Introduction
Weedy broomrape species belonging to the genus Phelipanche, are parasitic plants that are totally dependent on their hosts for development. They parasitize many agricultural crops, including red clover (Trifolium pratense L.), carrot (Daucus carota L.), potato (Solanum tuberosum L.), and tomato (Solanum lycopersicum L.), damaging them by attaching to the host roots and withdrawing nutrients and assimilates from the host (Parker Reference Parker2013). Two species of Phelipanche are economically important pests in several agricultural crops, and specifically in open-field tomatoes: branched broomrape [Phelipanche ramosa (L.) Pomel.] and Egyptian broomrape (Phelipanche aegyptiaca Pers.). Both species are multihost and are widespread in Mediterranean, North African, and South European countries, but can also be found in other regions, such as Australia and Chile (Parker Reference Parker2013). High tomato yield loss due to P. ramosa infestation has been reported in Chile, Italy, and the Sudan (Parker Reference Parker2013). Severe damage to tomato due to P. aegyptiaca infestation has been reported in Israel, ranging from high yield loss to the necessity of abandoning heavily infested fields (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009). Recently, P. aegyptiaca was detected for the first time in the United States, in a tomato field in California, prompting efforts for eradication to avoid its spread to other regions of the country (Miyao Reference Miyao2017).
The most common approaches for Phelipanche spp. management are based on resistant varieties or chemical control; this paper will focus on chemical control. To date, herbicides from two different modes of action are used to control Phelipanche spp. in different crops, namely acetolactate synthase inhibitors, from the chemical groups sulfonylureas and imidazolinones (Eizenberg et al. Reference Eizenberg, Hershenhorn, Ephrath Jhonathan and Kanampiu2013), and the 5-enolpyruvylshikimate-3-phosphate inhibitor glyphosate (Cochavi et al. 2016b). One of the herbicides used for control of P. aegyptiaca in tomatoes is sulfosulfuron, a sulfonylurea herbicide that has been used for selective control of autotrophic weeds in various crops (Eizenberg et al. Reference Eizenberg, Goldwasser, Achdary and Hershenhorn2003; Geiger and Stahlman Reference Geiger and Stahlman1996; Kutzior et al. Reference Kutzior, Spitainiak, Pawinska and Urbanowicz1999; Myhre et al. Reference Myhre, Loeppky and Stevenson2004). This herbicide is known to have long-term residual activity in neutral and low-alkaline soils, due to its slow degradation rate (Saha and Kulshrestha Reference Saha and Kulshrestha2002, Reference Saha and Kulshrestha2008), injuring sensitive plants up to 32 mo after its application (Kelley and Peeper Reference Kelley and Peeper2003; Lyon et al. Reference Lyon, Miller and Seifert-Higgins2003).
To design chemical management protocols of Phelipanche spp., several thermal time models of belowground development and parasitism dynamics have been developed and employed (Cochavi et al. Reference Cochavi, Rubin, Smirnov, Achdari and Eizenberg2016a; Ephrath and Eizenberg Reference Ephrath and Eizenberg2010; Ephrath et al. Reference Ephrath, Hershenhorn, Achdari, Bringer and Eizenberg2012). These models are necessary, because most of the life cycle of Phelipanche spp. takes place below the soil surface, and at this stage the parasite causes severe damage to the host. A thermal time model has been used to develop a management protocol for control of P. aegyptiaca in open-field tomatoes in Israel. This protocol, known as PICKIT, includes two steps: (1) PPI sulfosulfuron (37.5 g ha−1 to the top 10-cm soil layer; approximately 40ng g−1 soil) during winter, for control of the parasite’s seedlings after tomato planting, assuming winter rainfall will assist with dissolution of the herbicide into soil solution; and (2) application of imazapic after planting at 400, 600, and 800 growing degree days (GDD) (4.8, 7.2, and 7.2 g ha−1, respectively) for control of P. aegyptiaca attachments (Eizenberg et al. Reference Eizenberg, Achdari, Tal and Dagan2016). The PICKIT protocol has been implemented in commercial fields in Israel since 2014; however, reduced efficacy has been reported at some locations (Eizenberg et al. Reference Eizenberg, Achdari, Tal and Dagan2016).
In many reported cases, reduction in weed control efficacy with acetolactate synthase inhibitors is a result of herbicide-resistance evolution (Tranel and Wright Reference Tranel and Wright2002). However, this is unlikely the case in P. aegyptiaca control in Israel because of crop rotations (hosts of P. aegyptiaca are not grown more than once every 3 yr) and because the unique biology of the parasite allows germination of a minimal portion of the seeds yearly (Eizenberg et al. Reference Eizenberg, Hershenhorn, Ephrath Jhonathan and Kanampiu2013). Because sulfosulfuron is applied several weeks before tomato planting, and according to the thermal time model the critical timing for control (when initial parasitism occurs) is at 200 GDD (Ephrath et al. Reference Ephrath, Hershenhorn, Achdari, Bringer and Eizenberg2012), we hypothesize that cases of reduced control efficacy of P. aegyptiaca in open-field tomatoes were due to rapid sulfosulfuron dissipation from the soil. Dissipation could be a result of herbicide degradation, leaching below the main root zone, or both. The goal of this study was to investigate the co-effect of application timing and variable sulfosulfuron degradation rate on P. aegyptiaca control efficacy when using the PICKIT protocol. The following objectives were defined: (1) quantify degradation rates and residual activity of sulfosulfuron in three soils in batch experiments and (2) simulate daily residual soil concentrations of sulfosulfuron in selected tomato fields and relate concentration to the control efficacy of P. aegyptiaca.
Materials and Methods
Herbicides
Unless stated otherwise, commercial formulations of sulfosulfuron (Monitor™ WG, 75% ai, Monsanto, St. Louis, MO, USA) and imazapic (Cadre® LC, 240 g ai L, BASF, Catano, PR, USA) were used throughout the study. Doses and concentrations in this paper refer to the active ingredient.
Soil Sampling
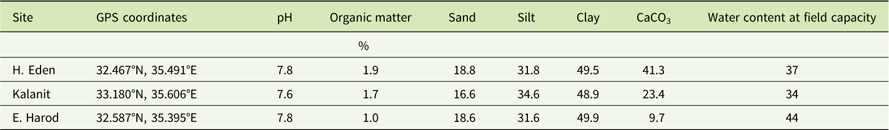
To quantify sulfosulfuron degradation rate and residual activity in selected soils, soil was sampled from three sites, H. Eden, Kalanit, and E. Harod, during the summer of 2015. The sites were selected to represent three different regions in northern Israel where tomatoes are typically grown. At each site, the soil was sampled with a shovel from the top 10-cm soil layer, in 20 random locations, and combined into one 50-L batch. The soil samples from the three sites were characterized as clayey soils, with low-alkaline pH and low organic matter content (Table 1). Samples from each site were air-dried, sieved (<4mm), and thoroughly mixed for 10 min in a cement mixer before being used in the relevant experiments.
