Case summary
A 35-year-old G2P1 female presented to a tertiary care centre at 30 weeks gestation for surgical management of a fetus with hypoplastic left heart syndrome.
Prenatal echocardiographic findings
Foetal echocardiogram showed hypoplastic left heart syndrome (mitral stenosis, aortic atresia) and left ventricular coronary fistulae, discrete narrowing of the distal portion of the ductus arteriosus (Supplementary Videos 1 and 2). Peak systolic velocity was 2.5 m/second and diastolic velocity was 0.5 m/second (Supplementary Fig 1). There was mild right ventricular hypertrophy and tricuspid regurgitation. In addition, there was marked systolic blunting of the middle cerebral artery Doppler, decreased umbilical artery systolic velocity, and abnormal pulsatility in the ductus venosus. Review of foetal echocardiograms at 27 weeks gestation and earlier showed normal ductus arteriosus velocity. The patient was not taking non-steroidal anti-inflammatory medications and was advised to eliminate polyphenol-rich foods from her diet. Repeat foetal echocardiograms were performed every 2 weeks until delivery, as well as weekly assessment with the maternal foetal specialist. Serial echocardiograms until 38 weeks showed progressive right ventricular hypertrophy and intermittent flow reversal in the ductus venosus.
Cerebral and umbilical Doppler findings
There was marked abnormality in all Doppler parameters. The umbilical artery pulsatility index was <2.5% throughout all foetal studies. The ductus venosus peak velocity index was >2.5 SD below the mean at 33 and 36 weeks gestation. All middle cerebral artery pulsatility indices were >2.5 SD below the mean.
Postnatal course
The neonate was delivered by spontaneous vaginal delivery at 38 weeks and weighed 3.2 kg. Neonatologist and cardiologist were present at delivery. The infant was born vigorous with normal saturations. Prostaglandin was initiated. An echocardiogram was carried out within the first hours while on prostaglandin, which showed narrowing of ductus arteriosus to 3 mm at the insertion to the aorta without significant pressure gradient. Complete post-natal imaging showed hypoplastic left heart syndrome with mitral stenosis and aortic atresia. There was severe left ventricular hypoplasia with coronary to left ventricle fistula. In addition, there was right ventricular hypertrophy (Supplementary Videos 3a–c). The ascending aorta measured 3.5 mm. The majority of the ductus arteriosus was widely patent, but with narrowing at the aortic insertion (Supplementary Fig 2). The infant was stable with pulmonary over-circulation in the pre-operative period and had consistently high oxygen saturations of 99%. Genetic assessment was not concerning for abnormalities. The infant underwent Norwood/Sano operation at day 5 of life. Intraoperatively, the neonate had significant ventricular fibrillation and ventricular tachycardia with no clear cause before commencement of bypass, as well as post-operative ventricular tachycardia and intermittent complete heart block. Intraoperatively, the right atrium was noted to be heavily muscularised. Within the first 24 hours after surgery, the infant was deployed onto ECMO for right ventricular dysfunction and had prolonged ICU stay with open chest owing to poor right ventricular function and elevated diastolic pressure. The infant ultimately succumbed to necrotising enterocolitis.
Foetal brain MRI studies
Two foetal MRI scans were performed at the time of foetal echo and Doppler studies (at 34.2 weeks gestational age, and repeated at 37.5 weeks gestational age, respectively) on a 1.5 T MRI scanner (GE Healthcare, Waukesha, Wisconsin, United States of America) using an eight-channel receiver coil (USAI, Aurora, Ohio, United States of America). The pulse sequences used to obtain anatomic images were T2-weighted multi-planar single-shot fast spin echo sequences performed as follows: TR/TE=1100/160 ms; voxel size=1.25×1.66×2 mm3–1.32×1.4×2 mm3 in all three axial, coronal, and sagittal planes with an acquisition time of 2–3 minutes per plane. Proton magnetic resonance spectroscopy, 1H-MRS, was performed with a single-voxel technique, and the spectra were acquired with a point-resolved spectroscopy sequence: echo time=144 ms, repetition time=1500 ms, 128 signal averages, scan time=3 minutes. Maternal sedation was not administered.
The reconstructed foetal brain volumes were segmented using a previously validated semi-automatic atlas-based image segmentation approach.Reference Evangelou, du Plessis, Vezina, Noeske and Limperopoulos 1 We measured total brain volume using ITK-SNAP software by multiplying the voxel count by the voxel unitary volume and converting to cm3.
Using 1H-MRS we quantified N-acetyl aspartate, a neuronal marker, creatine (Cre) which plays an important role in energy transfer, choline (Cho) a marker of cell membrane turnover, and lactate (Lac) a product of anaerobic metabolism.Reference Evangelou, du Plessis, Vezina, Noeske and Limperopoulos 1 Conventional foetal brain MRI studies were structurally normal. Total foetal brain volume measures were found to be progressively impaired compared with our normative foetal brain growth curvesReference Andescavage, du Plessis and McCarter 2 (Fig 1). 1H-MRS spectra were successfully obtained. An elevated lactate peak at 1.33 ppm was detected in both foetal MRI studies (Fig 2).

