INTRODUCTION
Neglected tropical diseases (NTDs) are increasingly being recognized as an important part of the global health agenda (Molyneux et al. Reference Molyneux, Hotez, Fenwick, Newman, Greenwood and Sachs2009). The increase in the profile of NTDs is a much needed step forward in the fight against some of the most devastating diseases affecting the poorest populations in the developing world. Increasing awareness has led to a better appreciation by governments and funding agencies as to how even limited resources directed at NTDs can have a major health impact. Likewise the integration of vertical control strategies and delivery systems into one package promises to bring improved benefits in terms of treatment for those in most need. NTDs incoporate both helminth, protist and bacterial pathogens and are grouped under this generic umbrella for the convenience of international health advocacy, there are several disease-specific issues that need attention.
Currently, and the list must surely grow, WHO lists 14 diseases as NTDs (see www.who.int/neglected_diseases/en/ ) and 5 are due to parasitic helminths: dracunculiasis, lymphatic filariasis, onchocerciasis, soil transmitted helminthiases (STHs) and schistosomiasis. Work associated with each of these NTDs is at a slightly different stage in terms of ongoing research and in the development and implementation of sustainable control strategies. For example, considerable success has been achieved with reduction of dracunculiasis throughout its range. Despite pockets of transmission continuing in some areas, this disease can, and hopefully soon will, be eradicated, thanks mainly to social and environmental interventions. Whereas, despite the availability of safe and effective drugs, much still needs to be done to reduce both prevalence and intensity of infection of the STHs and schistosomiasis; these infections continue to persist and long-term sustainable control remains a significant challenge in many areas. In order to help advance schistosomiasis research, a comprehensive research agenda for schistosomiasis has been developed, which details many of the future opportunities that could be pursued (Colley and Secor, Reference Colley and Secor2007).
As this volume marks the centenary of Parasitology in 1908, it is interesting to note that it was in that very year that schistosomiasis due to Schistosoma mansoni and its lateral-spined egg was recognized in Brazil due to the work of Dr Piraja da Siva (see Bergquist, Reference Bergquist2008). Around this time, a great debate was in progress between the parasitologists of the day arguing about the possibility of 2 species of Schistosoma. The first discovery of a human schistosome was in 1851 when Theodor Bilharz recorded the finding of Distomum haematobium, the parasite now known as Schistosoma haematobium (see Rollinson and Johnston, Reference Rollinson and Johnston1996). However, Bilharz recorded both lateral and terminal spined eggs which then blurred the now known species distinctions. This interesting controversy in the early development of the subject is well documented by Katz (Reference Katz2008). The current review is a personal reflection, reflecting this theme of different schistosome species and indeed different diseases. My aim is to draw attention to some of the important and disease-specific aspects of urinary schistosomiasis and to argue that it is now timely to place greater research effort on improving the understanding of this form of schistosomiasis and its impact on human health.
THE IMPACT OF SCHISTOSOMIASIS
Schistosomiasis remains a major public health problem in many parts of the developing world. There is currently no vaccine available and treatment depends almost entirely on the anthelmintic drug praziquantel. Currently, 24 species of schistosomes are recognized (this currently includes 3 species of Orientobilharzia), 6 of which cause disease in man, the 3 main species being Schistosoma mansoni, S. japonicum and S. haematobium. The scale of the problem is enormous, with estimates of 200 million people infected, with 85% of cases occurring on the African continent (Engels et al. Reference Engels, Chitsulo, Montresor and Savioli2002). The impact of infection is difficult to ascertain but recent meta-analysis suggests that the disability-adjusted life years (DALY) is much greater than previously assumed (King et al. Reference King, Dickman and Tisch2005). Disease pathology associated with schistosomiasis is primarily due to egg deposition and immune–mediated responses causing damage to the liver and urino-genital system. While schistosomiasis is recognized as a debilitating disease causing considerable morbidity, it is also estimated that annual global mortality could be as high as 300 000.
Schistosomiasis in sub-Saharan Africa is often considered, mainly for convenience, as a single disease, when in fact there are 2 major forms of schistosomiasis, intestinal and urinary, reflecting the site of egg deposition by the adult worms. Urinary schistosomiasis caused by S. haematobium has wide distribution across Africa, Madagascar and adjacent regions. Infection is associated with haematuria (blood in urine) and damage and significant pathology to the urino-genital system, which may lead to bladder cancers and renal failure later in life. In comparison, S. mansoni in Africa, the Caribbean and South America and S. japonicum in China and the Philippines, are primarily associated with liver fibrosis, haematesis and hepatosplenomegaly. The intermediate host relationships differ for each species, and the distribution of the main snail genera Bulinus, Biomphalaria and Oncomelania reflect the distribution of the parasites and the diseases that they cause. So the term schistosomiasis includes diseases which are clinically and biologically quite distinct.
SCHISTOSOMA HAEMATOBIUM: THE NEGLECTED SCHISTOSOME?
Current estimates suggest that in sub-Saharan Africa 112 million people are infected with S. haematobium and 54 million are infected with S. mansoni (www.who.int/schistosomiasis/epidemiology/table/en). Quantifying the impact of the different species of schistosome from the literature is notoriously difficult, but this does form a basis for discussion and suggests that the pathology caused by S. haematobium could greatly outweigh that caused by S. mansoni. In attempting to quantify the clinical morbidity associated with schistosome infections in sub-Saharan Africa, van der Werf et al. (Reference van der Werf, De Vlas, Brooker, Looman, Nagelkerke, Habbema and Engels2003b) made the following estimates using published data: the annual mortality rate due to non-functioning kidney relating to S. haematobium is estimated to be around 150 000, while that for haematemesis due to S. mansoni is 130 000 (van der Werf et al. Reference van der Werf, De Vlas, Brooker, Looman, Nagelkerke, Habbema and Engels2003b). In total, 70 million individuals in sub-Saharan Africa were estimated to experience haematuria associated with S. haematobium infection and 32 million experience dysuria. Ultrasound studies, used to detect the serious consequences of S. haematobium, suggest prevalences of around 18 and 10 million people, respectively, with major bladder wall pathology and major hydronephrosis. On the other hand, infection with S. mansoni was estimated to cause diarrhoea in 0·78 million, blood in stools in 4·4 million and hepatomegaly in 8·5 million people, although, as the associations are not always clear, the authors pointeded out that these may be underestimates specifically for S. mansoni (van der Werf et al. Reference van der Werf, De Vlas, Brooker, Looman, Nagelkerke, Habbema and Engels2003b).
Despite the perceived differences in prevalence and disease outcome, to date, studies of S. haematobium pale in comparison with those that have been performed on S. mansoni and S. japonicum: a general analysis of the literature covering work related to the 3 schistosome species over the last 39 years shows an almost 10-fold difference between S. mansoni and S. haematobium in the number of papers published in those journals covered by SCOPUS (Fig. 1A). Undoubtedly, this reflects the widespread adoption of S. mansoni and Biomphalaria glabrata as the model system for studying schistosomiasis in the laboratory. The shear practical difficulties of maintaining S. haematobium in laboratory rodents has significantly impacted on associated research. Most isolates of this schistosome will not develop in laboratory mice, and the bulinid snail hosts tend to be more difficult to maintain than Biomphalaria. There are few examples of successful long-term passage in rodents, and even using the hamster model, isolated strains of S. haematobium tend to be lost after a few generations of laboratory passage. Closely related species such as S. bovis do develop in laboratory mice but they have not routinely been used as disease models for S. haematobium.
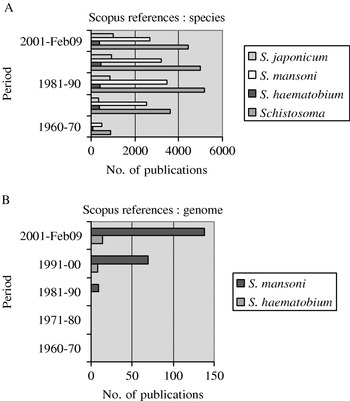
Fig. 1. SCOPUS search results for publications relating to (A) ‘Schistosoma’ overall and the three main species; (B) Schistosoma mansoni and Schistosoma haematobium and ‘genome’.
