Introduction
Prolyl endopeptidase (PREP; EC 3.4.21.26) is a protein belonging to the serine protease family, with post-proline cleavage activity (Morawski et al., Reference Morawski, Schulz, Zeitschel, Blosa, Seeger and Rossner2011). Its three-dimensional structure has been well characterized, revealing two domains: the catalytic triad (Ser554, His680, Asp641), arranged in an α/β-hydrolase fold, and this is covered by a so-called β-propeller domain (Fülöp et al., Reference Fülöp, Böcskei and Polgár1998). This peculiar structure is probably important to control access of substrates into the active site (Schulz et al., Reference Schulz, Gerhartz, Neubauer, Holloschi, Heiser, Hafner and Demuth2002). PREP is able to hydrolyse the peptide bond on the carboxyl side of proline residues in oligopeptides that are comprised of no more than about 30 amino acid residues (Szeltner & Polgár, Reference Szeltner and Polgár2008), as well as peptide hormones and neuropeptides (Wilk, Reference Wilk1983; Mentlein, Reference Mentlein1988). PREP has been detected in all mammalian tissues, including uterus (Walter et al., Reference Walter, Shlank, Glass, Schwartz and Kerenyi1971), heart, spleen, kidney, testis, liver and brain (Yoshimoto et al., Reference Yoshimoto, Ogita, Walter, Koida and Tsuru1979), where it shows the highest enzymatic activity (Taylor et al., Reference Taylor, Andrews, Henrikson and Dixon1980). Although the structural and enzymatic properties of this protein are well characterized, its biological functions are not well understood. PREP has been implicated in several processes including development, cell proliferation and differentiation (Ohtsuki et al., Reference Ohtsuki, Homma, Kurata, Komano and Natori1994; Matsubara et al., Reference Matsubara, Ono, Tsubuki, Irie and Kawashima1998; Suzuki et al., Reference Suzuki, Sakaguchi, Tanaka, Yoshimoto and Takaoka2014; Maruyama et al., Reference Maruyama, Matsubara and Kimura2017), cell death (Bär et al., Reference Bär, Rahfeld, Schulz, Gans, Ruiz-Carrillo, Manhart, Rosche and Demuth2006; Matsuda et al., Reference Matsuda, Sakaguchi, Tanaka, Yoshimoto and Takaoka2013), learning and memory (Irazusta et al., Reference Irazusta, Larrinaga, González-Maeso, Gil, Meana and Casis2002; D’Agostino et al., Reference D’Agostino, Kim, Liu, Jeong, Suyama, Calignano, Gao, Schwartz and Diano2013) and cognitive disorders (Rossner et al., Reference Rossner, Schulz, Zeitschel, Schliebs, Bigl and Demuth2005; Hannula et al., Reference Hannula, Myöhänen, Tenorio-Laranga, Männistö and Garcia-Horsman2013) and in both male and female reproduction-associated processes (Kimura et al., Reference Kimura, Ohnishi, Okimura, Hamabata and Takahashi1998, Reference Kimura, Matsui and Takahashi2002; Dotolo et al., Reference Dotolo, Kim, Pariante, Minucci and Diano2016; Venditti and Minucci, Reference Venditti and Minucci2018). The fact that PREP is able to inactivate several peptides, such as substance P (Kato et al., Reference Kato, Nakano, Kojima, Nagatsu and Sakakibara1980; Bellemère et al., Reference Bellemère, Morain, Vaudry and Jégou2003) and arginine–vasopressin (Walter, Reference Walter1976; Aoyagi et al., Reference Aoyagi, Wada, Nagai, Kojima, Harada, Takeuchi, Takahashi and Tsumita1990; Toide et al., Reference Toide, Okamiya, Iwamoto and Kato1995) implied that it mainly acts in the extracellular space, even though it lacks a secretion signal or a lipid anchor sequence (Venäläinen et al., Reference Venäläinen, Juvonen and Männistö2004). Instead, in several studies, it was detected in the cytoplasm, suggesting intracellular functions for this enzyme. A correlation between PREP activity and intracellular inositol triphosphate concentration has been demonstrated (Williams et al., Reference Williams, Eames, Ryves, Viggars and Harwood1999), suggesting its role in molecular pathways involving Ca2+ as a secondary messenger. Moreover, Schulz et al. (Reference Schulz, Zeitschel, Rudolph, Ruiz-Carrillo, Rahfeld, Gerhartz, Bigl, Demuth and Rossner2005) reported that PREP is located in close association