatory bulb pear-shaped with short, cone-shape sclerotized MCO (Fig. 8B). Germarium strongly lobate (especially in larger organisms) (see Fig. 8D); uterus expanded; vaginal opening ventro-lateral; vagina sinuous duct distally expanded, glandular. Vitellaria follicular, coextensive with caeca; vitelline commissure overlapping with branch of germarium; ootype, seminal receptacle not observed. Egg ovate, 67–108 (94; n = 3) long, 46–87 (71; n = 3) wide, with a short proximal filament (Fig. 8C).
Taxonomic summary:
Type host and locality: Sphyrna zygaena Montego Bay, Jamaica (Caribbean Sea)
Present hosts and localities: Sphyrna lewini and hybrids (S. lewini/S. gilberti) Bulls Bay, Awendaw, SC, USA (western North Atlantic Ocean)
Other hosts and localities: Sphyrna lewini Dakar, Senegal (eastern North Atlantic Ocean)
Site of infection: gill filaments
Specimens studied: syntypes USNM 1337561, USNM 1337564, HWML 1425; vouchers: USNM 1666662, USNM 1666663, USNM 1666664, USNM 1666665, USNM 1666666, USNM 1666668, USNM 1666669, USNM 1666670, HWML 216873, HWML 216874, HWML 216875, HWML 216876, HWML 216878, HWML 216879, HWML 216880, HWML 216881; hologenophores: USNM 1666667, HWML 216872, HWML 216877
Representative sequences: COI-Genbank OP342748, OP342749, OP342750, OP342751, OP342752, OP342753; 28S-Genbank OP348870, OP348871, OP348872, OP348873
Remarks: Bravo-Hollis (Reference Bravo-Hollis1970) noted that the main differences between L. parawilsoni and L. wilsoni were geographical location (L. parawilsoni described from S. lewini in the Pacific Ocean), and few morphological features. Loimosina parawilsoni depicts a sub-circular haptor with hooks embedded in the papillae, a ‘different [sic]’ shape of the anchors, the presence of a common genital pore and a MCO with sclerotized, blade-like lateral walls. Manter (Reference Manter1944) interpreted the MCO of L. wilsoni as ‘rudimentary, consisting of a very short, very thinly chitinized tube near the male pore [sic]’, uncertain of its position within or external to the ejaculatory bulb. Euzet and Maillard (Reference Euzet and Maillard1967) did not observe an MCO, instead considered this as the sclerotized distal wall of the ejaculatory bulb, protruding slightly ventrally. Syntype specimens from Manter (Reference Manter1944) were not mounted flat; structures were difficult to study and no MCO was visible in the syntypes. Unfortunately, there is no specimen from Euzet and Maillard (Reference Euzet and Maillard1967) in museum collections. Our specimens presented a short conical, sclerotized MCO at the distal extremity of the ejaculatory bulb (Fig. 8B) – thus, the presence of a sclerotized MCO projecting from the ejaculatory bulb is not a distinguishable diagnostic between the 2 species of Loimosina. Likewise, Bravo-Hollis (Reference Bravo-Hollis1970) mentions a difference in the shape of the anchors, but a difference between those of the syntype of L. parawilsoni (CNHE 154) and our specimens was not evident. Molecular sequences from specimens of Loimosina collected from S. lewini from the Pacific Ocean would allow clarification of this classification. However, as morphological descriptions stand, specimens of both Loimosina species are greatly similar which leaves the only differential criterion to be the shape of the haptor. However, the shape of non-sclerotized structures is often affected by fixation and mounting. Hence, given that both species are described from the same host, we strongly suggest that L. parawilsoni is a junior synonym of L. wilsoni. However, a definitive decision should await adequate restudy of specimens of L. parawilsoni and, preferably, a molecular delimitation of the species.
The same nucleotide composition of 28S from species of Loimosina redescribed in this study and that of Boeger et al. (Reference Boeger, Kritsky, Domingues and Bueno-Silva2014) also strongly supports that L. wilsoni is present in at least 1 species of Sphyrna in the South Atlantic Ocean (see discussion).