Table 1 Selected properties of the soils (top 10 cm) used for laboratory experiments.
Sulfosulfuron Degradation in Soil
Degradation rates were determined in the soil samples at two constant temperatures: 15 and 25 C. For each site (Table 1), 5-g subsamples of the soil batch were weighed into 20-ml vials and each fortified with sulfosulfuron (MON 35700, 99.9% sulfosulfuron, Monsanto, St. Louis, MO, USA) to achieve a concentration of 80ng sulfosulfuron g−1 dry soil and soil moisture of 70% of field capacity. For that, a stock solution of sulfosulfuron in deionized water was prepared (100µg ml−1), the solution was diluted with deionized water to prepare working solutions, and aliquots of the working solutions were used for fortification of the soil samples as stated earlier. Vials were capped and incubated at 15 or 25 C in the dark for a period of up to 224 d and were ventilated weekly by removing the cap for 20 min. Every 14 d (H. Eden at 25 C), 21 d (E. Harod and Kalanit at 25 C), or 28 d (all soils at 15 C), five replicate vials were randomly selected for extraction and determination of sulfosulfuron concentration by liquid chromatography–tandem mass spectrometry (LC-MS/MS); these intervals were selected based on preliminary observations of sulfosulfuron dissipation rate. Sulfosulfuron was extracted in an 8-ml solution of methanol and water (8:2 by volume) (HPLC Supra-gradient, Bio-Lab, Ashkelon, Israel) by shaking the vials for 16 h in a horizontal shaker. After extraction, vials were centrifuged for 10 min (2500 rpm), and the extract was filtered through a 0.45-μm PTFE filter (Membrane Solutions, Kent, WA, USA) and taken for LC-MS/MS analysis (Prominence high-speed HPLC, Shimadzu, Kyoto, Japan; QTRAP 4500 LC-MS/MS, AB Sciex, Singapore). Separation was done using a C18 column (Kinetex 5μ, C18, 250 by 4.6mm, Phenomenex, Torrance, CA, USA), with the mobile phase consisting of methanol and 0.1% acetic acid in water (8:2 by volume) at a flow rate of 1 ml min−1; under these conditions, sulfosulfuron retention time was 3.7 min. Sulfosulfuron concentrations in the soil extracts were calculated using a calibration curve of sulfosulfuron standards in methanol (1, 2.5, 10, 25, and 50ng ml−1) and normalized to the mass of dry soil in the original sample. The limit of detection in the soil sample, calculated as the mean concentration in the blank samples (soil without sulfosulfuron) plus 3 times the SD of the blank mean concentration, was 2.7ng g−1 soil. Sulfosulfuron recovery efficiency from the soil samples was 99% to 126%.
Sulfosulfuron Residual Activity in Soil
The residual activity of sulfosulfuron in the soil samples was quantified using a root-elongation bioassay commonly used to determine the effect of soil-applied herbicides on plants (Weiss et al. Reference Weiss, Rubin, Shulman, Ben Shir, Keinan and Wolf2006). In the current study, sorghum [Sorghum bicolor (L.) Moench.] hybrid STT12 (Sorghum Partners, Lubbock, TX, USA) was used as the test plant. For soil from each site (Table 1), a 1-kg subsample of the soil batch was weighed into a container. An aqueous solution of sulfosulfuron was applied to each container to achieve herbicide concentration of 80ng g−1 dry soil and soil moisture at 70% of water field capacity; deionized water was used instead of sulfosulfuron in the untreated control. After application, the soil was thoroughly mixed and packed into 10 square petri dishes (10 by 10 cm), and 10 sorghum seeds were sown in each plate and incubated for 4 d in the dark at 25 C as described by Weiss et al (Reference Weiss, Rubin, Shulman, Ben Shir, Keinan and Wolf2006). At the end of the incubation period, seedlings were carefully removed, and root length was measured. New sorghum seeds were seeded once every 2 wk into the same petri dishes, at different edges of the plate, as described by Saadi et al. (Reference Saadi, Raviv, Berkovich, Hanan, Aviani and Laor2013), and soil moisture was corrected with deionized water (by weight) once a week. In addition, the same sorghum bioassay was used to obtain a sorghum dose–response curve in coarse quartz. The coarse quartz sand medium (previously washed and air-dried) was selected because it was expected to weakly interact with the herbicide (Clausen et al. Reference Clausen, Fabricius and Madsen2001), and thus the majority of the applied sulfosulfuron was assumed to be available to the plants. Aqueous solutions of sulfosulfuron (0, 2, 5, 9, 19, 47, 140, and 420ng ml−1) were thoroughly mixed with the sand (0.1 ml g−1). For each concentration, the sulfosulfuron-treated quartz sand was packed into 3 square petri dishes; this was followed by sowing, incubation, and measurements of root length as described above. The whole experiment was replicated twice, and data from the two experiments were pooled. In this experimental setup the maximal inhibition of root growth (compared with the untreated control) was 85%; this value was used to normalize the data from the bioassay experiments (in quartz sand and soil) as follows (Equation 1):
where P is phytotoxicity (%) and I is inhibition of sorghum root growth (% of untreated control). A log-logistic three-parameter sigmoidal equation (Equation 2), commonly used for analysis of herbicide dose response (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995), was fit to the data of sulfosulfuron phytotoxicity to sorghum in quartz sand:
where P(C) is phytotoxicity (%) at sulfosulfuron concentration C (ng ml−1); a is maximum phytotoxicity (%); ED50 is the concentration (ng ml−1) at which a 50% reduction of maximum phytotoxicity occurred; and β is the slope at the inflection point.
Based on this dose–response curve, the residual activity of sulfosulfuron in the soil was predicted by simulating the daily residual concentrations in each soil using the laboratory-measured degradation rates. The predicted residual activity was compared with the measured residual activity in the sorghum bioassay.