The comparison of published work highlights the general shortage of research and resulting knowledge associated with S. haematobium in fields as diverse as molecular biology, genomics, immunology, epidemiology and clinical studies. The lack of molecular studies on S. haematobium compared with those on S. mansoni is particularly striking, as shown in Fig. 1B. The recent emphasis being placed on the selective elucidation of S. japonicum and S. mansoni transcriptomes and genomes (see Liu et al. Reference Liu, Chen, Cui, Wang and Han2008; Zerlotini et al. Reference Zerlotini, Heiges, Wang, Moraes, Dominitini, Ruiz, Kissinger and Oliveira2009; Verjovski-Almeida et al. Reference Verjovski-Almeida, DeMarco, Martins, Guimarães, Ojopi, Paquola, Piazza, Nishiyama, Kitajima, Adamson, Ashton, Bonaldo, Coulson, Dillon, Farias, Gregorio, Ho, Leite, Malaquias, Marques, Miyasato, Nascimento, Ohlweiler, Reis, Ribeiro, Sá, Stukart, Soares, Gargioni, Kawano, Rodrigues, Madeira, Wilson, Menck, Setubal, Leite and Dias-Neto2009) has led to new areas of schistosome biology being revealed, and the creation of new research areas benefiting from the rich datasets now available for identifying novel drug and immunoprophylactic candidates. Unfortunately, no similar large-scale sequencing projects have existed for S. haematobium. This is a striking omission and until similar databases are generated for this species, it is unlikely that more experimental or applied work will be forthcoming. Given the availability of a reference schistosome genome, the much higher throughput of second-generation DNA sequencing technology together with the cheaper cost per base sequence, it is hoped that this situation will soon be rectified. The biological differences between these important parasites should provide sufficient justification for this discrepancy to be corrected rapidly.
PHYLOGENY AND HYBRIDIZATION
In this short review, I raise the concern as to whether it is correct to extrapolate from one form of schistosomiasis to another, especially given their distinctly different pathology, ecology, epidemiology and response to control measures. The recent growth in studies relating to molecular phylogenies allows insight into the question – how closely related are the Schistosoma species?
The phylogeny of the Schistosomatidae has been investigated over the years using various approaches, initially based on morphology, intermediate snail host and geographical distribution (Rollinson and Southgate, Reference Rollinson, Southgate, Rollinson and Simpson1987). More sophisticated molecular tools have facilitated a greater understanding of the relationships between many of the 24 recognized species of Schistosoma (Lockyer et al. Reference Lockyer, Olson, Østergaard, Rollinson, Johnston, Attwood, Southgate, Horak, Snyder, Le, Agatsuma, McManus, Carmichael, Naem and Littlewood2003; Loker and Brant, Reference Loker and Brant2006; Webster et al. Reference Webster, Southgate, Timothy and Littlewood2006). All of the African species so far examined, including S. mansoni and S. haematobium, do share a common Mt DNA gene order that separates them from S. japonicum and related species. However, the closest living relatives to the S. haematobium group are not, as one might expect, the African species S. mansoni and S. rodhaini in the S. mansoni group but, in fact, a clade including the Asian species S. indicum, S. spindale and S. nasale (Webster et al. Reference Webster, Southgate, Timothy and Littlewood2006).
The S. haematobium species group currently contains 8 species, some of which are of some medical (S. haematobium, S. intercalatum, S. guineensis) and significant veterinary (S. bovis, S. mattheii and S. curassoni) importance; so close is their relationship, that it is clear that research aimed at S. haematobium may have added value in being pertinent to these other species. Additionally, many of the species within the S. haematobium group have been shown to hybridize both in nature and experimentally. Of particular note, is hybridization between S. mattheei and S. haematobium in southern Africa (Wright and Ross, Reference Wright and Ross1980) and S. guinieensis and S. haematobium in Cameroon (Webster et al. Reference Webster, Tchuem Tchuenté, Jourdane and Southgate2005). Phylogenetic results clearly treat S. intercalatum and S. guineensis, the two other schistosomes within the group that infect humans, as separate taxa, with each more closely related evolutionarily to S. haematobium than to each other (Webster et al. Reference Webster, Southgate, Timothy and Littlewood2006). A number of studies have shown the value of using microsatellites to study genetic variation within and between populations of S. mansoni but so far little progress has yet been made with the analysis of S. haematobium (see Rollinson et al. Reference Rollinson, Webster, Nyakaana and Stothard2009).
ASPECTS OF TRANSMISSION RELATING TO SCHISTOSOMA HAEMATOBIUM
Transmission of schistosomiasis occurs in freshwater habitats where both the intermediate snail hosts of the schistosome and people come together. In areas where low-level sanitation exists, urine and/or faeces containing schistosome eggs may often contaminate the water. Improved sanitation is therefore key to long-term control, but even when basic facilities such as pit latrines exist, transmission may continue when water contact remains high. It is probably the case in areas where limited sanitation exists that contamination of water bodies by urine is likely to be more common than faeces, a factor contributing to more frequent transmission and persistence of S. haematobium. Water contamination with urine clearly reflects human behaviour and this often seems remarkably resistant to change, despite efforts to improve sanitation and educate schoolchildren on the basics of the schistosome life cycle and basic hygiene. It is well recognized that the parasite is exquisitely adapted to enhance transmission from people to snails by the marked rhythm of egg excretion which tends to peak before mid-day. Another interesting factor is that S. haematobium infection, by reducing bladder elasticity, may increase the need to urinate. If this is further accentuated when an infected person is in contact with cool water, then the pathology caused by the schistosome may play a role in enhancing transmission (Rudge et al. Reference Rudge, Stothard, Basáñez, Mgeni, Khamis, Khamis and Rollinson2008).
S. haematobium is transmitted through snails primarily of the genus Bulinus, which contains around 36 species within 4 species groups. Bulinus spp. have an extensive distribution throughout much of Africa, Madagascar, parts of the Middle East and Mediterranean (Brown, Reference Brown1980). However, within any area, the intermediate host-parasite relationship is complex, in terms of specificity and compatibility, with both genetic and environmental factors playing a role in determining small-scale heterogeneities in schistosomiasis transmission, factors which need to be taken into account when focusing and improving schistosomiasis control at the local level (Rollinson et al. Reference Rollinson, Stothard and Southgate2001). The ability to identify transmission hotspots offers the potential for more effective, highly focal snail control and human chemotherapy in order to reduce transmission.
The distribution of potential intermediate snail hosts is key to understanding the distribution and transmission of schistosomes. The range and habitats of the intermediate snail hosts, Bulinus spp. and Biomphalaria spp., do not always overlap, with Bulinus being generally more successful in temporary habitats and more tolerant of higher temperatures. Where suitable conditions exist, the snails may be found together in the same or adjacent habitats and this may lead to mixed infections of S. haematobium (urinary) and S. mansoni (intestinal) in the human population, with consequences for disease pathology. Recent investigations of the local spatial and temporal transmission patterns for S. haematobium include those in Zanzibar (Stothard et al. Reference Stothard, Mgeni, Khamis, Seto, Ramsan and Rollinson2002a, Reference Stothard, Mgeni, Khamis, Seto, Ramsan, Hubbard, Kristensen and Rollinsonb; Rudge et al. Reference Rudge, Stothard, Basáñez, Mgeni, Khamis, Khamis and Rollinson2008), in Tanzania (Hamburger et al. Reference Hamburger, Hoffman, Kariuki, Muchiri, Ouma, Koech, Sturrock and King2004), in Niger (Ernould et al. Reference Ernould, Garba, Labbo, Kaman Kaman, Sidiki, Djibrilla and Chippaux2004; Labbo et al. Reference Labbo, Ernould, Djibrilla, Garba and Chippaux2008), in Kenya (Clennon et al. Reference Clennon, Mungai, Muchiri, King and Kitron2006, Reference Clennon, King, Muchiri and Kitron2007) in Nigeria (Oladejo and Ofoezie, Reference Oladejo and Ofoezie2006; Opara et al. Reference Opara, Udoidung and Ukpong2007), in the Senegal River Basin (Sène et al. Reference Sène, Southgate and Vercruysse2004), in Morocco (Yacoubi et al. Reference Yacoubi, Zekhnini, Moukrim and Rondelaud2007) and in Zimbabwe (Mukaratirwa et al. Reference Mukaratirwa, Siegismund, Kristensen and Chandiwana1996).