with tubulin, the main component of the cytoskeletal microtubules. They proposed that PREP might be involved in microtubule-associated process such as intracellular trafficking and vesicle sorting. These data suggest that PREP may have important functions inside the cells that are independent of its peptidase activity. , no reports have directly associated PREP with the epithelial cells of seminal vesicles (SV), exocrine glands that, together with the prostate and Cowper’s gland, constitute the male sex accessory system. During the exocytosis of molecules such as citric acid, prostaglandin, fructose and proteins, many cellular elements have an active role, such as microtubules for vesicle trafficking (Müsch, Reference Müsch2004; Park & Loh, Reference Park and Loh2008; Noordstra & Akhmanova, Reference Noordstra and Akhmanova2017) and Ca2+ for active exocytosis (Petersen & Tepikin, Reference Petersen and Tepikin2008; Raveh et al., Reference Raveh, Valitsky, Shani, Coorssen, Blank, Zimmerberg and Rahamimoff2012; Petersen, Reference Petersen2014). In a previous study, we reported the hypothesis that the formin DAAM1 may participate in SV physiology, suggesting its possible involvement as an actor in morphofunctional remodelling and organization of the epithelium and smooth muscle of these exocrine glands (Venditti et al., Reference Venditti, Fasano, Santillo, Aniello and Minucci2018). Therefore, to improve the understanding of the potential role of PREP in the cellular system, and in particular during exocytosis events, here we assessed its possible involvement in the secretory epithelium of mouse SV, also comparing the expression and localization with a fundamental component of cellular cytoskeleton, the microtubules.
Materials and methods
Animal care and tissue extraction
Male C57/BL mice (Mus musculus) were housed under defined conditions (12D:12L) and were fed standard food and provided with water ad libitum. Animals were perfused with phosphate-buffered saline (PBS; 13.6 mM NaCl; 2.68 mM KCl; 8.08 mM Na2HPO4; 18.4 mM KH2PO4; 0.9 mM CaCl2; 0.5 mM MgCl2; pH 7.4) and then sacrificed by decapitation under ketamine anaesthesia (100 mg/kg i.p.) in accordance with national and local guidelines covering experimental animals. For each animal, SV were dissected; one half was fixed in 10% formalin and embedded in paraffin for histological analysis, the other half was quickly frozen by immersion in liquid nitrogen and stored at −80°C until RNA and protein extraction. Additionally, testes were dissected, frozen and stored at −80°C to be used as a positive control.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the samples using TRIzol reagent (Sigma-Aldrich, St. Louis, MO, USA). First-strand cDNA was synthesized using 5 µg of total pooled RNA, 250 ng/ml oligo(dT) 0.8 mM primer (Promega, Heidelberg, Germany) and 100 U Super-script II RT enzyme (Invitrogen, Paisley, UK) in a total volume of 25 µl, according to the manufacturer’s instructions. As a negative control, the reaction was carried out on the same RNA without using the reverse transcriptase enzyme (RT−). In total, 2–3 µl of the obtained cDNA template were then used for the PCR reaction, as described by Chemek et al. (Reference Chemek, Venditti, Boughamoura, Mimouna, Messaoudi and Minucci2018). Based on the published sequence of Mus musculus PREP mRNA (NCBI GenBank accession no. NM_011156.3), specific oligonucleotide primers were designed as follows: PREP for: 5´-CCGTATTCTTGGCTTGAAGA-3´; and PREP rev: 5´-TCATGAACTTGATCGTCACC-3´. An appropriate region of Mus musculus α-tubulin mRNA (Tub; NCBI GenBank accession no. NM_011653.2) was amplified with specific oligonucleotide primers (Tub for 5´-CTCGCCTAGATCACAAGTTT-3´; Tub rev 5´-TATGAAAGCACACATTGCCA-3´) and used as the control. Amplifications were carried out for 35 cycles, with denaturation at 94°C for 30 s, annealing at 56°C for 45 s and extension at 72°C for 45 s. The expected PCR product sizes were 391 bp for PREP and 297 bp for Tub. The amplification products were electrophoresed on 1.2% agarose gel.