Phelipanche aegyptiaca Dose Response to Sulfosulfuron
To determine the response of P. aegyptiaca to a range of sulfosulfuron concentrations, a dose–response experiment was conducted in a net-house. Seeds of P. aegyptiaca were collected from an infested tomato field and stored at 4 C until being used in the following experiment. Seeds (10 mg kg−1 soil) were thoroughly mixed into air-dried soil from Newe Ya’ar Research Center (32.707°N, 35.179°E; 55% clay, 23% silt, 20% sand, 2.1% organic matter, pH 7.1) with a cement mixer for 10 min. The infested soil was used to fill 2-L pots (2.5 kg pot−1), into which tomato seedlings were subsequently planted. The pots were placed in a net-house, in a completely randomized design, and the seedlings were drip irrigated and fertilized as needed for normal development. Soil temperature was monitored using data loggers (HOBO Pendant® temperature/light data logger, Onset Computer, Bourne, MA, USA), and thermal time (GDD) was calculated as described by Ephrath et al. (Reference Ephrath, Hershenhorn, Achdari, Bringer and Eizenberg2012). For determining the P. aegyptiaca dose response to sulfosulfuron, 100 ml of the herbicide solution (0, 14, 57, 110, 230, 460, and 920ng ml−1; equivalent to 0, 1, 5, 10, 20, 40, and 80ng g−1 dry soil) was applied to the soil at 220 GDD (17 d after planting) in six replications per herbicide concentration. The experiment was terminated at 585 GDD (46 d after planting). Thereafter, the soil was removed from the plant roots by gentle washing under running tap water, and P. aegyptiaca attachments were removed and their dry weights recorded. The entire experiment was replicated twice, and data from the two experiments were pooled.
Control Efficacy of Phelipanche aegyptiaca Using PICKIT in the Field
Field studies were conducted to investigate the control efficacy of P. aegyptiaca in open-field tomatoes at H. Eden, Kalanit, and E. Harod (Table 1) using the PICKIT protocol. At H. Eden and E. Harod, two field studies were conducted in two consecutive years (2013 and 2014), in a different field plot in each year. At Kalanit, a single field study was conducted in 2015. All sites were naturally infested with P. aegyptiaca, according to data obtained from farm records.
The experiments were conducted in a complete randomized block design, with six replicates for treated or untreated control plots. Before the experiment, the field plots were plowed, compost was applied, and the soil was cultivated and leveled. Each plot (1.93-m wide, 10-m long) was planted with 2 rows of tomato plants, with 0.4-m spacing between rows and 0.2-m spacing between plots, at a rate of 25,000 plants ha−1. For PICKIT treatments, sulfosulfuron (37.5 g ha−1) was applied to the treated plots preplanting with a motorized sprayer (200 L ha−1; 300 kPa), equipped with a TeeJet® 8001E nozzle (Spraying Systems, Wheaton, IL, USA) and immediately incorporated with a rotavator into the top 10-cm soil layer. Sulfosulfuron was applied at a different timing in each of the five field experiments: in 2013, the preplant incorporation timing was 3 and 4 d before planting at H. Eden and E. Harod, respectively; in 2014, it was 41 and 54 d before planting at H. Eden and E. Harod, respectively; and in 2015, preplant incorporation timing at Kalanit was 59 d before planting. This variable timing was based on practical concerns of the farmers, who preferred early application, as mechanical incorporation in early winter is easier. Because it was initially presumed that sulfosulfuron dissipation would not affect the control efficacy, early sulfosulfuron applications were performed in 2014 and 2015. After planting, three applications of imazapic (4.8, 7.2, and 7.2gha−1) were made through the drip-irrigation system at different thermal times (400, 600, and 800 GDD, respectively), calculated according to Ephrath et al. (Reference Ephrath, Hershenhorn, Achdari, Bringer and Eizenberg2012). Drip-irrigation systems (Uni-ram or Drip-net, with a capacity of 1 Lh−1 every 0.5 m) were provided by Netafim Ltd. (Tel Aviv, Israel), and imazapic was injected into the irrigation system with a hydrostatic pump. The tomatoes were grown according to common practice in Israel. At the end of the growing season, P. aegyptiaca inflorescences were recorded in treated and untreated plots.
Model of Herbicide Degradation in Variable Temperatures
To test the correlation between P. aegyptiaca control efficacy and residual sulfosulfuron concentration at the critical period (200 GDD), predicted daily concentrations after preplant incorporation were calculated as follows: first, the sulfosulfuron degradation rate constant, at 15 and 25 C in each soil, was quantified from the constant temperature experiments. Based on previous reports on sulfosulfuron degradation in soil (Maheswari and Ramesh Reference Maheswari and Ramesh2007; Ramesh and Maheswari Reference Ramesh and Maheswari2003) first-order kinetics was assumed; hence, the integrated form of the first-order reaction equation was fit to the measured data and used to quantify the rate constant (Equation 3):
where C(t) is sulfosulfuron relative concentration (%) at time t (d); C(0) is the initial concentration (%), and k(T) is the degradation rate constant (d−1) at temperature T (C).
The activation energy (E a) of sulfosulfuron degradation was calculated based on the Arrhenius relationship between temperature and pesticide transformation (FOCUS 1997), using the data of first-order rate constants determined in laboratory experiments (Equation 4):
where k(T) and k ref are the degradation rate constant (d−1) under temperature T (K) and under a reference temperature, respectively [T ref=15 C (288.15 K)]; E a is the activation energy of sulfosulfuron degradation (J mol−1); and R is the universal gas constant (J mol−1K−1).
Next, the degradation rate constant under actual field temperatures [k(T)], was calculated from the daily mean temperatures at each field experiment (Equation 4). Finally, the concentration of sulfosulfuron throughout the season was calculated as follows (Equation 5):
where C(t) is sulfosulfuron concentration (ng g−1) at time t (d); Δt is the incremental time length (d); C(t −Δt) is sulfosulfuron concentration (ng g−1) at time t −Δt (d); and k(T) is the calculated rate constant (d−1) at the incremental time Δt calculated using Equation 4.
Statistical Analysis
A significance level of α=0.05 was used for all statistical analyses. Nonlinear regression with SigmaPlot v. 10.0 (Systat Software, USA), was used to quantify degradation rate and residual activity of sulfosulfuron in the soil samples, as well as sorghum and P. aegyptiaca dose response to sulfosulfuron. Comparison of regression models among experiments were conducted using an F-test. In the field experiments, P. aegyptiaca inflorescences were analyzed by JMP Pro v. 13.0.0 (SAS Institute, Cary, NC, USA) using ANOVA with a two-factorial design (treatment and site).
