Introduction
The fatty acid content of the majority of edible seed oils is primarily composed of molecules containing 16 or 18 carbons. Certain tropical oils such as coconut and palm oil are characterized by their shorter chain saturated fatty acids, in particular, dodecanoic acid (C12:0). Reports of fatty acids profiles have tended to ignore minor components, but with improvements in resolution due to the use of capillary columns in gas chromatography (GC), and sensitivity in mass spectroscopy (MS), it has been possible to better characterize fatty acids in oils.
Hexacosanoic acid (C26:0) is considered as a very long-chain fatty acid (VLFA), which is one containing at least 22 carbons. There has been some concern about dietary intakes of VLFA with the increased use of rapeseed in processed products such as spreads, which contains significant amounts of docos-13-enoic acid (C22:1; Bremer and Norum, Reference Bremer and Norum1982). It is known that in humans, hereditary diseases involving the oxidation of VLFA result in the build-up in tissues of VLFA, which is theorized to cause symptoms of adrenoleukodystrophy such as progressive myelination and adrenal cortex insufficiency (Moser and Moser, Reference Moser, Moser, Scriver, Beaudet, Sly and Valle1989). Peanut oil has been known to contain significant amounts of these fatty acids and has been used to study effects of dietary VLFA on tissues in animal models (Boles and Rizzo, Reference Boles and Rizzo1992). However, the dietary intake of VLFA was not found to influence the serum levels after feeding a high VLFA diet containing peanut oil. Despite this, it may be useful to be aware of VLFA in peanut seed oil for therapeutic purposes.
This study surveyed a subset of the peanut germplasm collection known as the ‘mini core’ or the ‘core of the core’ collection for hexacosanoic acid along with docosanoic and tetracosanoic acids, as these are the main VFLA found in peanut seed. The collection currently consists of 7432 accessions gathered from sources worldwide (http://www.ars-grin.gov/npgs/). The collection was reduced to a representative core and then further reduced to a representative mini core of 112 accessions based on morphological characteristics (Holbrook et al., Reference Holbrook, Anderson and Pittman1993; Holbrook and Dong, Reference Holbrook and Dong2005). Using descriptors of both above and below ground traits, the authors used cluster analysis to separate the data from the original core collection into groups that were theorized to be genetically similar. From that, a random 10% sample was taken to be representative and entitled the core of the core or the mini core. Of this mini core, 108 samples were made available for this study, allowing for a range of variety. The samples in this subset of the collection were sorted according to the global area that the original collection of the accession was made to look for relationships between the VLFA and origin.
Materials and methods
Plant materials and biochemicals
Peanut seed (Arachis hypogeae) were obtained from the Crop Genetics and Breeding Research Unit of the USDA in Tifton, GA, USA. All seeds were grown in 2005. The collected seeds were stored at − 15°C in plastic food storage bags until analyzed. Authentic standards of fatty acid methyl esters (FAMEs) were purchased from Sigma Chemical Corporation (St Louis, MO, USA) either in mixtures or as the individual fatty acids as was boron trifluoride (14% in methanol) used for the formation of the derivatives. Methanol, sodium hydroxide and hexane were purchased from the Thermo Fisher Corporation (Fairlawn, NJ, USA).
Lipid content
The seed was analyzed for total oil content using low-resolution nuclear magnetic resonance at 40°C using a Bruker MQ One Seed Analyzer (Bruker Optics, Billerica, MA, USA) with peanut seed oil used as a reference. In addition, total moisture was determined by the loss on drying in a forced air oven at 130°C for 6 h.
Lipid analysis
The seeds were cleaned of shells and other debris. The oil was expressed using a Carver Hydraulic Press (Carver, Inc., Wabash, IN, USA). The fatty acids were converted to their methyl esters using American Oil Chemists Society (AOCS) method Ce 2-66 (Firestone, Reference Firestone2004). The final hexane extract was analyzed for the FAME content using a Perkin Elmer Autosampler XL GC system (Perkin Elmer, Shelton, CN, USA). The separation was done on a BPX-070 column (SGE, Inc., Austin, TX, USA) that contained 70% cyanopropyl polysilphenylene-siloxane as the stationary phase (30 m × 0.25 mm, 0.25 μm film thickness). The carrier gas was helium at a flow rate of 40 psi. The temperature programme was 60°C with a hold time of 2 min increased to 180°C at a rate of 4°C/min, and then increased to 235°C at a rate of 10°C/min for a total run time of 27.7 min. The injection was split at 150 mL/min and detection was by flame ionization detection (FID).