Protein extraction and western blot analysis
Mouse SV and testes were lysed in a specific buffer (1% NP-40, 0.1% SDS, 100 mM sodium orthovanadate, 0.5% sodium deoxycholate in PBS) in the presence of protease inhibitors (4 mg/ml of leupeptin, aprotinin, pepstatin A, chymostatin, PMSF and 5 mg/ml of TPCK). The homogenates were sonicated twice by three pulses (20 Hz for 20 s each); after centrifugation for 30 min at 10,000 g, the supernatants were stored at −80°C. Proteins (40 µg) were separated by 9% SDS-PAGE and transferred to Hybond-P polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia Biotech, Buckinghamshire, UK) at 280 mA for 2.5 h at 4°C. The filters were treated for 3 h with blocking solution [5% skimmed milk in TBS (10 mM Tris–HCl pH 7.6, 150 mM NaCl)] containing 0.25% Tween-20 (Sigma-Aldrich Corp., Milan, Italy) before the addition of anti-PREP (Abcam cat. no. ab58988), or anti-tubulin (Sigma-Aldrich, Milan, Italy) antibodies diluted respectively 1:3000 and 1:10000, and incubated overnight at 4°C. After three washes in TBST (TBS including 0.25% Tween-20), the filters were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (Sigma- Aldrich, Milan, Italy) for the rabbit anti-PREP antibody diluted 1:10000 in the blocking solution, or with anti-mouse IgG (ImmunoReagents, Inc., Raleigh, NC, USA) for the mouse anti-tubulin diluted 1:10000 in the same blocking solution. Then, the filters were washed again three times in TBST and the immunocomplexes were revealed using the ECL-western blotting detection system (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Histological analysis
To assess the quality and characterize the histology of the tissue samples, 7-µm thick mouse SV sections were obtained, stained with haematoxylin and eosin (H&E), and examined under a light microscope (Leica DM 2500) for histological evaluation. Photographs were taken using a Leica DFC320 R2 digital camera.
Immunofluorescence analysis
For the co-localization experiments for PREP + tubulin, 7-µm thick SV sections were dewaxed, rehydrated and processed as described by Pariante et al. (Reference Pariante, Dotolo, Venditti, Ferrara, Donizetti, Aniello and Minucci2016). Briefly, antigen retrieval was performed by pressure-cooking slides for 3 min in 0.01 M citrate buffer (pH 6.0). Non-specific binding sites were blocked with an appropriate normal serum diluted 1:5 in PBS containing 5% (w/v) BSA before the addition of anti-PREP and anti-tubulin antibodies diluted 1:100 in the blocking solution, for overnight incubation at 4°C. After washing in PBS, the slides were incubated for 1 h with the appropriate secondary antibody (Alexa Fluor 488, Invitrogen; FITC-Jackson, ImmunoResearch, Pero MI, Italy; anti-Mouse IgG 568, Sigma-Aldrich, Milan, Italy) diluted 1:500 in the blocking mixture. The slides were mounted with Vectashield + DAPI (Vector Laboratories, Peterborough, UK) for nuclear staining, and then observed under an optical microscope (Leica DM 5000 B + CTR 5000) with a UV lamp and images were viewed and saved using IM-1000 software.
Results and discussion
Exocytosis is a secretory trafficking process during which molecules are transported to the cell surface, where they can be either inserted into the plasma membrane or released into the extracellular space. Secretory transport is a multi-step process: after their formation from the Golgi, exocytotic vesicles travel along cytoskeletal elements towards the cell periphery and dock and fuse with the plasma membrane, thanks to the presence of soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors (SNAREs). Secretion can occur constitutively to provide components of extracellular matrix and cell adhesion structures, aimed at maintaining cell homeostasis (constitutive exocytosis). Conversely, this process is regulated, controlled spatially and temporally by various signalling pathways, with the release of specific products in many types of differentiated cells (Noordstra & Akhmanova, Reference Noordstra and Akhmanova2017). Regulated exocytosis plays an important role in multiple processes such as the release of hydrolytic enzymes by intestinal cells and leukocytes, and in synaptic neurotransmission, and endocrine, paracrine or exocrine signalling (Burgess & Kelly, Reference Burgess and Kelly1987; Wu et al., Reference Wu, Hamid, Shin and Chiang2014).