The presence of Bulinus snails does not necessarily mean that they are involved in transmission, for example in Zanzibar, transmission of S. haematobium is only associated with water bodies harbouring B. globosus and not those in which B. nasutus is found (Stothard et al. Reference Stothard, Mgeni, Khamis, Seto, Ramsan, Hubbard, Kristensen and Rollinson2002b). It is important therefore to be able to discriminate between the various bulinid species where these are sympatric. Rollinson et al. (Reference Rollinson, Stothard and Southgate2001) used 2 molecular assays, allele-specific amplification (ASA) and SNaPshot, in case studies in Senegal, where B. globosus, B. umbilicatus, B. truncatus and B. senegalensis may act as intermediate hosts for S. haematobium, and in Zanzibar to discriminate between B. globosus and B. nasutus. The molecular evolution, genetic markers and compatibility of Bulinus species with S. haematobium on Madagascar was reported by Stothard et al. (Reference Stothard, Brémond, Andriamaro, Sellin, Sellin and Rollinson2001). Barcoding using the CO1 gene is now contributing to a much better understanding of the diversity within the genus and providing an alternative means for identification (Kane et al. Reference Kane, Stothard, Emery and Rollinson2008). Another important need is the differentiation of schistosome species within the snail; it is common for transmission of human and animal schistosomiasis to overlap, and morphological identification of cercariae is not a simple task. PCR methods based on the amplification of short repetitive regions have been developed for detecting and monitoring intermediate snail hosts infected with S. haematobium, and may prove valuable for evaluating residual transmission after control interventions (Hamburger et al. Reference Hamburger, Hoffman, Kariuki, Muchiri, Ouma, Koech, Sturrock and King2004; Abbasi et al. Reference Abbasi, King, Sturrock, Kariuki, Muchiri and Hamburger2007).
TREATMENT AND CONTROL
The challenges and prospects for the implementation of human schistosomiasis control were reviewed recently by Fenwick et al. (Reference Fenwick, Keiser and Utzinger2006). Since 2000, there has been a greater interest in the health and well-being of Africans, instigated largely by the World Health Organization (WHO, 2002), with the 2001 World Health Assembly resolution 54.19, together with funding from the Bill and Melinda Gates Foundation, the Millennium Development Goals, and the report of the Commission for Africa. In particular, the Schistosomiasis Control Initiative (SCI) has been an African success story, by helping to implement schistosomiasis control programmes and by distributing praziquantel with the specific aim of reducing morbidity. SCI was piloted in 2000, to implement and evaluate 6 national control programmes for schistosomiasis in sub-Saharan Africa and has now been expanded to integrate treatment of STHs and other NTDs (www.schisto.org, Fenwick et al. Reference Fenwick, Keiser and Utzinger2006).
The shift in the global control strategy for schistosomiasis from transmission containment to morbidity control was facilitated by the availability of safe and effective anti-schistosomal drugs, notably praziquantel (PZQ) (Fenwick et al. Reference Fenwick, Keiser and Utzinger2006; Ribeiro-dos-Santos et al. Reference Ribeiro-dos-Santos, Verjovski-Almeida and Leite2006; Caffrey, Reference Caffrey2007; Danso-Appiah et al. Reference Danso-Appiah, Utzinger, Liu and Olliaro2008). PZQ, a pyrazinoisoquinoline derivative, remains the drug of choice to treat both urinary and intestinal schistosomiasis (Hagan et al. Reference Hagan, Appleton, Coles, Kusel and Tchuem-Tchuenté2004), especially since the recent sharp reductions in price. However, although reasonably effective following a single dose, PZQ does not protect from re-infection, especially in children (Kabatereine et al. Reference Kabatereine, Vennervald, Ouma, Kemijumbi, Butterworth, Dunne and Fulford1999) and it is minimally effective against larval stages of the parasite (Cioli and Pica-Mattoccia, Reference Cioli and Pica-Mattoccia2003). Furthermore, response to the drug can vary and, although the clinical relevance of this remains to be determined, worryingly low cure rates of S. mansoni have been recorded in some studies in Africa (Doenhoff and Pica-Mattoccia, Reference Doenhoff and Pica-Mattoccia2006). The mechanism of action of PZQ is poorly understood, although there is indirect evidence that calcium ion channels are the molecular target (Doenhoff et al. Reference Doenhoff, Cioli and Utzinger2008). More recently Aragon et al. (Reference Aragon, Imani, Blackburn, Cupit, Melman, Goronga, Webb, Loker and Cunningham2009) developed an assay based on the transcriptional response of S. mansoni to heat shock to confirm that while 6-week post-infection (p.i.) schistosomes are sensitive to PZQ, 4-week p.i. schistosomes are not. It would be very interesting to confirm whether a similar age of worm-related differences occurred in S. haematobium.
In S. mansoni, PZQ has been shown to bind actin, induce worm muscle contractions and tegumental disruption, followed by exposure of the parasite's surface membrane antigens to the host immunological defense mechanisms (Tallima and El Ridi, Reference Tallima and El Ridi2007). PZQ treatment boosts anti-schistosome immune responses, with a Th2 bias that may contribute to immune-mediated killing and to protection against re-infection. However, pregnancy suppresses the potentially beneficial boost in cytokine responses associated with praziquantel treatment (Tweyongyere et al. Reference Tweyongyere, Mawa, Ngom-wegi, Ndibazza, Duong, Vennervald, Dunne, Katunguka-Rwakishaya and Elliott2008). The impact of chemotherapy on morbidity due to schistosomiasis was reviewed by Richter (Reference Richter2003), and there is good evidence for reversal of organ pathology in urinary schistosomiasis, but more data are required (Ramarakoto et al. Reference Ramarakoto, Leutscher, van Dam and Christensen2008).
The relative success of PZQ has rather stalled the advancement of other NCEs (new chemical entities), except for recent evaluations of the artemisinins and their derivatives (Utzinger et al. Reference Utzinger, Shu-Hua, Tanner and Keiser2007). Artemisinin (qinghaosu) is a naturally occurring 1,2,4-trioxane from Artemisia annua, and has potent anti-malarial activity and anti-tumour activity, in addition to anti-schistosomal effects (Jung et al. Reference Jung, Lee, Kim and Park2004). Artemether is the methyl ether derivative of artemisinin, while artesunate is the sodium salt, and both have been evaluated in clinical trials (Shuhua et al. Reference Shuhua, Tanner, N'Goran, Utzinger, Chollet, Bergquist, Minggang and Jiang2002; Borrmann et al. Reference Borrmann, Szlezák, Faucher, Matsiegui, Neubauer, Binder, Lell and Kremsner2001; N'goran et al. Reference N'goran, Utzinger, Gnaka, Yapi, N'guessan, Kigbafori, Lengeler and Tanner2003). These drugs need to be administered every 2–3 weeks (rather than once for PZQ) and have been used in combination with PZQ. Results, however, have been mixed and highlight the danger of assuming that one can extrapolate reliably between schistosome species (reviewed by Danso-Appiah et al. Reference Danso-Appiah, Utzinger, Liu and Olliaro2008). The protective efficacy of artemether against S. haematobium in schoolchildren in Cote d'Ivoire was reported to be significantly lower (patent infection 49% vs 65% for placebo) than that reported against S. mansoni and S. japonicum (N'goran et al. Reference N'goran, Utzinger, Gnaka, Yapi, N'guessan, Kigbafori, Lengeler and Tanner2003). Similarly, in a double-blind, placebo-controlled study of artesunate and PZQ in the treatment of S. haematobium in Gabon, Borrmann et al. (Reference Borrmann, Szlezák, Faucher, Matsiegui, Neubauer, Binder, Lell and Kremsner2001) reported cure rates of only 27% for artesunate compared with 73% for PZQ, but 81% for the combination, and so were also unable to confirm the earlier reports of efficacy against S. mansoni and S. japonicum. De Clercq et al. (Reference De Clercq, Vercruysse, Kongs, Verlé, Dompnier and Faye2002) also reported that PZQ was consistently more effective than artesunate against S. haematobium-infected school children in Senegal. On the other hand, Inyang-Etoh et al. (Reference Inyang-Etoh, Ejezie, Useh and Inyang-Etoh2009), who evaluated the combined effects of praziquantel and artesunate in the treatment of urinary schistosomiasis in schoolchildren in Adim Nigeria, reported similar cure rates for the artesunate group (70·5%) and the praziquantel group (72·7%) while the artesunate plus praziquantel group had the highest cure rate (88·6%). An interesting point to note is that the drugs may be acting on worms of different ages.
