Introduction
Dermatitis is a nearly ubiquitous toxicity of radical radiation therapy, the severity of which depends on pre-existing patient factors, skin care approaches and a variety of technical treatment parameters.Reference Chan, Larsen and Chan1–Reference Lee, Chuang and Quivey4 Self-care instructions for radiation dermatitis include regular application of skin products to hydrate, reduce pain and inflammation, alleviate puritis or provide a protective barrier for compromised areas.Reference McQuestion5–Reference Rizza, D'Agostino, Girlando and Puglia7 Although there is little evidence to support recommending specific products, evidence does suggest that gentle washing and regular moisturising are beneficial.Reference Chan, Larsen and Chan1, Reference D'Haese, Van Roy, Bate, Bijdekerke and Vinh-Hung3, Reference Gosselin, Schneider, Plambeck and Rowe8–Reference Roy, Fortin and Larochelle13 Maintaining a regular hygiene routine and skin hydration may also help patients cope with their treatment, alleviate anxiety and promote patient comfort.Reference D'Haese, Van Roy, Bate, Bijdekerke and Vinh-Hung3, Reference Aistars14 Wound care for more advanced reactions, such as moist desquamation, includes application of products that provide protection from further trauma, reduce moisture loss and reduce pain in the affected area.Reference McQuestion5, Reference McQuestion6, Reference Bolderston, Lloyd, Wong, Holden and Robb-Blenderman15 Encouraging gentle hygiene and appropriate skin product use during radiation therapy is therefore an important goal of self-care instructions.Reference Chan, Larsen and Chan1, Reference Dendaas2
Counselling patients to avoid applying products to their skin lies in opposition to these self-care instructions. Patients are commonly instructed to avoid applying products before treatment delivery, and staff may remove cream they consider to be excessive, potentially causing further trauma. Other researchers have reported that such practices are common and seem to be based more on tradition than evidence.Reference McQuestion5, Reference Aistars14, Reference Bieck and Phillips16, Reference Burch, Parker, Vann and Arazie17 Surveys among UK practitioners reveal a range of clinical practices often based on historical practice and personal preference.Reference Harris, Probst and Beardmore10, Reference Lavery18 The rationale for discouraging application of skin products before radiation delivery may be largely due to concerns that skin products create a dose build-up effect that increases skin reactions unnecessarily.
Discussions with patients in the author's department revealed that some patients may reduce or eliminate the use of skin products as a result of cautions to avoid having product on the skin during treatment delivery. Some patients avoid applying skin products in non-treatment areas; other patients avoid all skin care routines until after their treatment is complete for the day. Similar behaviour has been observed with pharmaceuticals, where patients facing inconsistent instructions may favour the least intervention that they believe to be effective.Reference Vermeire, Hearnshaw, Van Royen and Denekens19 Such practices may therefore complicate patient education, reduce adherence and compete with other self-care management instructions. Adherence with a skin care regimen may be lower when instructions are not consistent, repeated and uniform.Reference Mannix, Bartholomay, Doherty, Lewis and Bilodeau11, Reference Vermeire, Hearnshaw, Van Royen and Denekens19
Several authors have measured the surface dose implications of materials used during radiation delivery. Thin materials on the skin such as a thermoplastic immobilisation mask used for head-and-neck (H&N) treatment produce sufficient additional skin dose that some centres elect to remove portions of the mask in the neck area. The dose increase from such a device is estimated to be 18% for H&N intensity-modulated radiation therapy (IMRT),Reference Lee, Chuang and Quivey4 and may be higher for particular mask and beam conditions.Reference Hadley, Kelly and Lam20 A study from the author's institution confirmed that removal of portions of the mask in the neck area provided visual evidence of a meaningful reduction in skin toxicity without compromising treatment accuracy.Reference Velec, Waldron and O'Sullivan21 Unfortunately, neither of these studies quantified the dose increase to the skin due to the presence of a thermoplastic mask.
Dosimetric concerns regarding the presence of skin products appear to be of two types. First, applications may be sufficiently thick to create a clinically meaningful dose increase to the skin, particularly when the product is viscous, non-absorbent and/or applied in reservoirs such as crevices or skin folds. The second concern is that even small amounts of skin product may result in a meaningful dose increase to the skin owing to the beam geometry (i.e., tangential beams increasing the effective depth of the skin epithelium) or the product composition (i.e., small amounts of high atomic weight components creating additional superficial scatter dose).