Available partial sequences of the monocotylids Empruthotrema orashken Irigoitia et al., Reference Irigoitia, Braicovich, Rossin, Canel, Levy, Farber and Timi2019, Empruthotrema dorae Irigoitia et al., Reference Irigoitia, Braicovich, Rossin, Canel, Levy, Farber and Timi2019 and Empruthotrema aoneken Irigoitia et al., Reference Irigoitia, Braicovich, Rossin, Canel, Levy, Farber and Timi2019 of the COI mitochondrial DNA gene grouped with the sequences produced herein. Sequences of L. wilsoni cluster together with significant bootstrap support and present short intraspecific distances (0.01–0.02, see Table 5) and significantly greater interspecific distances (0.22–0.32; Fig. 4).
Discussion
Age-dependent parameters can produce high variability when averaging measurements (or providing ranges), and it would aid in the validation of measurements and resolve diagnostic issues related to the genera of Hexabothriidae if these variables were identified (see Vaughan and Christison, Reference Vaughan and Christison2012). For example, the extension of the vitellaria into the haptoral appendix is disputed in the literature as to whether it is a reliable character of a species or genera, or if it is age-dependent for individuals (Price, Reference Price1942; Euzet and Maillard, Reference Euzet and Maillard1967). Originally used by Price (Reference Price1942) as a diagnostic in the genera of Hexabothriidae, the extension of the vitellaria was noted to vary with the maturity of the specimen by Cerfontaine (Reference Cerfontaine1899), Sproston (Reference Sproston1946) and by Euzet and Maillard (Reference Euzet and Maillard1967) in their respective redescriptions of E. sphyrnae. Accordingly, Boeger and Kritsky (Reference Boeger and Kritsky1989) did not mention this aspect of the vitellaria distribution in their revision of Hexabothriidae. In our specimens, the vitellaria visibly extended into the haptor and the haptoral appendix in all intact mature specimens of both species of Erpocotyle (n = 16), but was not observed to do so in 3 juvenile representatives of E. sphyrnae, indicating that this character is age-dependent and not a reliable generic or specific diagnosis.
This study also revealed differences in the host repertoires of monogenoids – indicating different abilities to infect the spectrum of available hosts. Monogenoids typically demonstrate narrow host repertoires being either monoxenous – infecting a single host species – or stenoxenous – infecting a few related host species (Euzet and Combes, Reference Euzet and Combes1980; Rohde, Reference Rohde1994; Whittington et al., Reference Whittington, Cribb, Hamwood and Halliday2000). In this study, L. wilsoni presented a narrow host repertoire, as it was found only on S. lewini and the hybrids and did not infect any of the 44 S. gilberti examined. Host repertoire is typically determined by the opportunity of encounter and the compatibility of the involved host/parasite species (Combes, Reference Combes2001; Araujo et al., Reference Araujo, Braga, Brooks, Agosta, Hoberg, von Hartenthal and Boeger2015; Brooks et al., Reference Brooks, Hoberg and Boeger2019). Factors involved in the success of the establishment of a parasite may depend on biological and behavioural aspects of the host, such as season of reproduction, stage of maturation, physiology, feeding behaviour and immunity, to list a few (MacDonald, Reference Macdonald1975; Tinsley and Jackson, Reference Tinsley and Jackson2002; Glennon et al., Reference Glennon, Chisholm and Whittington2006; Ohashi et al., Reference Ohashi, Umeda, Hirazawa, Ozaki, Miura and Miura2007; Whittington and Kearn, Reference Whittington and Kearn2011).
Hosts for L. wilsoni have been identified as S. zygaena and S. lewini [syns: Sphyrna diplana (Springer, Reference Springer1941) and Sphyrna couardi (Cadenat, 1950)] (Manter, Reference Manter1944; Hargis, Reference Hargis1955; Euzet and Maillard, Reference Euzet and Maillard1967). However, there remains some uncertainty in the literature over host identity. Hargis (Reference Hargis1955) first suggested that the type host, S. zygaena, was possibly misidentified by Manter (Reference Manter1944), a doubt also mentioned by Euzet and Maillard (Reference Euzet and Maillard1967), and that the host could have been S. diplana (syn. S. lewini). This is supported by the fact that Manter (Reference Manter1944) never observed first-hand the shark specimens as they were collected and provided by Dr C. B. Wilson (for whom L. wilsoni was named). Euzet and Maillard (Reference Euzet and Maillard1967) also indicated the morphological similarities of S. couardi and S. lewini in their own report, which have been later synonymized in McEachran and Séret (Reference McEachran and Séret1987). Paradoxically, as advances in molecular research have improved species delimitation, cryptic host species are often uncovered, such as the case of S. gilberti and S. lewini. Thus, it becomes even more intriguing that in this study that L. wilsoni remained specific to S. lewini and admixed individuals with at least 50% S. lewini DNA (S. lewini F1 and BX) and that no S. gilberti or S. gilberti backcrosses (S. gilberti BX) were infected by L. wilsoni. The exclusion of S. gilberti BX from the host repertoire of L. wilsoni remains to be verified, however, given the small number of specimens of S. gilberti BX (n = 4). The exclusion of species S. gilberti, in contrast, from the host repertoire of L. wilsoni appears concrete given the large number of specimens of this species (n = 44).