The molecular pathway controlling regulated exocytosis has been extensively investigated and, in this process, there are two components that play a pivotal role: the microtubules and intracellular Ca2+. Microtubules are dynamic hollow tube-like structures with an outer diameter of 25 nm and lengths in the order of tens of microns. They have intrinsic polarity, with fast growing plus ends and slowly growing minus ends. Microtubules represent the ‘rails’ for motor proteins such as kinesins and dynein, and allow the movement of organelles, vesicles and other structures through their plus ends and minus ends, respectively (Noordstra & Akhmanova, Reference Noordstra and Akhmanova2017). During regulated exocytosis, vesicles, after their formation at the trans-Golgi, move to the apical cytoplasm, where they are stockpiled until the arrival of the appropriate signal (Messenger et al., Reference Messenger, Falkowski and Groblewski2014). In general, these stimuli provoke the release of Ca2+ from intracellular storage, in particular the endoplasmic reticulum, via the activation of inositol 1,4,5-trisphosphate (IP3) channels (Mikoshiba, Reference Mikoshiba1993; Yoo, Reference Yoo2011). The increase in [Ca2+] promotes the final step of vesicular exocytosis, namely the fusion of vesicles with the plasma membrane that requires specific heterotrimeric interactions between membrane-associated SNARE proteins (Verhage & Sorensen, Reference Verhage and Sorensen2008; James & Martin, Reference James and Martin2013).
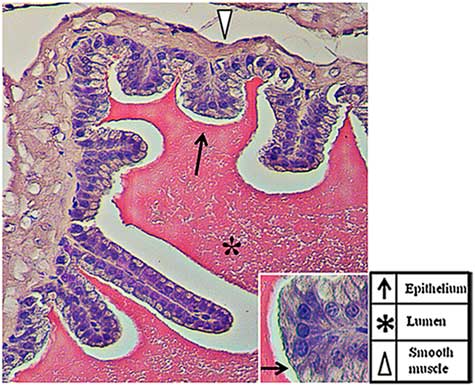
The SV are exocrine glands that belong to the male sex accessory glands. Their secretions, including sugars (for example fructose and glucose), peptides (for example glutathione) ions (for example Na+, K+, Zn+) and, above all, highly concentrated proteins, represent more than 60–70% of the seminal plasma (Spring-Mills & Hafez, Reference Spring-Mills and Hafez1979). The secretion is testosterone dependent (Aumüller & Seitz, Reference Aumüller and Seitz1990) as, once synthesized, these components are stored in vesicles at the cell periphery until the hormonal stimulus starts their fusion with the plasma membrane and their release in the lumen of the gland. As shown in Fig. 1, SV has a typical exocrine gland structure made by a pseudostratified epithelium with columnar secretory cells (arrows) and smaller basal cells. The epithelium is highly folded and deep narrow crypts lined with epithelium extend from the main sac-like lumen (asterisk). A lamina propria of connective tissue and thick coat of smooth muscle surround the epithelial lining (arrowhead). The nucleus of each columnar cell is located in the basal portion and is elongated in the direction of the long axis of the cell (Flickinger, Reference Flickinger1974). Given the involvement of both microtubules and intracellular Ca2+ during vesicles exocytosis and, as prolyl endopeptidase (PREP), has been associated with both of these (Williams et al., Reference Williams, Eames, Ryves, Viggars and Harwood1999; Schulz et al., Reference Schulz, Gerhartz, Neubauer, Holloschi, Heiser, Hafner and Demuth2002, Reference Schulz, Zeitschel, Rudolph, Ruiz-Carrillo, Rahfeld, Gerhartz, Bigl, Demuth and Rossner2005; Myöhänen et al., Reference Myöhänen, Venäläinen, Garcia-Horsman and Männistö2008), the major aim of the present study was to investigate the possible association of PREP and tubulin in the SV epithelium and its role during regulated exocytosis. PREP is a member of the serine peptidase group and is involved in several physiological and pathological processes through its ability to digest small peptides. However, its physiological role remains to be elucidated. Many reports have shown that the protein may have a very important role in the central nervous system, where it cleaves neuroactive peptides (Wilk, Reference Wilk1983; Mentlein, Reference Mentlein1988), but it may also have a direct or indirect function in other regions, including reproductive organs (Walter et al., Reference Walter, Shlank, Glass, Schwartz and Kerenyi1971; Yokosawa et al., Reference Yokosawa, Miyata, Sawada and Ishii1983; Yoshida et al. Reference Yoshida, Inaba, Ohtake and Morisawa1999; Kimura et al., Reference Kimura, Matsui and Takahashi2002; Valdivia et al., Reference Valdivia, Irazusta, Fernández, Múgica, Ochoa and Casis2004; Myöhänen et al., Reference Myöhänen, Pyykkö, Männistö and Carpen2012; Dotolo et al., Reference Dotolo, Kim, Pariante, Minucci and Diano2016).