Some of these concerns have been addressed by other authors.Reference Bieck and Phillips16, Reference Burch, Parker, Vann and Arazie17 Skin products with a variety of chemical constituents such as aluminium and zirconium have been shown to produce little dose increase (5·4% or less) for ‘reasonable’ amounts of product, but higher dose increases (24% or less) for ‘highly excessive’ amounts.Reference Burch, Parker, Vann and Arazie17 This evidence alleviates concerns regarding product composition but leaves room for questions regarding how thick a product must be before it has a meaningful clinical impact.
This study aims to determine the thickness of skin product necessary to produce a clinically meaningful dose increase to the skin for common treatment geometries. Conclusions about the likelihood of such a scenario occurring are drawn from the data and inform recommendations for evidence-based self-care instructions.
Methods
Departmental ethics research board approval was not required as there were no human subjects involved. The study involved two methods to generate a relative dose versus product thickness curve: metal oxide semiconductor field effect transistor (MOSFET) dose measurements and Monte Carlo simulation. Simulation provided dose data for very thin layers of skin product, which would otherwise be difficult to measure, whereas MOSFET measurements provided relative dose data necessary to scale the curve and data using a non-solid immobilisation material, which would be difficult to simulate.
Determining a threshold of clinical relevance
Selecting such a threshold of clinical relevance presented two major challenges: first, the dose increase in a particular reference scenario will vary depending on the beam geometry and the thickness and type of skin product; second, the relevance of any specific dose threshold will vary with the prescription dose. As previously discussed, the additional dose to the patient surface due to the presence of a thermoplastic immobilisation device for treatments in the H&N area is generally considered acceptable; however, in some instances (such as the authors’ institution) steps are taken to remove portions of the mask to reduce the skin dose. In other words, this is a scenario where clinicians may or may not take steps to avoid the increased skin dose.
Determining common treatment scenarios
Photon beam energy of 6 MV was selected as it is the lowest and therefore least skin-sparing energy available in our department. Two field sizes (10 × 10 cm2 and 1 × 1 cm2) were used to approximate conditions for large open fields and small, IMRT-like fields. A range of gantry angles (0°, 20°, 40°, 60° and 80° incident to the surface) were selected to test the effect of beam obliquity.
Simple water-based moisturisers (WBM) and silicone-based barrier creams (SBBC) are the most commonly used non-prescription products recommended to patients in our centre. Mass density and atomic constituent data required for Monte Carlo simulation were obtained from Healthpoint Inc. for Proshield® Plus Skin Protectant (an SBBC product) and from WellSpring Pharmaceutical Canada Corp. for Glaxal Base Cream® (a WBM product).
MOSFET Measurements
Control conditions
Dosimetry experiments were carried out on a Varian TrueBeam linear accelerator using the TN-1002RD MOSFET detector (Thomson and Nielsen Electronic, Ottawa, Ontario, Canada). The MOSFET detector was inset into a layer of tissue-equivalent Superflab plastic bolus (density = 1·02 g/cm3) such that the flat surface of the detector was flush with the bolus surface and aligned with the beam isocenter. A block of Solid Water (SW-457, Gammex RMI, Middleton, WI, USA; area = 25 × 25 cm2) was placed underneath to provide adequate backscatter. Photographs of this physical set-up are provided in Figure 1. A 1 mm sheet of polymethyl methacrylate (PMMA) was then placed on top to position the MOSFET at the approximate depth of the epithelial layer of the skin, acknowledging that in vivo skin thickness and other properties vary somewhat by location, age, skin condition and other factors.Reference Takema, Yorimoto, Kawai and Imokawa22 A source-to-surface distance was set at 100 cm to the PMMA surface. Three dose measurements were obtained for each field size and gantry angle using 100 MU at 600 MU/min calibrated to 1 cGy/MU at d max (depth of the maximum dose) for the 10 × 10 cm2 field size.

Figure 1 Metal oxide semiconductor field effect transistor (MOSFET) set-up. a: Physical set-up for MOSFET measurements showing solid water, bolus and detector in place. b: S-frame mask cutout. c: 1.5 mm layer of SBBC product.