The identification of certain fatty acids was determined using MS in tandem with the GC. A Perkin Elmer Turbo Mass Gold Spectrophotometer fitted with an electron impact source was used at 70 eV. The column conditions were similar to those used with FID above with the flow rate of helium serving as the carrier gas set to 1.5 mL/min. The mass scan range was 50–500. The transfer line was heated to 180°C and the ion source was heated to 230°C. Confirmation of the peak identities was done using the NIST library (NIST Version 2.0).
Fatty acid profile analysis
The fatty acids were identified from the GC chromatograms by comparison of retention time with authentic standard materials. Confirmation of hexacosanoic acid and other unknowns was done using MS. The fatty acid profiles were calculated as the percentage of the total fatty acids present as per AOCS method Ce 1f-96 (Firestone, Reference Firestone2004). All samples were analyzed in triplicate.
Statistical analysis
The data were expressed as the mean of three measurements ± standard deviation. Significant differences between the means were computed by the Tukey's method using the general linear models method (SAS, Version 9.1, SAS, Inc., Cary, NC, USA). Differences for total VLFA and C26:0 across the areas of origin were determined.
Results
All of the samples in this study were found to contain measurable amounts of the VLFAs, docosanoic (C22:0), tetracosanoic (C24:0) and hexacosanoic (C26:0) acids. The chromatogram in Fig. 1 is representative of all the samples. The quantities of the fatty acids present decreased with increasing chain length. The highest total level was 7.96% found in a sample from Zimbabwe (PI471952), and the lowest was 4.57% in a sample from Israel (PI343384). Table 1 gives the means with standard deviations for the VLFA of the accessions sorted according to global area of origin. This was done to reduce the size of the table, but the full table of data is available in the Supplementary Table S1, available online only at http://journals.cambridge.org.

Fig. 1 Chromatogram of the FAMEs analyzed by GC. The arrows indicate the VLFA.
Table 1 Means and standard deviations of the very long-chain fatty acid (VLFA) grouped according to global area of origins
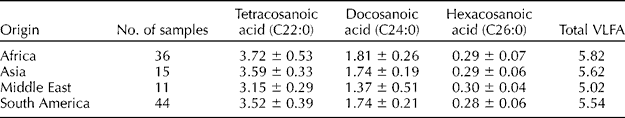
Values are reported as percent of the total fatty acids.
Discussion
Core collections are very important to condense large collections of germplasm to workable numbers and still maintain representative variance from the accessions (Holbrook and Dong, Reference Holbrook and Dong2005). The production areas listed for this study are the global locations where the seeds were originally gathered for inclusion into the germplasm collection. Although all peanut seeds are prodigy of South American origin, only 44 of the samples here were collected (Hammons, Reference Hammons, Pattee and Young1982). These were 18 samples from Argentina, 6 from Boliva, 5 from Brazil, 5 from Peru, 2 each from Cuba, Venezuela and Uruguay and 1 each from Columbia, Ecuador, Paraguay and Mexico, respectively. From Africa, the study included 36 of which, there were 2 from Burkina Faso, 10 from Zambia, 2 each from Malawi and Sudan, respectively, 6 from Zimbabwe, 5 from Nigeria, 4 from South Africa and 1 each from Madagascar, Senegal, Uganda, Cote D'Ivoire and Morocco. Ten samples were collected in Israel and one from Pakistan and were grouped together as representative of the Middle East. From Asia, eight samples were from India, four from China and one each from Japan, Taiwan and Thailand, respectively. Out of the 108 samples, only 2 had no listing in the Germplasm Information Network listing (http://www.ars-grin.gov/npgs/) as to location of the accession, and therefore was not discussed in this work. Fatty acid development is affected by maturity of the seed and since the peanut plant is indeterminate flowering, not all seeds in a single planting would be expected mature at the same time (Sanders, Reference Sanders1980). These samples were harvested at several time intervals to insure that optimum maturity was reached (Holbrook, 2007, private communication). Fatty acid development is also affected by climate conditions (Brown et al., Reference Brown, Cater, Mattil and Darroch1975; Andersen and Gorbet, Reference Andersen and Gorbet2002), but this variation was minimized as the seeds were produced for this study in a single location over one growing season. These controls helped to insure that differences among the fatty acid development could be attributed to traits of the seeds themselves. Comparisons were made between the total VLFA and the hexacosanoic acid. Figure 2 shows the relationship between C26:0 and the total of the VLFA content when the samples are grouped according to continent of seed origin. The means of each global area were plotted and then circles were drawn to enclose the standard deviations for the area of origin. It is found, that, while there is overlap due to the lack of significant differences among the sample means, the means for the different areas are distinct from each other. This indicates that there is some relationship between the origins on the VLFA content of the samples.