Given the potentially additive effect of PZQ and artemisinin derivatives, Laurent et al. (Reference Laurent, Boissier, Coslédan, Gornitzka, Robert and Meunier2008) have synthesized drugs which combine moieties from both classes of drugs, termed trioxaquantels, and have evaluated same in the S. mansoni mouse model, while Keiser and Utzinger (Reference Keiser and Utzinger2007) have reported on combination therapy with artemisinins and synthetic trioxolanes. Historically, the different response of S. haematobium and S. mansoni to drugs such as metrifonate and oxamniquine again highlights the differences between the schistosome species (see Marshall, Reference Marshall, Rollinson and Simpson1987). Obviously, from the chemotherapy perspective, a broad-spectrum anthelmintic such as PZQ or a combination of drugs that impact on both S. haematobium and S. mansoni is preferable for control. Recently, treatment with oxadiazoles in mice was shown to have activity against the 3 major schistosome species (Sayed et al. Reference Sayed, Simeonov, Thomas, Inglese, Austin and Williams2008). A potentially important development is the recent announcement that Glaxo Smith Kline will provide access to a pool of some 800 patents for third parties to develop treatments for 16 NTDs http://www.gsk.com/research/patent-pool.htm.
Anti-schistosomal vaccines are not yet available and progress towards such a goal has been slow: the challenge of developing vaccines against large metazoan parasites such as schistosomes is significant (Hagan and Sharaf, Reference Hagan and Sharaf2003; Bergquist, Reference Bergquist2008). A recent overview of vaccines for schistosomiasis is provided by McManus and Loukas (Reference McManus and Loukas2008). Vaccination could provide a complementary approach to schistosomiasis control that can be integrated into, and could help sustain, chemotherapy-based control programs (Bergquist et al. Reference Bergquist, Leonardo and Mitchell2005). The rationale is that drug treatment will provide short-term reduction of worm burdens, while vaccination would provide long-term protective immune responses. Vaccination can either be targeted towards the prevention of infection or to the reduction of parasite fecundity. A reduction in worm numbers is the ‘gold standard’ for anti-schistosome vaccine development but, as schistosome eggs are responsible for both pathology and transmission, a vaccine targeted at parasite fecundity and egg viability (such as the GST vaccine mentioned below) also appears to be entirely relevant (Capron et al. Reference Capron, Capron and Riveau2002).
It is recognized that complex immune mechanisms lead to the slow acquisition of immune resistance, through adaptive responses, though innate factors also play a part (Capron et al. Reference Capron, Riveau, Capron and Trottein2005; Wilson and Coulson, Reference Wilson and Coulson2006). Protective immunity has been difficult to demonstrate in humans, particularly in children, and there is the added level of complexity from co-infections with other pathogens, and understanding how these might affect the adaptive response and the establishment of immunological memory. For instance, people living in NTD endemic areas generally are more skewed towards a Th2 profile (Troye-Blomberg and Berzins, Reference Troye-Blomberg and Berzins2008). A key issue lies in identifying appropriate protective antigens to elicit immune responses that will attack the parasite but that will not cross-react with egg antigens and thus increase the chance of developing severe chronic disease in individuals that have already been infected (Lebens et al. Reference Lebens, Sun, Czerkinsky and Holmgren2004).
Clinical trial development has been initiated with an S. haematobium vaccine, Bilhvax, and this has now progressed past Phase II, with further trials planned in Senegal (Pearce, Reference Pearce2003, Capron et al. Reference Capron, Riveau, Capron and Trottein2005). This vaccine is based on recombinant glutathione S-transferase, GST, Sh28GST, and encouraging animal model data can be found in Boulanger et al. (Reference Boulanger, Warter, Sellin, Lindner, Pierce, Chippaux and Capron1999). It appears that most effort currently on identifying potential vaccine antigens is focused on S. mansoni, primarily because of the ability to trawl the S. mansoni genomic data base resource, http://schistoDB.net (Zerlotini et al. Reference Zerlotini, Heiges, Wang, Moraes, Dominitini, Ruiz, Kissinger and Oliveira2009). The skew in favour of S. mansoni vaccine work is particularly evident when searching the literature: the Scopus database gives 771 ‘hits’ for S. mansoni+vaccine but only 90 for S. haematobium+vaccine.
DIAGNOSIS OF URINARY SCHISTOSOMIASIS
When planning schistosomiasis control programmes, it is necessary to understand the extent of the disease problem, which means being able accurately to identify the individuals infected and the intensity of their infection, as well as the source of the infection, particularly the potential intermediate snail hosts involved and their location. Van der Werf et al. (Reference van der Werf, Bosompem and de Vlas2003a) reported that, in Ghana, health workers often do not recognize the symptoms of schistosomiasis, and Stothard et al. (Reference Stothard, Mgeni, Khamis, Seto, Ramsan and Rollinson2002a) found that the general level of understanding of kichocho (Zanzibarian term for urinary schistosomiasis) was low and that individual self-diagnosis was poor. Thus, there remains a need for rapid, routine, cost-effective methods of diagnosis of schistosomiasis, especially in the field. Effective field-applicable methods are needed to track both the dynamics of infection and alleviation of morbidity, to demonstrate the impact of intervention, and to provide reliable data to revise anthelmintic requirements through time, to ensure optimal drug allocations (French et al. Reference French, Rollinson, Basáñez, Mgeni, Khamis and Stothard2007; Stothard, Reference Stothard2009). Bayesian analysis of questionnaire data concerning self-reported schistosomiasis and self-reported blood in urine has been shown to be of value for targeting control programmes in Tanzania (Clements et al. Reference Clements, Brooker, Nyandindi, Fenwick and Blair2008).
To date, the gold standard for diagnosing S. haematobium in humans has been urine analysis. This involves observing urine colour for gross haematuria or using a dipstick (Hemastix) to detect microhaematuria and/or proteinuira/microalbuminuria. Diagnosis is then confirmed by identification and quantitation of schistosome eggs in filtered urine. Whilst reasonably sensitive and specific, confirmation nonetheless relies on laboratory analysis following collection of urines in the field (Stothard et al. Reference Stothard, Mgeni, Khamis, Seto, Ramsan, Hubbard, Kristensen and Rollinson2002b). Furthermore, parasitological measurement may not be sufficiently sensitive as eggs will not always be detectable using the standard single 10 ml syringe filtration.