Figure 1 Serial foetal brain volumetric measures over time in a fetus with hypoplastic left heart syndrome and prenatal ductal constriction as compared with normal values. Blue circles represent normative foetal brain volume measuresReference Andescavage, du Plessis and McCarter 2 and red triangle represents a foetal CHD case that was studied at two time points (gestation age: 34.2 and 37.5 weeks).
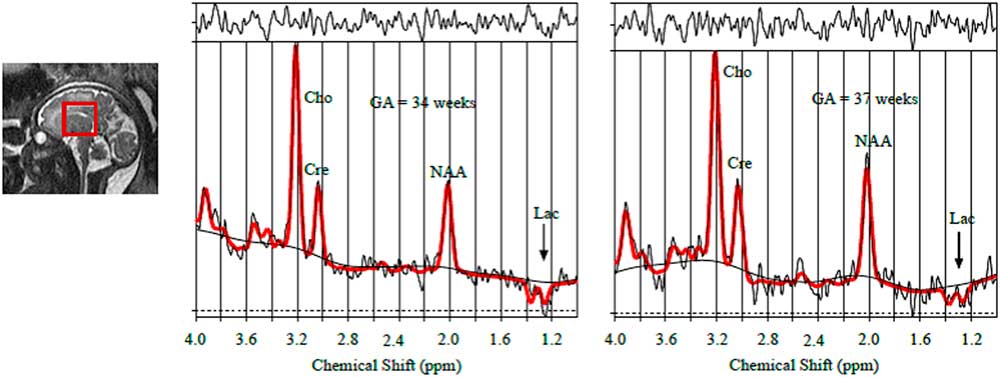
Figure 2 Spectral analysis result of foetal brain magnetic resonance spectroscopy indicating the presence of foetal lactate (at 1.3 ppm) in both scans (gestation age: 34.2 and 37.5 weeks).
Discussion
Although in utero constriction of the ductus arteriosus is a common phenomenon, it has not been reported earlier in a fetus with hypoplastic left heart syndrome or other forms of ductal-dependent systemic or pulmonary blood flow to our knowledge. In this patient, the ductal constriction was associated with marked right ventricular hypertrophy, decreased foetal brain growth on quantitative MRI, and the presence of lactate, that is, anaerobic metabolism, on foetal magnetic resonance spectroscopy.
Ductal constriction can occur as a congenital malformation early in pregnancy or be associated with exposures to maternal non-steroidal anti-inflammatory drugs, or cyclo-oxygenase inhibitors present in the diet.Reference Zielinsky and Busato 3 , Reference Zielinsky, Piccoli and Manica 4 It is diagnosed by increased systolic velocity, increased diastolic velocity, and decreased pulsatility index.Reference Huhta, Moise and Fisher 5 In this fetus, there was no change after dietary removal of polyphenols; however, eliminating them may have slowed or stopped the process. Postnatally, in the absence of congenital heart disease, ductal constriction can cause hydrops fetalis, right ventricular dysfunction, tricuspid regurgitation, and pulmonary hypertension. In the fetus with ductal-dependent systemic or pulmonary blood flow, pulmonary hypertension, and right ventricular dysfunction are potentially lethal. In this case, there was a benign in utero and postnatal course. However, the neonate had significant ventricular arrhythmias and right ventricular dysfunction in the perioperative period which may have been related to the altered right ventricular myocardium.
Conventional brain MRI did not detect abnormalities in the brain of this fetus, while advanced techniques highlighted the presence of lactate. Lactate is not expected in the normal brain.Reference Limperopoulos, Tworetzky and McElhinney 6 The presence of cerebral lactate has been described in growth-restricted fetuses as well as in the newborn, and is associated with adverse perinatal outcomes.Reference Andescavage, du Plessis and McCarter 2 , Reference Cetin, Barberis and Brusati 7 – Reference Shu, Ashwal, Holshouser, Nystrom and Hinshaw 10 Earlier publications have delineated the presence of lactate in fetuses with hypoplastic left heart syndrome, and this case adds further support to the literature.
This case provides supportive evidence for the theory that ductal constriction may be an aetiology of right ventricular hypertrophy and tricuspid regurgitation in infants with hypoplastic left heart syndrome, as has previously been described for infants with a two ventricle circulation.Reference Huhta, Vick, Carpenter and Gutgesell 11 In these cases, given the high risk for ventricular dysfunction, centres offering hybrid palliation may consider this option. In addition, discussion about transplantation should be included in pre- and post-natal counselling.
Despite the fact that the conventional brain MRI study was interpreted as normal, using quantitative three-dimensional volumetric and metabolic studies,Reference Limperopoulos, Tworetzky and McElhinney 6 we report decreased brain volume and abnormal metabolism. Quantitative MRI may provide deeper insight into the timing of insults that might disrupt normal brain development. This report offers promise that quantitative MRI will advance our understanding of the brain growth and biochemical disturbances which is likely to result from altered foetal haemodynamics.
Conclusions
Pre-natal ductal constriction in the setting of single ventricle physiology was associated with ventricular thickening and diastolic dysfunction. Altered haemodynamics may be associated with altered brain structure and function; in this case, it was only detected with advanced imaging techniques. Early consideration of transplantation and appropriate counselling about morbidity and mortality is important.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951118001701
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
All patient identifiers were removed from this case report.