Results and Discussion
Sulfosulfuron Degradation and Residual Activity in Laboratory Experiments
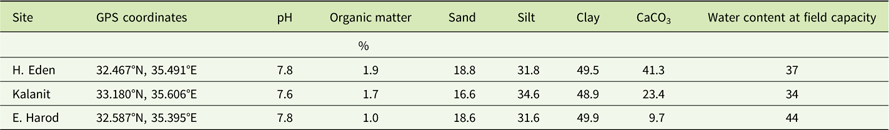
Degradation of sulfosulfuron at constant temperatures followed a first-order kinetics (Figure 1). At 15 C (Figure 1A), comparison of curves using the F-test for regression comparison revealed significant differences between the soils from different locations. Degradation rate constant (k±SE) was highest in the soil from H. Eden (0.012±0.001 d−1), intermediate in the soil from Kalanit (0.010±0.001 d−1), and lowest in the soil from E. Harod (0.008±0.001 d−1). At 25 C (Figure 1B), a higher degradation rate constant was found in all three soils, compared with the degradation rate constant at 15 C (Figure 1A), with a similar hierarchy (H. Eden > Kalanit > E. Harod). However, at this temperature, differences between sulfosulfuron degradation in H. Eden and Kalanit were not significant. First-order kinetics degradation is assumed for many pesticides (Beulke et al. Reference Beulke, Dubus, Brown and Gottesburen2000; FOCUS 1997) and has been previously reported for sulfosulfuron degradation in the soil (Maheswari and Ramesh Reference Maheswari and Ramesh2007; Ramesh and Maheswari Reference Ramesh and Maheswari2003). The temperature dependence of pesticide degradation in the soil has also been reported, and its quantification has been used for simulation of persistence in field conditions (Beulke et al. Reference Beulke, Dubus, Brown and Gottesburen2000; FOCUS 1997). Simulations of herbicide persistence could be used to predict the residual activity, assuming it reflects the degradation to nonphytotoxic metabolites.
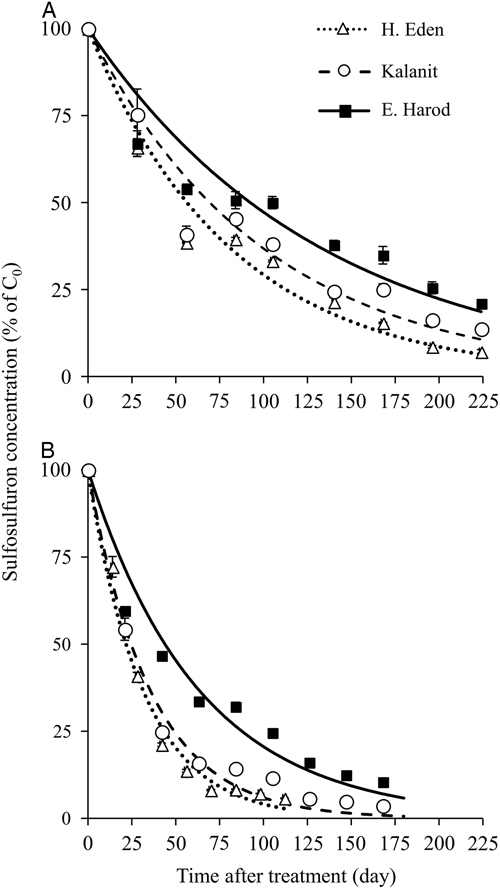
Figure 1 Degradation of sulfosulfuron in soil samples incubated at (A) 15C and (B) 25C. Sulfosulfuron concentration was quantified in soil extract using liquid chromatography–tandem mass spectrometry (LC-MS/MS) and presented as percentage of initial measured concentration (C 0). An exponential decay curve was fit to the mean concentrations at different incubation periods at each temperature: 15C: H. Eden: Y=100×e −0.012X , Kalanit: Y=100×e −0.010X , E. Harod: Y=100×e −0.008X ; 25C: H. Eden: Y=100×e −0.032X , Kalanit: Y=100×e −0.028X , E. Harod: Y=100×e −0.016X . Error bars represent the SE (n=5).
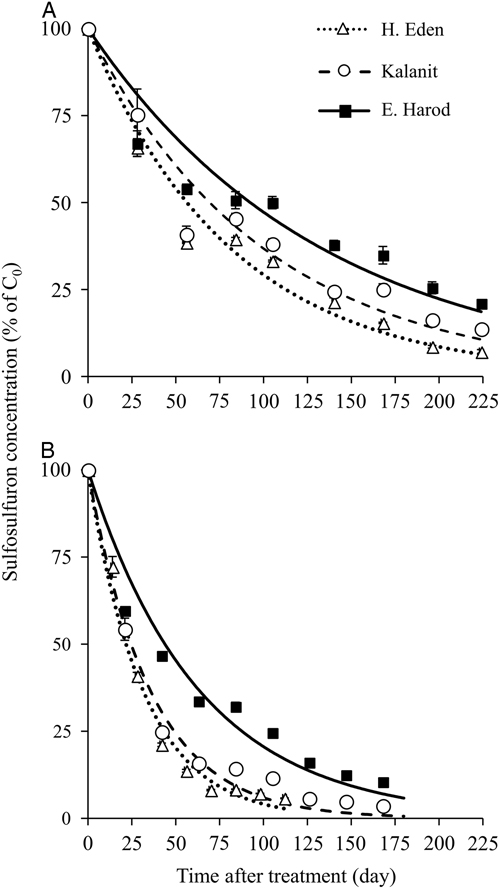
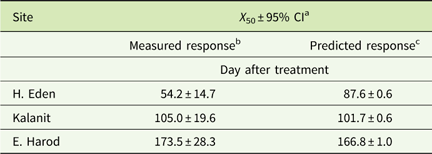
The residual activity of sulfosulfuron in the soils from H. Eden, Kalanit, and E. Harod was quantified at 25 C by using a sorghum root-elongation bioassay. A log-logistic three-parameter sigmoidal equation was fit to the phytotoxicity of sulfosulfuron over time in each soil sample (Figure 2). At the first monitoring date (at the day of application) low phytotoxicity was detected in all three soils, probably due to the nonequilibrium state of the herbicide in the soil solution, and therefore the data for this date were not used for the curve fitting. The maximal estimated sulfosulfuron phytotoxicity (±SE) was 97.1±8.9, 79.2±5.0, and 85.4±4.7% for soil samples from H. Eden, Kalanit, and E. Harod, respectively, while the time after treatment at which 50% reduction of initial phytotoxicity was reached (X 50±SE) was 54.2±6.2, 105.0±8.9, and 173.5±13.5 d, respectively. A comparison of regressions using the F-test showed significant differences between sites, with the longest period of sulfosulfuron residual activity in the soil from E. Harod, followed by an intermediate period of residual activity in the soil from Kalanit, and the shortest period of residual activity in the soil from H. Eden.