Rollinson et al. (Reference Rollinson, Klinger, Mgeni, Khamis and Stothard2005) evaluated 2 novel diagnostic assays, one involving the direct detection of haemoglobin in urine, denoted HemoCue Plasma/Low Hb, and the other for detecting microalbuminuria, the HemoCue Urine Albumin assay, and compared these assays with the dipstick, Hemastix. Although the HemoCue assays can be conducted in the field, they require the use of portable equipment such as a microcentrifuge and a photometer for quantifying the turbidity readout, as well as a power source. The HemoCue Plasma/Low Hb assay proved much less sensitive than the Hemastix, which can detect as little as 0·15–0·62 mgL−1, compared with 0·1 gL−1 for the photometer. The HemoCue Urine Albumin assay, however, proved both sensitive and specific and able to characterize infection status correctly 86% of the time, showing this to be useful as a rapid, robust, operational field diagnostic tool for determining levels of microalbuminuria and proxy of infection status with S. haematobium, especially since it does not require laborious urine filtration which is needed for parasitological investigation. Notwithstanding this, other complicating factors such as menses, other urogenital infections/dysfunctions or scarification practices, mean that not all raised albumin levels will be due solely to schistosome infections. As a follow up to this study, Stothard et al. (Reference Stothard, Sousa-Figueiredo, Simba Khamis, Garba and Rollinson2009b) used urine albumin-to-creatinine ratio (UACR) reagent strips (Microalbustix®) to detect urinary tract pathology (UTP) associated with urinary schistosomiasis in schoolchildren from Zanzibar. They concluded that abnormal and severely abnormal UACRs were strongly associated with egg-patent urinary schistosomiasis and UTP, although via different mechanisms, and that from a control perspective, Microalbustix® reagent strips were best applied in pre-screening protocols allowing selection, or rather confident exclusion, of schoolchildren with urinary schistosomiasis for more detailed investigations.
In an evaluation of Hemastix to detect microhaematuria in school children in Zanzibar, French et al. (Reference French, Rollinson, Basáñez, Mgeni, Khamis and Stothard2007) reported that diagnostic scores were generally stronger for boys than girls, likely due to the confounding effects of menstruation which masks the accuracy of detecting blood in urine caused by S. haematobium infection. However, the reasonable cost of Haemastix (ca. £0.20p per strip) combined with their rapidity and ease of use, makes them a useful tool for surveillance in S. haematobium control programmes, although there is anecdotal evidence to suggest that the quality of dipstick varies markedly between manufacturers, so some caution may be required. Recently, Stothard et al. Reference Stothard, Sousa-Figueiredo, Simba Khamis, Garba and Rollinson2009 evaluated 2 commercially available diagnostic tests for the detection of urinary schistosomiasis, the urine-circulating cathodic antigen (CCA) strip and the soluble egg antigen enzyme-linked immunosorbent assay (SEA-ELISA), in 150 schoolchildren from Zanzibar. While the SEA-ELISA holds promise as a complementary field-based method for monitoring infection dynamics in schoolchildren over and above standard parasitological methods, the CCA strip had very poor sensitivity in this setting and also in Ethiopia (Ayele et al. Reference Ayele, Erko, Legesse, Hailu and Medhin2008), while in Zimbabwe it was shown to have an acceptable role in diagnosis (Midzi et al. Reference Midzi, Butterworth, Mduluza, Munyati, Deelder and van Dam2009).
In populations with low worm burdens, and where sero-epidemiological data are sought, the ELISA has proved an effective immunodiagnostic tool, measuring total IgG and IgG4 against the 27–29 kDa antigen, cysteine proteinase, from S. haematobium (El Amir, Reference El Amir2008). The fact that the many species of schistosomes share cross-reacting as well as differential antigens was highlighted by Losada et al. (Reference Losada, Chacón, Colmenares, Bermúdez, Lorenzo, Pointier, Theron and Noya2005), and this will be relevant for diagnosis as well as for phylogenetic studies and vaccine purposes.
A review on the utility in diagnosis of schistosome antigens from cercariae, adult worms and eggs, can be found in Hamilton et al. (Reference Hamilton, Klinkert and Doenhoff1998), who concluded that antibody detection methods will likely find increasing use in situations of low infection intensity because of the relative insensitivity of both parasitological and antigen detection methods in low infection situations. Bosompem et al. (Reference Bosompem, Bentum, Otchere, Anyan, Brown, Osada, Takeo, Kojima and Ohta2004a), using a monoclonal antibody (MoAb)-based dipstick for diagnosing urinary schistosomiasis in Ghana, reported prevalence of 78% compared with the estimate from microscopy of 60·3%, and that there was no interference from S. mansoni which had a prevalence in this population of schoolchildren of 7·8%. In another study (Bosompem et al. Reference Bosompem, Owusu, Okanla and Kojima2004b) in infants in Ghana, prevalence of urinary schistosomiasis according to the MoAb dipstick was 30% compared with 11·2% by microscopy; 15/71 egg-negative individuals tested dipstick-positive, giving a dipstick specificity of 78·9%.
In developing rapid and robust methods for screening large numbers of urine samples, Balog et al. (Reference Balog, Hensbergen, Derks, Verweij, van Dam, Vennervald, Deelder and Mayboroda2009) have recently identified haemoglobin-derived peptides in urine from S. haematobium-infected individuals where the microhaematuria test was negative, using reverse-phase cation exchange fractionation followed by mass spectrometry. While suited to high through-put, this assay is not readily transferable to the field.
While ultrasonography, using portable ultrasound machines, has undoubtedly revolutionalized the assessment of schistosomiasis-related morbidity, its key value lies in large-scale community-based studies and post-therapeutic follow-up of populations in endemic areas (King, Reference King2002; Koukounari et al. Reference Koukounari, Fenwick, Whawell, Kabatereine, Kazibwe, Tukahebwa, Stothard and Webster2006); it is not well suited to individual diagnosis, and the poor specificity of some images is a major limitation in zones of low transmission (Boisier, Reference Boisier2000).
EFFECTS OF SCHISTOSOMA HAEMATOBIUM ON THE REPRODUCTIVE SYSTEM: FEMALES
Poggensee et al. (Reference Poggensee, Feldmeier and Krantz1999) highlighted the fact that morbidity caused by the presence of schistosome eggs in the lower and upper genital tract had been almost completely neglected during the previous two decades. Female genital schistosomiasis (FGS) caused by S. haematobium infections of the female urogenital tract is common where the parasite is endemic and may result in serious complications such as ectopic pregnancy and infertility, but reports are mostly limited to isolated case studies (see for example Garba et al. Reference Garba, Almoustapha, Garba and Nouhou2004; Bahrami et al. Reference Bahrami, Alatassi, Slone and O'Connor2006). This was acknowledged by WHO and, in 1997, the Gender Task Force of the WHO's Tropical Disease Research Programme (TDR) included FGS in a list of scientific areas that deserved high research priority. Notwithstanding this, the fact that the most recent overviews of the parasitological, clinical and epidemiological aspects of FGS are around 10 years old emphasizes the continuing neglected nature of FGS (Poggensee and Feldmeier, Reference Poggensee and Feldmeier2001) and the need to assess the current situation. However, there has been a recent informal recommendation by WHO to treat schistosome-infected pregnant and lactating women (Friedman et al. Reference Friedman, Mital, Kanzaria, Olds and Kurtis2007).
FGS is also associated with considerable suffering related to menstrual disorders, sexual intercourse, chronic abdominal pain and social/psychological problems because infertility is particularly distressing in African societies (Helling-Giese et al. Reference Helling-Giese, Kjetland, Gundersen, Poggensee, Richter, Krantz and Feldmeier1996). The high incidence of infertility recently reported in the Middle East (Serour, Reference Serour2008) was attributed in part to schistosomiasis. Retel-Lauretin (Reference Retel-Lauretin1978) reported that bilharzia caused multiple miscarriage and sterility in a study of 284 women in Upper Volta. El-Mahgoub (Reference El-Mahgoub1982) used laparoscopy to diagnose asymptomatic pelvic schistosomiasis in 13 infertile women, and found dense pelvic adhesions in all cases, a defective luteal phase in 23% and anovulation in 15%. A detailed clinical study to assess morbidity associated with S. haematobium was carried out in Madagascar by Leutscher et al. (Reference Leutscher, Ravaoalimalala, Raharisolo, Ramarakoto, Rasendramino, Raobelison, Vennervald and Feldmeier1998), and this was followed up by Ramarakoto et al. (Reference Ramarakoto, Leutscher, van Dam and Christensen2008) using ultrasonography. Although others have suggested that schistosomiasis alone does not appear to play a major role in infertility (Morice et al. Reference Morice, Chapron, Vacher Lavenu, Terrasse and Dubuisson1996), there appears to be general agreement that severe tubal bilharziosis may result in ectopic pregnancy (Hoffmann and Bauerfeind, Reference Hoffmann and Bauerfeind2003; Laxman et al. Reference Laxman, Adamson and Mahmood2008). Kjetland et al. (Reference Kjetland, Kurewa, Ndhlovu, Midzi, Gwanzura, Mason, Gomo and Gundersen2008a) provide evidence indicating that early treatment, before the age of 20, may be more efficient for gynaecologic morbidity control.