Boeger et al. (Reference Boeger, Kritsky, Domingues and Bueno-Silva2014) reported a specimen of Loimosina sp. from an unidentified hammerhead individual Sphyrna sp. off the coast of southeast Brazil. Although these authors were unable to identify this parasite to the species level based on morphology, its 28S rDNA sequence (GB Accession KF908848) was 98–100% identical to our specimens, suggesting that it was L. wilsoni. As S. gilberti appears not to be in the host repertoire of L. wilsoni, but only in S. lewini and hybrids, it is likely that the Sphyrna host species from Brazil was S. lewini, which is known from those coasts (Compagno, Reference Compagno1984), or yet another species, such as Sphyrna media Springer, 1940, S. mokarran (Rüppell, 1837), Sphyrna tiburo (Linnaeus, 1758), S. tudes (Valenciennes, 1822) or S. zygaena, all reported from south Brazil (Compagno, Reference Compagno1984). Whereas it is not known if L. wilsoni can infect other Sphyrna species, its presence on hybrids of S. lewini and S. gilberti indicates the possibility of it being stenoxenous and thus potentially using any of these hammerheads as host. Previous studies of the molecular phylogeny of family Sphyrnidae has found the globally distributed S. lewini to be more closely related to smaller, range-restricted (eastern Pacific and western Atlantic) sharks S. media, S. tudes and S. tiburo than to other globally distributed large sharks S. mokarran and S. zygaena (Lim et al., Reference Lim, Motta, Mara and Martin2010). This is further supported by Pinhal et al. (Reference Pinhal, Shivji, Vallinoto, Chapman, Gadig and Martins2012), which showed the cryptic Atlantic lineage, now known as S. gilberti, to be more closely related to S. lewini than the other smaller sharks. This further draws doubt to S. zygaena being included in the host repertoire of this parasite.
The hexabothriids E. microstoma and E. sphyrnae have wider host ranges than L. wilsoni. Both have been originally described from specimens of S. zygaena from the western North Atlantic along the US coast (Woods Hole, MA and Beaufort, NC, respectively) and included in their repertoire are other hammerheads: E. sphyrnae has been reported in S. zygaena and S. lewini from Dakar, Senegal (Euzet and Maillard, Reference Euzet and Maillard1967), S. lewini in Hawaii (Yamaguti, Reference Yamaguti1968), S. mokarran from Nuweibaa, Egypt (Maillard and Paperna, Reference Maillard and Paperna1978) and now in S. lewini, S. gilberti and their hybrids from the SC coast of the USA (present study). Erpocotyle microstoma has been reported also in S. mokarran but from the Panama Canal (Pacific Ocean; Caballero et al., Reference Caballero, Hidalgo and Grocott1956), S. tudes from Punta del Este, Uruguay (Suriano and Labriola, Reference Suriano and Labriola1998) and now in S. lewini, S. gilberti and their hybrids from the SC coast of the USA (present study). Maillard and Paperna (Reference Maillard and Paperna1978) were the first to recognize the wider repertoires of both these Erpocotyle species, and the vast geographic range of E. sphyrnae. Here, we further expand the host repertoire and geographic ranges of these 2 species.
Bullard et al. (Reference Bullard, Warren and Dutton2021) updated the list of monocotylids in S. lewini to include C. selachii, which they found infecting the olfactory cavities of S. lewini in the northern Gulf of Mexico. The present study did not investigate the olfactory cavities of the specimens of S. lewini, S. gilberti and hybrids collected from the western North Atlantic. However, C. selachii, the only described species and thus the type for the genus, is noted as having a very broad host repertoire; it has been found to infect several Sphyrna spp., including S. lewini, as well as species of Carcharhinus Blainville, 1816, Rhizoprionodon Whitley, 1929 (both Carcharhinidae) and Alopias Rafinesque, 1810 (Alopiidae), in the Gulf of Mexico and in the western North Atlantic. Thus, it is entirely possible that C. selachii infects S. lewini and/or S. gilberti in the western North Atlantic.