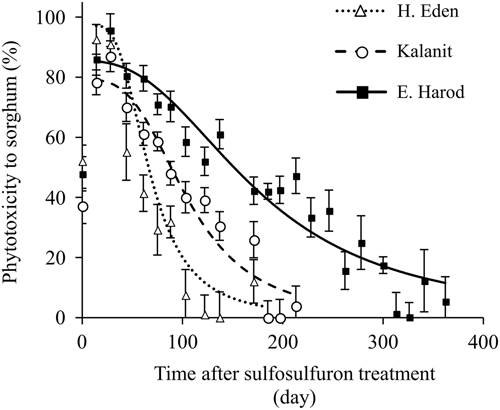
Figure 2 Sulfosulfuron residual activity on sorghum root elongation, in soil samples from three sites (Table 1). Sorghum was sown following different incubation periods at 25 C, after application of 80 ng sulfosulfuron g−1 soil. Root length was measured at 4 d after sowing, and phytotoxicity was calculated using Equation 1. A log-logistic three-parameter sigmoidal equation was fit to the mean phytotoxicity at different incubation periods: H. Eden: Y=97.1/(1+(X/54.2)2.8), Kalanit: Y=79.2/(1+(X/105.0)3.1), E. Harod: Y=85.4/(1+(X/173.5)2.5). Error bars represent the SE (n=10).
To test whether sorghum response over time is associated with degradation of sulfosulfuron, the daily concentration of the herbicide throughout the experiment was simulated using the degradation rate constant at a temperature of 25C (Equation 3), and the expected daily phytotoxicity to sorghum was subsequently calculated according to the dose response in quartz sand (Figure 3). Predicted and measured residual activity curves in the three soils followed a similar order (H. Eden < Kalanit < E. Harod) (Table 2; unpublished data). However, in the soil from H. Eden, the predicted residual activity was overestimated, as can be observed by the deviation of the predicted inflection point (X 50) from the measured inflection point (Table 2). Nonetheless, these findings show a close link between the degradation of sulfosulfuron, as obtained from analytical measurements, and the residual activity of the herbicide, as measured in the bioassay, supporting the assumption of degradation to nonphytotoxic metabolites. When sulfosulfuron is applied PPI in tomato, degradation to nonphytotoxic metabolite is expected to result in reduced injury to P. aegyptiaca. The extent of this effect, however, depends on the parasite’s dose response to sulfosulfuron.
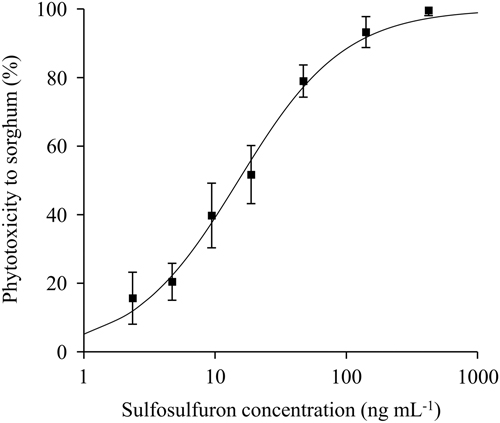
Figure 3 Sorghum dose response to sulfosulfuron in quartz sand. Root length was measured 4 d after treatment and phytotoxicity was calculated using Equation 1. A log-logistic three-parameter sigmoidal curve was fit to the mean phytotoxicity of the different herbicide concentrations: Y=100/(1+(X/15.1)−1.1). Error bars represent the SE (n=6).
Table 2 Time to 50% reduction of initial phytotoxicity (X 50) of sulfosulfuron in the sorghum bioassay.
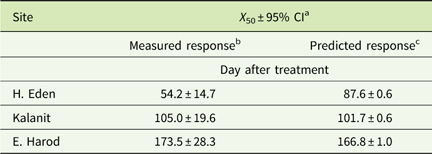
a 95% CI, 95% confidence interval.
b Curves are presented in Figure 2.
c Based on calculation of daily residual concentration of sulfosulfuron at 25C (between 0 to 400 d after treatment; Equation 3) and predicted sorghum response for each concentration according to the dose–response curve (Figure 3). The predicted curves are not presented.
Phelipanche aegyptiaca Dose Response to Sulfosulfuron
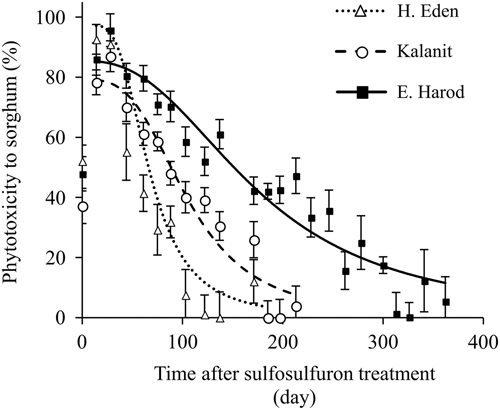
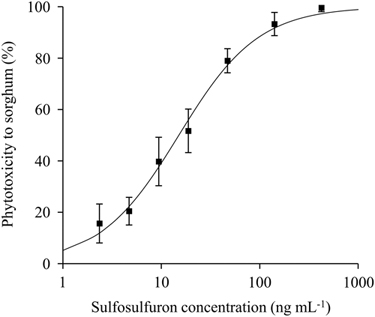
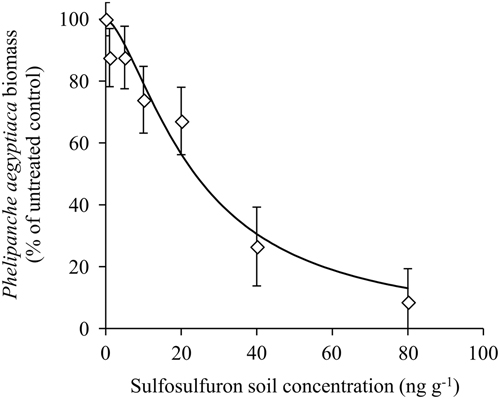
A dose–response experiment was conducted in a net-house to test the response of P. aegyptiaca to a range of sulfosulfuron concentrations in the soil. The P. aegyptiaca biomass as affected by sulfosulfuron fit a three-parameter log-logistic equation (Figure 4). The parasite was affected by low concentrations of the herbicide (applied at 220 GDD), with a 10% reduction of biomass at a sulfosulfuron concentration of 5ng g−1 soil. The ED50±SE was 23.6±4.4ng g−1 soil, which is lower than the expected soil concentration of sulfosulfuron following recommended preplant incorporation in the field (37.5 gha−1 to a 10-cm soil layer; approximately 40ng g−1 soil). Previous reports have demonstrated a reduction in parasitism of P. aegyptiaca to tomato using sulfonylurea (Eizenberg et al. Reference Eizenberg, Goldwasser, Golan, Plakhine and Hershenhorn2004) and imidazolinone herbicides (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009). Specifically, Eizenberg et al. (Reference Eizenberg, Goldwasser, Golan, Plakhine and Hershenhorn2004) reported on control of the parasite with PPI sulfonylurea, including sulfosulfuron, but did not investigate the possible effect of dissipation on the control efficacy. The current study revealed that, in the present application method (preplant incorporation of 37.5 gha−1 to a 10-cm soil layer; approximately 40ng g−1 soil), reduction in herbicide soil concentration due to dissipation could substantially reduce the injury to P. aegyptiaca seedlings at 200 GDD, the critical period for control (Figure 4).