A diagnostic algorithm was proposed by Helling-Giese (Reference Helling-Giese1997). There are indications that cervical schistosomiasis lesions could become cofactors for viral infection such as HIV and HPV-cervical cancer (Poggensee and Feldmeier, Reference Poggensee and Feldmeier2001; Swai et al. Reference Swai, Poggensee, Mtweve and Krantz2006). Bichler et al. (Reference Bichler, Feil, Zumbrägel, Eipper and Dyballa2001), in their review of genito-urinary schistosomiasis, proposed the novel diagnostic tool of ECP (eosinophil cationic protein) as a correlate of inflammation of the GU tract.
In a pilot study of FGS in an Egyptian community setting, Talaat et al. (Reference Talaat, Watts, Mekheimar, Ali and Hamed2004) combined clinical assessment with an in-depth study of the social context of reproductive health. S. haematobium ova were found in 16·7% of women in the study (21/126). Half of the women who agreed to a full gynecological examination (43 of 86) had evidence of reproductive morbidity due to schistosomiasis, either schistosome eggs in the cervix or sandy patches, tissue changes in the reproductive tract. Other reproductive tract morbidities included infections (vaginitis 40%, chronic cervicitis 75%, pelvic inflammation 9%) and prolapse (54%). FGS was associated with dysparunia, abnormal vaginal discharge, vaginal or cervical polyps, contact bleeding, vulval itching and chronic cervicitis. Community members recognized S. haematobium as a health problem, but did not believe that it affected reproductive health, underlining the need for educational programmes.
Kjetland et al.'s study (2005) in 527 women aged 20–49 in rural Zimbabwe aimed to describe the prevalence of gynecological S. haematobium infection and to differentiate the disease from sexually transmitted infections (STIs). They found that up to 75% of women with urinary schistosomiasis have S. haematobium ova in the genitals. Genital homogenous yellow and/or grainy sandy patches, the commonest type of genital pathology, were identified in 243 (46%) women. Grainy sandy patches were significantly associated with S. haematobium ova only. Genital S. haematobium ova were also significantly associated with homogenous yellow sandy patches, mucosal bleeding, and abnormal blood vessels. The presence of ova was not a predictor for ulcers, papillomata, leukoplakia, polyps or cell atypia. Mucosal sandy patches seem to be pathognomonic for S. haematobium infection in the female genitals. Coexistence of ova and other lesions may not be causal. In a further study in Zimbabwean women, Kjetland et al. (Reference Kjetland, Kurewa, Ndhlovu, Midzi, Gwanzura, Mason, Gomo and Gundersen2008a) reported that genital schistosomiasis was associated with stress incontinence and pollakisuria, but not with menstrual irregularities, current or previous ulcers. Kjetland et al. (Reference Kjetland, Mduluza, Ndhlovu, Gomo, Gwanzura, Midzi, Mason and Gundersen2006b; Reference Kjetland, Ndhlovu, Kurewa, Midzi, Gomo, Mduluza, Friis and Gundersen2008b) considered that sandy patches may be an important risk factor for both the acquisition and transmission of HIV.
EFFECTS OF SCHISTOSOMA HAEMATOBIUM ON THE REPRODUCTIVE SYSTEM: MALES
Schistosomiasis has been recognized as a cause of significant male urogenital morbidity in Africa (Heyns and Bornman, Reference Heyns and Bornman2008). Leutscher et al. (Reference Leutscher, Reimert, Vennervald, Ravaoalimalala, Ramarakoto, Serieye, Raobelison and Esterre2000) detected S. haematobium eggs in 43% of semen samples with increased levels of eosinophil cationic protein, suggesting that the genital organs of men are frequently affected with schistosomiasis. In their efforts to elucidate the consequences of male genital schistosomiasis (MGS) for reproductive health, Leutscher et al. (Reference Leutscher, Ramarakoto, Hoffmann, Jensen, Ramaniraka, Randrianasolo, Raharisolo and Christensen2008) evaluated various potential markers for MGS, including eosinophil cationic protein (ECP) and soluble egg antigen (SEA) in urine and semen, and circulating anodic antigen (CAA) in serum. Egg counts, ECP, and SEA in urine and CAA in serum correlated positively, but urine egg counts, as an indirect marker of MGS, remained their preferred diagnostic method from a public health perspective. In a study of semen quality in men in Madagascar, Leutscher et al. (Reference Leutscher, Høst and Reimert2009) found that S. haematobium infection was associated with sperm apoptosis and reduced production of seminal fluid. Egg-induced inflammation in the seminal vesicles and the prostate could be an underlying mechanism for both observations.
URINARY SCHISTOSOMIASIS: BLADDER CANCER AND KIDNEY FAILURE
The association between schistosomiasis and cancer was reviewed by Palumbo (Reference Palumbo2007). The incidence of bladder cancer, which ranks ninth in worldwide cancers overall, varies considerably between countries, and a number of risk factors have been identified, especially smoking and exposure to environmental carcinogens. While accurate epidemiological data on the incidence of and mortality due to bladder cancer are unavailable for most African countries, bladder cancer is nevertheless the most commonly diagnosed malignancy in many tropical and subtropical countries where S. haematobium is endemic, with estimates of schistosome-associated bladder cancer incidence of 3–4 cases per 100 000 (Shiff et al. Reference Shiff, Veltri, Naples, Quartey, Otchere, Anyan, Marlow and Bosompem2006), being more common in men than women (Schwartz, Reference Schwartz1981; Murta-Nascimento et al. Reference Murta-Nascimento, Schmitz-Dräger, Zeegers, Steineck, Kogevinas, Real and Malats2007; Wu et al. Reference Wu, Ros, Gu and Kiemeney2008). In Mozambique, Ebert (Reference Ebert1987) reported incidences of 24 and 19 cases per 100 000 for men and women, respectively, and Kitinya et al. (Reference Kitinya, Laurèn, Eshleman, Paljärvi and Tanaka1986) reported that the geographical distribution of bladder cancer closely corresponded to the prevalence of S. haematobium infection, similarly in Sudan (Malik et al. Reference Malik, Veress, Daoud and El Hassan1975).