Fig. 2 Means of the levels of hexacosanoic acid (C26:0) present for each global area plotted against the total VLFA content as percentage of the total fatty acids present. The circles represent the standard deviations from the means.
Current literature describes the presence of VLFA in only a select number of plant materials. Tables of fatty acid profiles for nutritional labelling often do not include the minor components or simply report all the fatty acids according to saturation levels (Gebhart and Thomas, Reference Gebhart and Thomas2002). Hexacosanoic acid has been found in significant quantities in the seed of the Owala nut (African oil bean), which is consumed as food in West Africa (Jones et al., Reference Jones, Robinson and Southwell1987), and in olive skins (Milosevic et al., Reference Milosevic, Ashton and Cocksedge2002). It has also been reported to be a component of peanut oil (Kuemmel, Reference Kuemmel1964; Dorschel, Reference Dorschel2008), but since the levels are so low, it is usually not reported in studies describing fatty acid content of peanuts (Özcan and Seven, Reference Özcan and Seven2003; Venkatachalam and Sathe, Reference Venkatachalam and Sathe2006). In an early study, the position of VLFA on triglycerides was always found to be with unsaturated fatty acids (Sempore and Bezard, Reference Sempore and Bezard1986). They were most often seen with two molecules of linoleic acid (C18:2) in peanut triglycerides.
The study of VLFA has focused on their metabolism as these fatty acids make up a large group of those found in lipids of the nervous system such as cerebrosides, sulphatides and especially sphingomylein (Boles and Rizzo, Reference Boles and Rizzo1992). The focus of early dietary studies of VLFA was erucic acid (C22:1) due to the high content of it in rapeseed oil (40–60% of the fatty acids) and fish oils that had been hydrogenated to make spreads, resulting in the double bond being in the trans position (Sebedio et al., Reference Sebedio, Langman, Eaton and Ackman1981). Our study showed some very minor amount of C22:1, but always < 0.03% of the total fatty acids, if it was present at all. Later work focused specifically on the effects of the saturated VLFA, such as those reported here for peanuts, and found that although the fatty acids were absorbed from the diet by the study animal, in this case, mice, and digestibility was over 95%, brain lipid composition did not show changes (Boles and Rizzo, Reference Boles and Rizzo1992). Liver saturated VLFA did, however, increase. These changes were not considered to be harmful, as additional peroxisomal fatty acid oxidation did not occur in the liver compared with the diet higher in monounsaturated fatty acids. In conclusion, it was found that actual changes in liver VLFA were better related to the monounsaturated fatty acids in the diet undergoing β oxidation. From such studies, VLFA is assumed to be relatively innocuous, even in persons afflicted with syndromes involving VLFA.
It would be useful to be aware of the VLFA content for possible enhancement by conventional breeding if the effects of them in the diet proved beneficial. In cultures of human fibroblast cells, cholesterol synthesis has been found to be inhibited by them (Rizzo, Reference Rizzo1998). The actual clinical association of the intake of VLFA with cholesterol lowering is lacking. As discussed above, the effects of intake of VLFA are still being considered (Moser and Borel, Reference Moser and Borel1995), so it would serve well to monitor levels in peanuts due to their popularity in the human diet. For other uses, VLFA have been proposed as organogelators (Daniel and Rajasekharan, Reference Daniel and Rajasekharan2003). Saturated VLFA are thought to be capable of aligning in a linear fashion so that the triglycerides become entangled in lattice structures. These structures are then stabilized by their internal hydrogen bonding that enables them to hold liquids resulting in gelling. Since peanut oil was found to contain these compounds at 5% or more, it may be of interest for such applications.
Peanut seed oil appears to be one of the few plant oils that contain measurable amounts of VLFA. For food applications, it may be useful to be aware of the presence of these fatty acids, when the oil is used for functional or nutraceutical applications. Knowledge of peanut as a source of VLFA for industrial applications may also prove useful. There is potential for peanut breeding programmes to increase this trait and this should be considered if the positive aspects of VLFA are confirmed.
Acknowledgements
The authors gratefully thank Dr Corley C. Holbrook of the USDA ARS Crop Genetics and Breeding Research Unit for the gift of the seed, Mr Roger Thompson of the USDA ARS Food Science Unit for his assistance with the statistical analysis and Dr Jack Davis of the USDA ARS Market Quality Unit for his assistance with the figures. Mr James Schaefer and Mr Keith Hendrix of the USDA ARS Market Quality Unit are acknowledged for their excellent technical assistance.