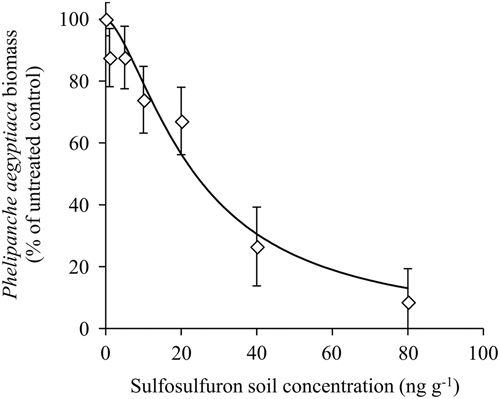
Figure 4 Phelipanche aegyptiaca dose response to sulfosulfuron in the soil in pots in which tomatoes were grown as host plants. Sulfosulfuron was applied to the soil at 220 growing degree days (GDD) (17 d after planting), and the experiment was terminated at 585 GDD (46 d after planting). A log-logistic three-parameter sigmoidal curve was fit to the mean biomass of P. aegyptiaca at the different herbicide concentrations: Y=100/(1+(X/23.6)1.5. Error bars represent the SE (n=12).
Control Efficacy of Phelipanche aegyptiaca in the Field and Its Association with Sulfosulfuron Degradation
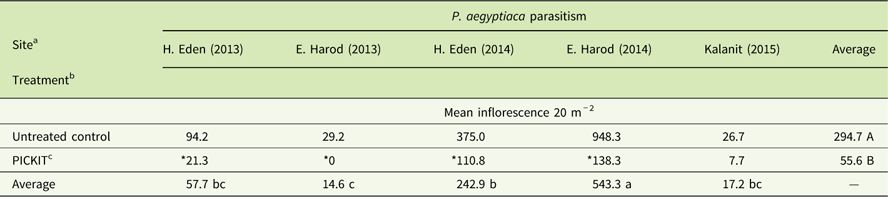
Over the course of three tomato growing seasons (2013 to 2015) five independent field studies were performed to test the efficacy of the PICKIT protocol (e.g., sulfosulfuron PPI + imazapic post–P. aegyptiaca attachment). PICKIT treatment had a significant effect in reducing P. aegyptiaca inflorescence emergence in the treated plots (Table 3). The site had a significant effect on inflorescence emergence, probably due to the wide range of infestation at the different sites: a mean of 26 to 948 inflorescences 20 m−2 in the untreated control plot (Table 3). The statistical analyses also revealed a significant interaction of the herbicide treatment and site on inflorescence emergence, indicating the varied effect of the treatment on the different sites (Table 3).
Table 3 Phelipanche aegyptiaca control with PICKIT in open-field tomatoes.
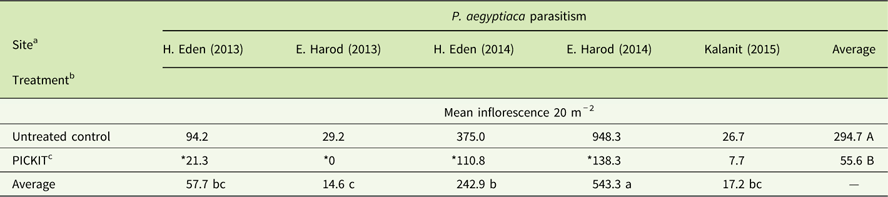
a Site had a significant major effect on P. aegyptiaca inflorescence emergence; different lowercase letters indicate statistically different mean inflorescences among sites at α = 0.05, as determined by the Tukey-Kramer test.
b Treatment had a significant major effect on P. aegyptiaca inflorescence emergence; different uppercase letters indicate statistically different mean inflorescences among treatments at α = 0.05, as determined by the Tukey-Kramer test.
c “Site” and “Treatment” interaction had a significant effect on P. aegyptiaca inflorescence emergence; among each site, means marked with an asterisk (*) had significantly lower numbers of P. aegyptiaca inflorescences (α = 0.05), as determined by specific comparisons using ANOVA. PICKIT treatment: 37.5 g ha-1 PPI sulfosulfuron followed by herbigation with 4.8, 7.2, and 7.2 g ha−1 imazapic at 400, 600, and 800 growing degree days, respectively.
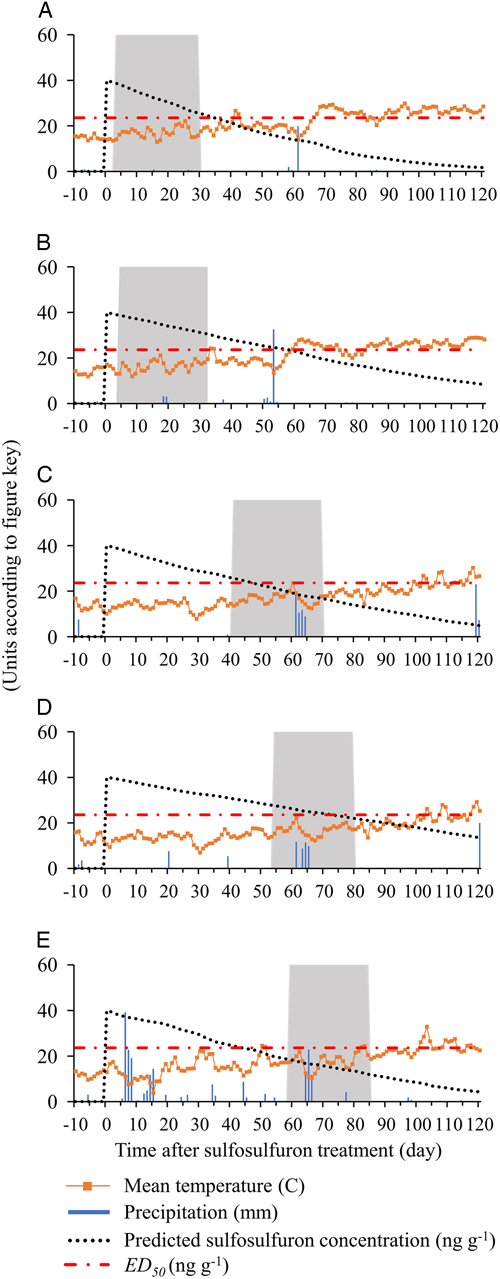
The association between residual sulfosulfuron concentration in the soil and observed control efficacy in the field studies was examined by simulating daily sulfosulfuron degradation in the field. To do so, the activation energy (E a) of sulfosulfuron in each soil was calculated (Equation 4), using the laboratory-measured degradation rates presented in Figure 1 (data for activation energy are not shown). Next, the degradation rate constant, k(T), was calculated for each day (Equation 4), based on actual field temperatures (Figure 5), and used for calculation of daily sulfosulfuron concentration (Equation 5; Figure 5). Leaching of sulfosulfuron was expected to have a minor effect on dissipation and control efficacy of P. aegyptiaca, as leaching of sulfonylureas from the rhizosphere in field conditions is considered limited (Grey and McCullough Reference Grey and McCullough2012), and the parasite is able to germinate, attach to the host roots, and complete its life cycle even when seeds are buried in the soil to a depth of 30 cm (Eizenberg et al. Reference Eizenberg, Lande, Achdari, Roichman and Hershenhorn2007). Moreover, low amounts of precipitation were recorded in the field experiment of the current study: in H. Eden and E. Harod field experiments, a total of 3 to 56mm of rainfall was recorded, from application to 200 GDD, and in Kalanit, a total of 202mm was recorded, with no extreme rainfall events during this period (Figure 5). Because leaching of sulfosulfuron from the rhizosphere has been shown to be restricted even after 9 mo with an average monthly rainfall of 82mm (Brown et al. Reference Brown, Dubus, Fogg, Spirlet and Gustin2004), dissipation through leaching in the current study was assumed to be negligible.