Moreover, the pathology of the disease differs in these areas – while in Western countries, bladder cancers are predominantly transitional cell carcinomas (TCC) (Shaw et al. Reference Shaw, Elder, Abbas and Knowles1999), squamous cell carcinomas (SCC) are the well-documented sequelae of chronic infection with S. haematobium (Thomas et al. Reference Thomas, Bassett, Sigola and Taylor1990), although a recent case study in Egypt highlighted that schistosomiasis may be associated with non-squamous cell forms of bladder cancer (Kim et al. Reference Kim, Robertson, Belanger and Mai2008). Bedwani et al. (Reference Bedwani, Renganathan, El Kwhsky, Braga, Abu Seif, Abul Azm, Zaki and La Vecchia1998) reported that a clinical history of urinary schistosomiasis was significantly, but modestly, associated with increased bladder cancer risk, explaining some 16% of bladder cancer cases in the Egyptian population they analysed from 1994 to 1996. An analysis of cancer registry data from Bulawayo, Zimbabwe, for the period 1963–77, attributed 28% of bladder cancer cases to schistosomiasis, and 71% were SCC (Vizcaino et al. Reference Vizcaino, Parkin, Boffetta and Skinner1994). The odds ratios for increased risk of bladder cancer associated with a history of schistosomiasis were 3·9 for men and 5·7 for women. With increased urbanization, industrialization, and cigarette smoking in many African countries, there is an increasing incidence of TCC relative to SCC of the bladder (Heyns and van der Merwe, Reference Heyns and van der Merwe2008), and this has been documented in Egypt, where, over the past 26 years, TCC has now become the most frequent type (Wishahi, Reference Wishahi1997; Felix et al. Reference Felix, Soliman, Khaled, Zaghloul, Banerjee, El-Baradie, El-Kalawy and Wilson2008). Interestingly, there has been a concomitant decrease in the prevalence of S. haematobium in Egypt in recent years, which may also be a factor in this change in tumour type (Abol-Enein, Reference Abol-Enein2008).
Although the aetiological role of S. haematobium in bladder cancer is widely accepted (Abol-Enein, Reference Abol-Enein2008), the mechanism[s] of carcinogenesis are still unclear, and the overall severity of schistosomal infection is unlikely to be the sole factor in the pathogenesis of carcinoma of the bladder (Zarzour et al. Reference Zarzour, Selim, Abd-Elsayed, Hameed and AbdelAziz2008). The presence of N-nitroso compounds and N-nitrosodimethylamine in the urine of S. haematobium-infected patients both before and after the development of cancer, and the observation that these compounds also occur in bladder cancer patients with no history of schistosomal infection, suggested that these compounds might have a role not only in the initiation of the carcinogenic process, but also in its progression (Mohsen et al. Reference Mohsen, Hassan, El-Sewedy, Aboul-Azm, Maganitti and Airoldi1999). There have been a number of attempts to identify specific gene associations (Armengol et al. Reference Armengol, Eissa, Lozano, Shoman, Sumoy, Caballín and Knuutila2007). Al-Qahtani and Aly (Reference Al-Qahtani and Aly2007) reported that aberrations of chromosome 9 were observed in 90% of squamous cell carcinoma, but only in 51% of transitional cell carcinoma, and aberrations of chromosome 17 were detected in only 25% of squamous cell carcinoma, compared with 82% in transitional cell carcinoma, but p53 gene amplification was similar in both types. Hammam et al. (Reference Hammam, Aziz, Roshdy and Abdel Hadi2008) have shown that cyclooxygenase-2 (COX-2) is overexpressed in schistosomal-associated bladder cancer, and that COX-2 may be of significance to the development and proliferation of bladder TCC. Although Bcl-2 and Bcl-XL are frequently overexpressed in bladder cancer, and overexpression of Bcl-XL is associated with tumor progression in schistosoma-related SCC bladder cancer, the prognostic value of Bcl-2 expression remains obscure, according to immunohistochemical studies by Hameed et al. (Reference Hameed, Abdel Raheem, Mosad, Hammouda, Kamel and Abdel Aziz2008). El-Meghawry et al. (Reference El-Meghawry El-Kenawy, El-Kott, Hamed and Kuroki2006) reported that MK-1 is a prognostic marker for recurrence of schistosomiasis-associated squamous cell carcinoma of the urinary bladder.
Sheweita et al. (Reference Sheweita, El-Shahat, Bazeed, Abu El-Maati and O'Connor2004) identified alterations in the activities of drug-metabolizing enzymes in human bladder tissues as a result of S. haematobium infection that may change the bladder's capacity to detoxify endogenous compounds and/or potentiate the deleterious effects of bladder carcinogens. Evidence has shown that soluble egg antigen (SEA) has been associated with increased urothelial cell proliferation and higher expression of cell cycle genes. This lead was followed up by El Awady et al. (Reference El Awady, Mohammad, Shousha, El-Mahdy and Khalil2003) who tried to identify genes relevant to the mitogenic effect of SEA, and isolated and characterized a putative tumour suppressor gene that is believed to be a member of the mevalonate pathway.
Hodder et al. (Reference Hodder, Mahmoud, Sorenson, Weinert, Stein, Ouma, Koech and King2000) examined urine cytology findings among residents of the S. haematobium-endemic Msambweni area of Coast Province, Kenya. Overall, S. haematobium infection was strongly associated with increased risk for cytological abnormality, and the data suggested an age-dependent progression of cellular abnormalities in the urinary epithelium which becomes independent of concurrent infection intensity as subjects grew older. Other biomarkers have been described by Shiff et al. (Reference Shiff, Veltri, Naples, Quartey, Otchere, Anyan, Marlow and Bosompem2006), including a BLCA-4 test (urine) and nuclear morphometry or quantitative nuclear grading (QNG) of epithelial cells (urine sediment), which quantifies DNA ploidy status and nuclear morphometric descriptors. Akinwale et al. (Reference Akinwale, Oliveira, Ajayi, Akande, Oyebadejo and Okereke2008) quantified squamous cell abnormalities in exfoliated cells from the urine of S. haematobium-infected adults in a rural fishing community in Nigeria, and found this to be a specific but insensitive screening tool for detecting bladder cancer. Sabe et al. (Reference Sabe, Mangoud, Elalfy, Elsayed, Shaaban, Hafez, El Sherbini and Morsy2008) described the pathological manifestations of schistosomiasis pre-cancerous lesions in the bladder.
There is a paucity of information on treatments for schistosomiasis-associated bladder cancer reflecting, no doubt, the paucity of diagnosis and available treatments in Africa for this condition. In Egypt, bladder cancer was largely treated by surgery until the mid-90s, with chemotherapeutic drugs being evaluated thereafter (Khaled et al. Reference Khaled, Gad El-Mawla, El-Said, Hamza, Gaafar, El-Attar, Abu Rabia and Magrath1996).
Although multiple aetiologies are associated with renal diseases in the tropics, Gerold et al. (Reference Gerold, Werner and Sperschneider1999) reported that schistosomiasis was the most common specific entity for chronic end-stage renal failure (CERF) in younger patients. Obstructive/reflux nephropathy, attributed to urinary schistosomiasis, was common in Egypt, Libya and Southern Algeria (Barsoum, Reference Barsoum2003). Indeed, as indicated earlier, using the very limited data available, a mortality rate due to non-functioning kidney of 150 000 per year has been attributed to S. haematobium (http://www.who.int/immunization/topics/schistosomiasis/en/index.html). An important question is whether genetic strains of the parasite exist which are associated with particular severe pathologies. Much of the work relating to bladder cancer seems to be in areas where the intermediate snail host is Bulinus truncatus.
SCHISTOSOMIASIS AND HIV
There have been a number of reports on the interactions between schistosomiasis and human immunodeficiency virus (HIV) with, in general, the effects of HIV being more profound on schistosomiasis than the effects of schistosomiasis on HIV-1 progression, in Western Kenya at least (Secor and Sundstrom, Reference Secor and Sundstrom2007). Co-infections may affect the pathology and progression of each infection, and these may be further confounded by other concomitant infections/parasites, such as the causal virus of genital herpes, HSV, and tuberculosis. Moreover, there may be differences between the different species of schistosome, particularly in view of their differing tissue tropisms: urogenital in the case of S. haematobium and intestinal for S. mansoni. There have even been reports of antigenic mimicry between schistosomes and HIV regulatory proteins (Capron and Dessaint, Reference Capron and Dessaint1992).