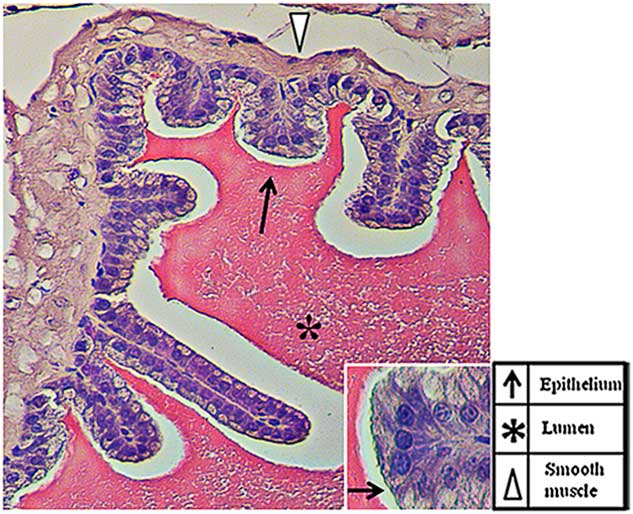
Figure 1 Haematoxylin–eosin staining of a tissue section in which the normal histology of the adult mouse seminal vesicles is highlighted.
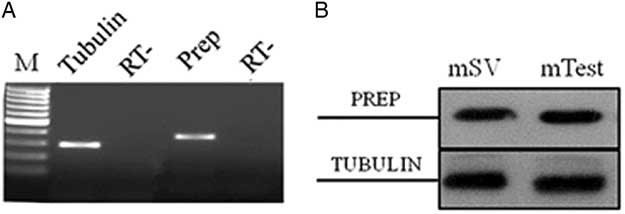
The first encouraging evidence about PREP expression in SV comes from PCR and western blot expression data. The presence of PREP mRNA was first determined in adult mouse SV by semiquantitative relative RT-PCR analysis. Agarose gel electrophoresis of amplification products showed a single band of the expected size (391 bp) in cDNA from SV (Fig. 2A). The amplified cDNA band corresponded to the PREP transcript, as demonstrated by successive cloning and sequencing (not shown). Quality control of cDNA was performed using specific primers for tubulin mRNA, showing a band of the expected size (297 bp) (Fig. 2A). PCR analysis of the negative control (RT−) samples did not show any signal. PREP expression was also evidenced by western blot analysis using polyclonal anti-PREP antibody: an 80 kDa band (corresponding to PREP) was present in total extracts from adult mouse SV and testis, used as a control (Fig. 2B, top section). The same samples were used for the analysis of tubulin. As expected, its band was detected in both samples as a confirmation of the expression of protein in mouse SV and testis (Fig. 2B, bottom section). Therefore, these data represent the first report that demonstrated PREP expression in the SV of adult mice.
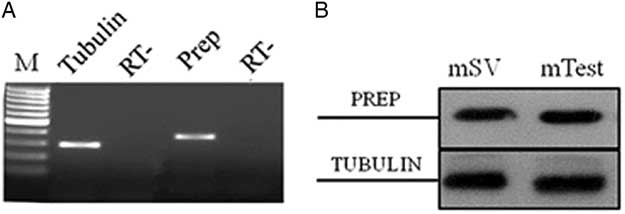
Figure 2 Expression of PREP mRNA and protein in adult mouse seminal vesicles. (A) Agarose gel electrophoresis of RT-PCR products performed on total RNA from adult mouse seminal vesicles. The amplification products were obtained using specific primers for mouse PREP and tubulin mRNA. M: molecular weight marker XIV (Roche Diagnostics); RT−: negative control. (B) Western blot analysis for PREP (80 kDa, top section) and tubulin (50 kDa, bottom section) performed on total protein extracts from adult mouse seminal vesicles (mSV) and testis (mTest), used as a positive control (Venditti & Minucci, Reference Venditti and Minucci2018).