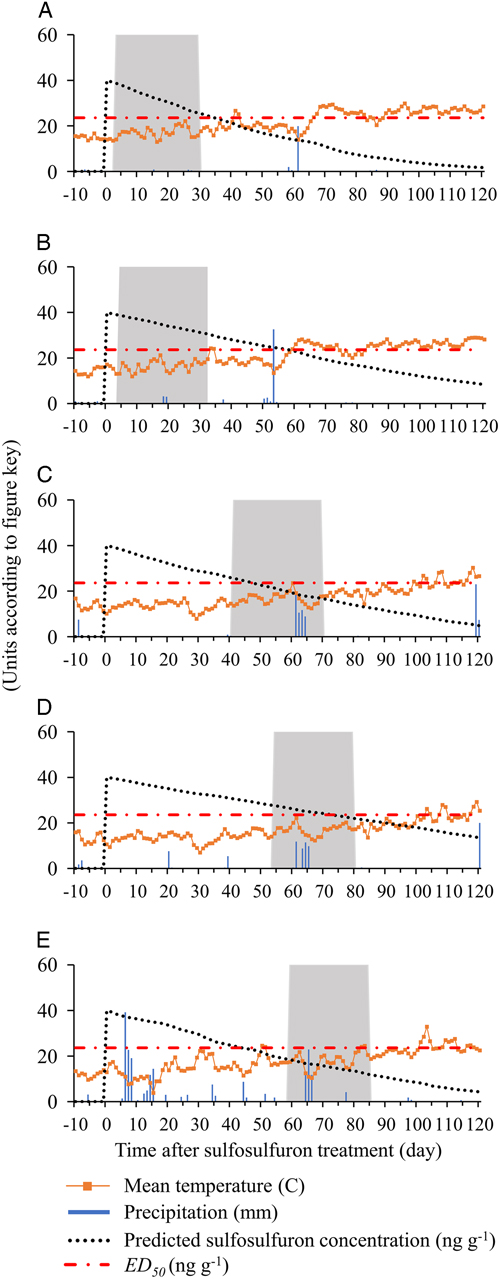
Figure 5 Simulation of residual sulfosulfuron concentrations under actual field conditions during the tomato growing season at (a) H. Eden 2013, (b) E. Harod 2013, (c) H. Eden 2014, (d) E. Harod 2014, and (e) Kalanit 2015. Mean temperature and precipitation data for each study site were obtained from the Israeli Meteorological Service Database (https://ims.data.gov.il, accessed: December 2017); sulfosulfuron concentrations were calculated using Equations 4 and 5; ED50 was taken from the Phelipanche aegyptiaca dose–response curve (Figure 4); the gray rectangle represents the time between tomato planting and the critical timing to control P. aegyptiaca seedlings (200 growing degree days); GDD was calculated according to Ephrath et al (Reference Ephrath, Hershenhorn, Achdari, Bringer and Eizenberg2012).
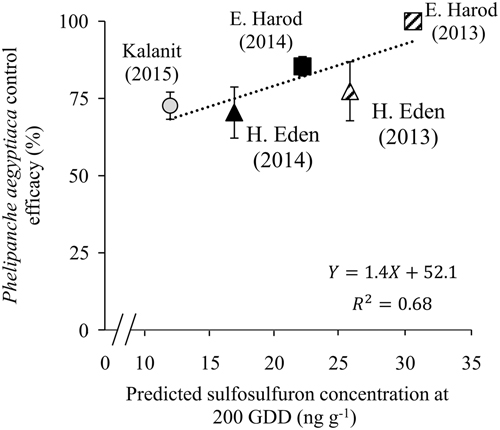
According to the simulations of sulfosulfuron concentration, substantial differences in concentrations were expected to be found in the different tomato fields at the critical timing to control P. aegyptiaca seedlings (Figure 5). The wide range of expected concentrations, from 30.6ng g−1 at E. Harod in 2013 (Figure 5B) to only 12.0ng g−1 at Kalanit in 2015 (Figure 5E), results partly from the timings of sulfosulfuron PPI (3 to 59 d before tomato planting) and partly from the differences in degradation rates in each soil under specific seasonal temperatures. The effect of the variable degradation rate is evident when examining cases of similar sulfosulfuron PPI timing: At H. Eden in 2013 (Figure 5A) and E. Harod in 2013 (Figure 5B), sulfosulfuron was applied 4 and 3 d before planting, respectively, and higher concentrations were expected to be found during the critical timing in the latter case. At E. Harod in 2014 (Figure 5D) and Kalanit in 2015 (Figure 5C), sulfosulfuron was applied 54 and 59 d before planting, respectively, while higher concentrations during the critical timing were expected to be found in the former case. The variable concentrations of sulfosulfuron at the critical period (12.0 to 30.6ng g−1) are expected to result in substantial differences in injury to P. aegyptiaca (26% to 60% injury) according to the dose–response curve (Figure 4). This could affect the final control efficacy in terms of inflorescence emergence in the field. Indeed, the recorded P. aegyptiaca control efficacy in the field studies—defined as the percent reduction of inflorescences in PICKIT compared with the untreated control—and predicted concentration at the critical period are positively correlated (Figure 6). Generally, high control efficacy was achieved in E. Harod, which had soil characterized by slow degradation and long-term residual activity of sulfosulfuron in the laboratory experiments (Figures 1 and 2). In contrast, at H. Eden and Kalanit, for which degradation was faster and residual activity lasted for a shorter period (Figures 1 and 2), generally lower control efficacies were recorded in the field (Figure 6). Because a single inflorescence of P. aegyptiaca can produce 10,000 to 500,000 seeds annually, with high seed longevity (Joel Reference Joel2013), reduced control efficacy may enrich the seedbank and infestation levels in the soil over the years (Rubiales et al. Reference Rubiales, FernÁndez-Aparicio, Wegmann and Joel2009), as has been shown for several autotrophic weeds (Buhler Reference Buhler1999).