Erikstrup et al. (Reference Erikstrup, Kallestrup, Zinyama-Gutsire, Gomo, Van Dam, Deelder, Butterworth and Ullum2008), in rural Zimbabwe, showed that schistosomiasis treatment (for both S. mansoni and S. haematobium) may attenuate HIV replication by decreasing systemic inflammation, as measured by plasma levels of various cytokines. However, Brown et al. (Reference Brown, Kizza, Watera, Quigley, Rowland, Hughes, Whitworth and Elliott2004) concluded that their data from Uganda did not support the hypothesis that treating schistosomiasis (in his case S. mansoni) would be beneficial in slowing HIV progression in co-infected adults, corroborating the earlier report by Elliott et al. (Reference Elliott, Mawa, Joseph, Namujju, Kizza, Nakiyingi, Watera and Whitworth2003) that helminth infection (S. mansoni among others) does not exacerbate HIV infection. Similarly, Hosseinipour et al. (Reference Hosseinipour, Napravnik, Joaki, Gama, Mbeye, Banda, Martinson and Cohen2007) reported that helminth infections were more common in HIV-uninfected than in HIV-infected persons in Malawi and that successful treatment of parasitic infections had no effect on HIV RNA levels. Similarly, in Kenya, Lawn et al. (Reference Lawn, Karanja, Mwinzi, Andove, Colley, Folks and Secor2000) reported that treatment of schistosomiasis was not associated with a reduction in plasma HIV-1 load.
On the other hand, Kjetlan et al. (Reference Kjetland, Ndhlovu, Gomo, Mduluza, Midzi, Gwanzura, Mason and Gundersen2006b) reported that women with genital schistosomiasis (S. haematobium) in a rural Zimbabwean community had an almost 3-fold enhanced risk of having HIV, and Ndhlovu et al. (Reference Ndhlovu, Mduluza, Kjetland, Midzi, Nyanga, Gundersen, Friis and Gomo2007) found that women over 35 years old and infected with urinary schistosomiasis had a significantly higher HIV prevalence (37·5%) than those without schistosomiasis (16·8%), although this difference was less marked in younger women. This again highlights the difference between S. haematobium and S. mansoni, and the danger of extrapolating from one species to the other.
There are differing reports regarding the impact of HIV infection on schistosome infection. An early paper by N'Zoukoudi-N'Doundou et al. (Reference N'Zoukoudi-N'Doundou, Dirat, Akouala, Penchenier, Makuwa and Rey1995) reported that HIV infection limited schistosome development and decreased antibody production. Lloyd-Smith et al. (Reference Lloyd-Smith, Poss and Grenfell2008) have reviewed the data on how HIV-1 co-infection may amplify parasite transmission and thus influence the emergence of novel parasite strains. It is well known that HIV and schistosomes interact with host CD4 T cells (Mwinzi et al. Reference Mwinzi, Karanja, Colley, Orago and Secor2001; Secor, Reference Secor2006) and that helminth parasites bias the immune system towards the Th2-type, as well as drive immune anergy (Da'Dara et al. Reference Da'Dara, Lautsch, Dudek, Novitsky, Lee, Essex and Harn2006; van Riet et al. Reference van Riet, Hartgers and Yazdanbakhsh2007). This immune bias is likely to have an adverse impact on the potential efficacy of viral vaccines which, as a rule, depend upon the generation of Th1/CD8 cellular immune responses for clearing viral infections (Robinson and Boyer, Reference Robinson and Boyer2004). The fact that acute HIV-1 infection causes early and massive depletion of CD4 T cells in the gut, together with the proximity of helmith infections to gastrointestinal mucosal sites, may be the basis of the interaction in the case of S. mansoni. Impairment of the S. mansoni-specific immune response elicited by treatment with praziquantel in Ugandans with HIV-1 coinfection, reported by Joseph et al. (Reference Joseph, Jones, Laidlaw, Mohamed, Mawa, Namujju, Kizza and Elliott2004) may account for the previously reported increased susceptibility to infection and re-infection with S. mansoni in coinfected individuals. Karanja et al. (Reference Karanja, Hightower, Colley, Mwinzi, Galil, Andove and Secor2002) showed in a study of schistosomiasis in car washers along the shores of Lake Victoria that decreased CD4 T-cell counts in HIV-1-positive individuals corresponded to increased susceptibility to S. mansoni infections.
This is in contrast to the situation reported for S. haematobium in Zambia, where Mwanakasale et al. (Reference Mwanakasale, Vounatsou, Sukwa, Ziba, Ernest and Tanner2003) found that resistance to re-infection with S. haematobium was not altered in subjects co-infected with HIV (although asymptomatic for AIDS). However, in this case, individuals with co-infection excreted fewer eggs and complained less of haematuria than those without HIV infection, and the sensitivity and positive predictive value of reported haematuria as an indication of heavy infection were lower in the group co-infected with HIV, which may have implications for the use of haematuria for diagnosis of schistosomiasis in areas where HIV is prevalent. Significantly lower CD4 cell counts in HIV-1-positive and in S. mansoni-infected HIV-1-negative individuals, in comparison with S. haematobium-infected subjects in Zimbabwe, were reported by Kallestrup et al. (Reference Kallestrup, Zinyama, Gomo, Butterworth, Van Dam, Erikstrup and Ullum2005). They also reported that the intensities of schistosome infections (S. haematobium and S. mansoni) did not differ between HIV-1-negative and HIV-1-positive subjects, suggesting that adult HIV-1 related immunodeficiency did not impair the ability to excrete schistosome eggs in low intensity infection, and concluded that HIV infection may not have major implications for diagnosis and surveillance of schistosomiasis. However, Fontanet et al. (Reference Fontanet, Woldemichael, Sahlu, Van Dam, Messele, Rinke de Wit, Masho and Van Lieshout2000), working in Ethiopia, found that, in the case of S. mansoni, egg output was significantly lower in the HIV-positives than in the HIV-negatives, although CCA concentrations (circulating cathodic antigens, i.e. worm loads) were found to be similar for these two groups. Karanja et al. (Reference Karanja, Boyer, Strand, Colley, Nahlen, Ouma and Secor1998) tested the hypothesis that immunodeficiencies, such as infection HIV-1, might render praziquantel less effective in treating schistosomiasis, and found that persons with high levels of S. mansoni infection, who were or were not also infected with HIV-1, responded equally to praziquantel therapy. Individuals with low percentages (<20%) of CD4+ T cells did not differ from individuals with higher CD4 cell percentages, so demonstrating that persons with HIV-1 infection can be treated effectively for S. mansoni schistosomiasis with praziquantel. However, it remains to be established whether this also holds true for S. haematobium infections.
CONCLUSIONS
Current and past research on S. mansoni will remain the main foundation on which to build future studies on schistosomiasis in Africa. There are many reasons why this should be, including the availability of experimental material together with the growing genome database. This short review is intended to highlight urinary schistosomiasis as an important disease in its own right with the aim of promoting further research studies on S. haematobium that, judging by the research literature, is in danger of becoming a neglected parasite. There are clear biological differences between the schistosome species in terms of distribution, epidemiology and transmission and considerable differences in disease outcomes. S. haematobium is associated with severe pathological implications for the urino-genital tract, including bladder cancer, and it is becoming increasingly obvious that interactions with other pathogens, especially HIV, are an important part of the disease landscape. In order better to understand urinary schistosomiasis and its many interactions, more detailed research is required, both in the field and in the laboratory. Because of the exquisite specificities and compatibilities of schistosomes and snails and other known biological differences between the species, extrapolating between S. mansoni and S. haematobium may have limitations. The search for new drug targets in combination with selection of viable vaccine candidates is an important priority in developing novel strategies to combat both urinary and intestinal schistosomiasis. However, the lack of foreseeable new drugs or vaccines on the horizon does not bode well for the future control of these important neglected tropical diseases. Greater research effort is required for S. haematobium to bring studies in line with the other major schistosomes infecting humans and to provide those unexpected insights which may provide new tools for control.
Many of the views expressed result from collaborations and discussions with friends and colleagues working with schistosomiasis. I would especially like to thank my colleagues at the Natural History Museum and all those currently involved with EU CONTRAST-(FP6 STREP contract no: 032203, http://www.eu-contrast.eu). I would also like to acknowledge and congratulate editors past and present for maintaining the high quality of Parasitology and occasionally publishing my research papers, one of the first being in 1979 on the detection of S. haematobium.