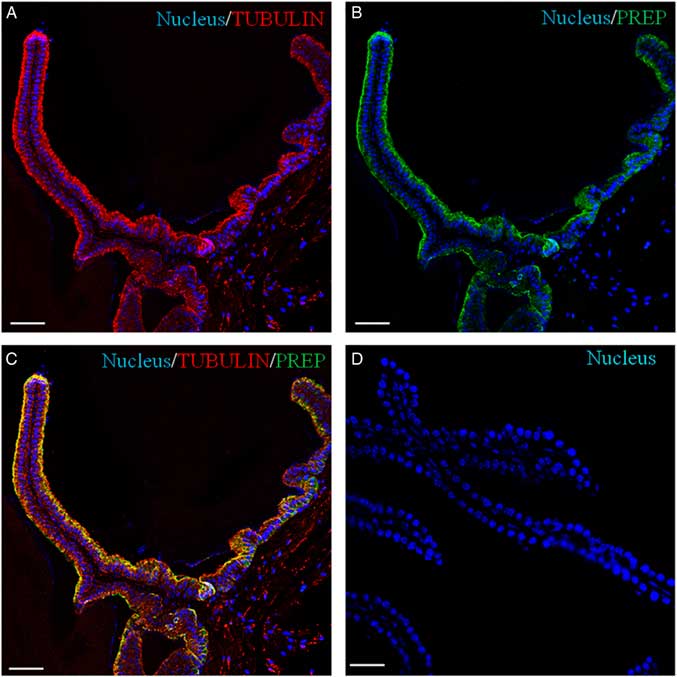
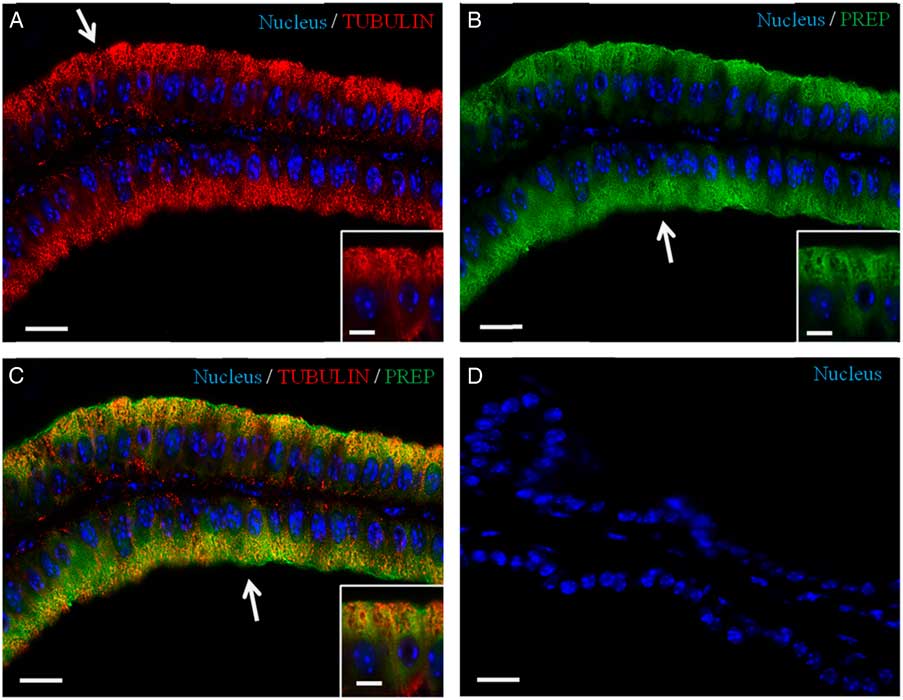
Recently, we have demonstrated the association of PREP with microtubules during morphofunctional remodelling, which occurs in the first wave of rat spermatogenesis in which during mitosis the peptidase shares its localization with tubulin in Sertoli cells, gonocytes and spermatogonia. During meiosis, both proteins are found in spermatocytes, and in the cytoplasm of Sertoli cells; during spermiogenesis, they localize in the cytoplasm of round and elongated spermatids. Finally, they are expressed in the flagellum of mature gametes (Venditti & Minucci, Reference Venditti and Minucci2018). To obtain more detailed data, a co-localization experiment of PREP + tubulin was studied using immunofluorescence analysis (Figs 3 and 4). The results showed that both proteins had cytoplasmic localization inside epithelial cells, but with slight differences in their expression pattern. Tubulin is evident in the cytoplasm, where the microtubules are orientated in linear arrays parallel to the long axis of the cell, with a more intense signal at the apical half part of the cytoplasm (arrows, Figs 3A and 4A; better highlighted in the inset). PREP had a more diffused localization, but was also prominent at the same cytoplasmic region (Fig. 4A, B; better highlighted in the inset). Figures 3C and 4C showed that the PREP signal noticeably overlapped with fluorescence produced by tubulin (see arrows), confirming their association in the apical half of the cytoplasm, where the two co-localization signals produced the intermediate yellow–orange tint.