Reference conditions
A section of S-frame immobilisation device material (WFR Industries, Trenton, NJ, USA) was obtained from the neck region of a formed mask and flattened gently in warm water. The thickness and stretch of the mask sample was estimated by the investigators to be representative of that typically present in a real clinical scenario. Dose measurements were obtained with the mask section placed on the PMMA with and without a 1·5 mm gap. Positioning of the holes in the material was not considered as this is expected to result in <1% dose variation.Reference Hadley, Kelly and Lam20
Experimental conditions
Experimental conditions added 1·5 mm of either SBBC product or WBM product on top of the PMMA. A uniform layer of skin product was created by filling a 12 × 12 × 0·15 cm3 well of a plastic frame, eliminating bubbles and smoothing the surface as much as possible. It was noted that this amount of product appeared much thicker than the investigators have observed clinically on a patient's skin. Experimental measurements were divided by control measurements to produce relative doses (i.e., % dose increase relative to the dose with nothing on top of the PMMA).
Additional MOSFET measurements were taken with 5, 10 or 15 mm Superflab bolus placed on top of the PMMA. These measurements were used to test the reliability of the Monte Carlo simulation model. All measurements were done in triplicate, averaged and performed in a single sitting to minimise variations that might otherwise reduce the integrity of the relative dose measures.
Monte Carlo simulation
A Monte Carlo simulation using the Electron Gamma Shower (EGSnrc) code was constructed as shown in Figure 2 to mimic the conditions in the physical set-up above.Reference Kawrakow and Rogers23–Reference Rogers, Faddegon, Ding, Ma, We and Mackie25 Five hundred million particle histories were used in each simulation run with the electron and photon cut-off energy set to 0·7 and 0·01 MeV. The relative dose error (uncertainty as a fraction of dose in the voxel) is ∼1% based on the Monte Carlo output files.Reference Rogers, Faddegon, Ding, Ma, We and Mackie25 Skin products of variable thickness between 0 and 1·5 mm were created in the model. Experimental measurements were divided by control measurements to produce relative doses. Simulation data were then scaled by the corresponding MOSFET measurements to correct for approximations in the simulation model, consistent with other published methods.Reference Chow26–Reference Chow, Grigorov and Barnett28 The resulting dose–thickness curve was used to determine the thickness of product necessary to create a relative dose increase equivalent to that of the S-frame mask present for every treatment fraction. For reference, other research has used 0·3 mm moisturiser thickness on the skin as a realistic amount.Reference Rietschel29

Figure 2 Monte Carlo simulation. Abbreviation: PMMA, polymethyl methacrylate.
Results
MOSFET measurements had good reproducibility with <4% difference between repeated measures. Relative doses differed by <2% for the 5, 10 and 15 mm Superflab bolus, but as much as 5·3% with 1·5 mm SBBC product in place. The difference seen for 1·5 mm SBBC was expected because of differences between the physical set-up and simulation conditions, particularly with regard to the presence of the MOSFET detector itself in the physical set-up.Reference Chow and Leung30 Sample data are shown in Figure 3.

Figure 3 Sample uncorrected relative dose data. Note: Relative dose data for 10 × 10 cm2 field size, gantry 0°. Monte Carlo simulation data is not yet scaled. Abbreviation: MOSFET, metal oxide semiconductor field effect transistor.
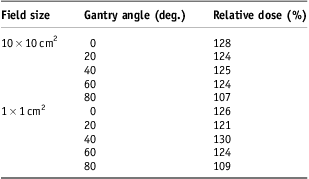
Dose under the S-frame mask material was obtained with and without a 1·5 mm gap. These measurements varied by <2%. The relative dose under the mask for all tested geometries is shown in Table 1. At a gantry angle of 80°, the relative % dose increase was strikingly lower because of a marked increase in dose with no product. This may be because the mask sample is neither solid nor perfectly flat. As such a geometry is unlikely to be the primary source of dose to the patient's skin in any typical H&N IMRT treatment, we elected to average those readings for gantry <80° to obtain a threshold of clinical relevance of 125% (i.e., a 25% or greater relative dose increase due to the presence of skin product would be considered clinically significant if present for the entire course of treatment).
Table 1 Relative dose under the S-frame mask
The dose thickness curves for SBBC are shown in Figures 4 and 5; similar curves for WBM (not shown) were also produced. The thickness of SBBC product necessary to equal a 125% relative dose was found to be 0·7 to 1·1 mm for large fields and >1·5 mm for small fields as shown in Table 2. The thickness of WBM product required was 1·2 mm for large (10 × 10 cm2 field size) direct (gantry 0° and 20°) fields and ≥1·5 mm for other geometries. Although the experimental design did not permit calculation of specific thicknesses >1·5 mm, it was observed during preparation of these layers of product that such thicknesses would be impractical to apply in a clinical scenario.