In conclusion, this is the first study to compare monogenoid fauna between cryptic Sphyrna species and their hybrids in the western North Atlantic Ocean, and the first report of monogenoids collected from the gills of individuals of S. gilberti and hybrids with S. lewini. Genetic sequencing using nuclear ribosomal ITS2 sequences showed that species S. lewini and S. gilberti diverged around 4.5 million years ago (Pinhal et al., Reference Pinhal, Shivji, Vallinoto, Chapman, Gadig and Martins2012) yet can be found sympatrically and can produce reproductive and viable hybrids. This study demonstrated that these 2 hammerheads have differences in monogenoid fauna of the gills, which in addition to the number of vertebrae, further supports a distinction between species.
Lastly, Loimoidae or Loimoinae Price, Reference Price1936 – a subfamily of Monocotylidae – was previously composed of species of 3 genera: Loimos McCallum, 1917, Loimosina and Loimopapillosum Hargis, 1955. The more comprehensive morphological analysis of the specimens provided in this study and the recognition that the sequence of the 28S rDNA fragment of L. wilsoni presented herein is identical to that of Loimosina sp. published by Boeger et al. (Reference Boeger, Kritsky, Domingues and Bueno-Silva2014). This corroborates the decision that the species of these genera are allocated within the Monocotylidae and do not compose a monophyletic assemblage within the family, following the suggestion of Boeger et al. (Reference Boeger, Kritsky, Domingues and Bueno-Silva2014) and Chero et al. (Reference Chero, Cruces, Sáez, Oliveira, Santos and Luque2021). Although there is no sequence currently available for the species of Loimos, molecular phylogenetic analyses using sequences of Loimosina from the present study and from Boeger et al. (Reference Boeger, Kritsky, Domingues and Bueno-Silva2014), and Loimopapillosum (see Chero et al., Reference Chero, Cruces, Sáez, Oliveira, Santos and Luque2021), strongly indicate that these are members of distinct clades within the Monocotylidae. The classification of Loimos and a more robust decision on the validity of Loimoidae/Loimoinae, however, should await sequencing of corresponding DNA fragments and phylogenetic analysis. Likewise, sequences of Loimosina specimens from the Pacific Ocean (in combination with morphological examination) would clarify whether L. parawilsoni is in fact a synonym for L. wilsoni, as suggested in the present study.
Acknowledgements
The authors thank Yolanda Villacampa at the Scientific Imaging Lab at the National Museum of Natural History, Smithsonian Institution (Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington DC), and Luis García Prieto, collections manager of CHNE, who rapidly provided photographs of the syntype specimens following 2 years of closures and delays related to the COVID-19 pandemic.
Data availability
All sequences and representative specimens are available from respective repositories, as stated in the manuscript.
Author's contribution
I. d. B. conceived the idea of the initial study, which was carried out by K. M. D.; sharks were captured and provided by B. S. F. and A. S. G.; all molecular sequencing was done by A. B. and K. M. H.-S.; D. S. P. provided assistance in data manipulation and in writing; W. A. B. assisted in taxonomy, data analysis and in writing.
Financial support
College of Charleston funding provided by the Undergraduate Research and Creative Activities Major Academic Year Support (MAYS) Grant #MA 2019-02. Funding for this research conducted at the Universidade Federal do Paraná was provided in the form of a scholarship by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Brasil – finance Code 001). This work was partially supported by Grant ‘O Paradigma de Estocolmo: explorando as previsões do paradigma sobre os padrões e processos em sistemas parasitos e hospedeiros’ from the Conselho Nacional de pesquisa e Desenvolvimento, Brazil (No. 302708/2020-0). Funding for the capture and research of sharks was provided by the Competitive State Wildlife Grant SC-U2-F15AP00050.
Conflict of interest
None.
Ethical standards
All sharks were collected and sampled by authorized staff under official permits or scientific exemptions of US state government agencies (SCDNR Scientific Permit no. 2212).