Figure 6 The correlation between predicted sulfosulfuron concentration at the critical timing at 200 growing degree days (Figure 5) and Phelipanche aegyptiaca control efficacy when using the PICKIT management protocol. Phelipanche aegyptiaca control efficacy (defined as the percent reduction of inflorescences in PICKIT compared with the untreated control) was calculated from inflorescence counts from the field studies (Table 3). Error bars represent the SE (n=6).
The correlation between sulfosulfuron concentration and P. aegyptiaca control efficacy emphasizes the key role of sulfosulfuron treatment in the PICKIT protocol, that is, controlling P. aegyptiaca seedlings before attachment. Later herbigations with imazapic (after planting) to control P. aegyptiaca attachments are likely responsible for maintaining moderate control efficacy levels at low residual sulfosulfuron concentration; however, they do not entirely compensate for the presumed reduction in injury to the seedlings. Combining preplanting or PRE treatments of residual herbicides with herbicide treatments after planting has been shown to have a positive effect on weed control in other cropping systems: Nurse et al. (Reference Nurse, Swanton, Tardif and Sikkema2006) found that treatment with the residual herbicides flufenacet and metribuzin before POST application of glyphosate enhanced the control of broadleaf weeds in glyphosate-resistant maize. Whitaker et al. (Reference Whitaker, York, Jordan, Stanley and Sosnoskie2011) showed an increase in control of Palmer amaranth (Amaranthus palmeri S. Watson) in glyphosate-resistant cotton (Gossypium hirsutum L.) when integrating PRE residual herbicide into the management system, and suggested this strategy as means of reducing the establishment of glyphosate-resistant A. palmeri populations. Price et al. (Reference Price, Koger, Wilcut, Miller and Santen2008) found that integrating a long-term residual herbicide in layby treatment of either glyphosate, glufosinate, or MSMA increased weed control in cotton. The results in the current paper suggest that, although sulfosulfuron is considered a residual herbicide in neutral to alkaline soils, its persistence in some fields may not be sufficiently high to maintain the concentrations required for control of P. aegyptiaca at the critical timing. Continued research on the mechanisms that dictate the fate and residual activity of sulfosulfuron, and other residual herbicides in the soil, combined with information on the critical concentration and timing for control of each weed, would help optimize current application protocols and foster development of new strategies for management of parasitic and autotrophic weeds.
Two main mechanisms dictate sulfonylurea degradation in the soil: chemical hydrolysis and biodegradation by soil microorganisms (Beyer et al. Reference Beyer, Duffy, Hay and Schlueter1988). The soils used for this study have similar pH levels (7.6 to 7.8) at which chemical hydrolysis is slow and degradation is largely due to microbial breakdown (Beyer et al. Reference Beyer, Duffy, Hay and Schlueter1988). Recent studies reported on the isolation of different sulfosulfuron-degrading microorganisms (Arya et al. Reference Arya, Mishra and Sharma2016; Yadav and Choudhury Reference Yadav and Choudhury2014). It is thus most likely that differences in the biodegradation rate of sulfosulfuron are the cause for varied degradation rates and residual activity recorded in the current study (Figures 1 and 2). However, using the current data, we could not elucidate the factors responsible for such variable biodegradation rates. Other studies have reported several agricultural practices that enhance the biodegradation of sulfonylureas in soil, all of which are common in Israeli farms: Dvorkin et al. (Reference Dvorkin, Manor, Sibony, Chefetz and Rubin2012) showed an increase in the degradation rate of trifloxysulfuron following long-term irrigation with treated wastewater. Delgado-Moreno and Peña (Reference Delgado-Moreno and Peña2007) reported on a significant increase in the degradation rate of bensulfuron following application of organic amendments to the soil. Ravelli et al. (Reference Ravelli, Pantani, Calamai and Fusi1997) revealed a decrease in chlorsulfuron half-life in the soil following repeated applications. Moreover, Arbeli and Fuentes (Reference Arbeli and Fuentes2007) summarized in a review many reported cases of enhanced biodegradation due to repeated soil application of a pesticide or other pesticides from the same chemical group. It is possible that in the current study the agricultural practices on each farm influenced the observed variation in sulfosulfuron degradation rate (Figure 1), but further work is needed to confirm this premise.
In conclusion, there are substantial differences in sulfosulfuron degradation in the tested soils that are most likely due to differences in biodegradation rate. When sulfosulfuron is applied PPI for control of P. aegyptiaca in open-field tomatoes, rapid degradation reduces the control efficacy of the parasite. Furthermore, early preplant incorporation (before the critical period for P. aegyptiaca control), which is favored by farmers, enhances the effect of rapid degradation on control efficacy. Increasing sulfosulfuron PPI rate would likely reduce the negative co-effect of rapid degradation rate and early preplant incorporation on the control efficacy; however, this bears clear environmental and economic disadvantages. Understanding the factors affecting varied sulfosulfuron degradation rates in different soils will allow knowledge-based decision making concerning herbicide application rate and timing in each field and, consequently, optimization of control of P. aegyptiaca as well as nonparasitic weeds.
Acknowledgments
This study was funded by the Office of the Chief Scientist, Israel Ministry of Agriculture and Rural Development, Project No. 301-0852-16. The authors would like to thank Dr. Ahmed Nasser, from the Interinstitutional Analytical Instrumentation Unit in the Agricultural Research Organization at Volcani Center, Israel, for his valuable assistance in the development of the analytical procedures. The authors would also thank Dr. Yaakov Goldwasser and Dr. On Rabinovitz for providing data of P. aegyptiaca counting in the Kalanit field study. No conflicts of interest have been declared.