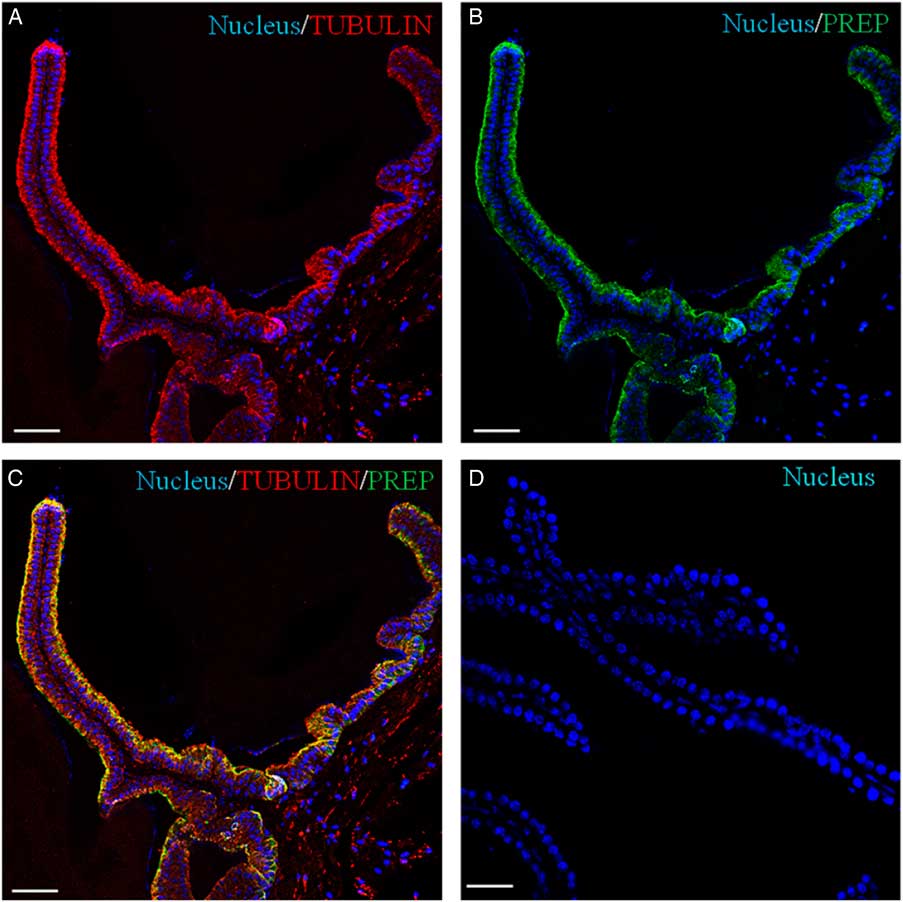
Figure 3 Immunolocalization of PREP in adult mouse seminal vesicles. General appearance of PREP (green, B, C) and co-localization by double immunofluorescence with tubulin (red, A, C) on mouse seminal vesicles sections. The nucleus is marked in blue (A–D). (D) Negative control, obtained by omitting the primary antibodies. The scale bars represent 40 mm.