Figure 4 Dose versus silicone-based barrier creams product thickness for small fields.

Figure 5 Dose versus silicone-based barrier creams product thickness for large fields.
Table 2 Clinically relevant thickness of skin product for various geometries
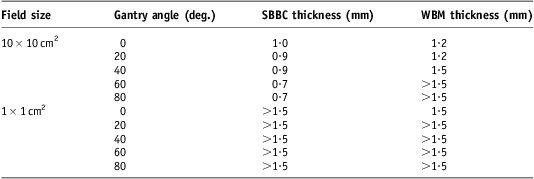
Note: Thickness is the amount that must be present during every treatment fraction to equal a 125% dose increase relative to the dose with no material on the skin.
Abbreviations: SBBC, silicone-based barrier cream; WBM, water-based moisturizer.
Discussion
A combination of MOSFET and Monte Carlo simulation approaches was required for this study. There was minimal variation between repeated measurements and good agreement between the two methods for control conditions, indicating that the data are reliable. As the uncorrected simulation data showed less dose increase due to skin product than did the more technically sensitive MOSFET measurements, this approach should tend to overestimate rather than underestimate the dose implication.
Determining a threshold of clinical significance was a challenging aspect of this study. Using S-frame mask material, a 125% relative dose was relevant. This seems reasonable given that others have reported 119% surface (not epithelial) dose due to the S-frame mask for H&N IMRT.Reference Lee, Chuang and Quivey4
A reasonable application of WBM is on the order of 0·3 mm, much less than the 0·7 mm required to create a significant dose increase to the skin under the least favourable geometry tested. This result is consistent with Burch et al.Reference Burch, Parker, Vann and Arazie17 who found that large dose increases required much more skin product than would normally be encountered in clinical practice. It may be possible to apply a thicker layer of sticky, non-absorbent SBBC product; however, such products would also typically be used for only a small portion of a patient's treatment (i.e., during the management of moist desquamation) and therefore have a proportional effect on skin dose. Other scenarios where a thicker amount of product might be applied include crevices and skin folds, such as abdominal and pelvic folds, under the breast or in the anal cleft. Although these scenarios were not tested in this study, the skin in these areas already receives higher than surface dose owing to self-bolusing; the addition of skin product within the fold should have no additional dosimetric effect. Furthermore, the use of skin products to minimise friction and maximise cleanliness and comfort may be particularly important in these areas.
Important limitations of this study include the approximation of skin epithelium depth and imperfections in the physical model used for MOSFET measurements. The MOSFET detectors required to determine the extent of this variability by repeating the physical measurements would have been costly. Measurements were performed using 6 MV photon energies, and therefore these results should be taken cautiously when considering lower energy photons. The dosimetric effects for electron treatments would be largely irrelevant as the skin is a target tissue in such cases. For very low energy photon treatments (i.e., kilovoltage), beam attenuation would be a greater concern than dose build-up. The beam properties tested in this study are therefore not exhaustive, but do reflect the most common treatment scenarios for external beam radiation therapy treating non-skin targets.
To put these results into context, one can calculate the amount of cream a patient would need to use under the least favourable conditions (product, geometry and details of application) in order to create clinically significant dose increase to the skin. Applying 0·7 mm of product once daily for 25 fractions to a 10 × 10 cm2 area of skin would require more than 15 tubes of 4 oz Proshield® Plus. This amount is many times higher if a patient applies cream more often, to a larger area, in thicker amounts, etc. The likelihood of a patient applying such large amounts of skin product in practice seems remote. Dosimetric concerns should therefore not play a role in self-care instructions for the management of skin changes during radiation therapy. Avoiding dosimetry-based restrictions on when and how much skin product patients can use during treatment may improve the clarity and effectiveness of self-care instructions.
Conclusions
It seems unrealistic to anticipate patients applying enough skin product in the treatment area on a daily basis to be of clinical concern, even for a variety of geometries ranging from a large direct beam to small obliquely incident beam segments. We therefore recommend that there are no dosimetric reasons to restrict the use of these types of skin products during radiation therapy for common treatment geometries using megavoltage energy X-rays.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
This work did not involve human and/or animal experimentation.