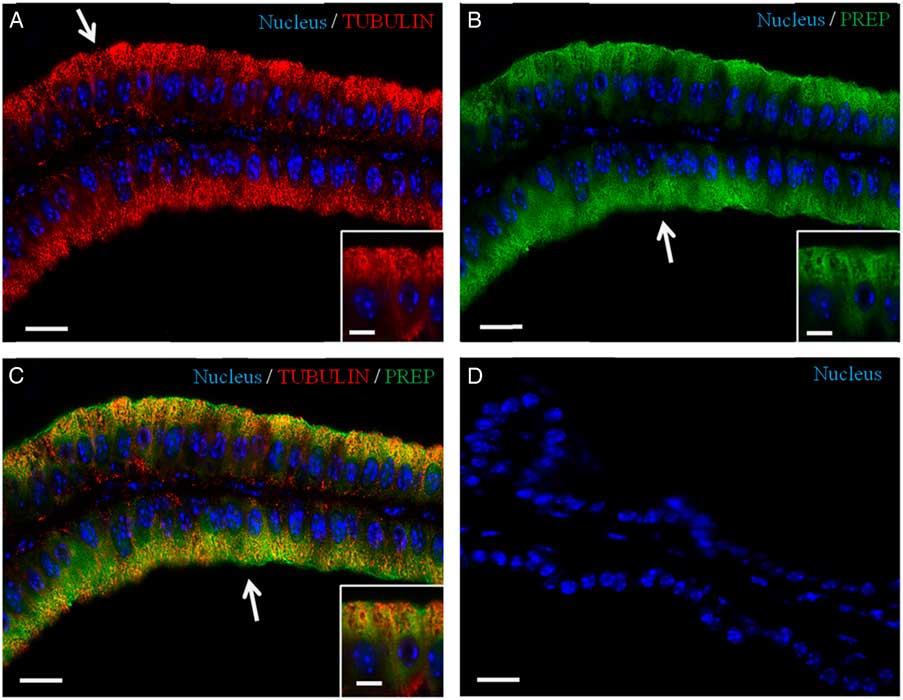
Figure 4 Co-localization of PREP and tubulin in adult mouse seminal vesicles. (A, C) DAPI-fluorescent nuclear staining (blue) and tubulin staining (red). Tubulin is evident in the cytoplasm, with a more intense signal at the apical half part of the cell (arrow in A). (B, C) DAPI-fluorescent nuclear staining (blue) and PREP staining (green). PREP has a more diffused localization, but is also prominent at the apical cytoplasm (arrows in B, C). (C) Merged fluorescent channels (blue/red/green), this enhances the co-localization of PREP and tubulin at the apical half cytoplasm (arrow). (D) Negative control, obtained by omitting the primary antibody. The scale bars represent 20 µm, except for the insets, where they represent 10 µm.
Taking our results together, we hypothesized a double role for PREP during exocytosis regulation in the SV. PREP has been known as a possible member of the inositol 1,4,5-trisphosphate pathway in regulation of multiple inositol polyphosphate phosphatase activity, which results in modulation of cytosolic Ca2+ levels (Szeltner & Polgár, Reference Szeltner and Polgár2008). Therefore, we suggested that PREP, through its involvement in Ca2+ signalling, might take part in the regulation in vesicle exocytosis in mouse SV. Moreover, we can confirm that PREP and tubulin co-localize in the SV epithelium. This peptidase has already been associated with tubulin in glioma cell lines (Schulz et al., Reference Schulz, Zeitschel, Rudolph, Ruiz-Carrillo, Rahfeld, Gerhartz, Bigl, Demuth and Rossner2005), and the authors found that PREP localization in these cells is strictly comparable with those of microtubules, above all in the perinuclear space, indicating the importance of physical binding of PREP to tubulin in microtubules-dependent cellular transport independent of its peptidase function. These last findings led us to hypothesize an involvement of PREP in the intracellular transport along these cytoskeletal elements, such as intracellular trafficking and vesicle sorting in SV epithelium. In conclusion, our work shows the expression and the localization of PREP in adult mouse SV. Although further investigation is required to better understand the possible implications of such varied pattern, our current data provide a profile of PREP co-localization with microtubules suggesting its possible involvement as a component of morphofunctional remodelling and organization of the epithelium of these exocrine glands.
Financial support
The authors received financial support for the research, authorship, and publication of this article from the Department of Experimental Medicine, University of Campania ‘Luigi Vanvitelli’, Italy (2018).
Conflicts of interest
The authors declare no conflict of interest.
Ethical standards
This study was carried out in accordance with the Italian guidelines (D. Lvo 116/92) and authorized by the local Animal Care Committee (ASL44, Prot. Vet. 22/95). All efforts were made to reduce animal suffering and the number of animals.